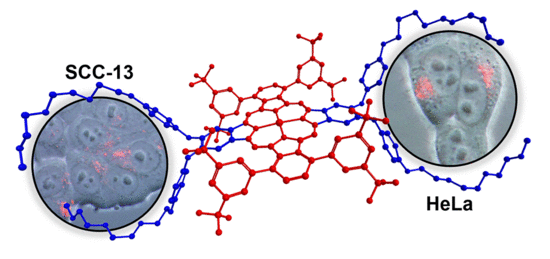
Assessing Amphiphilic ABAB Zn(II) Phthalocyanines with Enhanced Photosensitization Abilities in In Vitro Photodynamic Therapy Studies Against Cancer
Abstract
1. Introduction
2. Results and Discussion
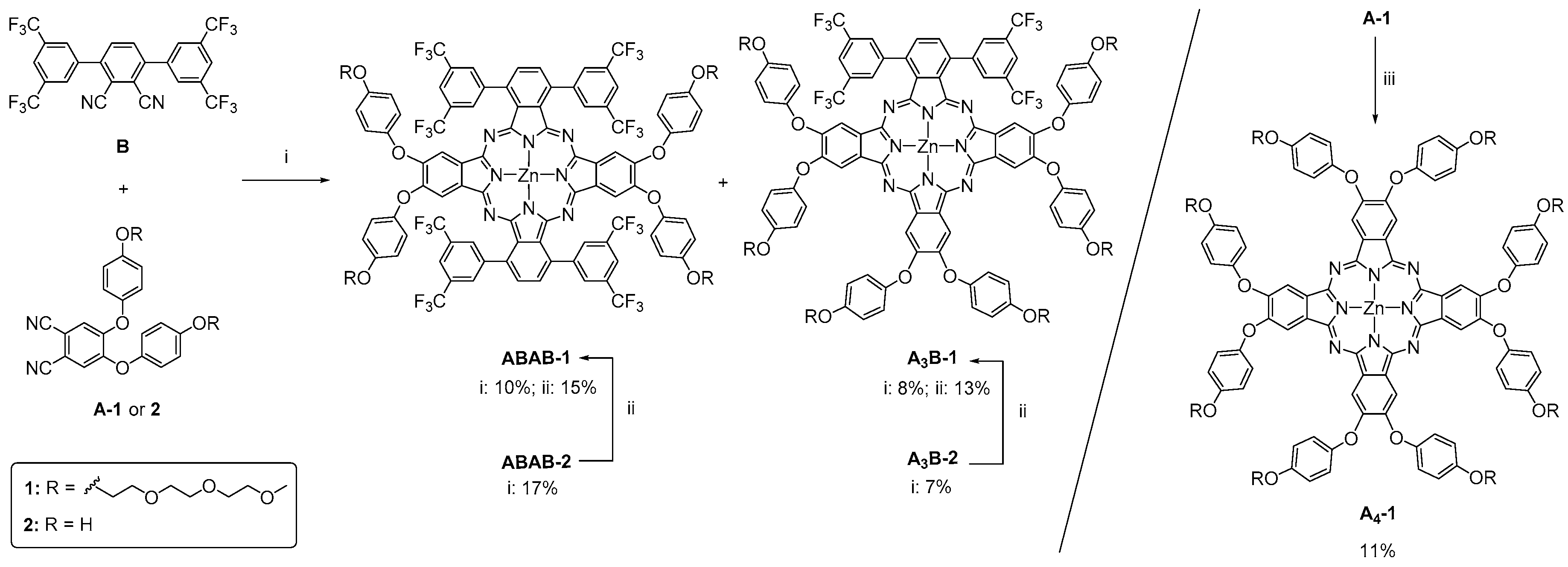
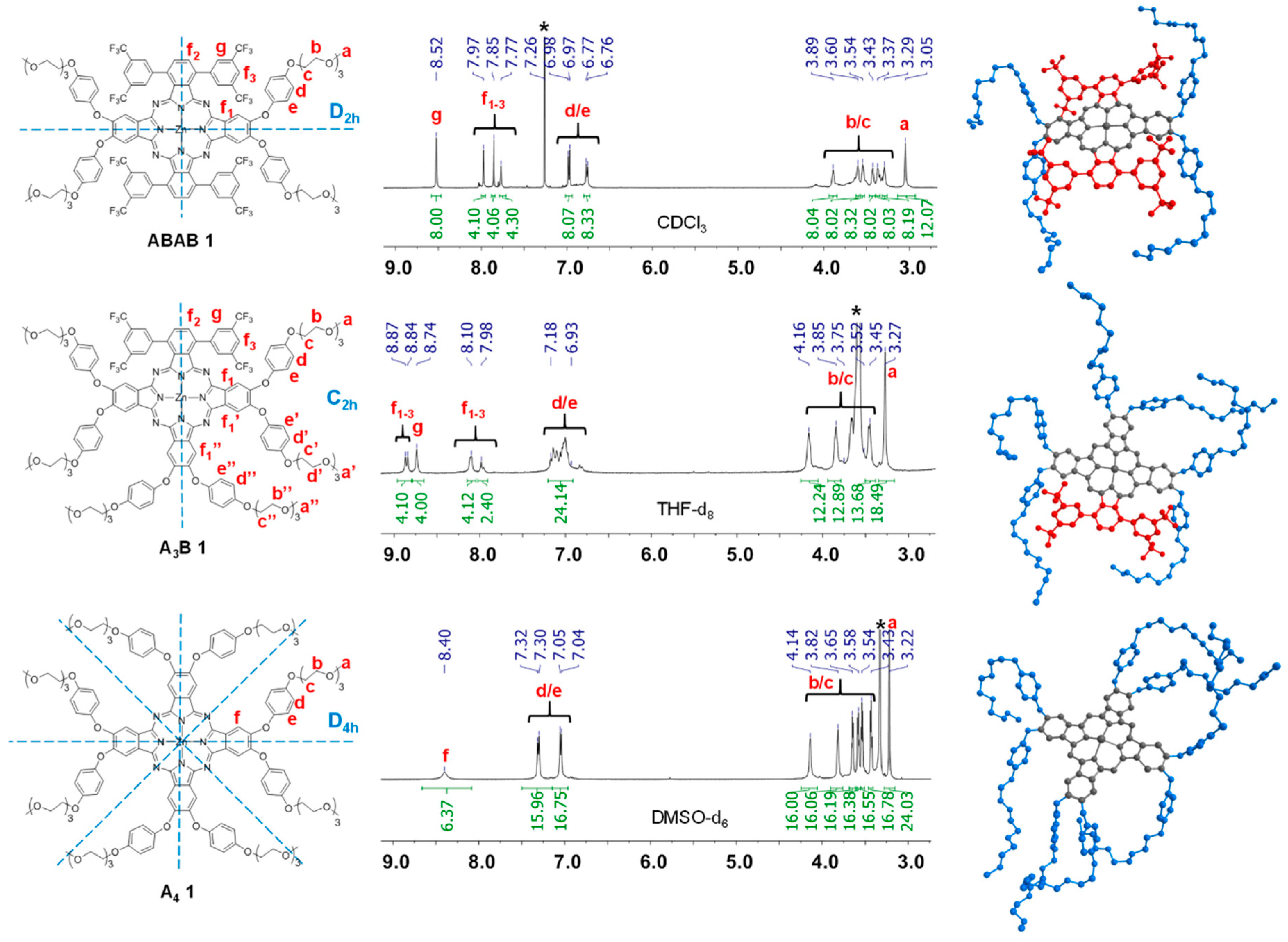
2.1. Synthesis
2.2. Photophysical Characterization
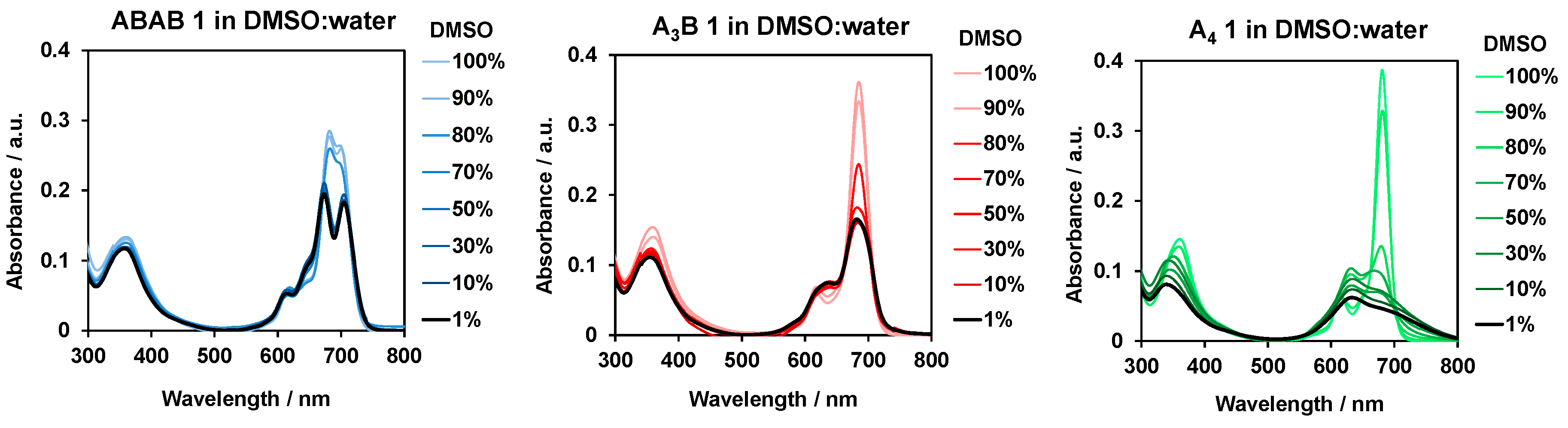
2.3. Aggregation Experiments
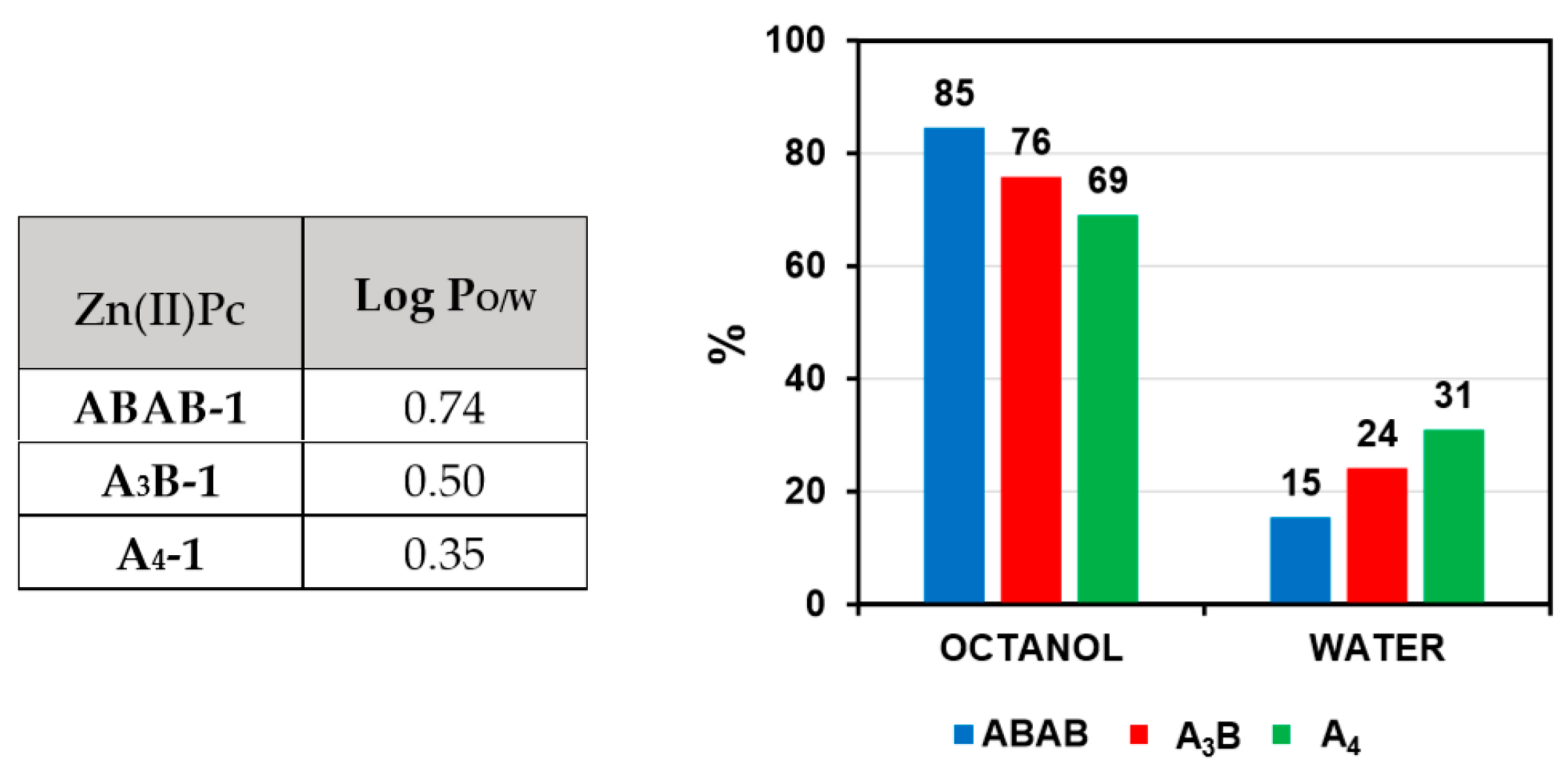
2.4. Determination of the Log Po/w
2.5. Biological Studies
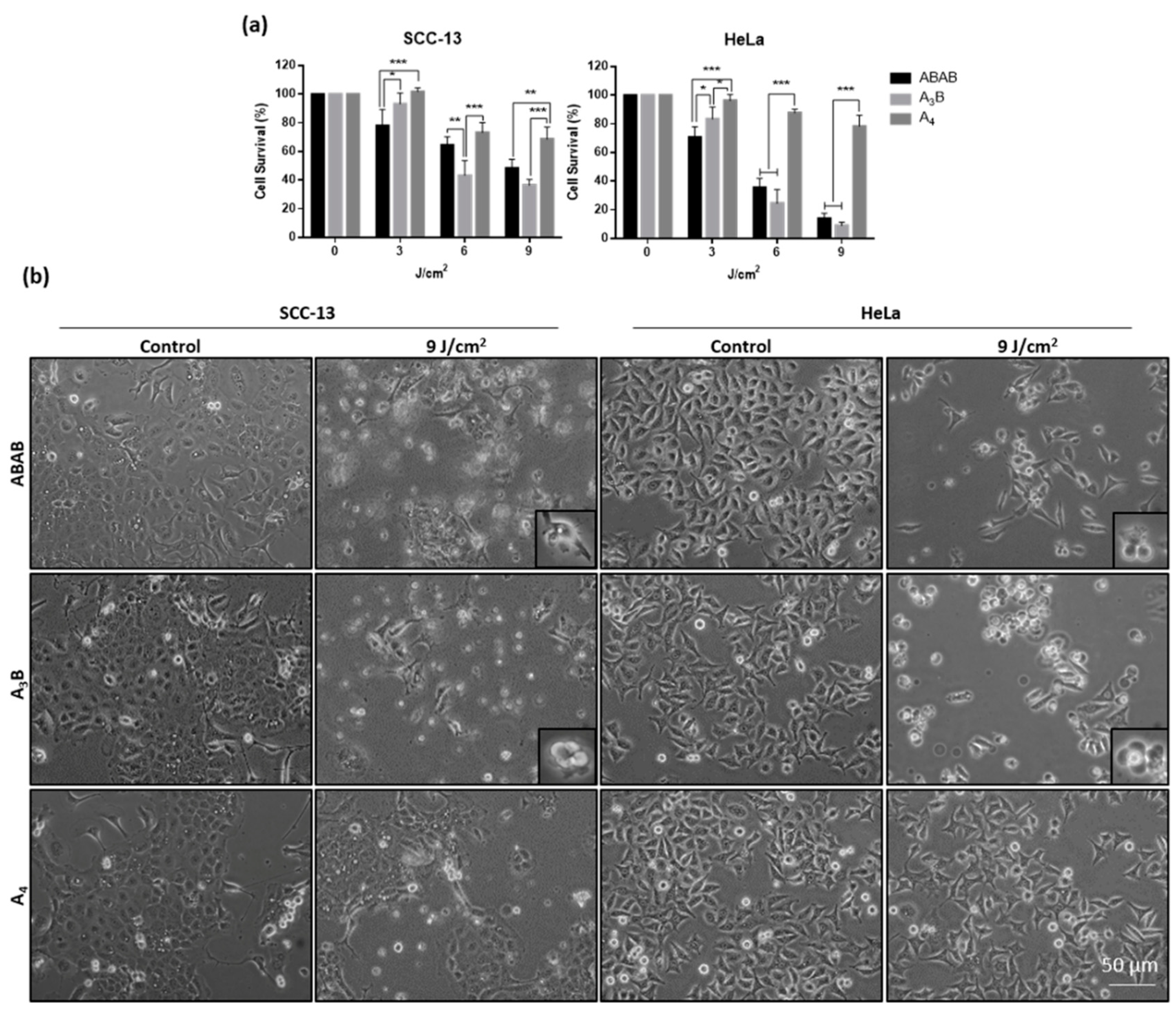
2.5.1. Cytotoxicity Studies
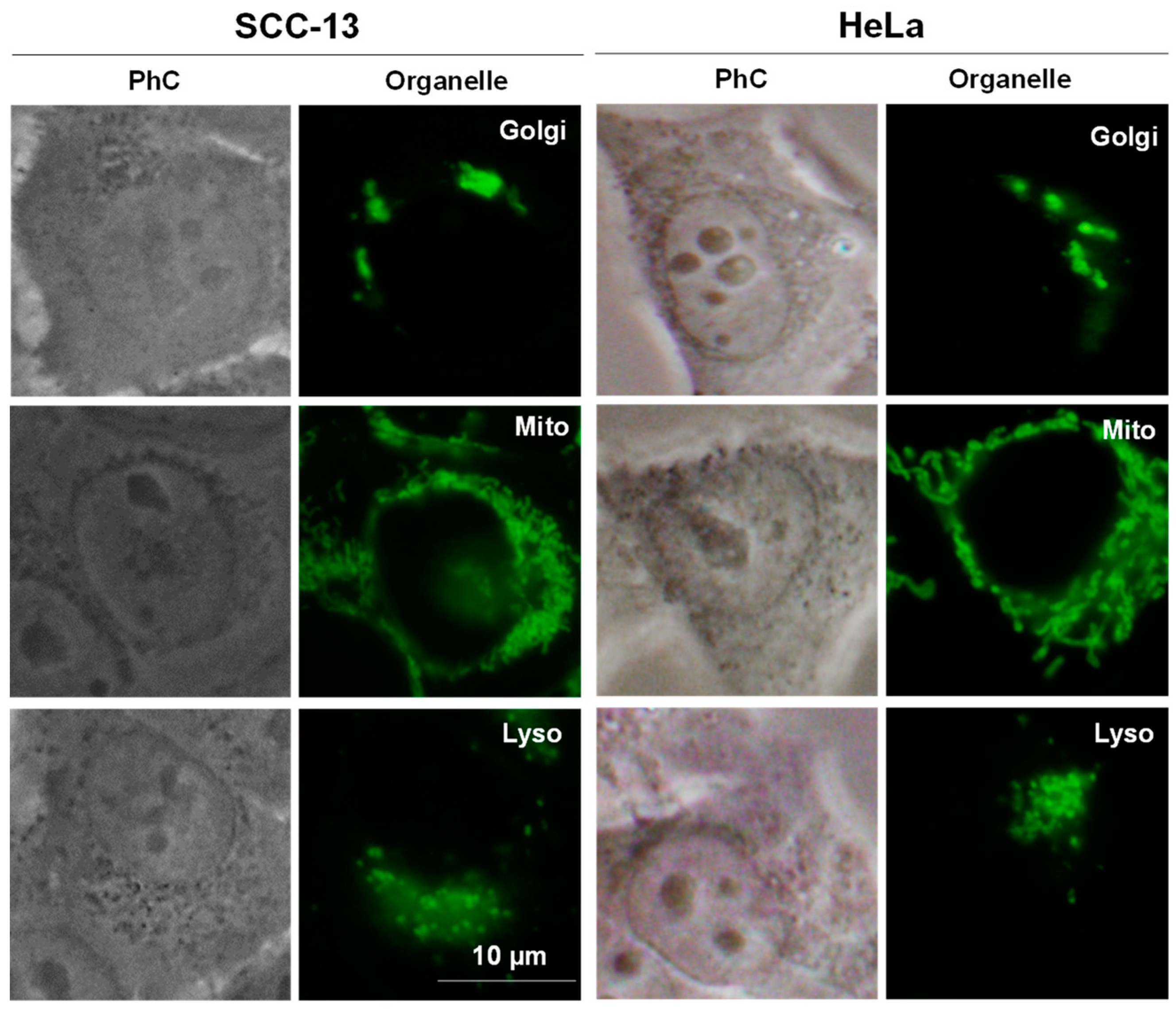
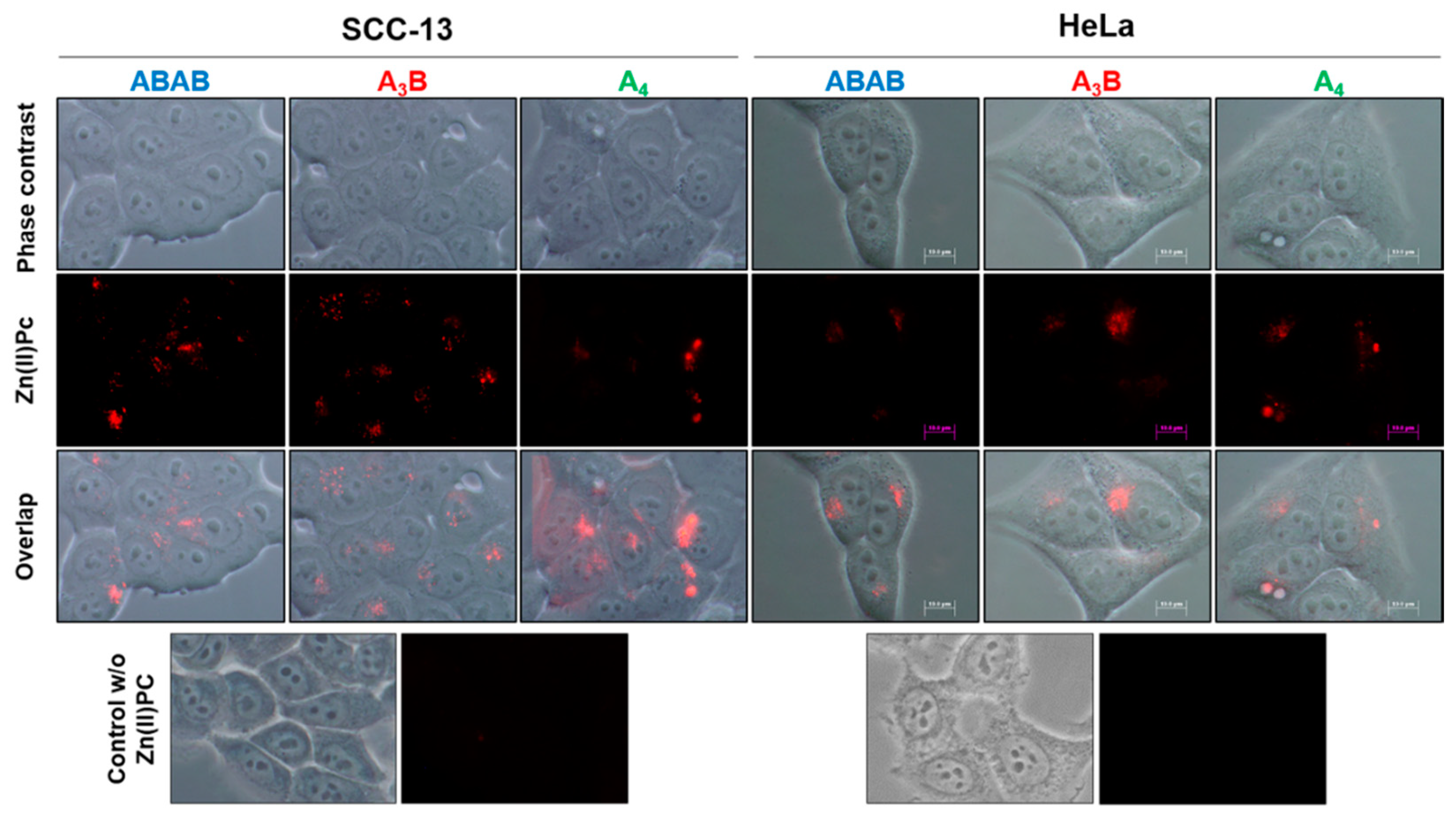
2.5.2. Localization Study
3. Materials and Methods
3.1. General Methods and Characterization Techniques
3.2. Synthesis
3.3. Spectroscopic Techniques
3.4. Determination of the Log Po/w
3.5. Biological Assays
3.5.1. Cell Culture
3.5.2. Photodynamic Treatment
3.5.3. Cell Viability
3.5.4. Measurement of Intracellular ROS
3.5.5. Optical Microscopy and Statistical Analysis
3.5.6. Subcellular Localization
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Leznoff, C.C.; Lever, A.B.P. Phthalocyanines: Properties and Aplications; VCH Publishers: New York, NY, USA, 1989; Volume 1–4. [Google Scholar]
- Allen, C.M.; Sharman, W.M.; Van Lier, J.E. Current status of phthalocyanines in the photodynamic therapy of cancer. J. Porphyr. Phthalocyanines 2001, 5, 161–169. [Google Scholar] [CrossRef]
- Chen, Z.; Lohr, A.; Saha-Möller, C.R.; Würthner, F. Self-assembled π-stacks of functional dyes in solution: Structural and thermodynamic features. Chem. Soc. Rev. 2009, 38, 564–584. [Google Scholar] [PubMed]
- Wasielewski, M.R. Self-Assembly Strategies for Integrating Light Harvesting and Charge Separation in Artificial Photosynthetic Systems. Acc. Chem. Res. 2009, 42, 1910–1921. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, F.; Durmuş, M.; Ahsen, V.; Nyokong, T. Synthetic pathways to water-soluble phthalocyanines and close analogs. Co-ord. Chem. Rev. 2010, 254, 2792–2847. [Google Scholar] [CrossRef]
- Revuelta-Maza, M.A.; Hally, C.; Nonell, S.; De La Torre, G.; Torres, T. Crosswise Phthalocyanines with Collinear Functionalization: New Paradigmatic Derivatives for Efficient Singlet Oxygen Photosensitization. ChemPlusChem 2019, 84, 673–679. [Google Scholar] [CrossRef]
- Zorlu, Y.; Ermeydan, M.A.; Dumoulin, F.; Ahsen, V.; Savoie, H.; Boyle, R.W. Glycerol and galactose substituted zinc phthalocyanines. Synthesis and photodynamic activity. Photochem. Photobiol. Sci. 2009, 8, 312. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, G.; Peng, Y.; Lin, H.; Zheng, X.; Xie, M. Zinc Phthalocyanine Tetrasulfonate (ZnPcS4): A New Photosensitizer for Photodynamic Therapy in Choroidal Neovascularization. J. Ocul. Pharmacol. Ther. 2007, 23, 377–386. [Google Scholar] [CrossRef]
- Lamch, Ł.; Kulbacka, J.; Dubińska-Magiera, M.; Saczko, J.; Wilk, K.A. Folate-directed zinc (II) phthalocyanine loaded polymeric micelles engineered to generate reactive oxygen species for efficacious photodynamic therapy of cancer. Photodiagn. Photodyn. Ther. 2019, 25, 480–491. [Google Scholar] [CrossRef]
- Wei, G.; Huang, L.; Jiang, Y.; Shen, Y.; Huang, Z.; Huang, Y.; Sun, X.; Zhao, C. Lenvatinib-zinc phthalocyanine conjugates as potential agents for enhancing synergistic therapy of multidrug-resistant cancer by glutathione depletion. Eur. J. Med. Chem. 2019, 169, 53–64. [Google Scholar] [CrossRef]
- Fazio, E.; Jaramillo-Garcia, J.; De La Torre, G.; Torres, T. Efficient Synthesis of ABAB Functionalized Phthalocyanines. Org. Lett. 2014, 16, 4706–4709. [Google Scholar] [CrossRef]
- Van Vlerken, L.E.; Vyas, T.K.; Amiji, M.M. Poly(ethylene glycol)-modified Nanocarriers for Tumor-targeted and Intracellular Delivery. Pharm. Res. 2007, 24, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-Y.; Jiang, X.-J.; Fong, W.-P.; Ng, D.K.P. Highly photocytotoxic 1,4-dipegylated zinc(ii) phthalocyanines. Effects of the chain length on the in vitro photodynamic activities. Org. Biomol. Chem. 2008, 6, 4560. [Google Scholar] [CrossRef] [PubMed]
- Kroon, J.M.; Koehorst, R.B.M.; Van Dijk, M.; Sanders, G.M.; Sudhölter, E.J.R. Self-assembling properties of non-ionic tetraphenylporphyrins and discotic phthalocyanines carrying oligo(ethylene oxide) alkyl or alkoxy units. J. Mater. Chem. 1997, 7, 615–624. [Google Scholar] [CrossRef]
- Tuncel, S.; Dumoulin, F.; Gailer, J.; Sooriyaarachchi, M.; Atilla, D.; Durmuş, M.; Bouchu, D.; Savoie, H.; Boyle, R.W.; Ahsen, V. A set of highly water-soluble tetraethyleneglycol-substituted Zn(II) phthalocyanines: Synthesis, photochemical and photophysical properties, interaction with plasma proteins and in vitro phototoxicity. Dalton Trans. 2011, 40, 4067–4079. [Google Scholar] [CrossRef]
- Uslan, C.; Köksoy, B.; Durmuş, M.; Öztürk, Y.; Çakar, Z.P.; Gürsel, Y.H.; Sesalan, B.S.; Işleyen, N.D. The synthesis and investigation of photochemical, photophysical and biological properties of new lutetium, indium, and zinc phthalocyanines substituted with PEGME-2000 blocks. JBIC J. Boil. Inorg. Chem. 2019, 24, 191–210. [Google Scholar] [CrossRef]
- Foley, S.; Jones, G.; Liuzzi, R.; McGarvey, D.J.; Perry, M.H.; Truscott, T.G. The synthesis and photophysical properties of polyether substituted phthalocyanines of potential use in photodynamic therapy. J. Chem. Soc. Perkin Trans. 2 1997, 29, 1725–1730. [Google Scholar] [CrossRef]
- Bandera, Y.; Burdette, M.K.; Shetzline, J.A.; Jenkins, R.; Creager, S.E.; Foulger, S.H. Synthesis of water soluble axially disubstituted silicon (IV) phthalocyanines with alkyne & azide functionality. Dyes Pigments 2016, 125, 72–79. [Google Scholar]
- Huang, J.-D.; Wang, S.; Lo, P.-C.; Fong, W.-P.; Ko, W.-H.; Ng, D.K.P. Halogenated Silicon (IV) phthalocyanines with axial poly(ethylene glycol) chains. Synthesis, spectroscopic properties, complexation with bovine serum albumin and in vitro photodynamic activities. New J. Chem. 2004, 28, 348–354. [Google Scholar] [CrossRef]
- Uslan, C.; Öztürk, Y.; Yıldız, B.T.; Çakar, Z.P.; Göksel, M.; Durmus, M.; Gursel, Y.H.; Sesalan, B.Ş.; Işleyen, N.D. A novel of PEG-conjugated phthalocyanine and evaluation of its photocytotoxicity and antibacterial properties for photodynamic therapy. J. Porphyr. Phthalocyanines 2018, 22, 10–24. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, X.; Zhang, B.; Kang, H.; Du, L.; Li, M. Nanostructures of an amphiphilic zinc phthalocyanine polymer conjugate for photodynamic therapy of psoriasis. Colloids Surf. B Biointerfaces 2015, 128, 405–409. [Google Scholar] [CrossRef]
- Revuelta-Maza, M.A.; Nonell, S.; De La Torre, G.; Torres, T. Boosting the singlet oxygen photosensitization abilities of Zn(II) phthalocyanines through functionalization with bulky fluorinated substituents. Org. Biomol. Chem. 2019, 17, 7448–7454. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Khoshdel, E.; Haddleton, D.M. Synthesis of water soluble PEGylated (copper) phthalocyanines via Mitsunobu reaction and Cu(i)-catalysed azide–alkyne cycloaddition (CuAAC) “click” chemistry. Polym. Chem. 2013, 4, 4405. [Google Scholar] [CrossRef]
- Revuelta-Maza, M.Á.; González-Jiménez, P.; Hally, C.; Agut, M.; Nonell, S.; de la Torre, G.; Torres, T. Fluorine-substituted tetracationic ABAB-phthalocyanines for efficient photodynamic inactivation of Gram-positive and Gram-negative bacteria. Eur. J. Med. Chem. 2020, 187, 111957. [Google Scholar] [CrossRef] [PubMed]
- Ajiro, H.; Takahashi, Y.; Akashi, M. Thermosensitive Biodegradable Homopolymer of Trimethylene Carbonate Derivative at Body Temperature. Macromolecules 2012, 45, 2668–2674. [Google Scholar] [CrossRef]
- Garcia, A.M.; Weerasekera, H.D.A.; Pitre, S.P.; McNeill, B.; Lissi, E.; Edwards, A.M.; Alarcon, E.I.; Edwards-Mujica, A.M. Photodynamic performance of zinc phthalocyanine in HeLa cells: A comparison between DPCC liposomes and BSA as delivery systems. J. Photochem. Photobiol. B Boil. 2016, 163, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Yee, M.; Kim, H.; Cheung, J.; Chino, T.; Düzgüneş, N.; Konopka, K. Phototoxicity of Liposomal Zn- and Al-phthalocyanine Against Cervical and Oral Squamous Cell Carcinoma Cells In Vitro. Med. Sci. Monit. Basic Res. 2016, 22, 156–164. [Google Scholar] [CrossRef]
- Li, L.; Luo, Z.; Chen, Z.; Chen, J.; Zhou, S.; Xu, P.; Hu, P.; Wang, J.; Chen, N.; Huang, J.; et al. Enhanced Photodynamic Efficacy of Zinc Phthalocyanine by Conjugating to Heptalysine. Bioconjug. Chem. 2012, 23, 2168–2172. [Google Scholar] [CrossRef]
- Çakır, D.; Göksel, M.; Çakır, V.; Durmuş, M.; Biyiklioglu, Z.; Kantekin, H. Amphiphilic zinc phthalocyanine photosensitizers: Synthesis, photophysicochemical properties and in vitro studies for photodynamic therapy. Dalton Trans. 2015, 44, 9646–9658. [Google Scholar] [CrossRef]
- Rello, S.; Stockert, J.C.; Moreno, V.; Pacheco, M.; Juarranz, A.; Villanueva, A.; Cañete, M.; Gamez, A. Morphological criteria to distinguish cell death induced by apoptotic and necrotic treatments. Apoptosis 2005, 10, 201–208. [Google Scholar] [CrossRef]
- Makhseed, S.; Tuhl, A.; Samuel, J.; Zimcik, P.; Al-Awadi, N.; Novakova, V. New highly soluble phenoxy-substituted phthalocyanine and azaphthalocyanine derivatives: Synthesis, photochemical and photophysical studies and atypical aggregation behavior. Dyes Pigments 2012, 95, 351–357. [Google Scholar] [CrossRef]
- Kuznetsova, N.A.; Gretsova, N.S.; Kalmykova, E.A.; Makarova, E.A.; Dashkevich, S.N.; Negrimovsky, V.M.; Lukyanets, E.A. Structure-photochemical properties relationship for porphyrins and related compounds. Zh. Obshch. Khim 2000, 70, 140–148. [Google Scholar]
- Jiménez-Banzo, A.; Ragàs, X.; Kapusta, P.; Nonell, S. Time-resolved methods in biophysics. 7. Photon counting vs. analog time-resolved singlet oxygen phosphorescence detection. Photochem. Photobiol. Sci. 2008, 7, 1003. [Google Scholar] [CrossRef] [PubMed]
- Nonell, S.; Braslavsky, S.E. [4] Time-resolved singlet oxygen detection. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2000; Volume 319, pp. 37–49. ISBN 0076-6879. [Google Scholar]
- Martí, C.; Jürgens, O.; Cuenca, O.; Casals, M.; Nonell, S. Aromatic ketones as standards for singlet molecular oxygen O2(1Δg) photosensitization. Time-resolved photoacoustic and near-IR emission studies. J. Photochem. Photobiol. A Chem. 1996, 97, 11–18. [Google Scholar] [CrossRef]
- Schmidt, R.; Tanielian, C.; Dunsbach, R.; Wolff, C. Phenalenone, a universal reference compound for the determination of quantum yields of singlet oxygen O2(1Δg) sensitization. J. Photochem. Photobiol. A Chem. 1994, 79, 11–17. [Google Scholar] [CrossRef]
- Blázquez-Castro, A.; Carrasco, E.; Calvo, M.I.; Jaén, P.; Stockert, J.C.; Juarranz, Á.; Sanz-Rodriguez, F.; Espada, J. Protoporphyrin IX-dependent photodynamic production of endogenous ROS stimulates cell proliferation. Eur. J. Cell Boil. 2012, 91, 216–223. [Google Scholar] [CrossRef] [PubMed]
| Sample | Solvent | Logε (λ/nm) | λf/nm | ΦF | τS/ns | τT/μs | ΦΔ |
|---|---|---|---|---|---|---|---|
| ABAB-1 | toluene | 4.73 (328), 5.09 (674), 5.12 (712) a | 715 | 0.13 | 1.7 | 0.35 b | 0.77 |
| A3B-1 | toluene | 4.63 (349), 4.97 (679) a, 4.94 (697) | 702 | 0.17 | 2.1 | 0.47 b | 0.71 |
| A4-1 | toluene | 4.50 (349), 5.03 (682) a | 684 | 0.43 | 3.0 | 0.45 b | 0.61 |
| ABAB-1 | THF | 4.82 (349), 5.08 (671), 5.09 (703) a | 709 | 0.13 | 1.7 | 0.30 c | 0.83 |
| A3B-1 | THF | 4.71 (348), 5.03 (675) a | 692 | 0.28 | 2.2 | 0.23 c | 0.74 |
| A4-1 | THF | 4.61 (349), 5.12 (676) a | 682 | 0.25 | 3.0 | 0.37 c | 0.68 |
| Compound | Concentration [M] | Surviving Fraction (% ± SD) 1 | |
|---|---|---|---|
| SCC-13 | HeLa | ||
| No Zn(II)Pc – Control dark | 101 ± 2.4 | 100 ± 1.1 | |
| No Zn(II)Pc – Control dark + 1% DMSO | 102 ± 4.3 | 98 ± 3.7 | |
| No Zn(II)Pc – Control light (9 J/cm2) | 100 ± 1.7 | 99 ± 2.3 | |
| No Zn(II)Pc – Control light (9 J/cm2) + 1% DMSO | 101 ± 2.8 | 101 ± 1.5 | |
| ABAB-1 | 1 × 10−6 | 98 ± 5.4 | 101 ± 5.5 |
| 1 × 10−7 | 102 ± 3.1 | 95 ± 2.8 | |
| A3B-1 | 1 × 10−6 | 97 ± 6.3 | 97 ± 3.5 |
| 1 × 10−7 | 99 ± 5.5 | 99 ± 3.6 | |
| A4-1 | 1 × 10−6 | 99 ± 4.5 | 97 ± 5.5 |
| 1 × 10−7 | 98 ± 5.0 | 98 ± 2.8 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revuelta-Maza, M.Á.; Mascaraque, M.; González-Jiménez, P.; González-Camuñas, A.; Nonell, S.; Juarranz, Á.; de la Torre, G.; Torres, T. Assessing Amphiphilic ABAB Zn(II) Phthalocyanines with Enhanced Photosensitization Abilities in In Vitro Photodynamic Therapy Studies Against Cancer. Molecules 2020, 25, 213. https://doi.org/10.3390/molecules25010213
Revuelta-Maza MÁ, Mascaraque M, González-Jiménez P, González-Camuñas A, Nonell S, Juarranz Á, de la Torre G, Torres T. Assessing Amphiphilic ABAB Zn(II) Phthalocyanines with Enhanced Photosensitization Abilities in In Vitro Photodynamic Therapy Studies Against Cancer. Molecules. 2020; 25(1):213. https://doi.org/10.3390/molecules25010213
Chicago/Turabian StyleRevuelta-Maza, Miguel Á., Marta Mascaraque, Patricia González-Jiménez, Arturo González-Camuñas, Santi Nonell, Ángeles Juarranz, Gema de la Torre, and Tomás Torres. 2020. "Assessing Amphiphilic ABAB Zn(II) Phthalocyanines with Enhanced Photosensitization Abilities in In Vitro Photodynamic Therapy Studies Against Cancer" Molecules 25, no. 1: 213. https://doi.org/10.3390/molecules25010213
APA StyleRevuelta-Maza, M. Á., Mascaraque, M., González-Jiménez, P., González-Camuñas, A., Nonell, S., Juarranz, Á., de la Torre, G., & Torres, T. (2020). Assessing Amphiphilic ABAB Zn(II) Phthalocyanines with Enhanced Photosensitization Abilities in In Vitro Photodynamic Therapy Studies Against Cancer. Molecules, 25(1), 213. https://doi.org/10.3390/molecules25010213