Silk/Natural Rubber (NR) and 3,4-Dihydroxyphenylalanine (DOPA)-Modified Silk/NR Composites: Synthesis, Secondary Structure, and Mechanical Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Preparation and Basic Properties of Silk/NR Composites
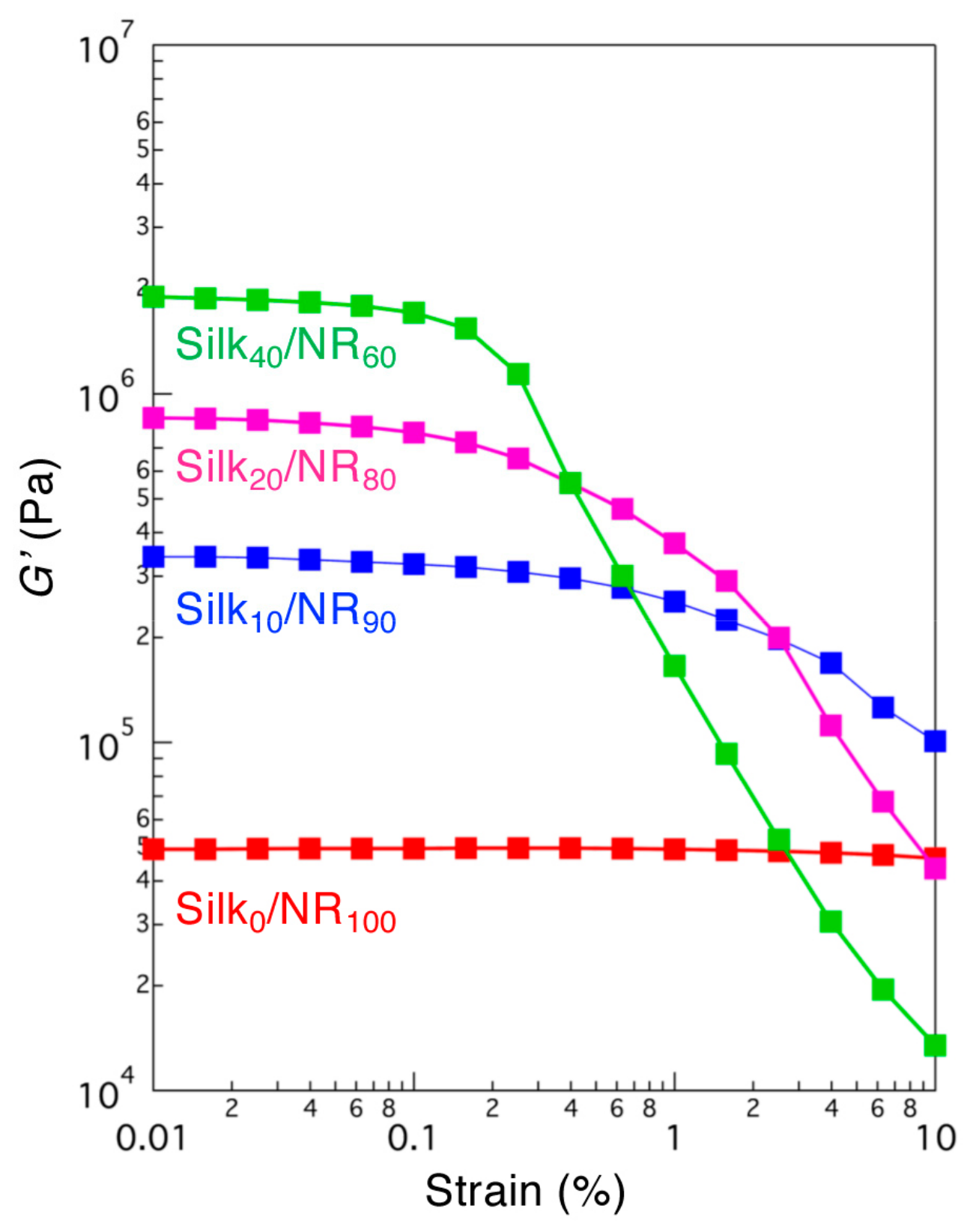
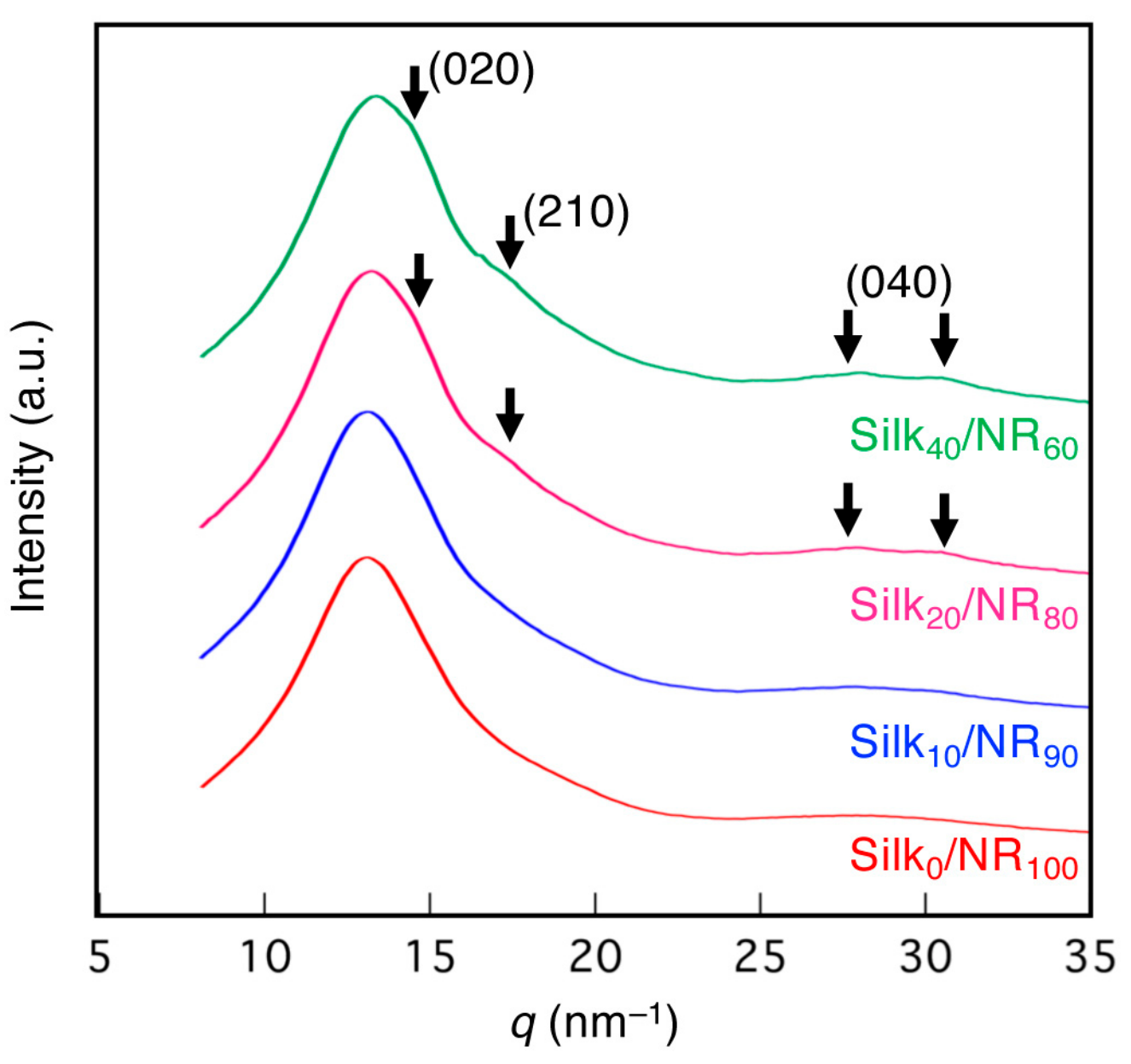
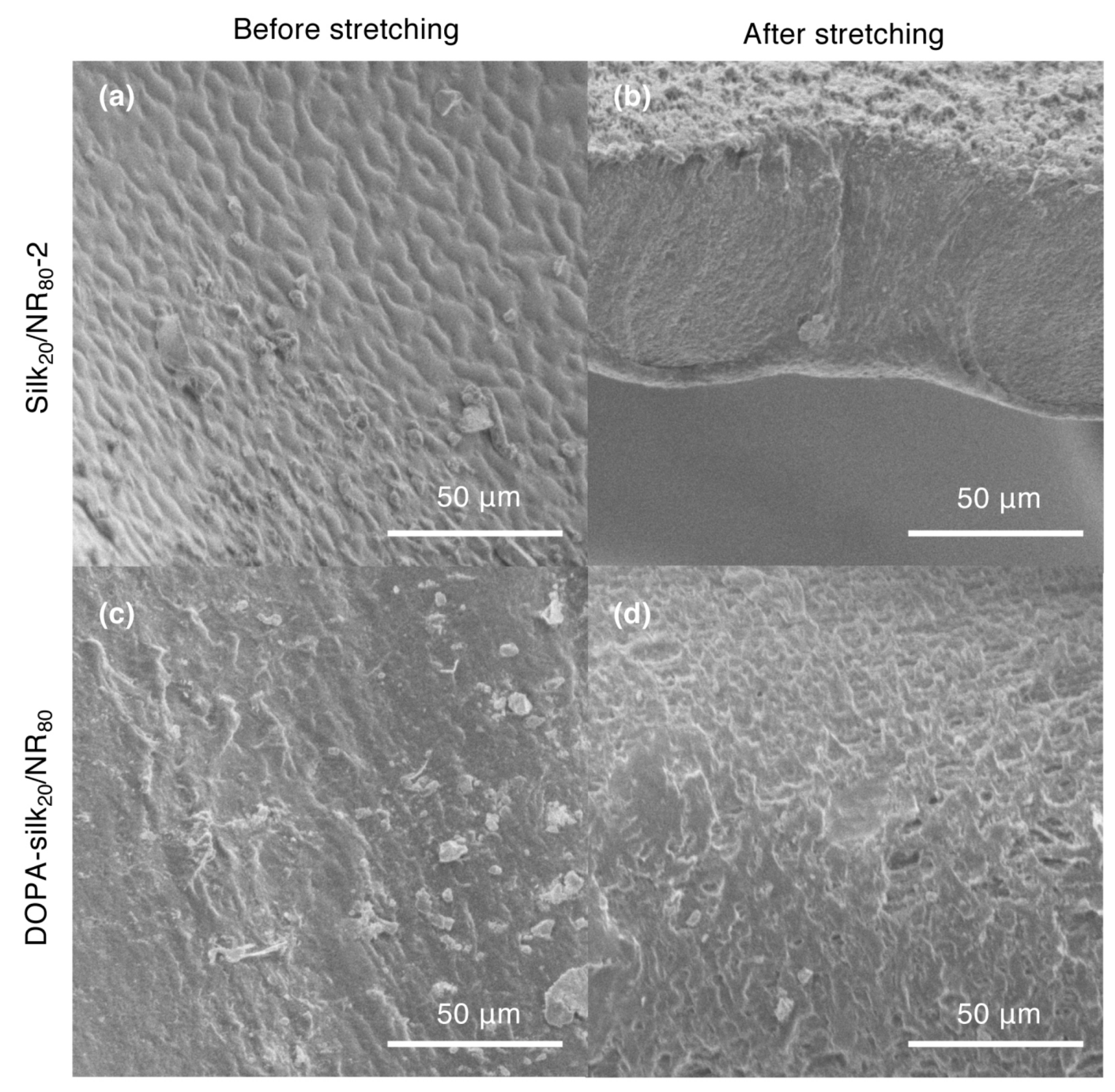
2.2. Structural Characterization of Silk/NR Composites
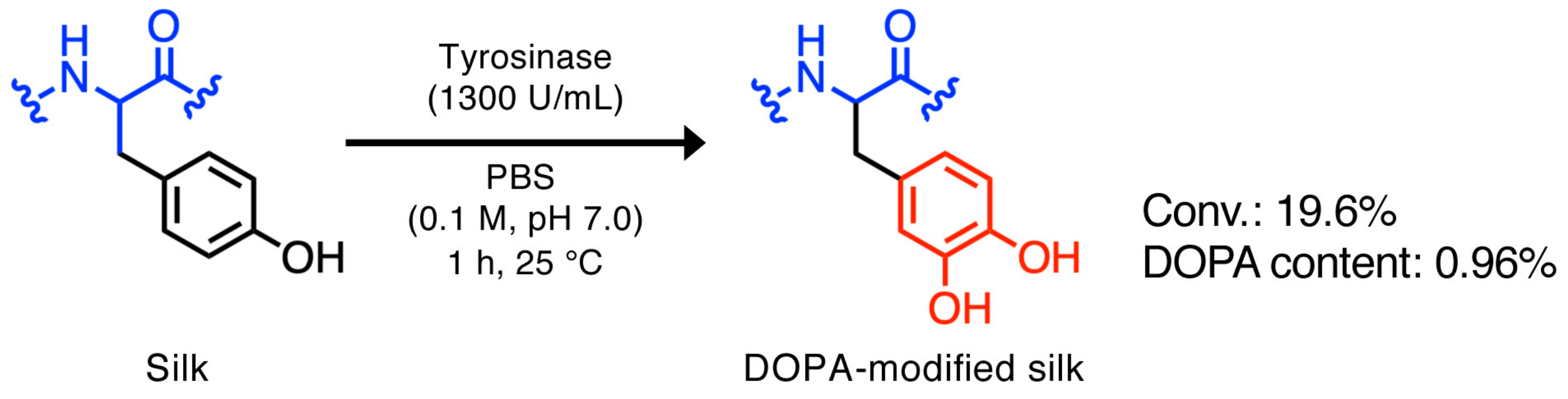
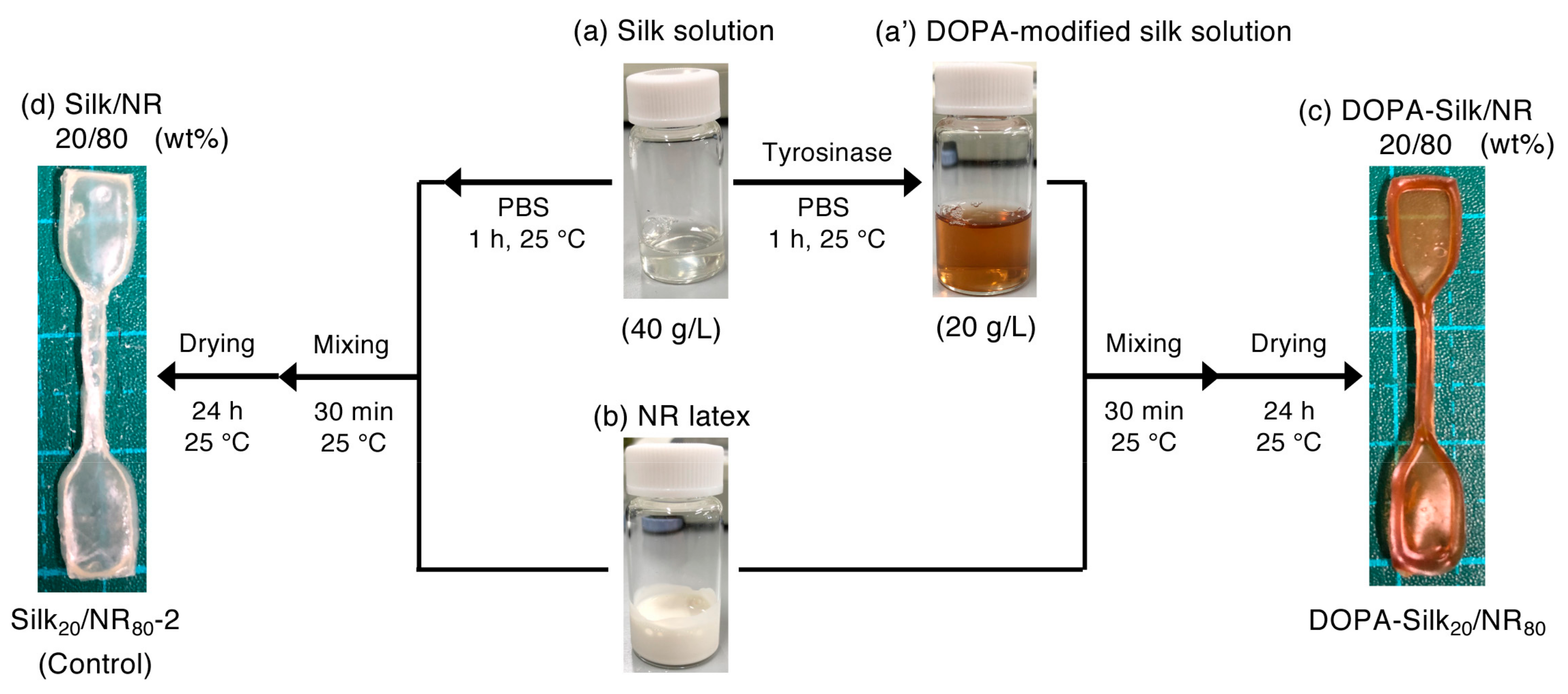
2.3. Preparation of the DOPA-silk/NR Composite
2.4. The Mechanical Properties of DOPA-Silk/NR
2.5. Structural Characterization of the DOPA-Silk/NR Composite
3. Materials and Methods
3.1. Materials
3.2. Preparation of Silk Solution
3.3. Preparation Protocol of Silk/NR Composites
3.4. Preparation Protocol of DOPA-Silk/NR Composites
3.5. Tensile Tests
3.6. Rheological Measurements
3.7. SEM Observations
3.8. ATR-FTIR Measurements
3.9. WAXD Measurements
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Vollrath, F.; Porter, D. Spider silk as a model biomaterial. Appl. Phys. A-Mater. 2006, 82, 205–212. [Google Scholar] [CrossRef]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2019, 8, e1800465. [Google Scholar] [PubMed] [Green Version]
- Huang, W.; Ling, S.; Li, C.; Omenetto, F.G.; Kaplan, D.L. Silkworm silk-based materials and devices generated using bio-nanotechnology. Chem. Soc. Rev. 2018, 47, 6486–6504. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Numata, K.; Kaplan, D.L. Silk-based delivery systems of bioactive molecules. Adv. Drug Deliver. Rev. 2010, 62, 1497–1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, A.R.; Kaplan, D.L. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef] [Green Version]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Malay, A.D.; Sato, R.; Yazawa, K.; Watanabe, H.; Ifuku, N.; Masunaga, H.; Hikima, T.; Guan, J.; Mandal, B.B.; Damrongsakkul, S.; et al. Relationships between physical properties and sequence in silkworm silks. Sci. Rep. 2016, 6, 27573. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.G.; Janin, J. Silk fibroin: structural implications of a remarkable amino acid sequence. Proteins 2001, 44, 119–122. [Google Scholar] [CrossRef]
- Saito, H.; Tabeta, R.; Asakura, T.; Iwanaga, Y.; Shoji, A.; Ozaki, T.; Ando, I. High-resolution carbon-13 NMR study of silk fibroin in the solid state by the cross-polarization-magic angle spinning method. Conformational characterization of silk I and silk II type forms of Bombyx mori fibroin by the conformation-dependent carbon-13 chemical shifts. Macromolecules 1984, 17, 1405–1412. [Google Scholar]
- Yang, K.; Guan, J.; Numata, K.; Wu, C.; Wu, S.; Shao, Z.; Ritchie, R.O. Integrating tough Antheraea pernyi silk and strong carbon fibres for impact-critical structural composites. Nat. Commun. 2019, 10, 3786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Yazawa, K.; Tsuchiya, K.; Numata, K.; Guan, J. Molecular Interactions and Toughening Mechanisms in Silk Fibroin–Epoxy Resin Blend Films. Biomacromolecules 2019, 20, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Ifuku, N.; Isogai, A. Silk composite with a fluoropolymer as a water-resistant protein-based material. Polymers 2018, 10, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchiya, K.; Masunaga, H.; Numata, K. Tensile Reinforcement of Silk Films by the Addition of Telechelic-Type Polyalanine. Biomacromolecules 2017, 18, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, S.; Guan, J.; Shao, Z.; Ritchie, R.O. Enhancing the Mechanical Toughness of Epoxy-Resin Composites Using Natural Silk Reinforcements. Sci. Rep. 2017, 7, 11939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, D.U.; Porter, D.; Vollrath, F. Can silk become an effective reinforcing fibre? A property comparison with flax and glass reinforced composites. Compos. Sci. Technol. 2014, 101, 173–183. [Google Scholar] [CrossRef]
- Numata, K.; Yamazaki, S.; Katashima, T.; Chuah, J.A.; Naga, N.; Sakai, T. Silk-pectin hydrogel with superior mechanical properties, biodegradability, and biocompatibility. Macromol. Biosci. 2014, 14, 799–806. [Google Scholar] [CrossRef]
- Heo, S.; Yun, Y.S.; Cho, S.Y.; Jin, H.-J. Flexible Bio-Composites Based on Silks and Celluloses. J. Nanosci. Nanotechno. 2012, 12, 811–814. [Google Scholar] [CrossRef]
- Kesenci, K.; Motta, A.; Fambri, L.; Migliaresi, C. Poly(ε-caprolactone-co-D,L-lactide)/silk fibroin composite materials: Preparation and characterization. J. Biomater. Sci. Polym. Ed. 2001, 12, 337–351. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Samani, S.; Shokrgozar, M.A.; Kundu, S.C.; Reis, R.L.; Fatahi, Y.; Kaplan, D.L. Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol. Adv. 2018, 36, 68–91. [Google Scholar] [CrossRef] [PubMed]
- Kharlampieva, E.; Kozlovskaya, V.; Gunawidjaja, R.; Shevchenko, V.V.; Vaia, R.; Naik, R.R.; Kaplan, D.L.; Tsukruk, V.V. Flexible Silk–Inorganic Nanocomposites: From Transparent to Highly Reflective. Adv. Funct. Mater. 2010, 20, 840–846. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Friis, T.; Xiao, Y. The osteogenic properties of CaP/silk composite scaffolds. Biomaterials 2010, 31, 2848–2856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, L.; Tsuchiya, K.; Numata, K. Chemoenzymatic modification of silk fibroin with poly (2, 6-dimethyl-1, 5-phenylene ether) using horseradish peroxidase. RSC Adv. 2016, 6, 28737–28744. [Google Scholar] [CrossRef] [Green Version]
- Freddi, G.; Anghileri, A.; Sampaio, S.; Buchert, J.; Monti, P.; Taddei, P. Tyrosinase-catalyzed modification of Bombyx mori silk fibroin: grafting of chitosan under heterogeneous reaction conditions. J. Biotechnol. 2006, 125, 281–294. [Google Scholar] [CrossRef]
- Furuzono, T.; Kishida, A.; Tanaka, J. Nano-scaled hydroxyapatite/polymer composite I. Coating of sintered hydroxyapatite particles on poly(γ-methacryloxypropyl trimethoxysilane)-grafted silk fibroin fibers through chemical bonding. J. Mater. Sci. Mater. Med. 2004, 15, 19–23. [Google Scholar] [CrossRef]
- Furuzono, T.; Ishihara, K.; Nakabayashi, N.; Tamada, Y. Chemical modification of silk fibroin with 2-methacryloyloxyethyl phosphorylcholine. II. Graft-polymerization onto fabric through 2-methacryloyloxyethyl isocyanate and interaction between fabric and platelets. Biomaterials 2000, 21, 327–333. [Google Scholar] [CrossRef]
- Tamada, Y.; Furuzono, T.; Taguchi, T.; Kishida, A.; Akashi, M. Ca-adsorption and apatite deposition on silk fabrics modified with phosphate polymer chains. J. Biomater. Sci. Polym. Ed. 1999, 10, 787–793. [Google Scholar] [CrossRef]
- Gotoh, Y.; Tsukada, M.; Minoura, N. Chemical modification of silk fibroin with cyanuric chloride-activated poly (ethylene glycol): Analyses of reaction site by proton NMR spectroscopy and conformation of the conjugates. Bioconjug. Chem. 1993, 4, 554–559. [Google Scholar] [CrossRef]
- Sogawa, H.; Ifuku, N.; Numata, K. 3,4-Dihydroxyphenylalanine (DOPA)-Containing Silk Fibroin: Its Enzymatic Synthesis and Adhesion Properties. ACS Biomater. Sci. Eng. 2019, 5, 5644–5651. [Google Scholar] [CrossRef] [Green Version]
- Burke, K.A.; Roberts, D.C.; Kaplan, D.L. Silk Fibroin Aqueous-Based Adhesives Inspired by Mussel Adhesive Proteins. Biomacromolecules 2016, 17, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.A.; Morse, D.E. The bioadhesive ofPhragmatopoma californica tubes: a silk-like cement containingL-DOPA. J. Comp. Physiol. B 1988, 158, 317–324. [Google Scholar] [CrossRef]
- Bokobza, L. Natural rubber nanocomposites: A review. Nanomaterials 2019, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.C. Chapter. 4: The Production of Natural Rubber from Hevea brasiliensis Latex: Colloidal Properties, Preservation, Purification and Processing. In Natural Rubber Materials: Volume 1: Blends and IPNs; The Royal Society of Chemistry: London, UK, 2014; Volume 1, pp. 73–106. [Google Scholar]
- Tanaka, Y. Structural Characterization of Natural Polyisoprenes: Solve the Mystery of Natural Rubber Based on Structural Study. Rubber Chem. Technol. 2001, 74, 355–375. [Google Scholar] [CrossRef]
- Song, K. 2-Micro- and nano-fillers used in the rubber industry. In Progress in Rubber Nanocomposites; Thomas, S., Maria, H.J., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 41–80. [Google Scholar]
- Salomez, M.; Subileau, M.; Intapun, J.; Bonfils, F.; Sainte-Beuve, J.; Vaysse, L.; Dubreucq, E. Micro-organisms in latex and natural rubber coagula of Hevea brasiliensis and their impact on rubber composition, structure and properties. J. Appl. Microbiol. 2014, 117, 921–929. [Google Scholar] [CrossRef]
- Danafar, F.; Kalantari, M. A Review of Natural Rubber Nanocomposites Based on Carbon Nanotubes. J. Rubber Res. 2018, 21, 293–310. [Google Scholar] [CrossRef]
- Feldman, D. Natural rubber nanocomposites. J. Macromol. Sci. A 2017, 54, 629–634. [Google Scholar] [CrossRef]
- Heinrich, G.; Klüppel, M.; Vilgis, T.A. Reinforcement of elastomers. Curr. Opin. Solid St. M 2002, 6, 195–203. [Google Scholar] [CrossRef]
- Karásek, L.; Sumita, M. Characterization of dispersion state of filler and polymer-filler interactions in rubber-carbon black composites. J. Mater. Sci. 1996, 31, 281–289. [Google Scholar] [CrossRef]
- Thomas, M.G.; Abraham, E.; Jyotishkumar, P.; Maria, H.J.; Pothen, L.A.; Thomas, S. Nanocelluloses from jute fibers and their nanocomposites with natural rubber: Preparation and characterization. Int. J. Biol. Macromol. 2015, 81, 768–777. [Google Scholar] [CrossRef]
- Trovatti, E.; Carvalho, A.J.; Ribeiro, S.J.; Gandini, A. Simple green approach to reinforce natural rubber with bacterial cellulose nanofibers. Biomacromolecules 2013, 14, 2667–2674. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Elbi, P.A.; Deepa, B.; Jyotishkumar, P.; Pothen, L.A.; Narine, S.S.; Thomas, S. X-ray diffraction and biodegradation analysis of green composites of natural rubber/nanocellulose. Polym. Degrad. Stabil. 2012, 97, 2378–2387. [Google Scholar] [CrossRef]
- Siqueira, G.; Tapin-Lingua, S.; Bras, J.; da Silva Perez, D.; Dufresne, A. Mechanical properties of natural rubber nanocomposites reinforced with cellulosic nanoparticles obtained from combined mechanical shearing, and enzymatic and acid hydrolysis of sisal fibers. Cellulose 2011, 18, 57–65. [Google Scholar] [CrossRef]
- Bendahou, A.; Kaddami, H.; Dufresne, A. Investigation on the effect of cellulosic nanoparticles’ morphology on the properties of natural rubber based nanocomposites. Eur. Polym. J. 2010, 46, 609–620. [Google Scholar] [CrossRef]
- Parambath Kanoth, B.; Claudino, M.; Johansson, M.; Berglund, L.A.; Zhou, Q. Biocomposites from natural rubber: Synergistic effects of functionalized cellulose nanocrystals as both reinforcing and cross-linking agents via free-radical thiol–ene chemistry. ACS Appl. Mater. Interfaces 2015, 7, 16303–16310. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.A.; Isaksson, P.; Lindström, T.; Nishino, T. Cellulose Nanopaper Structures of High Toughness. Biomacromolecules 2008, 9, 1579–1585. [Google Scholar] [CrossRef]
- Missoum, K.; Belgacem, M.N.; Bras, J. Nanofibrillated cellulose surface modification: a review. Materials 2013, 6, 1745–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.H.; Luo, Y.Y.; Yi, Z.F.; Feng, C.F.; Wang, Y.Q.; Peng, Z. Natural Rubber/Bombyx Mori Silk Fibroin Composites: Preparation and Properties. In Proceedings of Advanced Materials Research; Liu, S., Zuo, M., Eds.; Trans Tech Publications: Stafa-Zurich, Switzerland, 2011; pp. 894–900. [Google Scholar]
- Rajkumar, G.; Srinivasan, J.; Suvitha, L. Development of novel silk/wool hybrid fibre polypropylene composites. Iran. Polym. J. 2013, 22, 277–284. [Google Scholar] [CrossRef]
- Bazrafshan, Z.; Stylios, G.K. Custom-built electrostatics and supplementary bonding in the design of reinforced Collagen-g-P(methyl methacrylate-co-ethyl acrylate)/ nylon 66 core-shell fibers. J. Mech. Behav. Biomed. Mater. 2018, 87, 19–29. [Google Scholar] [CrossRef]
- Savetlana, S.; Sukmana, I.; Saputra, F. The effect of carbon black loading and structure on tensile property of natural rubber composite. In Proceedings of IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; p. 012009. [Google Scholar]
- Hu, X.; Kaplan, D.; Cebe, P. Determining beta-sheet crystallinity in fibrous proteins by thermal analysis and infrared spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Martel, A.; Burghammer, M.; Davies, R.J.; Di Cola, E.; Vendrely, C.; Riekel, C. Silk Fiber Assembly Studied by Synchrotron Radiation SAXS/WAXS and Raman Spectroscopy. J. Am. Chem. Soc. 2008, 130, 17070–17074. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Johnson, M.A.; Martin, D.C. Microstructural Characterization of Bombyx mori Silk Fibers. Macromolecules 1998, 31, 8857–8864. [Google Scholar] [CrossRef]
- Numata, K.; Masunaga, H.; Hikima, T.; Sasaki, S.; Sekiyama, K.; Takata, M. Use of extension-deformation-based crystallisation of silk fibres to differentiate their functions in nature. Soft Matter 2015, 11, 6335–6342. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Baker, P.J. Synthesis of Adhesive Peptides Similar to Those Found in Blue Mussel (Mytilus edulis) Using Papain and Tyrosinase. Biomacromolecules 2014, 15, 3206–3212. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C. Recent advances in crosslinking chemistry of biomimetic poly (ethylene glycol) hydrogels. RSC Adv. 2015, 5, 39844–39853. [Google Scholar] [CrossRef] [Green Version]
- Kang, G.D.; Lee, K.H.; Ki, C.S.; Nahm, J.H.; Park, Y.H. Silk fibroin/chitosan conjugate crosslinked by tyrosinase. Macromol. Res. 2004, 12, 534–539. [Google Scholar] [CrossRef]
- Herlinger, E.; Jameson, R.F.; Linert, W. Spontaneous autoxidation of dopamine. J. Chem. Soc. Perkin 1995, 259–263. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, X.; Knight, D.P.; Zong, X.-H.; Deng, F.; Yao, W.-H. Effects of pH and Calcium Ions on the Conformational Transitions in Silk Fibroin Using 2D Raman Correlation Spectroscopy and 13C Solid-State NMR. Biochemistry 2004, 43, 11302–11311. [Google Scholar] [CrossRef]
- Zhao, H.; Heusler, E.; Jones, G.; Li, L.; Werner, V.; Germershaus, O.; Ritzer, J.; Luehmann, T.; Meinel, L. Decoration of silk fibroin by click chemistry for biomedical application. J. Struct. Biol. 2014, 186, 420–430. [Google Scholar] [CrossRef]
- Canabady-Rochelle, L.L.; Belton, D.J.; Deschaume, O.; Currie, H.A.; Kaplan, D.L.; Perry, C.C. Bioinspired silicification of silica-binding peptide-silk protein chimeras: comparison of chemically and genetically produced proteins. Biomacromolecules 2012, 13, 683–690. [Google Scholar] [CrossRef]
- Oouchi, M.; Ukawa, J.; Ishii, Y.; Maeda, H. Structural Analysis of the Terminal Groups in Commercial Hevea Natural Rubber by 2D-NMR with DOSY Filters and Multiple-WET Methods Using Ultrahigh-Field NMR. Biomacromolecules 2019, 20, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Burger, C.; Toki, S.; Rong, L.X.; Hsiao, B.S.; Amnuaypornsri, S.; Sakdapipanich, J. Crystal and Crystallites Structure of Natural Rubber and Peroxide-Vulcanized Natural Rubber by a Two-Dimensional Wide-Angle X-ray Diffraction Simulation Method. II. Strain-Induced Crystallization versus Temperature-Induced Crystallization. Macromolecules 2013, 46, 9712–9721. [Google Scholar] [CrossRef]
- Che, J.; Burger, C.; Toki, S.; Rong, L.X.; Hsiao, B.S.; Amnuaypornsri, S.; Sakdapipanich, J. Crystal and Crystallites Structure of Natural Rubber and Synthetic cis-1,4-Polyisoprene by a New Two Dimensional Wide Angle X-ray Diffraction Simulation Method. I. Strain-Induced Crystallization. Macromolecules 2013, 46, 4520–4528. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. Photometric ninhydrin method for use in the chromatography of amino acids. J. Biol. Chem. 1948, 176, 367–388. [Google Scholar]
- Numata, K.; Sato, R.; Yazawa, K.; Hikima, T.; Masunaga, H. Crystal structure and physical properties of Antheraea yamamai silk fibers: Long poly(alanine) sequences are partially in the crystalline region. Polymer 2015, 77, 87–94. [Google Scholar] [CrossRef]
- Hammersley, A.P.; Svensson, S.O.; Hanfland, M.; Fitch, A.N.; Hausermann, D. Two-dimensional detector software: From real detector to idealised image or two-theta scan. High Pressure Res. 1996, 14, 235–248. [Google Scholar] [CrossRef]
Sample Availability: Not available. |










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sogawa, H.; Korawit, T.; Masunaga, H.; Numata, K. Silk/Natural Rubber (NR) and 3,4-Dihydroxyphenylalanine (DOPA)-Modified Silk/NR Composites: Synthesis, Secondary Structure, and Mechanical Properties. Molecules 2020, 25, 235. https://doi.org/10.3390/molecules25010235
Sogawa H, Korawit T, Masunaga H, Numata K. Silk/Natural Rubber (NR) and 3,4-Dihydroxyphenylalanine (DOPA)-Modified Silk/NR Composites: Synthesis, Secondary Structure, and Mechanical Properties. Molecules. 2020; 25(1):235. https://doi.org/10.3390/molecules25010235
Chicago/Turabian StyleSogawa, Hiromitsu, Treratanakulwongs Korawit, Hiroyasu Masunaga, and Keiji Numata. 2020. "Silk/Natural Rubber (NR) and 3,4-Dihydroxyphenylalanine (DOPA)-Modified Silk/NR Composites: Synthesis, Secondary Structure, and Mechanical Properties" Molecules 25, no. 1: 235. https://doi.org/10.3390/molecules25010235






