Correlation Study of Honey Regarding their Physicochemical Properties and Sugars and Cyclitols Content
Abstract
:1. Introduction
2. Results and Discussion
2.1. Role and Assessment of Physico-Chemical Properties of Honey
2.2. The Content of Sugars and Cyclitols in the Investigated Honey Samples
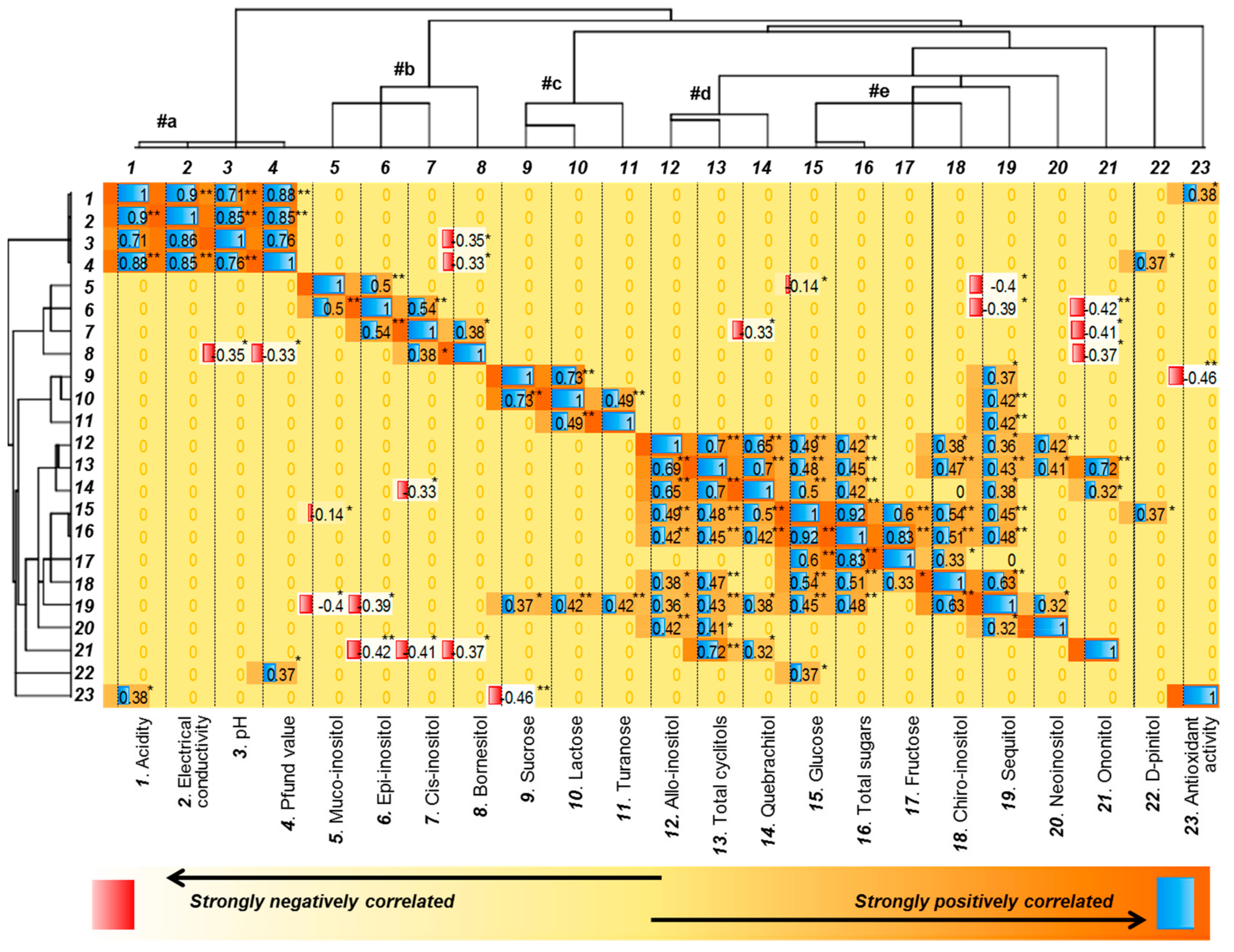
2.3. Correlation Analyses
2.3.1. Correlation Between the Investigated Variables
2.3.2. Correlation Between Honey Types
3. Materials and Methods
3.1. Honey Samples Collection and Chemicals Involved
3.2. Samples Analysis
3.3. Validation Parameters
3.4. Determination of Physico-Chemical Properties
3.4.1. Pfund Value and Honey Color
3.4.2. Acidity and pH
3.4.3. Electrical Conductivity
3.4.4. Antioxidant Activity
3.5. Statistical Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bueno-Costa, F.M.; Zambiazi, R.C.; Bohmer, B.W.; Chaves, F.C.; da Silva, W.P.; Zanusso, J.T.; Dutra, I. Antibacterial and antioxidant activity of honey from the state of Rio Grande do Sul, Brazil. LWT Food Sci. Technol. 2016, 65, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and Modern Uses of Natural Honey in Human Diseases: A Review. Iran. J. Basic Med. Sci. 2013, 16, 731–742. [Google Scholar] [PubMed]
- Matzen, R.D.; Leth-Espensen, J.Z.; Jansson, T.; Nielsen, D.S.; Lund, M.N.; Matzen, S. The Antibacterial Effect In Vitro of Honey Derived from Various Danish Flora. Dermatol. Res. Pract. 2018, 7021713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Sohaimy, S.A.; Masry, S.H.D.; Shehata, M.G. Physicochemical characteristics of honey from different origins. Ann. Agric. Sci. 2015, 60, 279–287. [Google Scholar]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Clearwater, M.J.; Revell, M.; Noe, S.; Manley-Harris, M. Influence of genotype, floral stage, and water stress on floral nectar yield and composition of mānuka (Leptospermum scoparium). Ann. Bot. 2018, 121, 501–512. [Google Scholar] [CrossRef] [Green Version]
- Sanz, M.L.; Sanz, J.; Martınez-Castro, I. Presence of some cyclitols in honey. Food Chem. 2004, 84, 133–135. [Google Scholar] [CrossRef]
- Al-Suod, H.; Ligor, M.; Ratiu, I.A.; Rafinska, K.; Górecki, R.; Buszewski, B. A window on cyclitols: Characterization and analytics of inositols. Phytochem. Lett. 2017, 20, 507–519. [Google Scholar] [CrossRef]
- Naseer, S.; Khan, S.A.; Azim, M.K. Identification of cultivable bacteria from natural honey of different botanical origin. Pak. J. Biochem. Mol. Biol. 2015, 48, 53–56. [Google Scholar]
- Irungu, J.; Raina, S.; Torto, B. Determination of pesticide residues in honey: A preliminary study from two of Africa’s largest honey producers. Int. J. Food Contam. 2016, 3, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kamal, M.A.; Klein, P. Determination of sugars in honey by liquid chromatography. Saudi J Biol Sci. 2011, 18, 17–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ligor, M.; Ratiu, I.A.; Kiełbasa, A.; Al-Suod, H.; Buszewski, B. Extraction approaches used for the determination of biologically active compounds (cyclitols, polyphenols and saponins) isolated from plant material. Electrophoresis 2018, 39, 1860–1874. [Google Scholar] [CrossRef] [PubMed]
- Ratiu, I.A.; Al-Suod, H.; Ligor, M.; Ligor, T.; Krakowska, A.; Górecki, R.; Buszewski, B. Simultaneous determination of cyclitols and sugars following a comprehensive investigation of 40 plants. Food Anal. Methods 2019, 12, 1466–1478. [Google Scholar] [CrossRef] [Green Version]
- Al-Suod, H.; Ratiu, I.A.; Ligor, M.; Ligor, T.; Buszewski, B. Determination of sugars and cyclitols isolated from various morphological parts of Medicago sativa L. J. Sep. Sci. 2018, 41, 1118–1128. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Al-Suod, H.; Ligor, M.; Ligor, T.; Railean-Plugaru, V.; Buszewski, B. Complex investigation of extraction techniques applied for cyclitols and sugars isolation from different species of Solidago genus. Electrophoresis 2018, 39, 1966–1974. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Zeković, Z.; Jerković, J.; Zengin, G.; Gašić, U.; Tešić, Ž.; Mašković, P.; Soares, C.; Barroso, M.F.; et al. The influence of the extraction temperature on polyphenolic profiles and bioactivity of chamomile subcritical water extracts. Food Chem. 2019, 271, 328–337. [Google Scholar] [CrossRef] [Green Version]
- Cvetanović, A.; Švarc-Gajić, J.; Zeković, Z.; Gašić, U.; Tešić, Ž.; Zengin, G.; Mašković, P.; Mahomoodally, M.F.; Đurović, S. Subcritical water extraction as a cutting edge technology for the extraction of bioactive compounds from chamomile: Influence of pressure on chemical composition and bioactivity of extracts. Food Chem. 2018, 266, 389–396. [Google Scholar] [CrossRef]
- Al-Suod, H.; Ratiu, I.A.; Górecki, R.; Buszewski, B. Pressurized liquid extraction of cyclitols and sugars: Optimization of extraction parameters and selective separation. J. Sep. Sci. 2019, 42, 1117–1298. [Google Scholar] [CrossRef]
- Al-Suod, H.; Ratiu, I.A.; Krakowska-Sieprawska, A.; Lahuta, L.; Górecki, R.; Buszewski, B. Supercritical fluid extraction in isolation of cyclitols and sugars from chamomile flowers. J. Sep. Sci. 2019, 42, 3243–3252. [Google Scholar] [CrossRef]
- Al-Suod, H.; Gadzała-Kopciuch, R.; Buszewski, B. Simultaneous HPLC-ELSD determination of sugars and cyclitols in different parts of Phacelia tanacetifolia. Benth. Biochem. Syst. Ecol. 2018, 80, 32–38. [Google Scholar] [CrossRef]
- Majewska, E.; Druzynska, B.; Wołosiak, R. Determination of the botanical origin of honeybee honeys based on the analysis of their selected physicochemical parameters coupled with chemometric assays. Food Sci. Biotechnol. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Council EU. Council directive 2001/110/EC of 20 December 2001 relating to honey. Off. J. Eur. Commun. L. 2001, 10, 47–52. [Google Scholar]
- Oroian, M.; Ropciuc, S. Honey authentication based on physicochemical parameters and phenolic compounds. Comput. Electron. Agric. 2017, 138, 148–156. [Google Scholar] [CrossRef]
- Oroian, M.; Amariei, S.; Leahu, A.; Gutt, G. Multi-elements composition of honey as a suitable tool for its authenticity analysis. Pol. J. Food Nutr. Sci. 2015, 62, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Dzugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules. 2018, 23, 2069. [Google Scholar] [CrossRef] [Green Version]
- Chua, L.S.; Rahaman, N.L.A.; Adnan, N.A.; Tjih, T.; Tan, E. Antioxidant Activity of Three Honey Samples in relation with Their Biochemical Components. J. Anal. Meth. Chem. 2013, 313798. [Google Scholar] [CrossRef]
- Al-Suod, H.; Pomastowski, P.; Ligor, M.; Railean-Plugaru, V.; Buszewski, B. New approach for fast identification of cyclitols by MALDI-TOF mass spectrometry. Phytochem Anal. 2018, 29, 528–537. [Google Scholar] [CrossRef]
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M.C. Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef]
- Rybak-Chmielewska, H. Changes in the carbohydrate composition of honey undergoing during storage. J. Apic. Sci. 2007, 51, 39–48. [Google Scholar]
- Moreira, R.F.A.; Maria, C.A.B.; Pietroluongo, M.; Trugo, L.C. Chemical changes in the volatile fractions of Brazilian honeys during storage under tropical conditions. Food Chem. 2010, 121, 697–704. [Google Scholar] [CrossRef]
- Kaskoniene, V.; Venskutonis, P.R.; Ceksteryte, V. Composition of volatile compounds of honey of various floral origin and beebread collected in Lithuania. Food Chem. 2008, 111, 988–997. [Google Scholar] [CrossRef]




| No | Honey Type | Pfund Value (mm) | Color According to Pfund Scale | pH | Acidity (meq/kg) | Electrical Conductivity (mS/cm) | Antioxidant Activity | Manufacturer/ Place & Origin Country/ Year |
|---|---|---|---|---|---|---|---|---|
| 1 | Bush | 129.66 | dark amber | 4.20 ± 0.03 | 26.0 | 0.677 ± 0.03 | 4.43 ± 0.4 | “Peter & Trisha Norris”, Lenah Valley, Tasmania, Australia, 2016 |
| 2 | Leatherwood | 88.81 | amber | 4.32 ± 0.03 | 21.0 | 0.704 ± 0.02 | 4.25 ± 0.05 | |
| 3 | Clover | 20.10 | yellow- white | 3.60 ± 0.01 | 22.0 | 0.255 ± 0.01 | 3.95 ± 0.27 | |
| 4 | Multiflower | 77.05 | light amber | 4.30 ± 0.02 | 12.0 | 0.067 ± 0.00 | 2.95 ± 0.16 | “Fragrant Greece”, Greece, 2017 |
| 5 | Multiflower | 183.73 | dark amber | 4.15 ± 0.05 | 114.0 | 0.322 ± 0.04 | 4.9 ± 0.06 | “Adam Gardynik”, Nyaoundere Kameron, 2018 |
| 6 | Acacia | 33.10 | white | 3.80 ± 0.02 | 20.0 | 0.103 ± 0.04 | 2.53 ± 0.21 | Private beekeeper, Cluj County, Romania, 2017 |
| 7 | Rapeseed | 94.13 | amber | 3.66 ± 0.03 | 36.6 | 0.266 ± 0.05 | 4.75 ± 0.41 | Private beekeeper, Cluj County, Romania, 2017 |
| 8 | Multiflower | 100.45 | amber | 3.49 ± 0.01 | 24.0 | 0.073 ± 0.01 | 2.74 ± 0.11 | “Lume de miel”, certified by Famille Michand, France, 2018 |
| 9 | Raspberry | 101.56 | amber | 3.53 ± 0.02 | 30.0 | 0.076 ± 0.03 | 3.15 ± 0.19 | “Medokomerc”, Čestín, Czech Republic, 2017 |
| 10 | Clover | 18.99 | yellow- white | 3.74 ± 0.04 | 20.0 | 0.046 ± 0.06 | 4.09 ± 0.24 | “Pykoht”, Dubna, Russia, 2017, |
| 11 | Multiflower | 170.76 | dark amber | 4.04 ± 0.02 | 44.0 | 0.140 ± 0.01 | 4.63 ± 0.33 | “Isis Mel”, Embu-Guaçu, Brazil, 2018 |
| 12 | Sunflower | 62.07 | light amber | 3.88 ± 0.01 | 21.0 | 0.404 ± 0.04 | 3.8 ± 0.33 | Private beekeeper, Olekszyn, Wielkopolskie, Poland, 2016 |
| 13 | Raspberry | 91.29 | amber | 3.65 ± 0.02 | 21.0 | 0.292 ± 0.03 | 3.48 ± 0.09 | Private beekeeper, Olekszyn, Wielkopolskie, Poland, 2016 |
| 14 | Buckwheat | 190.7 | dark amber | 3.59 ± 0.03 | 72.5 | 0.556 ± 0.02 | 6.01 ± 0.03 | “Pasieka Andrzej Kuś”, Kujawsko-Pomorskie, Poland, 2016 |
| 15 | Rapeseed | 34.34 | white | 4.02 ± 0.06 | 12.8 | 0.187 ± 0.01 | 3.85 ± 0.09 | Private beekeeper, Solec Kujawski, Kujawsko- Pomorskie, Poland, 2016 |
| 16 | Buckwheat | 107.5 | amber | 3.89 ± 0.02 | 35.0 | 0.310 ± 0.03 | 5.38 ± 0.29 | “Sądecki Bartnik”, Stróże, Małopolskie, Poland, 2016 |
| 17 | Goldenrod | 104.53 | amber | 3.67 ± 0.01 | 49.0 | 0.669 ± 0.05 | 5.36 ± 0.08 | “Jakubiec gospodarstwo”, Bielsko-Biała,Śląskie, Poalnd, 2015 |
| 18 | Sunflower | 114.44 | dark amber | 3.68 ± 0.03 | 25.0 | 0.374 ± 0.04 | 3.91 ± 0.22 | “Sądecki Bartnik”, Stróże, Małopolskie, Poland, 2016 |
| 19 | Acacia | 10.2 | extra white | 3.58 ± 0.05 | 22.0 | 0.235 ± 0.01 | 3.55 ± 0.2 | Private beekeeper, Karczowiska Górne, Warmińsko-Mazurskie, Poland, 2017 |
| 20 | Acacia | 21.47 | white | 3.61 ± 0.02 | 16.5 | 0.213 ± 0.05 | 4.08 ± 0.16 | Private beekeeper, Janowiec, Lubelskie, Poland 2017 |
| 21 | Linden | 68.01 | light amber | 4.02 ± 0.01 | 23.0 | 0.678 ± 0.03 | 4.46 ± 0.1 | “Sądecki Bartnik”, Stróże, Małopolskie, Poland, 2018 |
| 22 | Multifloral/linden | 59.72 | light amber | 4.27 ± 0.03 | 30.0 | 0.854 ± 0.02 | 4.75 ± 0.21 | Private beekeeper, Wilga Mazowieckie, Poland, 2016 |
| 23 | Goldenrod | 58.85 | light amber | 3.31 ± 0.02 | 42.7 | 0.331 ± 0.01 | 4.43 ± 0.06 | “Słoneczna Pasieka”, Stryków, Łódzkie, Poland, 2016 |
| 24 | Dandelion | 101.31 | amber | 3.99 ± 0.00 | 22.0 | 0.050 ± 0.04 | 3.57 ± 0.14 | Private beekeeper, Białowieża, Podlaskie, Poland, 2017 |
| 25 | Ivy vine | 514.55 | green | 3.80 ± 0.02 | 53.0 | 0.258 ± 0.01 | 5.29 ± 0.23 | “Piotr Nowakowski”, Wrocław, Dolnośląskie, Poland, 2018 |
| 26 | Buckwheat | 117.04 | dark amber | 3.2 ± 0.01 | 100.0 | 0.100 ± 0.02 | 5.33 ± 0.14 | Private beekeeper, Białystok, Podlaskie, Poland, 2018 |
| 27 | Raspberry | 87.45 | amber | 3.82 ± 0.03 | 33.0 | 0.061 ± 0.02 | 6.01 ± 0.22 | Private beekeeper, Białowieża, Podlaskie, Poland 2017 |
| 28 | Buckwheat | 182.15 | dark amber | 3.78 ± 0.04 | 65.0 | 0.088 ± 0.01 | 6.24 ± 0.06 | Private beekeeper, Białowieża, Podlaskie, Poland 2017 |
| 29 | Multiflower | 219.42 | dark amber | 3.66 ± 0.01 | 80,0 | 0.087 ± 0.05 | 5.62 ± 0.02 | Private beekeeper, Lubelskie, Poland, 2017 |
| 30 | Spring flowers | 39.42 | very light amber | 3.60 ± 0.03 | 23.0 | 0.041 ± 0.02 | 2.76 ± 0.11 | Private beekeeper, Janowiec, Lubelskie, Poland, 2018 |
| 31 | Buckwheat | 184.63 | dark amber | 3.59 ± 0.02 | 44.0 | 0.393 ± 0.02 | 5.73 ± 0.21 | “Barć Świętokrzyska” Daleszyce, Świętokrzyskie, Poland, 2016 |
| 32 | Honeydew | 151.08 | dark amber | 4.49 ± 0.01 | 31.0 | 1.221 ± 0.05 | 5.21 ± 0.27 | “Sądecki Bartnik”, Stróże, Małopolskie, Poland, 2017 |
| 33 | Rape | 114.07 | amber | 3.64 ± 0.04 | 15.0 | 0.181 ± 0.02 | 2.72 ± 0.19 | “Sądecki Bartnik”, Stróże, Małopolskie, Poland, 2018 |
| 34 | Honeydew/buckwheat | 408.08 | dark amber | 3.58 ± 0.02 | 84.0 | 0.114 ± 0.03 | 7.03 ± 0.17 | Private beekeeper, Karczowiska Górne, Warmińsko-Mazurskie, Poland, 2017 |
| 35 | Buckwheat | 160.12 | dark amber | 3.28 ± 0.03 | 95.0 | 0.106 ± 0.01 | 5.17 ± 0.07 | Private beekeeper, Sosnówka, Dolnośląskie, Poland, 2018 |
| 36 | Rape | 85.47 | light amber | 3.59 ± 0.02 | 24.0 | 0.035 ± 0.02 | 3.48 ± 0.27 | Private beekeeper, Miłków, Dolnośląskie, Poland, 2018 |
| 37 | Buckwheat | 231.05 | dark amber | 4.04 ± 0.01 | 29.0 | 0.462 ± 0.03 | 4.97 ± 0.28 | Private beekeeper, Krzeczyn Mały k. Lubina, Dolnośląskie, Poland, 2016 |
| 38 | Linden/Multiflora | 76.8 | light amber | 4.27 ± 0.02 | 33.3 | 0.812 ± 0.03 | 4.21 ± 0.16 | Private beekeeper, Bobrowniki, Kujawsko- Pomorskie, Poland, 2016 |
| Standard | Retention Time (Rt) | Retention Index (Ri) | Regression Equation | R2 | RSD% | LOD (ng*mL−1) | LOQ (ng*mL−1) |
|---|---|---|---|---|---|---|---|
| Xylose | 9.51 | 1728 | y = 0.1136x − 0.0836 | 0.9990 | 2.42 | 10.81 | 32.75 |
| 9.68 | 1735 | y = 0.2014x + 0.0347 | 0.9998 | 2.34 | 19.92 | 60.35 | |
| D-fructose | 10.08 | 1832 | y = 0.274x − 1.3427 | 0.9992 | 1.03 | 8.66 | 26.25 |
| 10.14 | 1840 | y = 0.1245x − 0.5283 | 0.9995 | 2.15 | 3.68 | 11.14 | |
| 10.24 | 1847 | y = 0.0788x − 0.5207 | 0.9990 | 1.90 | 3.87 | 11.73 | |
| D-Pinitol | 10.44 | 1861 | y = 0.4234x + 0.0917 | 0.9998 | 0.15 | 2.72 | 8.25 |
| Quabrachitol | 10.86 | 1878 | y = 0.1074x − 0.0521 | 0.9993 | 1.08 | 4.12 | 12.47 |
| Allo-inositol | 10.98 | 1902 | y = 0.4181x − 0.3954 | 0.9983 | 1.07 | 19.06 | 57.76 |
| D-Glucose | 11.15 | 1922 | y = 0.2911x − 4.8439 | 0.9977 | 1.11 | 2.69 | 8.17 |
| 12.25 | 2006 | y = 0.0807x − 0.4037 | 0.9983 | 3.43 | 4.12 | 12.47 | |
| Neo-inositol | 11.25 | 1934 | y = 0.2969x − 0.1251 | 0.9995 | 0.93 | 10.89 | 33.00 |
| Muco-inositol | 11.49 | 1941 | y = 0.2338x − 0.1819 | 0.9994 | 1.12 | 7.29 | 22.09 |
| D-Chiro-inositol | 11.93 | 1965 | y = 1.279x + 0.8057 | 0.9994 | 0.10 | 6.02 | 18.25 |
| Sequoyitol | 12.01 | 1973 | y = 0.167x − 0.0591 | 0.9993 | 1.46 | 7.13 | 21.60 |
| Ononitol | 12.12 | 1988 | y = 0.0929x − 0.0288 | 0.9996 | 1.15 | 4.12 | 12.47 |
| Bornesitol | 12.51 | 2035 | y = 0.2315x − 0.0338 | 0.9996 | 1.16 | 11.22 | 33.99 |
| Epi-inositol | 12.70 | 2070 | y = 0.2038x + 0.1189 | 0.9993 | 0.53 | 5.61 | 17.00 |
| Cis-inositol | 13.00 | 2095 | y = 0.1617x + 0.0601 | 0.9979 | 0.84 | 9.72 | 29.44 |
| Myo-inositol | 13.53 | 2120 | y = 1.0889x − 0.1878 | 0.9997 | 0.56 | 19.42 | 58.88 |
| Sucrose | 18.67 | 2686 | y = 0.0259x + 0.0075 | 0.9988 | 2.52 | 2.69 | 8.17 |
| Maltose | 18.77 | 2702 | y = 0.1744x + 0.048 | 0.9981 | 1.86 | 6.16 | 18.65 |
| Lactose | 18.91 | 2730 | y = 0.0151x + 0.0138 | 0.9987 | 1.90 | 1.50 | 4.55 |
| D-(+)-turanose | 19.14 | 2747 | y = 0.1222x − 0.0637 | 0.9996 | 3.08 | 1.75 | 5.31 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratiu, I.A.; Al-Suod, H.; Bukowska, M.; Ligor, M.; Buszewski, B. Correlation Study of Honey Regarding their Physicochemical Properties and Sugars and Cyclitols Content. Molecules 2020, 25, 34. https://doi.org/10.3390/molecules25010034
Ratiu IA, Al-Suod H, Bukowska M, Ligor M, Buszewski B. Correlation Study of Honey Regarding their Physicochemical Properties and Sugars and Cyclitols Content. Molecules. 2020; 25(1):34. https://doi.org/10.3390/molecules25010034
Chicago/Turabian StyleRatiu, Ileana Andreea, Hossam Al-Suod, Małgorzata Bukowska, Magdalena Ligor, and Bogusław Buszewski. 2020. "Correlation Study of Honey Regarding their Physicochemical Properties and Sugars and Cyclitols Content" Molecules 25, no. 1: 34. https://doi.org/10.3390/molecules25010034






