Effect of Heterocyclic Ring on LnIII Coordination, Luminescence and Extraction of Diamides of 2,2′-Bipyridyl-6,6′-Dicarboxylic Acid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
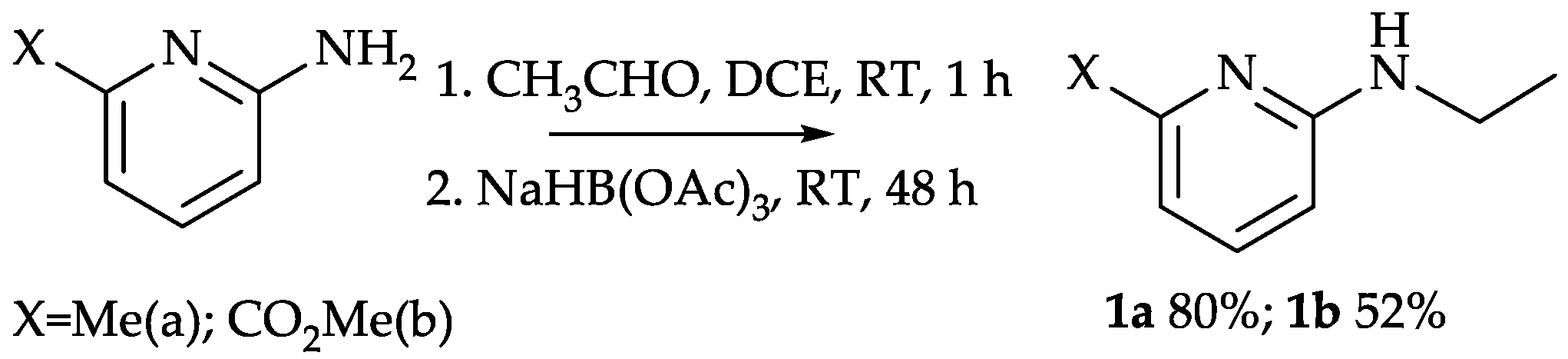
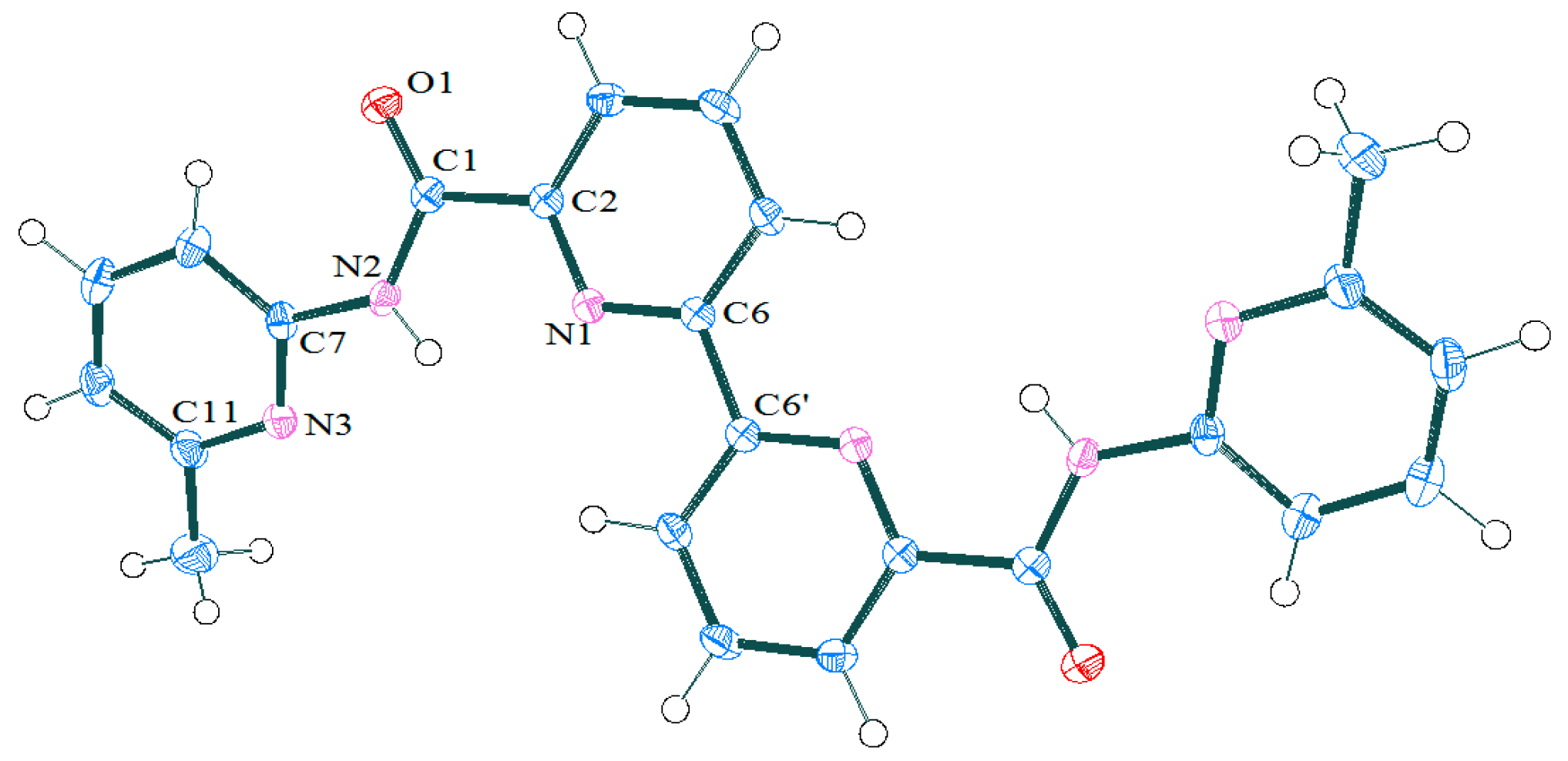
2.1.1. Preparation and Structure of Diamide-Based Ligands
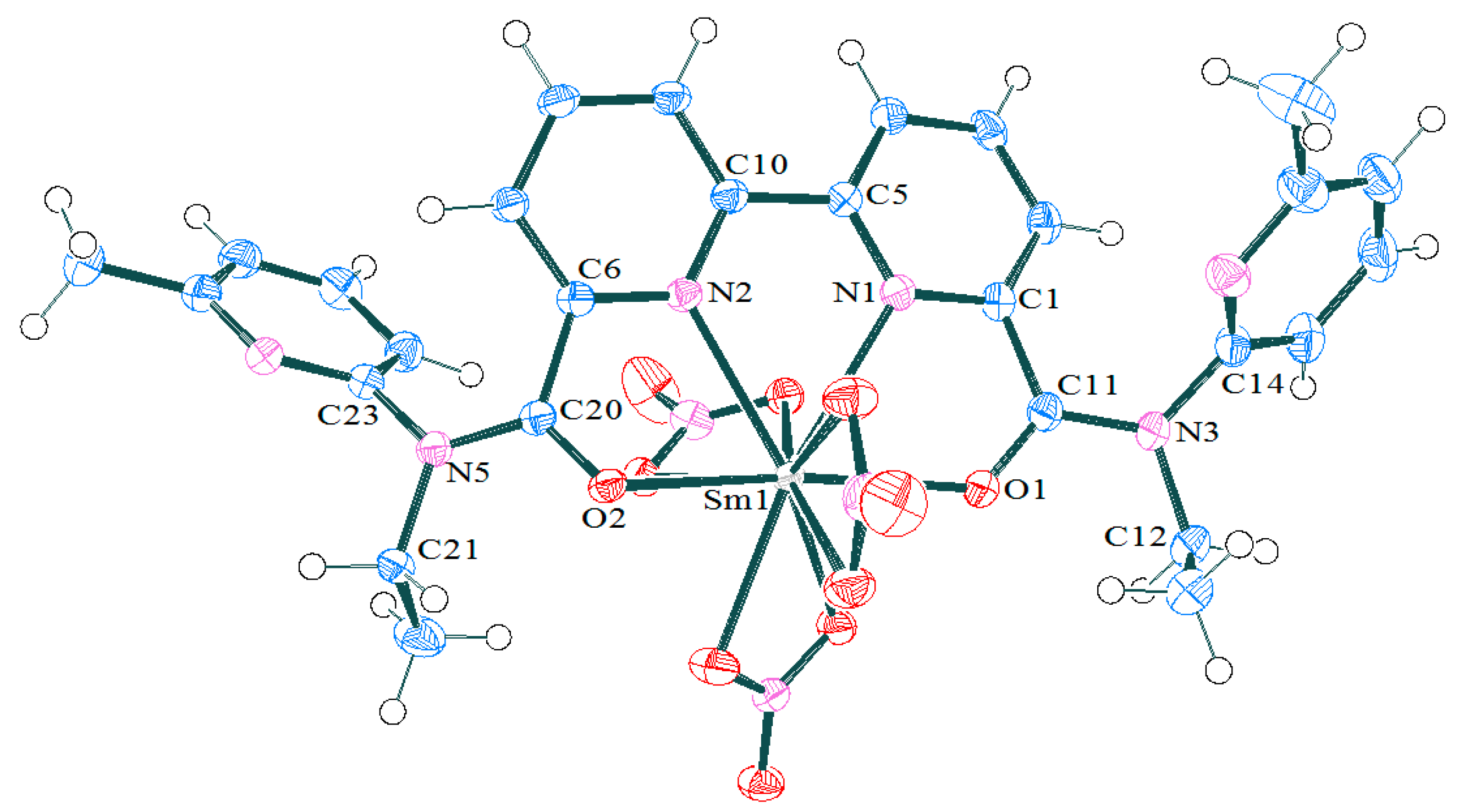
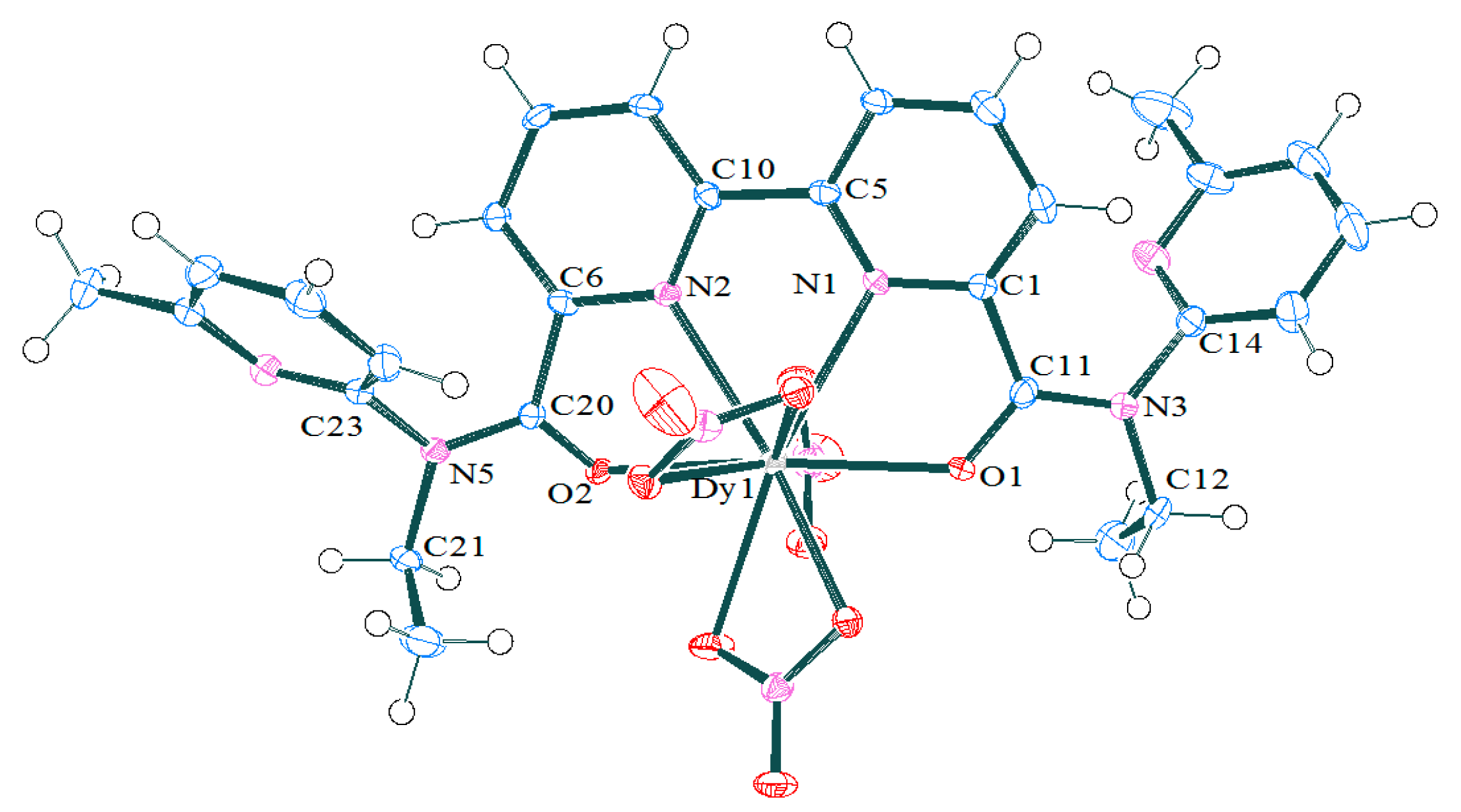
2.1.2. Synthesis and Structures of LnIII Complexes
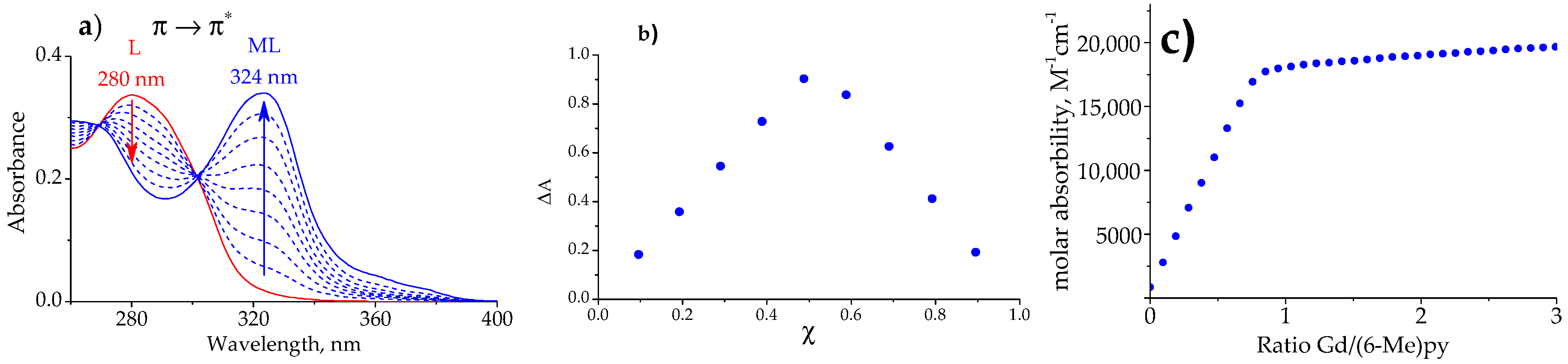
2.2. Stability of the LnIII Complexes and Extraction Efficiency of the Ligands
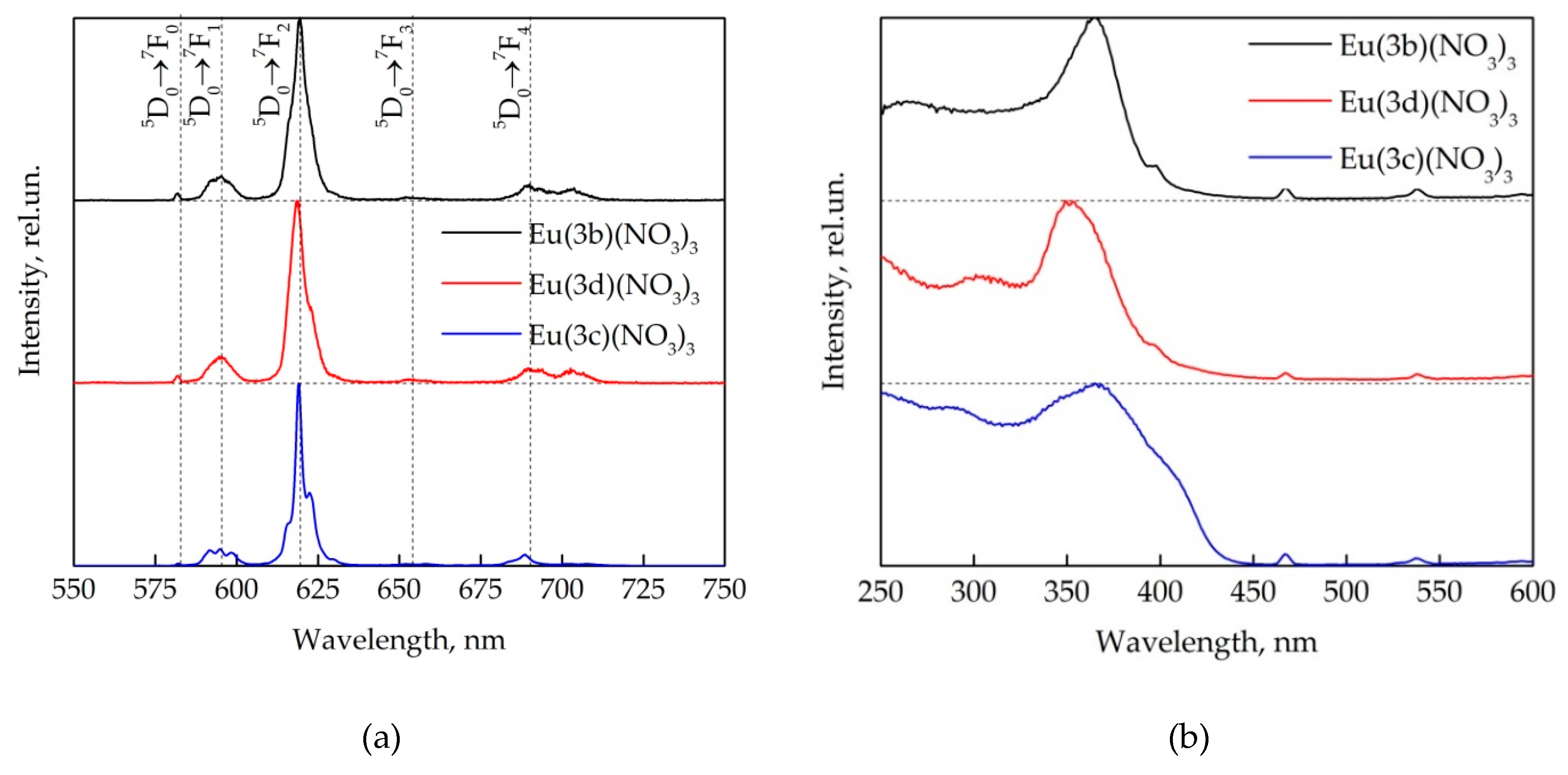
2.3. Photophysical Properties of Ln(3)(NO3)3 Complexes
3. Materials and Methods
3.1. General
3.2. Spectrophotometric Titration
3.3. Luminescent Measurements
3.4. Reductive Amination Using Sodium Triacetoxyborohydride
3.5. Alkylation and Hydrolysis of the N-acetylaminopyridines
3.5.1. General Method 1
3.5.2. General Method 2
3.6. Synthesis of 2,2′-bipyridyl-6,6′-dicarboxylic acid diamides (General Method)
3.7. Synthesis of Complexes with Lanthanides Nitrates (General Method)
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kodama, K.; Kobayashi, A.; Hirose, T. Synthesis and Spectral Properties of Ruthenium(II) Complexes Based on 2,2′-Bipyridines Modified by a Perylene Chromophore. Tetrahedron Lett. 2013, 54, 5514–5517. [Google Scholar] [CrossRef]
- Güden-Silber, T.; Klein, K.; Seitz, M. 4,4′-Bis(Trifluoromethyl)-2,2′-Bipyridine – a Multipurpose Ligand Scaffold for Lanthanoid-Based Luminescence/19F NMR Probes. Dalton Trans. 2013, 42, 13882–13888. [Google Scholar] [CrossRef] [PubMed]
- Kenausis, G.; Taylor, C.; Katakis, I.; Heller, A. “Wiring” of Glucose Oxidase and Lactate Oxidase within a Hydrogel Made with Poly(Vinyl Pyridine) Complexed with [Os(4,4′-Dimethoxy-2,2′-Bipyridine)2Cl]+/2+. J. Chem. Soc. Faraday Trans. 1996, 92, 4131–4136. [Google Scholar] [CrossRef]
- Reddy, B.N.K.; Raju, B.D.R.; Thyagarajan, K.; Ramanaiah, R.; Jho, Y.D.; Reddy, B.S. Optical characterization of Eu3+ ion doped alkali oxide modified borosilicate glasses for red laser and display device applications. Ceram. Int. 2017, 43, 8886–8892. [Google Scholar] [CrossRef]
- Sukegawa, A.; Sekiguchi, H.; Matsuzaki, R.; Yamane, K.; Okada, H.; Kishino, K.; Wakahara, A. Self-Organized Eu-Doped GaN Nanocolumn Light-Emitting Diode Grown by RF-Molecular-Beam Epitaxy. Phys. Status Solidi A 2019, 216, 1800501. [Google Scholar] [CrossRef]
- Xie, W.; Wang, J.; Cao, M.; Hu, Z.; Feng, Y.; Chen, X.; Jiang, N.; Dai, J.; Shi, Y.; Babin, V.; et al. Fabrication and properties of Eu:Lu2O3 transparent ceramics for X-ray radiation detectors. Opt. Mater. 2018, 80, 22–29. [Google Scholar] [CrossRef]
- Zych, E.; Wόjtowicz, M.; Dobrowolska, A.; Kȩpiński, L. Radioluminescence and photoluminescence of hafnia-based Eu-doped phosphors. Opt. Mater. 2009, 31, 1764–1767. [Google Scholar] [CrossRef]
- Ekberg, C.; Löfström-Engdahl, E.; Aneheim, E.; Foreman, M.R.S.; Geist, A.; Lundberg, D.; Denecke, M.; Persson, I. The structures of CyMe4-BTBP complexes of americium(III) and europium(III) in solvents used in solvent extraction, explaining their separation properties. Dalton Trans. 2015, 44, 18395–18402. [Google Scholar] [CrossRef] [Green Version]
- Lewis, F.W.; Harwood, L.M.; Hudson, M.J.; Distler, P.; John, J.; Stamberg, K.; Núñez, A.; Galán, H.; Espartero, A.G. Synthesis and evaluation of lipophilic BTBP ligands for An/Ln separations in nuclear waste treatment: The effect of alkyl substitution on extraction properties and implications for ligand design. Eur. J. Org. Chem. 2012, 8, 1509–1519. [Google Scholar] [CrossRef] [Green Version]
- Heine, J.; Müller-Buschbaum, K. Engineering metal-based luminescence in coordination polymers and metal–organic frameworks. Chem. Soc. Rev. 2013, 42, 9232–9242. [Google Scholar] [CrossRef]
- Borisova, N.E.; Ivanov, A.V.; Matveev, P.I.; Smirnova, A.A.; Belova, E.V.; Kalmykov, S.N.; Myasoedov, B.F. Screening of the structure of americium extractants based on a 2,2′-bipyridyl scaffold: A simple way to a N2,O2-tetradentate ligands library for rational design of An/Ln extractants. ChemistrySelect 2018, 3, 1983–1989. [Google Scholar] [CrossRef]
- Borisova, N.E.; Sumyanova, T.B.; Kharcheva, A.V.; Matveev, P.I.; Ivanov, A.V.; Razumova, E.A.; Patsaeva, S.V. The lanthanide complexes of 2,2′-bipyridyl-6,6′-dicarboxylic dimethylanilides: The influence of a secondary coordination sphere on the stability, structure, luminescence and f-element extraction. Dalton Trans. 2018, 47, 16755–16765. [Google Scholar] [CrossRef] [PubMed]
- Borisova, N.E.; Kostin, A.A.; Eroshkina, E.A.; Reshetova, M.D.; Lyssenko, K.A.; Spodine, E.N.; Puntus, L.N. Lanthanide complexes with tetradentate N,N′,O,O′-dipyridyl-based ligands: Structure, stability, and photophysical properties. Eur. J. Inorg. Chem. 2014, 13, 2219–2229. [Google Scholar] [CrossRef]
- Ikawa, T.; Fujita, Y.; Mizusaki, T.; Betsuin, S.; Takamatsu, H.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Selective N-alkylation of amines using nitriles under hydrogenation conditions: Facile synthesis of secondary and tertiary amines. Org. Biomol. Chem. 2012, 10, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Li, D.; Jiang, Z.; Li, Z. A Facile N-monoalkylation of aminopyridines. J. Chem. Res. 2011, 35, 628–629. [Google Scholar] [CrossRef]
- Okamoto, I.; Terashima, M.; Masu, H.; Nabeta, M.; Ono, K.; Morita, N.; Katagiri, K.; Azumaya, I.; Tamura, O. Acid-induced conformational alteration of cis-preferential aromatic amides bearing N-methyl-N-(2-pyridyl) moiety. Tetrahedron 2011, 67, 8536–8543. [Google Scholar] [CrossRef]
- Devi, P.; Barry, S.M.; Houlihan, K.M.; Murphy, M.J.; Turner, P.; Jensen, P.; Rutledge, P.J. Synthesis and structural characterisation of amides from picolinic acid and pyridine-2,6-dicarboxylic acid. Sci. Rep. 2015, 5, 9950. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coordin. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Kharcheva, A.V.; Ivanov, A.V.; Borisova, N.E.; Kaminskaya, T.P.; Patsaeva, S.V.; Popov, V.V.; Yuzhakov, V.I. Luminescent solutions and films of new europium complexes with chelating ligands. In Proceedings of the Saratov Fall Meeting 2014, Optical Technologies in Biophysics and Medicine, Laser Physics and Photonics XVI, and Computational Biophysics XVI, Saratov, Russia, 23–26 September 2014. [Google Scholar]
- Aebischer, A.; Gumy, F.; Bunzli, J.C. Intrinsic quantum yields and radiative lifetimes of lanthanide tris(dipicolinates). Phys. Chem. Chem. Phys. 2009, 11, 1346–1353. [Google Scholar] [CrossRef]
- Bünzli, J.C.G.; Eliseeva, S.V. Basics of Lanthanide Photophysics. Lanthanide Luminescence: Photophysical, Analytical and Biological Aspects; Spinger Series on Fluorescence; Hänninen, P., Härmä, H., Eds.; Springer Verlag: Berlin, Germany, 2010; Vol. 8. [Google Scholar]
- Ferrarini, P.L.; Mori, C.; Badawneh, M.; Calderone, V.; Greco, R.; Manera, C.; Martinelli, A.; Nieri, P.; Saccomanni, G. Synthesis and β-blocking activity of (R,S)-(E)-oximeethers of 2,3-dihydro-1,8-naphthyridine and 2,3-dihydrothiopyrano[2,3-b]pyridine:potential antihypertensive agents—Part IX. Eur. J. Med. Chem. 2000, 35, 815–826. [Google Scholar] [CrossRef]
- Pan, S.; Matsuo, Y.; Endo, K.; Shibata, T. Cationic iridium-catalyzed enantioselective activation of secondary sp3 C–H bond adjacent to nitrogen atom. Tetrahedron 2012, 68, 9009–9015. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, K.; Xu, C.; Miao, H.; Ding, Y. Synthesis, structures of benzoxazolyl iridium(III) complexes, and applications on C–C and C–N bond formation reactions under solvent-free conditions: Catalytic activity enhanced by noncoordinating anion without silver effect. ACS Catal. 2014, 4, 3910–3918. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, R.; Wan, H.; Yang, Y.; Wang, D. Alanine triazole iridium-catalyzed C–N bond formation through borrowing hydrogen strategy. Tetrahedron Lett. 2016, 57, 4588–4591. [Google Scholar] [CrossRef]
- Krein, D.M.; Lowary, T.L. A Convenient synthesis of 2-(alkylamino)pyridines. J. Org. Chem. 2002, 67, 4965–4967. [Google Scholar] [CrossRef]
- Sajiki, H.; Ikawa, T.; Hirota, K. Reductive and catalytic monoalkylation of primary amines using nitriles as an alkylating reagent. Org. Lett. 2004, 6, 4977–4980. [Google Scholar] [CrossRef]
- Wallaca, K.J.; Belcher, W.J.; Turner, D.R.; Syed, K.F.; Steed, J.W. Slow anion exchange, conformational equilibria, and fluorescent sensing in venus flytrap aminopyridinium-based anion hosts. J. Am. Chem. Soc. 2003, 125, 9699–9715. [Google Scholar] [CrossRef]
- Watanabe, Y.; Morisaki, Y.; Kondo, T.; Mitsudo, T.A. Ruthenium complex-controlled catalytic N-mono- or N,N-dialkylation of heteroaromatic amines with alcohols. J. Org. Chem. 1996, 61, 4214–4218. [Google Scholar] [CrossRef]
- Yakhontov, L.N.; Marshalkin, M.F. Dokl. Akad. Nauk SSSR 1971, 199, 625–627.
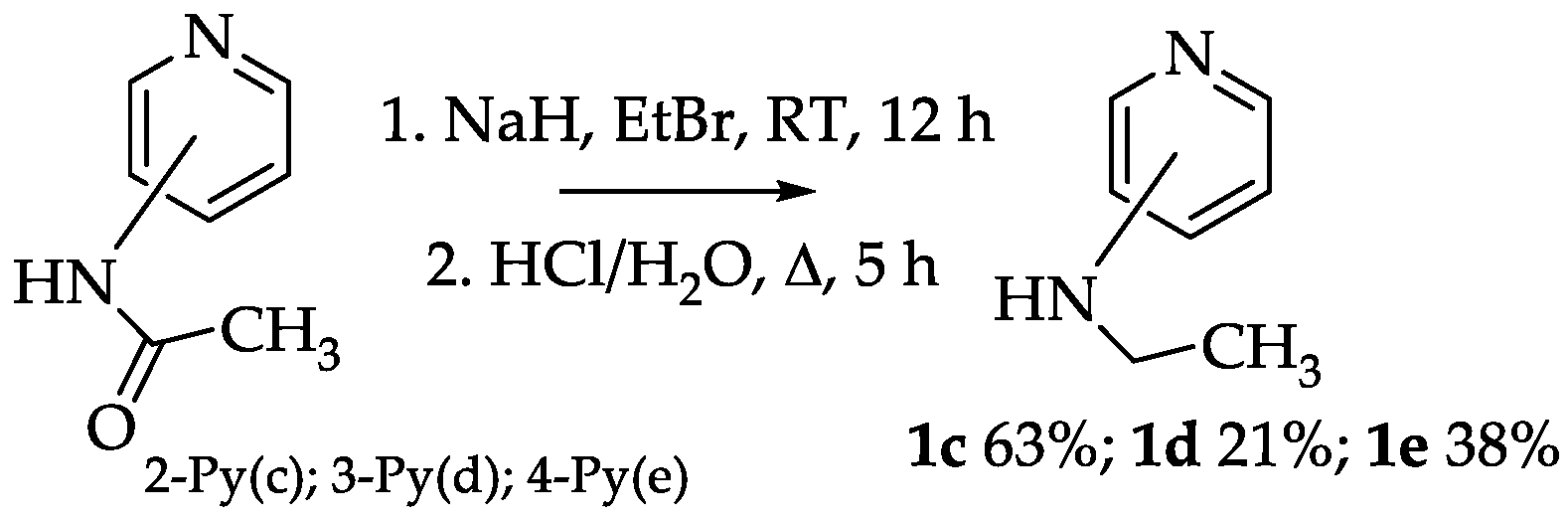



Compound Number and Structure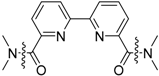 | Acid Moiety | Amidic Moiety | |||||
|---|---|---|---|---|---|---|---|
| 3Py | 4Py | 5Py | 3Py′ | 4Py′ | 5Py′ | ||
| 2a | 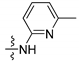 | 8.3 d | 8.4 br.s | 8.8 dd | 7.0 dd | 7.7 t | 8.2 dd |
| 2b | 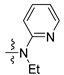 | 7.49 d | 7.65 t | 7.78 dd | 6.93 d | 7.45 td | 6.96 ddd |
| 2c | 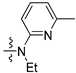 | 7.51 br.d | 7.64 t | 7.76 d | 6.64 br.d | 7.30 t | 6.80 d |
| 2d |  | 7.32 d | 7.70 t | 7.81 d | 8.44 d | - | 8.44 d |
| 2e | 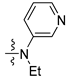 | 7.4 | 7.7 | 7.7 | - | 8.3 | 8.4 |
| Sm(3c)(NO3)3 | 8.35 d | 7.97 t | 7.02 d | 7.33 d | 7.63 t | 6.91 d | |
| Eu(3c)(NO3)3 | 4.25br.s | 5.84 br.s | 4.34 br.s | 7.25 br.s | 7.83 br.s | 7.97 br.s | |
 BPDA (R = H); F-BPDA (R = 2F); Bridge-BPDA (R = 3,4-(CH2)4 | ||||
|---|---|---|---|---|
| Bonds/Angles | 2a | F-BPDA [11] | Bridge-BPDA [11] | BPDA [13] |
| C1-C2 | 1.512(2) | 1.519(3) | 1.502(2) | 1.511(2) |
| C1-O1 | 1.224(2) | 1.239(3) | 1.222(2) | 1.230(1) |
| C1-N2 | 1.361(2) | 1.371(3) | 1.358(2) | 1.357(2) |
| N2-H | 0.85(2) | - | - | - |
| N2-C7 | 1.411(2) | 1.447(3) | 1.436(2) | 1.435(2) |
| N1-C6-C6′-N1′ | 180.0(1) | 180.0(2) | 180.0(2) | 180.0(1) |
| N1-C2-C1-O1 | 177.2(1) | 142.0(2) | -151.4(2) | 138.7(1) |
| Angle between amidic Ar and Py planes | 23.19 | 61.0 | 68.8 | 63.8 |
| Angle between O=CNC and Ar-plane | 20.05 | 63.3 | 68.4 | 64.6 |
| Angle between O=CNC and Py-plane | 3.64 | 35.7 | 24.7 | 37.5 |
| Bonds/Angles | NdBPDA(NO3)3 [13] | TbBPDA(NO3)3 [13] | Sm(3c)(NO3)3 | Dy(3c)(NO3)3 | |
|---|---|---|---|---|---|
| M–O1 M–O2 | 2.481(1) 2.409(1) | 2.402(3) 2.325(3) | 2.447(2) 2.405(2) | 2.424(3) 2.387(3) | 2.407(4) 2.346(5) |
| dM-O1–dM-O2 | 0.072(1) | 0.077(3) | 0.042(2) | 0.037(3) | 0.61(5) |
| M–N1 M–N2 | 2.605(2) 2.683(2) | 2.514(3) 2.611(3) | 2.553(2) 2.649(2) | 2.529(3) 2.636(3) | 2.497(6) 2.603(5) |
| dM–N1–dM–N2 | 0.078(2) | 0.097(3) | 0.096(2) | 0.107(3) | 0.106(6) |
| O1–M–O2 | 161.48(5) | 154.66(8) | 158.78(6) | 157.36(9) | 155.9(2) |
| N1–M–N2 | 61.30(5) | 62.65(6) | 61.64(6) | 61.9(1) | 62.4(2) |
| Deviation of caps from normal to the square planes | 15 | 4.821 9.277 | 5.531 9.456 | 6.749 10.049 | |
| Angle between two planes of antiprism | 1.2–1.7 | 1.67 | 2.23 | 3.32 | |
| Mean deviation of atoms from the square planes | - | 0.089 0.189 | 0.086 0.184 | 0.073 0.174 | |
| Ln3+ | La | Ce | Pr | Nd | Sm | Gd | Er | Tm | Yb |
|---|---|---|---|---|---|---|---|---|---|
| 2c | 6.02 ± 0.04 | 5.89 ± 0.04 | 5.97 ± 0.04 | 6.05 ± 0.05 | 6.18 ± 0.06 | 6.36 ± 0.05 | 6.6 ± 0.1 | 6.7 ± 0.1 | 7.0 ± 0.1 |
| 2,5-diMeBPDA [12] | 6.11 | 6.42 | 6.59 | 6.82 | 6.71 | 6.30 | 6.7 | 6.33 | 6.5 |
| 2,4-diMeBPDA [12] | 6.45 | 6.41 | 7.11 | 7.36 | 6.96 | 6.59 | 7.29 | 6.91 | 7.05 |
| 3,4-diMeBPDA [12] | 5.6 | 6.91 | 7.6 | 8.3 | 7.6 | 6.76 | 7.35 | 7.28 | 6.9 |
| 3,5-diMeBPDA [12] | 6.0 | 6.51 | 7.19 | 7.6 | 6.89 | 6.69 | 7.3 | 7.23 | 6.86 |
| BPDA [13] | 6.2 | 5.6 | 5.9 | 5.9 | 5.7 | 5.7 | 5.4 | 5.9 | 5.6 |
| Europium Complex | Eu(3b)(NO3)3 | Eu(3d)(NO3)3 | Eu(3c)(NO3)3 |
|---|---|---|---|
| Wavelength of absorption maximum, nm | 322 | 320 | 323 |
| Asymmetry ratio | 11.95 | 11.94 | 11.90 |
| Observed luminescence lifetime, ms | 1.30 | 1.21 | 1.23 |
| Radiative lifetime, ms | 1.91 | 1.91 | 1.92 |
| Internal luminescence quantum yield, % | 65 | 63 | 64 |
| External luminescence quantum yield, % | 5 | 14 | 18 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borisova, N.E.; Ivanov, A.V.; Kharcheva, A.V.; Sumyanova, T.B.; Surkova, U.V.; Matveev, P.I.; Patsaeva, S.V. Effect of Heterocyclic Ring on LnIII Coordination, Luminescence and Extraction of Diamides of 2,2′-Bipyridyl-6,6′-Dicarboxylic Acid. Molecules 2020, 25, 62. https://doi.org/10.3390/molecules25010062
Borisova NE, Ivanov AV, Kharcheva AV, Sumyanova TB, Surkova UV, Matveev PI, Patsaeva SV. Effect of Heterocyclic Ring on LnIII Coordination, Luminescence and Extraction of Diamides of 2,2′-Bipyridyl-6,6′-Dicarboxylic Acid. Molecules. 2020; 25(1):62. https://doi.org/10.3390/molecules25010062
Chicago/Turabian StyleBorisova, Nataliya E., Alexey V. Ivanov, Anastasia V. Kharcheva, Tsagana B. Sumyanova, Uliana V. Surkova, Petr I. Matveev, and Svetlana V. Patsaeva. 2020. "Effect of Heterocyclic Ring on LnIII Coordination, Luminescence and Extraction of Diamides of 2,2′-Bipyridyl-6,6′-Dicarboxylic Acid" Molecules 25, no. 1: 62. https://doi.org/10.3390/molecules25010062





