1. Introduction
Translational dynamics of molecules in porous materials can be efficiently studied by nuclear magnetic resonance (NMR), such that various groups of methods were proposed for this purpose [
1]. In general, all these methods are based on the use of magnetic field gradients. A notable technique for studying the translational dynamics is the pulse gradient spin-echo (PGSE) sequence in which two gradient pulses were superimposed in a spin-echo pulse sequence [
2]. In the case of unrestricted diffusion, for example, for free diffusion of molecules in liquids, molecular displacements follow a normal distribution so as to the phases the molecules gain during the PGSE sequence. In the sequence, the two gradient pulses have an opposite effect, such that the phases produced by the gradient pulses are canceled for a stationary spin and result in a net phase shift for a non-stationary spin. For such a diffusion the signal attenuation is an exponential function of the phase standard deviation squared. For the PGSE sequence, this is proportional to the square of the product between the gradient pulse duration and its amplitude, with the time separation between the gradient pulses and to the diffusion coefficient. With all the instrumental parameters known, the diffusion coefficient can be calculated from the signal attenuation.
When diffusion is restricted, like in porous materials, then the previous approach for measurement of the diffusion coefficient can lead to different results, depending on the choice of parameters used for the measurement. An important parameter is the time separation between the gradient pulses, which can also be considered as the diffusion observation time. When the observation time is short, such that the diffusing molecules on average do not displace enough to reach the walls of the porous structure, the measured diffusion coefficient corresponds to that of the unrestricted diffusion. However, when the observation time is sufficiently long, such that the diffusing molecules displace enough to experience the restriction of the porous structure, this results in the reduction of the measured diffusion coefficient compared to the diffusion coefficient of unrestricted diffusion. The ratio between the two extreme cases, i.e., between the unrestricted and restricted diffusion coefficients, corresponds also to the tortuosity of the porous medium, which is more generally defined as the ratio between the length of the shortest curve connecting two points following the porous structure and the straight line distance between the points [
1,
3].
In the case of restricted diffusion, the distribution of molecular displacements is, in general, no longer Gaussian. Therefore, the result for the measured diffusion coefficient at a given diffusion observation time also depends on the selection of the gradient amplitude in the PGSE measurement. In addition, the measurement of just one parameter is insufficient for the description of non-Gaussian displacements distributions. Therefore, a set of PGSE sequences are often run with the amplitudes of gradient pulses that increase in a step-wise manner. With this approach, a set of NMR signals with an increasing signal attenuation is henceforth acquired. From the set of signals, an average propagator can be calculated using the Fourier transformation [
4]. The average propagator can be considered as the spatial average probability function for the spin to have a certain dynamic displacement over the diffusion observation time [
5,
6]. The average propagator in the case of unrestricted diffusion equals to the normal distribution and is equal to more complex functions when diffusion is restricted [
7]. The study of translational dynamics, therefore, can be considered as a time-based analysis due to its dependency on the diffusion observation time.
In another group of methods, the molecular translational dynamics are analyzed in the frequency domain [
8,
9,
10,
11], such that the diffusion is considered as a function of the frequency of translational molecular motion. In theory, the diffusion coefficient as a function of frequency, also known as the diffusion spectrum, is defined as the Fourier transform of the molecular velocity autocorrelation function [
12]. In this method, the diffusion spectrum is measured by periodically causing spin dephasing and rephrasing using an oscillating gradient. The time integral of the gradient is known as the spin dephasing function, and its Fourier transform as the dephasing spectrum. It can be shown mathematically that the molecular velocity autocorrelation function is associated with the spin-echo signal attenuation via the spin dephasing function. More specifically, the spin-echo signal attenuation is proportional to the frequency integral of the product between the diffusion spectrum and the spin dephasing spectrum power [
8,
9]. From this relation, the diffusion spectrum cannot be expressed explicitly unless the dephasing spectrum is shaped in a Dirac’s delta-like function. This can be experimentally attained by using modulated gradients (MG) which harmonically oscillate and the spectra meet the above conditions. The frequency of gradient modulation defines the position of the delta function in the frequency domain and therefore also the frequency at which the diffusion spectrum is measured. For measuring one modulated-gradient spin-echo (MGSE) sequence, the diffusion spectrum is obtained for a single point; therefore, the MGSE sequence must be repeated several times to measure the diffusion spectrum in a sufficiently large number of frequency points. Harmonically oscillating gradients can be attained by using the time-varying magnetic field gradients, such as the OGSE (oscillating gradient spin-echo) method [
10,
13] or they can be obtained by the constant gradient MGSE method in which the gradient is superimposed to the Carr-Purcell-Meiboom-Gill (CPMG) radiofrequency (RF) pulse sequence [
11,
14]. The second approach results in an effective oscillating gradient with a period of oscillation equal to the double inter-echo time.
A popular porous material often studied by NMR is cement. Several different NMR methods were used in these studies, including the solid-state NMR spectroscopy [
15] of different nuclei in cement compounds, e.g.,
29Si [
16],
27Al [
17] and
2H [
18]. This method can also be optimized to allow in situ applications [
19]. The previously reported cement materials are most often used in civil engineering, like different modifications of Portland cement. However, few practical studies were also demonstrated in dentistry, i.e., glass ionomer cements [
20]. The porous structure of the cement materials can also be probed by NMR of hyperpolarized gases such as
129Xe [
21]. A large group of cement NMR studies have been focused on studying cement hydration. This process can be followed by the sequential measurements of NMR relaxation times in cement [
22,
23]. The imaging modality of this method can also be performed [
24]. Lastly, the diffusion measurements of water in cement is another option for studying cement hydration, especially in the early stages of hydration [
25]. The other applications of NMR in civil engineering can be found in moisture content measurements [
26], detection of water depth profiles in concrete or wood materials [
27], and determination of porosity and pore size distribution in soils [
28].
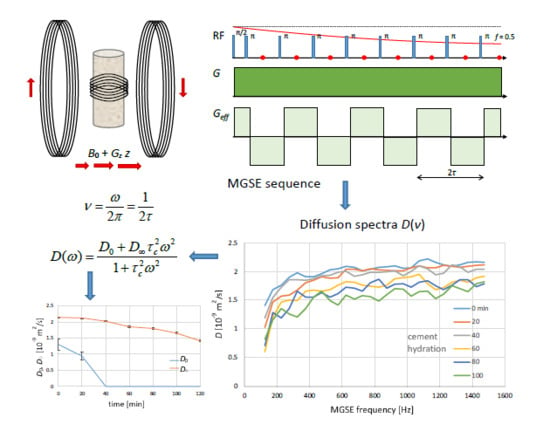
In this study, the constant gradient MGSE method was tested for acquisition of the diffusion spectra, first on a sample with unrestricted diffusion and then on a sample with restricted diffusion. The latter was a hydrating white cement paste sample. Due to the cement hydration process, the porous structure of the sample changed with time. This process was followed by the sequential acquisition of the diffusion spectra, which were also analyzed by the corresponding mathematical model function, and the model parameters were extracted for each spectrum using the best-fit model analysis. In comparison to our previous MGSE studies [
11,
14,
29,
30], which were mainly theoretical or advocated the proof of concept, this is the first MGSE study aiming to more practical applications of the method for the construction materials. The MGSE technique has a clear advantage of acquiring spectral information over the traditional methods for the assessment of cement hydration, e.g., hydration heat under isothermal condition [
31]. The diffusion coefficient is measured in the range of frequencies corresponding to different dynamics of translational molecular motion.
2. Results and Discussion
An example of the diffusion spectrum, the calculation for a single frequency point of 1 kHz, is shown in
Figure 1. The spectrum was calculated for the white cement paste sample at the beginning of hydration.
Figure 1a shows a sequence of
N = 40 zero-frequency filtered signals of spin-echoes acquired at a magnetic field gradient of
GN = 3.21 T/m using the MGSE sequence. It can be seen that the echo signals approach to the noise threshold line set to tenfold the noise level. As the signals were acquired in the constant magnetic field gradient, they originated from a thin slice located in the center of the sample. Due to the orientation of the magnetic field gradient, the slice was orientated longitudinally with respect to the sample. Its thickness is inversely proportional to the gradient amplitude
and was calculated to 57 μm. The corresponding signals of spin-echoes acquired using the same sequence and parameters without a magnetic field gradient were approximately 70 times higher (
Figure 1b). This is because the signals originated from the entire sample of 5 mm in diameter. In
Figure 1c, the sequence of
N normalized measured echo signals
(blue dots) is shown. These were obtained by dividing each echo signal measured with the magnetic field gradient against the corresponding echo signal measured without the magnetic field gradient. The normalized echoes were then analyzed using the model function
(gray curve) to find the corresponding best-fit model parameters
A and
D (Equation (3)). The void dots in the graph correspond to the measured signals below the noise threshold line and were not included in the best-fit model analysis.
The constant gradient MGSE method was applied first to the bulk water sample to test the method for its stability. The diffusion of water molecules in the sample was unrestricted, and the displacements of water molecules for any diffusion observation time should have the Gaussian distribution. The equivalent of this result in the frequency domain is a flat diffusion spectrum
, where
is dependent on temperature. As the experiments were performed at room temperature (23 °C), the expected value for diffusion is 2.2 × 10
−9 m
2/s [
32]. The measured diffusion spectrum for the water sample in
Figure 2a meets the expectations quite well. The spectrum is flat over the entire range of measured frequencies, i.e., from 50 to 1500 Hz, and its value is in the expected range, including the error bars for each measured frequency point. However, the error is increasing with an increasing frequency of measurement. In order to avoid the existence of coherence pathways other than the direct one, the signal in this method is acquired only from the narrow frequency band of the width equal to the reciprocal signal acquisition time
[
29]. To keep the signal attenuation with all frequencies identical, the gradient amplitude is set proportional to the frequency of the MGSE experiment, such that the signal originates from a progressively thinner slice with the increasing frequencies. This explains why the measurements with higher frequencies have more noise. For the last experimental point at 1500 Hz, where the gradient was highest (4.81 T/m), the signal was originated only from a 38 μm thick slice, while for the first experimental point at 50 Hz, the gradient was 30 times lower (0.16 T/m) so that 30 times more signal was originated from 30 times thicker slice of 1.14 mm.
The same method was also applied to the hydrating white cement paste sample. As the sample was changing over time, the diffusion spectra were acquired sequentially every 20 min. In
Figure 2b, the six measured diffusion spectra of the white cement paste sample are illustrated. The acquisition parameters for the spectra were identical to those in the experiment with water; the measured frequency range, all timing parameters, and gradient amplitudes were the same. From the spectra, it can be inferred that all of them exhibited a similar trend, which was significantly different compared to the spectrum of the bulk water sample. The spectra were no longer flat and they all start with low diffusion values at low frequencies which gradually increased to a plateau at higher frequencies. With an increasing cement hydration time, the diffusion values of the spectra decreased over the entire frequency range (in the beginning as well as in the plateau). This result can be better seen in
Figure 3a, where the diffusion spectra were analyzed for its dependence on the cement hydration time over three different frequency ranges: low, with frequencies up to 500 Hz, medium, with frequencies between 500 and 1000 Hz, and high, with frequencies between 1000 and 1500 Hz. In the analysis, the frequency range values were calculated as an average diffusion value of the respective frequency range for diffusion spectra of all hydration times. In
Figure 3a, the diffusion values decreased with an increasing hydration time for all three frequency ranges and the values for each hydration time were the lowest in the case of a low-frequency range, and steadily increased for medium to high-frequency ranges, such that the values for medium frequency range were only slightly lower than for the high-frequency range.
The shape of the cement diffusion spectra can be explained by the porous structure of the cement paste sample and with the hydration, time decreasing the porosity of it. At higher frequencies, diffusion spectra are only sensitive to small pores, whereas, at lower frequencies, they are sensitive both to small and larger pores. This explains the shape of diffusion spectra that was increasing with frequency. Another way of understanding the diffusion spectra is to examine the position of their inflection point. This is sensitive to the pore size as described by Equation (7). With larger pores, the inflection point is at low frequencies. However, with the decrease in porosity, the inflection point is shifting to the higher frequencies. According to the manufacturer’s specifications, the cement powder had a grain of up to 90 μm, so that the pore size order of the cement paste at the beginning of hydration was approximately 10 μm and decreased during the hydration for three orders of magnitude to approximately 10 nm at the end of hydration. In the spectra, the lowest measured frequency was equal to 50 Hz. According to Equation (7), this frequency corresponds to the characteristic time of 126 ms and the measurement at this frequency is sensitive to pores of 24 μm or smaller. Here, it is assumed that D is equal to the free water diffusion at room temperature. The same analysis for the highest measured frequency of 1500 Hz yielded a pore size of 4.3 μm or smaller. For the 10 nm pore size, the inflection point was already in the range of 300 MHz. Therefore, this method was able to detect the inflection point only at the initial stage of cement hydration, but not at later stages of hydration as the inflection point shifted to frequencies out of the measured range. However, the decreasing trend of diffusion spectrum at lower frequencies during the hydration was still possible to detect. There are two reasons for the inability to measure the diffusion spectrum at higher frequencies than 1500 Hz. One of which is that for such a detection much higher magnetic field gradients are needed, and the other is an increasingly shorter T2 of the cement paste sample during hydration. Longer T2 values (as for example in bulk water) enable detection of longer MGSE echo trains and also a more accurate measurement of diffusion at lower magnetic field gradients.
The reduction in porosity during the cement hydration can also be seen from graphs of transverse (
T2) and longitudinal (
T1) NMR relaxation times as a function of cement hydration time in
Figure 3b,c, respectively.
T2 relaxation was initially equal to 14 ms, and after two hours of hydration, it decreased to 8 ms in an almost linear pattern. A decrease from 120 ms to 80 ms was also observed in
T1 relaxation time. According to the Brownstein–Tarr equation (Equation (5)), both relaxation rates, transverse (1/
T2), and longitudinal (1/
T1) increase with the surface to volume ratio
S/
V. In a system with decreasing porosity, the surface to volume ratio is increasing and so do the relaxation rates (1/
T2 and 1/
T1), while the relaxation times (
T2 and
T1) are decreasing, as depicted in the graphs. No bleeding was observed during the hydration of cement paste samples, which may potentially spoil the measured relaxation times due to the two-component relaxation process. The first component belongs to the water in the pores and the second one belongs to the water film on the top of the sample. Both measured relaxation times of the cement paste sample were shorter than expected for a pure white cement paste sample. This is most likely because white cement was used for building construction and has, therefore, more likely the paramagnetic impurities present than very pure laboratory-grade white cement. The impurities, when mixed with water, act as
T1 and
T2 NMR contrast agents [
33].
Diffusion spectra of cement paste in
Figure 2b are noisier than the diffusion spectrum of water in
Figure 2a, especially with higher hydration times. There are several explanations for this. The first is that the cement paste contained 38% of water and 62% of cement (by weight ratios); therefore, the water concentration in the cement paste sample was lower than in the water sample. Another reason indicates a much shorter
T2 relaxation time of the cement paste sample than that of the water sample, i.e., 8–14 ms vs. 2000 ms. In the constant gradient MGSE sequence, the echo train signals were acquired up to 20 ms after the signal excitation. Due to the long
T2 relaxation time of water, the signals from all the echoes were used for the calculation of the water diffusion coefficient for all the frequencies. However, with the cement paste sample,
T2 was for all the measured spectra shorter than the signal echo-train, which was 20 ms long. In addition, the signals were also decaying during the echo train because of the diffusion in the constant external gradient, which generated late echoes approaching noise. This effect was higher for higher frequencies due to a thinner slice from which the signal originated and was higher for longer hydration times due to shorter
T2 relaxation times. As the noisy echoes could spoil the calculation of the diffusion coefficient, echoes with the signal to noise ratio lower than 10 were excluded from the best-fit analysis for calculation of the diffusion coefficient (see example in
Figure 1). Therefore, the diffusion coefficients were often calculated from fewer experimental points (echo signals), which reduced the precision of the calculation. An important factor that contributed to the signal reduction with longer hydration times was also the chemical transformation of water during the cement hydration process from free water to the compounds, e.g., hydrate phases such as ettringite, that do not produce detectable signals by the MGSE method. The signal reduction on account of the water transformation after two hours of hydration was 46%. Another problem, especially with low frequencies are the internal gradients. This is because, with low frequencies, the diffusion spectra are measured with longer inter-echo times and low external gradients. Therefore, the contribution of internal gradients to the signal attenuation of the echo train could become significant, especially in the systems with inclusions of highly paramagnetic or diamagnetic particles. Without compensation for the internal gradient effect, the corresponding extra signal attenuation would be incorrectly attributed to a faster diffusion. This problem is greatly reduced in the constant gradient MGSE method by normalization of the measured echoes prior to their best fit analysis. During normalization, each echo acquired with the external magnetic field gradient is divided by the corresponding echo acquired without the gradient. The normalized echoes have no
T2 relaxation time dependence and significantly reduced dependence on the internal gradients. If the signal attenuation due to the internal gradients is high, the normalized echoes are very noisy, which can ultimately prevent the calculation of the diffusion constants. For this reason, it was not possible to calculate the diffusion constant at 50 Hz in the diffusion spectra of the cement paste sample.
Figure 4 shows the modeled diffusion spectra that were obtained by the best-fit analysis using the model in Equation (6) and data form the measured spectra in
Figure 2b, while the dependence of the diffusion and characteristic time model parameters on the hydration time are shown in
Figure 4b and 4c, respectively. The quality of fit is demonstrated just for the initial spectrum where the experimental data points are shown along with the error bars, while for the remaining hydration times, the modeled spectra are shown. Diffusion parameters
and
are both decreasing with an increasing hydration time, which was expected. However, the decrease
was very fast; the parameter reached zero after 40 min only. In the case of fitting analysis, the negative values for
were obtained with hydration times larger than 40 min, which is unrealistic and possibly due to increased noise in spectra for longer hydration times. Therefore, for these times,
was assumed zero, and only the remaining two parameters were fitted. This change in the fitting analysis can consequently generate confusing results for the characteristic time parameter
. These are such that a decreasing trend for an increasing hydration time is anticipated possibly due to the reduction in porosity. However, this was not obtained.
decreased for the first two time points (0 and 20 min), and it subsequently increased at 40 min (change from three to two-parameter fitting) and consequently decreased again until the last point at 120 min. The increment at the last point can be attributed to high spectral noise.