Recent Advances in Chemical Biology Using Benzophenones and Diazirines as Radical Precursors
Abstract
:1. Introduction
2. Chemical Principles
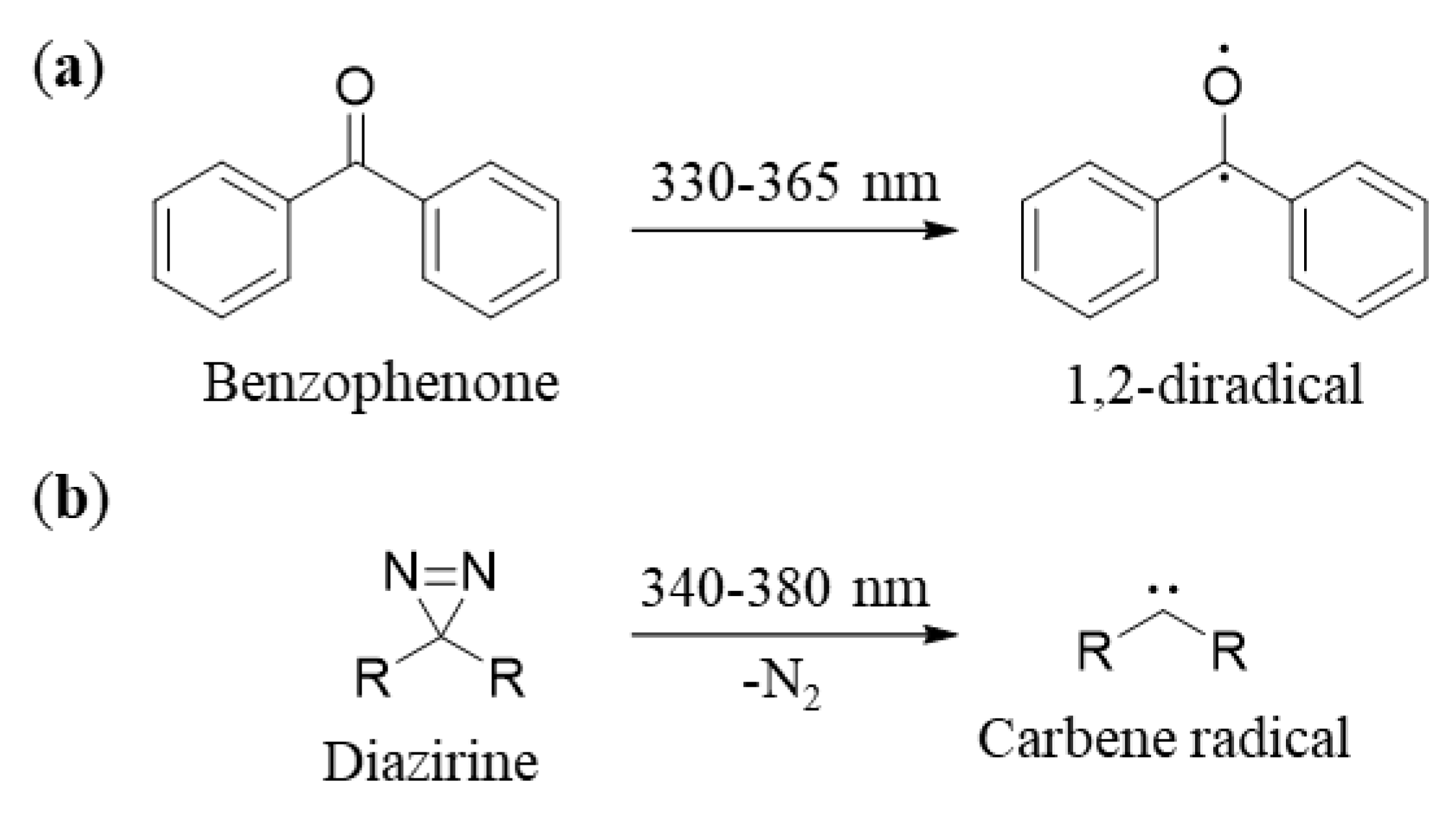
2.1. Benzophenones
2.2. Diazirines
2.2.1. Chemical and Physical Properties
2.2.2. Recent Synthetic Developments
3. Applications of Benzophenone and Diazirine Radical Precursors to Chemical Biology
3.1. Benzophenones
3.1.1. Understanding Biological Interactions and Mechanisms
3.1.2. Other Applications Incorporating BP Probes
3.2. Diazirines in Chemical Biology
3.2.1. Target Engagement and Discovery of Novel Bioactive Compounds
3.2.2. Studying Complex Protein Interactions
3.2.3. Probing Membrane Proteins and Other Protein–Biomolecule Interactions
3.2.4. Molecular Markers for Imaging Studies
4. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galardy, R.E.; Craig, L.C.; Jamieson, J.D.; Printz, M.P. Photoaffinity Labeling of Peptide Hormone Binding Sites. J. Biol. Chem. 1974, 249, 3510–3518. [Google Scholar] [CrossRef] [PubMed]
- Dormán, G.; Nakamura, H.; Pulsipher, A.; Prestwich, G.D. The Life of Pi Star: Exploring the Exciting and Forbidden Worlds of the Benzophenone Photophore. Chem. Rev. 2016, 116, 15284–15398. [Google Scholar] [CrossRef] [PubMed]
- Dormán, G.; Prestwich, G.D. Benzophenone Photophores in Biochemistry. Biochemistry 1994, 33, 5661–5673. [Google Scholar] [CrossRef] [PubMed]
- Kotzyba-Hibert, F.; Kapfer, I.; Goeldner, M. Recent Trends in Photoaffinity Labeling. Angew. Chem. Int. Ed. 1995, 34, 1296–1312. [Google Scholar] [CrossRef]
- Prestwich, G.D.; Dormán, G.; Elliott, J.T.; Marecak, D.M.; Chaudhary, A. Benzophenone Photoprobes for Phosphoinositides, Peptides and Drugs. Photochem. Photobiol. 1997, 65, 222–234. [Google Scholar] [CrossRef]
- Brown, R.D.; Hefferman, M.L. Study of Formaldehyde by a ‘Self-Consistent Electronegativity’ Molecular–Orbital Method. Trans. Faraday Soc. 1957, 54, 757–764. [Google Scholar] [CrossRef]
- Alabugin, I.V. Stereoelectronic Effects: A Bridge Between Structure and Reactivity; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- El-Sayed, M.A.; Zinsli, P. Triplet Spin Label and Molecular Dynamics. J. Lumin. 1976, 12–13, 389–395. [Google Scholar] [CrossRef]
- Aloïse, S.; Ruckebusch, C.; Blanchet, L.; Réhault, J.; Buntinx, G.; Huvenne, J.P. The Benzophenone S1(n,Π*) →T1(n,π *) States Intersystem Crossing Reinvestigated by Ultrafast Absorption Spectroscopy and Multivariate Curve Resolution. J. Phys. Chem. A 2008, 112, 224–231. [Google Scholar] [CrossRef]
- Yabumoto, S.; Sato, S.; Hamaguchi, H.O. Vibrational and Electronic Infrared Absorption Spectra of Benzophenone in the Lowest Excited Triplet State. Chem. Phys. Lett. 2005, 416, 100–103. [Google Scholar] [CrossRef]
- Spighi, G.; Gaveau, M.A.; Mestdagh, J.M.; Poisson, L.; Soep, B. Gas Phase Dynamics of Triplet Formation in Benzophenone. Phys. Chem. Chem. Phys. 2014, 16, 9610–9618. [Google Scholar] [CrossRef]
- Khalaf, A. Photochemistry and Free Radical Stabilisation of the Captodative Centre. Trends Photochem. Photobiol. 2010, 12, 7–15. [Google Scholar]
- Hopkinson, A.C. Radical Cations of Amino Acids and Peptides: Structures and Stabilities. Mass Spectrom. Rev. 2009, 28, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Gioannini, T.L. The Use of Benzophenone as a Photoaffinity Label, Labeling in p-Benzoylphenylacetyl Chymotrypsin at Unit Efficiency. Photochem. Photobiol. 1979, 29, 883–892. [Google Scholar] [CrossRef]
- Weber, P.J.A.; Beck-Sickinger, A.G. Comparison of the Photochemical Behavior of Four Different Photoactivatable Probes. J. Pept. Res. 1997, 49, 375–383. [Google Scholar] [CrossRef]
- Lapinsky, D.J. Tandem Photoaffinity Labeling-Bioorthogonal Conjugation in Medicinal Chemistry. Bioorg. Med. Chem. 2012, 20, 6237–6247. [Google Scholar] [CrossRef] [PubMed]
- Vodovozova, E.L. PhotoaffinityLabeling and Its Application in Structural Biology. Biokhimiya 2007, 72, 5–26. [Google Scholar] [CrossRef]
- Tian, Y.; Lin, Q. Recent Development of Photo-Cross-Linkers as Tools for Biomedical Research. CHIMIA Int. J. Chem. 2018, 72, 758–763. [Google Scholar] [CrossRef]
- Huang, W.C.C.; Kuo, K.T.T.; Adebayo, B.O.O.; Wang, C.H.H.; Chen, Y.J.J.; Jin, K.; Tsai, T.H.H.; Yeh, C.T.T. Garcinol Inhibits Cancer Stem Cell-like Phenotype via Suppression of the Wnt/β-Catenin/STAT3 Axis Signalling Pathway in Human Non-Small Cell Lung Carcinomas. J. Nutr. Biochem. 2018, 54, 140–150. [Google Scholar] [CrossRef]
- Liu, H.W.W.; Lee, P.M.M.; Bamodu, O.A.A.; Su, Y.K.K.; Fong, I.H.H.; Yeh, C.T.T.; Chien, M.H.H.; Kan, I.H.H.; Lin, C.M.M. Enhanced Hsa-Mir-181d/p-STAT3 and Hsa-MiR-181d/p-STAT5A Ratios Mediate the Anticancer Effect of Garcinol in STAT3/5A-Addicted Glioblastoma. Cancers 2019, 11, 1888. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Park, C.; Oh, E.; Sung, Y.; Lee, J.; Park, K.H.; Kang, H. Benzophenone Compounds, from a Marine-Derived Strain of the Fungus Pestalotiopsis Neglecta, Inhibit Proliferation of Pancreatic Cancer Cells by Targeting the MEK/ERK Pathway. J. Nat. Prod. 2019, 82, 3357–3365. [Google Scholar] [CrossRef]
- Bae, S.Y.Y.; Liao, L.; Park, S.H.H.; Kim, W.K.K.; Shin, J.; Lee, S.K.K. Antitumor Activity of Asperphenin A, a Lipopeptidyl Benzophenone from Marine-Derived Aspergillus Sp. Fungus, by Inhibiting Tubulin Polymerization in Colon Cancer Cells. Mar. Drugs 2020, 18, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boscá, F.; Miranda, M.A. Photosensitizing Drugs Containing the Benzophenone Chromophore. J. Photochem. Photobiol. B Biol. 1998, 43, 1–26. [Google Scholar] [CrossRef]
- Oya, A.; Tanaka, N.; Kusama, T.; Kim, S.Y.; Hayashi, S.; Kojoma, M.; Hishida, A.; Kawahara, N.; Sakai, K.; Gonoi, T.; et al. Prenylated Benzophenones from Triadenum Japonicum. J. Nat. Prod. 2015, 78, 258–264. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, L.; Alves, K.F.F.; Maciel-Rezende, C.M.M.; de Jesus, L.O.P.; Pires, F.R.R.; Junior, C.V.V.; Izidoro, M.A.A.; de Júdice, W.A.S.; dos Santos, M.H.H.; Marques, M.J.J. Benzophenone Derivatives as Cysteine Protease Inhibitors and Biological Activity against Leishmania(L.) Amazonensis Amastigotes. Biomed. Pharmacother. 2015, 75, 93–99. [Google Scholar] [CrossRef]
- Heo, J.; Shin, H.; Lee, J.; Kim, T.; Inn, K.S.S.; Kim, N.J.J. Synthesis and Biological Evaluation of N-Cyclopropylbenzamide-Benzophenone Hybrids as Novel and Selective P38 Mitogen Activated Protein Kinase (MAPK) Inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 3694–3698. [Google Scholar] [CrossRef]
- Georgakis, N.D.D.; Karagiannopoulos, D.A.A.; Thireou, T.N.N.; Eliopoulos, E.E.E.; Labrou, N.E.E.; Tsoungas, P.G.G.; Koutsilieris, M.N.N.; Clonis, Y.D.D. Concluding the Trilogy: The Interaction of 2,2′-Dihydroxy-Benzophenones and Their Carbonyl N-Analogues with Human Glutathione Transferase M1-1 Face to Face with the P1-1 and A1-1 Isoenzymes Involved in MDR. Chem. Biol. Drug Des. 2017, 90, 900–908. [Google Scholar] [CrossRef]
- Surana, K.; Chaudhary, B.; Diwaker, M.; Sharma, S. Benzophenone: A Ubiquitous Scaffold in Medicinal Chemistry. Med. Chem. Commun. 2018, 9, 1803–1817. [Google Scholar] [CrossRef]
- Hill, J.R.; Robertson, A.A.B. Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis. J. Med. Chem. 2018, 61, 6945–6963. [Google Scholar] [CrossRef]
- S. R. Paulsen. 3.3-Dialkyl-Diazacyclopropen-(1). Angew. Chem. Int. Ed. 1960, 72, 781–782.
- Smith, E.; Collins, I. Photoaffinity Labeling in Target-and Binding-Site Identification. Future Med. Chem. 2015, 7, 159–183. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, N.; Robb, M.A.; Wilsey, S.; Bernardi, F.; Bottom, A.; Olivucci, M. Mechanism of Carbene Formation from the Excited States of Diazirine and Diazomethane: An MC-SCF Study. J. Am. Chem. Soc. 1994, 116, 2064–2074. [Google Scholar] [CrossRef]
- Brunner, J.; Senn, H.; Richards, F.M. 3-Trifluoromethyl-3-Phenyldiazirine. A New Carbene Generating Group for Photolabeling Reagents. J. Biol. Chem. 1980, 255, 3313–3318. [Google Scholar]
- Rosenberg, M.G.; Brinker, U.H. Constrained Carbenes. Eur. J. Org. Chem. 2006, 2006, 5423–5440. [Google Scholar] [CrossRef]
- Singh, A.; Thornton, E.R.; Westheimer, F.H. The Photolysis of Diazo-Acetylchymotrypsin. J. Biol. Chem. 1962, 237, PC3006–PC3008. [Google Scholar]
- Bayley, H.; Knowles, J.R. Photogenerated Reagents for Membrane Labeling. 2. Phenylcarbene and Adamantylidene Formed within the Lipid Bilayer. Biochemistry 1978, 17, 2420–2423. [Google Scholar] [CrossRef] [PubMed]
- Platz, M.S. A Perspective on Physical Organic Chemistry. J. Org. Chem. 2014, 79, 2341–2353. [Google Scholar] [CrossRef]
- Pliego, J.R.; De Almeida, W.B.; Celebi, S.; Zhu, Z.; Platz, M.S. Singlet-Triplet Gap, and the Electronic and Vibrational Spectra of Chlorophenylcarbene: A Combined Theoretical and Experimental Study. J. Phys. Chem. A 1999, 103, 7481–7486. [Google Scholar] [CrossRef]
- Song, M.G.; Sheridan, R.S. Regiochemical Substituent Switching of Spin States in Aryl(Trifluoromethyl) Carbenes. J. Am. Chem. Soc. 2011, 133, 19688–19690. [Google Scholar] [CrossRef]
- Wang, J.; Kubicki, J.; Peng, H.; Platz, M.S. Influence of Solvent on Carbene Intersystem Crossing Rates. J. Am. Chem. Soc. 2008, 130, 6604–6609. [Google Scholar] [CrossRef]
- Zhang, Y.; Vyas, S.; Hadad, C.M.; Platz, M.S. An Ab Initio Study of the Ground and Excited State Chemistry of Phenyldiazirine and Phenyldiazomethane. J. Phys. Chem. A 2010, 114, 5902–5912. [Google Scholar] [CrossRef]
- Das, J. Aliphatic Diazirines as Photoaffinity Probes for Proteins: Recent Developments. Chem. Rev. 2011, 111, 4405–4417. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.A. Chemical Reagents in Photoaffinity Labeling. Tetrahedron 1995, 51, 12479–12520. [Google Scholar] [CrossRef]
- Miller, D.J.; Moody, C.J. Synthetic Applications of the O-H Insertion Reactions of Carbenes and Carbenoids Derived from Diazocarbonyl and Related Diazo Compounds. Tetrahedron 1995, 51, 10811–10843. [Google Scholar] [CrossRef]
- Ge, S.S.; Chen, B.; Wu, Y.Y.; Long, Q.S.; Zhao, Y.L.; Wang, P.Y.; Yang, S. Current Advances of Carbene-Mediated Photoaffinity Labeling in Medicinal Chemistry. RSC Adv. 2018, 8, 29428–29454. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Burdzinski, G.; Kubicki, J.; Vyas, S.; Hadad, C.M.; Sliwa, M.; Poizat, O.; Buntinx, G.; Platz, M.S. Study of the S1 Excited State of Para-Methoxy-3-Phenyl-3-Methyl Diazirine by Ultrafast Time Resolved UV-Vis and IR Spectroscopies and Theory. J. Am. Chem. Soc. 2009, 131, 13784–13790. [Google Scholar] [CrossRef]
- Zhang, Y.; Burdzinski, G.; Kubicki, J.; Platz, M.S. Direct Observation of Carbene and Diazo Formation from Aryldiazirines by Ultrafast Infrared Spectroscopy. J. Am. Chem. Soc. 2008, 130, 16134–16135. [Google Scholar] [CrossRef]
- Moss, R.A. Diazirines: Carbene Precursors Par Excellence. Acc. Chem. Res. 2006, 39, 267–272. [Google Scholar] [CrossRef]
- Dubinsky, L.; Krom, B.P.; Meijler, M.M. Diazirine Based Photoaffinity Labeling. Bioorg. Med. Chem. 2012, 20, 554–570. [Google Scholar] [CrossRef]
- Ichiishi, N.; Moore, K.P.; Wassermann, A.M.; Wolkenberg, S.E.; Krska, S.W. Reducing Limitation in Probe Design: The Development of a Diazirine-Compatible Suzuki-Miyaura Cross Coupling Reaction. ACS Med. Chem. Lett. 2019, 10, 56–61. [Google Scholar] [CrossRef]
- Luo, L.; Parrish, C.A.; Nevins, N.; McNulty, D.E.; Chaudhari, A.M.; Carson, J.D.; Sudakin, V.; Shaw, A.N.; Lehr, R.; Zhao, H.; et al. ATP-Competitive Inhibitors of the Mitotic Kinesin KSP That Function via an Allosteric Mechanism. Nat. Chem. Biol. 2007, 3, 722–726. [Google Scholar] [CrossRef]
- Wang, L.; Tachrim, Z.P.; Kurokawa, N.; Ohashi, F.; Sakihama, Y.; Hashidoko, Y.; Hashimoto, M. Base-Mediated One-Pot Synthesis of Aliphatic Diazirines for Photoaffinity Labeling. Molecules 2017, 22, 1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Murai, Y.; Yoshida, T.; Ishida, A.; Masuda, K.; Sakihama, Y.; Hashidoko, Y.; Hatanaka, Y.; Hashimoto, M. Alternative One-Pot Synthesis of (Trifluoromethyl)Phenyldiazirines from Tosyloxime Derivatives: Application for New Synthesis of Optically Pure Diazirinylphenylalanines for Photoaffinity Labeling. Org. Lett. 2015, 17, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Glachet, T.; Marzag, H.; Saraiva Rosa, N.; Colell, J.F.P.; Zhang, G.; Warren, W.S.; Franck, X.; Theis, T.; Reboul, V. Iodonitrene in Action: Direct Transformation of Amino Acids into Terminal Diazirines and 15N2-Diazirines and Their Application as Hyperpolarized Markers. J. Am. Chem. Soc. 2019, 141, 13689–13696. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.-E. Bifunctional Non-Canonical Amino Acids: Combining Photo-Crosslinking with Click Chemistry. Biomolecules 2020, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Olsen, L.B.; Lau, Y.H.; Jensen, C.H.; Rossmann, M.; Baker, Y.R.; Sore, H.F.; Collins, S.; Spring, D.R. Development of a Multifunctional Benzophenone Linker for Peptide Stapling and Photoaffinity Labelling. ChemBioChem 2016, 17, 689–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moiola, M.; Memeo, M.G.; Quadrelli, P. Stapled Peptides-a Useful Improvement for Peptide-Based Drugs. Molecules 2019, 24, 3654. [Google Scholar] [CrossRef] [Green Version]
- Robertson, N.S.; Spring, D.R. Using Peptidomimetics and Constrained Peptides as Valuable Tools for Inhibiting Protein-Protein Interactions. Molecules 2018, 23, 959. [Google Scholar] [CrossRef] [Green Version]
- Walensky, L.D.; Bird, G.H. Hydrocarbon-Stapled Peptides: Principles, Practice, and Progress. J. Med. Chem. 2014, 57, 6275–6288. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Cross, A.R.R.; Crowe-McAuliffe, C.; Weigert-Munoz, A.; Csatary, E.E.E.; Solinski, A.E.E.; Krysiak, J.; Goldberg, J.B.B.; Wilson, D.N.N.; Medina, E.; et al. The Natural Product Elegaphenone Potentiates Antibiotic Effects against Pseudomonas Aeruginosa. Angew. Chem. Int. Ed. 2019, 58, 8581–8584. [Google Scholar] [CrossRef]
- Venkatraman, R.K.; Orr-Ewing, A.J. Photochemistry of Benzophenone in Solution: A Tale of Two Different Solvent Environments. J. Am. Chem. Soc. 2019, 141, 15222–15229. [Google Scholar] [CrossRef]
- Dewanji, A.; Krach, P.E.; Rueping, M. The Dual Role of Benzophenone in Visible-Light/Nickel Photoredox-Catalyzed C−H Arylations: Hydrogen-Atom Transfer and Energy Transfer. Angew. Chem. Int. Ed. 2019, 58, 3566–3570. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, F.; Andreu, I.; Brunetti, M.; Schmallegger, M.; Gescheidt, G.; Neshchadin, D.; Miranda, M.A. Hydrogen Abstraction from the C15 Position of the Cholesterol Skeleton. J. Org. Chem. 2019, 84, 15184–15191. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.H.; Fetzer, C.; Sieber, S.A. Chemical Probes Unravel an Antimicrobial Defense Response Triggered by Binding of the Human Opioid Dynorphin to a Bacterial Sensor Kinase. J. Am. Chem. Soc. 2017, 139, 6152–6159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shouksmith, A.E.; Gawel, J.M.; Nawar, N.; Sina, D.; Raouf, Y.S.; Bukhari, S.; He, L.; Johns, A.E.; Manaswiyoungkul, P.; Olaoye, O.O.; et al. Class I/IIb-Selective HDAC Inhibitor Exhibits Oral Bioavailability and Therapeutic Efficacy in Acute Myeloid Leukemia. ACS Med. Chem. Lett. 2020, 11, 56–64. [Google Scholar] [CrossRef]
- Porter, N.J.; Christianson, D.W. Structure, Mechanism, and Inhibition of the Zinc-Dependent Histone Deacetylases. Curr. Opin. Struct. Biol. 2019, 59, 9–18. [Google Scholar] [CrossRef]
- Lopez, J.E.; Haynes, S.E.; Majmudar, J.D.; Martin, B.R.; Fierke, C.A. HDAC8 Substrates Identified by Genetically Encoded Active Site Photocrosslinking. J. Am. Chem. Soc. 2017, 139, 16222–16227. [Google Scholar] [CrossRef]
- Porter, N.J.; Christianson, D.W. Binding of the Microbial Cyclic Tetrapeptide Trapoxin A to the Class i Histone Deacetylase HDAC8. ACS Chem. Biol. 2017, 12, 2281–2286. [Google Scholar] [CrossRef]
- Klán, P.; Šolomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013, 113, 119–191. [Google Scholar] [CrossRef]
- Anhäuser, L.; Klöcker, N.; Muttach, F.; Mäsing, F.; Špaček, P.; Studer, A.; Rentmeister, A. A Benzophenone-Based Photocaging Strategy for the N7 Position of Guanosine. Angew. Chem. Int. Ed. 2020, 59, 3161–3165. [Google Scholar] [CrossRef]
- So, W.H.; Wong, C.T.T.; Xia, J. Peptide Photocaging: A Brief Account of the Chemistry and Biological Applications. Chin. Chem. Lett. 2018, 29, 1058–1062. [Google Scholar] [CrossRef]
- Jakubovska, J.; Tauraitė, D.; Meškys, R. A Versatile Method for the UVA-Induced Cross-Linking of Acetophenone- or Benzophenone-Functionalized DNA. Sci. Rep. 2018, 8, 16484. [Google Scholar] [CrossRef] [PubMed]
- Saaidin, A.S.; Murai, Y.; Ishikawa, T.; Monde, K. Design and Synthesis of Ligand-Tag Exchangeable Photoaffinity Probe Utilizing Nosyl Chemistry. Eur. J. Org. Chem. 2019, 2019, 7563–7567. [Google Scholar] [CrossRef]
- Hill, J.R.; Coll, R.C.; Schroder, K.; Robertson, A.A.B. Design, Synthesis and Evaluation of an NLRP3 Inhibitor Diazirine Photoaffinity Probe. Tetrahedron Lett. 2020, 151849. [Google Scholar] [CrossRef]
- Wales, J.A.; Chen, C.Y.; Breci, L.; Weichsel, A.; Bernier, S.G.; Sheppeck, J.E.I.I.; Solinga, R.; Nakai, T.; Renhowe, P.A.; Jung, J.; et al. Discovery of Stimulator Binding to a Conserved Pocket in the Heme Domain of Soluble Guanylyl Cyclase. J. Biol. Chem. 2018, 293, 1850–1864. [Google Scholar] [CrossRef] [Green Version]
- Jackson, P.; Lapinsky, D.J. Appendage and Scaffold Diverse Fully Functionalized Small-Molecule Probes via a Minimalist Terminal Alkyne-Aliphatic Diazirine Isocyanide. J. Org. Chem. 2018, 83, 11245–11253. [Google Scholar] [CrossRef]
- Denton, K.E.; Krusemark, C.J. Crosslinking of DNA-Linked Ligands to Target Proteins for Enrichment from DNA-Encoded Libraries. Med. Chem. Commun. 2016, 7, 2020–2027. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.; Xu, H.; Li, J.; Lu, F.; Ma, F.; Wang, S.; Xiong, H.; Wang, W.; Buratto, D.; Zonta, F.; et al. Functionality-Independent DNA Encoding of Complex Natural Products. Angew. Chem. Int. Ed. 2019, 58, 9254–9261. [Google Scholar] [CrossRef]
- Sannino, A.; Gironda-Martínez, A.; Gorre, É.M.D.; Prati, L.; Piazzi, J.; Scheuermann, J.; Neri, D.; Donckele, E.J.; Samain, F. Critical Evaluation of Photo-Cross-Linking Parameters for the Implementation of Efficient DNA-Encoded Chemical Library Selections. ACS Comb. Sci. 2020, 22, 204–212. [Google Scholar] [CrossRef]
- Che, Y.; Gilbert, A.M.; Shanmugasundaram, V.; Noe, M.C. Inducing Protein-Protein Interactions with Molecular Glues. Bioorg. Med. Chem. Lett. 2018, 28, 2585–2592. [Google Scholar] [CrossRef]
- Flaxman, H.A.A.; Chang, C.F.F.; Wu, H.Y.Y.; Nakamoto, C.H.H.; Woo, C.M.M. A Binding Site Hotspot Map of the FKBP12-Rapamycin-FRB Ternary Complex by Photoaffinity Labeling and Mass Spectrometry-Based Proteomics. J. Am. Chem. Soc. 2019, 141, 11759–11764. [Google Scholar] [CrossRef]
- Horne, J.E.E.; Walko, M.; Calabrese, A.N.N.; Levenstein, M.A.A.; Brockwell, D.J.J.; Kapur, N.; Wilson, A.J.J.; Radford, S.E.E. Rapid Mapping of Protein Interactions Using Tag-Transfer Photocrosslinkers. Angew. Chem. Int. Ed. 2018, 57, 16688–16692. [Google Scholar] [CrossRef]
- Mulder, M.P.C.; Witting, K.; Berlin, I.; Pruneda, J.N.; Wu, K.P.; Chang, J.G.; Merkx, R.; Bialas, J.; Groettrup, M.; Vertegaal, A.C.O.; et al. A Cascading Activity-Based Probe Sequentially Targets E1-E2-E3 Ubiquitin Enzymes. Nat. Chem. Biol. 2016, 12, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Pao, K.C.; Stanley, M.; Han, C.; Lai, Y.C.; Murphy, P.; Balk, K.; Wood, N.T.; Corti, O.; Corvol, J.C.; Muqit, M.M.K.; et al. Probes of Ubiquitin E3 Ligases Enable Systematic Dissection of Parkin Activation. Nat. Chem. Biol. 2016, 12, 324–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulder, M.P.C.; Merkx, R.; Witting, K.F.; Hameed, D.S.; El Atmioui, D.; Lelieveld, L.; Liebelt, F.; Neefjes, J.; Berlin, I.; Vertegaal, A.C.O.; et al. Total Chemical Synthesis of SUMO and SUMO-Based Probes for Profiling the Activity of SUMO-Specific Proteases. Angew. Chem. Int. Ed. 2018, 57, 8958–8962. [Google Scholar] [CrossRef] [PubMed]
- Pao, K.C.; Wood, N.T.; Knebel, A.; Rafie, K.; Stanley, M.; Mabbitt, P.D.; Sundaramoorthy, R.; Hofmann, K.; Van Aalten, D.M.F.; Virdee, S. Activity-Based E3 Ligase Profiling Uncovers an E3 Ligase with Esterification Activity. Nature 2018, 556, 381–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, C.C.; Kleinman, J.I.; Brittain, S.M.; Lee, P.S.; Chung, C.Y.S.; Kim, K.; Petri, Y.; Thomas, J.R.; Tallarico, J.A.; McKenna, J.M.; et al. Covalent Ligand Screening Uncovers a RNF4 E3 Ligase Recruiter for Targeted Protein Degradation Applications. ACS Chem. Biol. 2019, 14, 2430–2440. [Google Scholar] [CrossRef]
- Zhang, Y.; Hirota, T.; Kuwata, K.; Oishi, S.; Gramani, S.G.; Bode, J.W. Chemical Synthesis of Atomically Tailored SUMO E2 Conjugating Enzymes for the Formation of Covalently Linked SUMO-E2-E3 Ligase Ternary Complexes. J. Am. Chem. Soc. 2019, 141, 14742–14751. [Google Scholar] [CrossRef]
- Gupta, G.D.; Coyaud, É.; Gonçalves, J.; Mojarad, B.A.; Liu, Y.; Wu, Q.; Gheiratmand, L.; Comartin, D.; Tkach, J.M.; Cheung, S.W.T.; et al. A Dynamic Protein Interaction Landscape of the Human Centrosome-Cilium Interface. Cell 2015, 163, 1484–1499. [Google Scholar] [CrossRef] [Green Version]
- Paek, J.; Kalocsay, M.; Staus, D.P.; Wingler, L.; Pascolutti, R.; Paulo, J.A.; Gygi, S.P.; Kruse, A.C. Multidimensional Tracking of GPCR Signaling via Peroxidase-Catalyzed Proximity Labeling. Cell 2017, 169, 338–349. [Google Scholar] [CrossRef] [Green Version]
- Branon, T.C.; Bosch, J.A.; Sanchez, A.D.; Udeshi, N.D.; Svinkina, T.; Carr, S.A.; Feldman, J.L.; Perrimon, N.; Ting, A.Y. Efficient Proximity Labeling in Living Cells and Organisms with TurboID. Nat. Biotechnol. 2018, 36, 880–898. [Google Scholar] [CrossRef]
- Hill, Z.B.; Pollock, S.B.; Zhuang, M.; Wells, J.A. Direct Proximity Tagging of Small Molecule Protein Targets Using an Engineered NEDD8 Ligase. J. Am. Chem. Soc. 2016, 138, 13123–13126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Zheng, J.; Sun, W.; Huo, Y.; Zhang, L.; Hao, P.; Wang, H.; Zhuang, M. A Proximity-Tagging System to Identify Membrane Protein–Protein Interactions. Nat. Methods 2018, 15, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Chen, L.; Liu, S.; Zhao, J.; Zhang, H.; Chen, P.R. Enzyme-Mediated Intercellular Proximity Labeling for Detecting Cell-Cell Interactions. J. Am. Chem. Soc. 2019, 141, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Carroll, K.S.; Liebler, D.C. The Expanding Landscape of the Thiol Redox Proteome. Mol. Cell. Proteom. 2016, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Van Der Reest, J.; Lilla, S.; Zheng, L.; Zanivan, S.; Gottlieb, E. Proteome-Wide Analysis of Cysteine Oxidation Reveals Metabolic Sensitivity to Redox Stress. Nat. Commun. 2018, 9, 1581. [Google Scholar] [CrossRef]
- McCutcheon, D.C.; Lee, G.; Carlos, A.; Montgomery, J.E.; Moellering, R.E. Photoproximity Profiling of Protein-Protein Interactions in Cells. J. Am. Chem. Soc. 2020, 142, 146–153. [Google Scholar] [CrossRef]
- Ren, F.; Logeman, B.L.; Zhang, X.; Liu, Y.; Thiele, D.J.; Yuan, P. X-Ray Structures of the High-Affinity Copper Transporter Ctr1. Nat. Commun. 2019, 10, 1386. [Google Scholar] [CrossRef] [Green Version]
- Standfuss, J. Membrane Protein Dynamics Studied by X-Ray Lasers—Or Why Only Time Will Tell. Curr. Opin. Struct. Biol. 2019, 57, 63–71. [Google Scholar] [CrossRef]
- Deneka, D.; Sawicka, M.; Lam, A.K.M.; Paulino, C.; Dutzler, R. Structure of a Volume-Regulated Anion Channel of the LRRC8 Family. Nature 2018, 558, 254–259. [Google Scholar] [CrossRef]
- Xiao, P.; Bolton, D.; Munro, R.A.; Brown, L.S.; Ladizhansky, V. Solid-State NMR Spectroscopy Based Atomistic View of a Membrane Protein Unfolding Pathway. Nat. Commun. 2019, 10, 3867. [Google Scholar] [CrossRef] [Green Version]
- Pinto, C.; Mance, D.; Julien, M.; Daniels, M.; Weingarth, M.; Baldus, M. Studying Assembly of the BAM Complex in Native Membranes by Cellular Solid-State NMR Spectroscopy. J. Struct. Biol. 2019, 206, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bechara, C.; Robinson, C.V. Different Modes of Lipid Binding to Membrane Proteins Probed by Mass Spectrometry. J. Am. Chem. Soc. 2015, 137, 5240–5247. [Google Scholar] [CrossRef] [PubMed]
- Bolla, J.R.; Agasid, M.T.; Mehmood, S.; Robinson, C.V. Membrane Protein–Lipid Interactions Probed Using Mass Spectrometry. Annu. Rev. Biochem. 2019, 88, 85–111. [Google Scholar] [CrossRef]
- Teo, A.C.K.; Lee, S.C.; Pollock, N.L.; Stroud, Z.; Hall, S.; Thakker, A.; Pitt, A.R.; Dafforn, T.R.; Spickett, C.M.; Roper, D.I. Analysis of SMALP Co-Extracted Phospholipids Shows Distinct Membrane Environments for Three Classes of Bacterial Membrane Protein. Sci. Rep. 2019, 9, 1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, C.V. Mass Spectrometry: From Plasma Proteins to Mitochondrial Membranes. Proc. Natl. Acad. Sci. USA 2019, 116, 2814–2820. [Google Scholar] [CrossRef] [Green Version]
- Manzi, L.; Barrow, A.S.; Hopper, J.T.S.; Kaminska, R.; Kleanthous, C.; Robinson, C.V.; Moses, J.E.; Oldham, N.J. Carbene Footprinting Reveals Binding Interfaces of a Multimeric Membrane-Spanning Protein. Angew. Chem. Int. Ed. 2017, 129, 15069–15073. [Google Scholar] [CrossRef]
- Niphakis, M.J.; Lum, K.M.; Cognetta, A.B.; Correia, B.E.; Ichu, T.A.; Olucha, J.; Brown, S.J.; Kundu, S.; Piscitelli, F.; Rosen, H.; et al. A Global Map of Lipid-Binding Proteins and Their Ligandability in Cells. Cell 2015, 161, 1668–1680. [Google Scholar] [CrossRef] [Green Version]
- Müller, R.; Citir, M.; Hauke, S.; Schultz, C. Synthesis and Cellular Labeling of Caged Phosphatidylinositol Derivatives. Chem.-A Eur. J. 2020, 26, 384–389. [Google Scholar] [CrossRef]
- Wang, D.; Du, S.; Cazenave-Gassiot, A.; Ge, J.; Lee, J.S.S.; Wenk, M.R.R.; Yao, S.Q.Q. Global Mapping of Protein–Lipid Interactions by Using Modified Choline-Containing Phospholipids Metabolically Synthesized in Live Cells. Angew. Chem. Int. Ed. 2017, 56, 5829–5833. [Google Scholar] [CrossRef]
- Arguello, A.E.; Deliberto, A.N.; Kleiner, R.E. RNA Chemical Proteomics Reveals the N6-Methyladenosine (M6A)-Regulated Protein-RNA Interactome. J. Am. Chem. Soc. 2017, 139, 17249–17252. [Google Scholar] [CrossRef]
- Kim, D.; Shin, K.; Kwon, S.G.; Hyeon, T. Synthesis and Biomedical Applications of Multifunctional Nanoparticles. Adv. Mater. 2018, 30, 1802309. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.Y.Y.; Hou, Y.R.R.; Adak, A.K.K.; Waniwan, J.T.T.; Dela Rosa, M.A.C.A.C.; Low, P.Y.Y.; Angata, T.; Hwang, K.C.C.; Chen, Y.J.J.; Lin, C.C.C. Boronate Affinity-Based Photoactivatable Magnetic Nanoparticles for the Oriented and Irreversible Conjugation of Fc-Fused Lectins and Antibodies. Chem. Sci. 2019, 10, 8600–8609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, M.L.; Kalechofsky, N.; Belzer, A.; Rosay, M.; Kempf, J.G. Brute-Force Hyperpolarization for NMR and MRI. J. Am. Chem. Soc. 2015, 137, 8428–8434. [Google Scholar] [CrossRef] [PubMed]
- Kouřil, K.; Kouřilová, H.; Bartram, S.; Levitt, M.H.; Meier, B. Scalable Dissolution-Dynamic Nuclear Polarization with Rapid Transfer of a Polarized Solid. Nat. Commun. 2019, 10, 1733. [Google Scholar] [CrossRef] [Green Version]
- Salnikov, O.G.; Nikolaou, P.; Ariyasingha, N.M.; Kovtunov, K.V.; Koptyug, I.V.; Chekmenev, E.Y. Clinical-Scale Batch-Mode Production of Hyperpolarized Propane Gas for MRI. Anal. Chem. 2019, 91, 4741–4746. [Google Scholar] [CrossRef]
- Kovtunov, K.V.; Koptyug, I.V.; Fekete, M.; Duckett, S.B.; Theis, T.; Joalland, B.; Chekmenev, E.Y. Parahydrogen-Induced Hyperpolarization of Gases. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Lee, S.J.; Jeong, K.; Shim, J.H.; Lee, H.J.; Min, S.; Chae, H.; Namgoong, S.K.; Kim, K. SQUID-Based Ultralow-Field MRI of a Hyperpolarized Material Using Signal Amplification by Reversible Exchange. Sci. Rep. 2019, 9, 12422. [Google Scholar] [CrossRef] [Green Version]
- Kovtunov, K.V.; Kovtunova, L.M.; Gemeinhardt, M.E.; Bukhtiyarov, A.V.; Gesiorski, J.; Bukhtiyarov, V.I.; Chekmenev, E.Y.; Koptyug, I.V.; Goodson, B.M. Heterogeneous Microtesla SABRE Enhancement of 15N NMR Signals. Angew. Chem. Int. Ed. 2017, 56, 10433–10437. [Google Scholar] [CrossRef]
- Svyatova, A.; Skovpin, I.V.; Chukanov, N.V.; Kovtunov, K.V.; Chekmenev, E.Y.; Pravdivtsev, A.N.; Hövener, J.B.; Koptyug, I.V. 15N MRI of SLIC-SABRE Hyperpolarized 15N-Labelled Pyridine and Nicotinamide. Chem.-A Eur. J. 2019, 25, 8465–8470. [Google Scholar] [CrossRef]
- Truong, M.L.; Theis, T.; Coffey, A.M.; Shchepin, R.V.; Waddell, K.W.; Shi, F.; Goodson, B.M.; Warren, W.S.; Chekmenev, E.Y. 15N Hyperpolarization by Reversible Exchange Using SABRE-SHEATH. J. Phys. Chem. C 2015, 119, 8786–8797. [Google Scholar] [CrossRef] [Green Version]
- Theis, T.; Truong, M.L.; Coffey, A.M.; Shchepin, R.V.; Waddell, K.W.; Shi, F.; Goodson, B.M.; Warren, W.S.; Chekmenev, E.Y. Microtesla SABRE Enables 10% Nitrogen-15 Nuclear Spin Polarization. J. Am. Chem. Soc. 2015, 137, 1404–1407. [Google Scholar] [CrossRef] [PubMed]
- Colell, J.F.P.; Emondts, M.; Logan, A.W.J.; Shen, K.; Bae, J.; Shchepin, R.V.; Ortiz, G.X.; Spannring, P.; Wang, Q.; Malcolmson, S.J.; et al. Direct Hyperpolarization of Nitrogen-15 in Aqueous Media with Parahydrogen in Reversible Exchange. J. Am. Chem. Soc. 2017, 139, 7761–7767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, K.; Logan, A.W.J.W.J.; Colell, J.F.P.F.P.; Bae, J.; Ortiz, G.X.X.; Theis, T.; Warren, W.S.S.; Malcolmson, S.J.J.; Wang, Q. Diazirines as Potential Molecular Imaging Tags: Probing the Requirements for Efficient and Long-Lived SABRE-Induced Hyperpolarization. Angew. Chem. Int. Ed. 2017, 56, 12112–12116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Colell, J.F.P.P.; Glachet, T.; Lindale, J.R.; Reboul, V.; Theis, T.; Warren, W.S. Terminal Diazirines Enable Reverse Polarization Transfer from 15N2 Singlets. Angew. Chem. Int. Ed. 2019, 131, 11118–11124. [Google Scholar] [CrossRef]
- Park, H.; Zhang, G.; Bae, J.; Theis, T.; Warren, W.S.; Wang, Q. Application of 15N2 -Diazirines as a Versatile Platform for Hyperpolarization of Biological Molecules by d-DNP. Bioconjug. Chem. 2020, 31, 537–541. [Google Scholar] [CrossRef]























© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.M.; Olaoye, O.O. Recent Advances in Chemical Biology Using Benzophenones and Diazirines as Radical Precursors. Molecules 2020, 25, 2285. https://doi.org/10.3390/molecules25102285
Hassan MM, Olaoye OO. Recent Advances in Chemical Biology Using Benzophenones and Diazirines as Radical Precursors. Molecules. 2020; 25(10):2285. https://doi.org/10.3390/molecules25102285
Chicago/Turabian StyleHassan, Muhammad Murtaza, and Olasunkanmi O. Olaoye. 2020. "Recent Advances in Chemical Biology Using Benzophenones and Diazirines as Radical Precursors" Molecules 25, no. 10: 2285. https://doi.org/10.3390/molecules25102285
APA StyleHassan, M. M., & Olaoye, O. O. (2020). Recent Advances in Chemical Biology Using Benzophenones and Diazirines as Radical Precursors. Molecules, 25(10), 2285. https://doi.org/10.3390/molecules25102285





