PET Imaging of [11C]MPC-6827, a Microtubule-Based Radiotracer in Non-Human Primate Brains
Abstract
1. Introduction
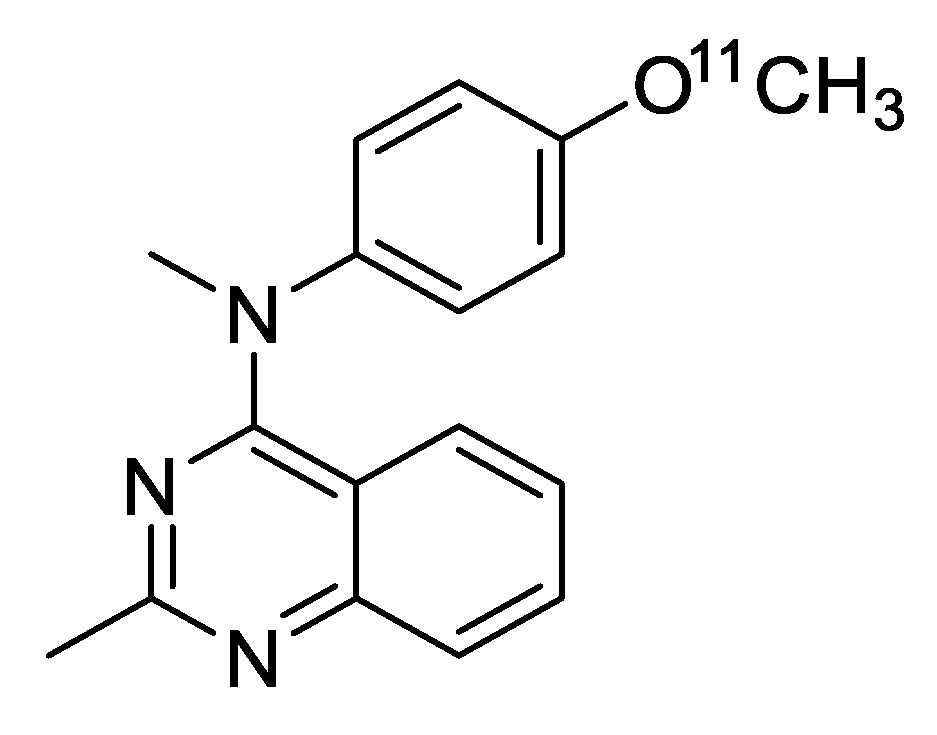
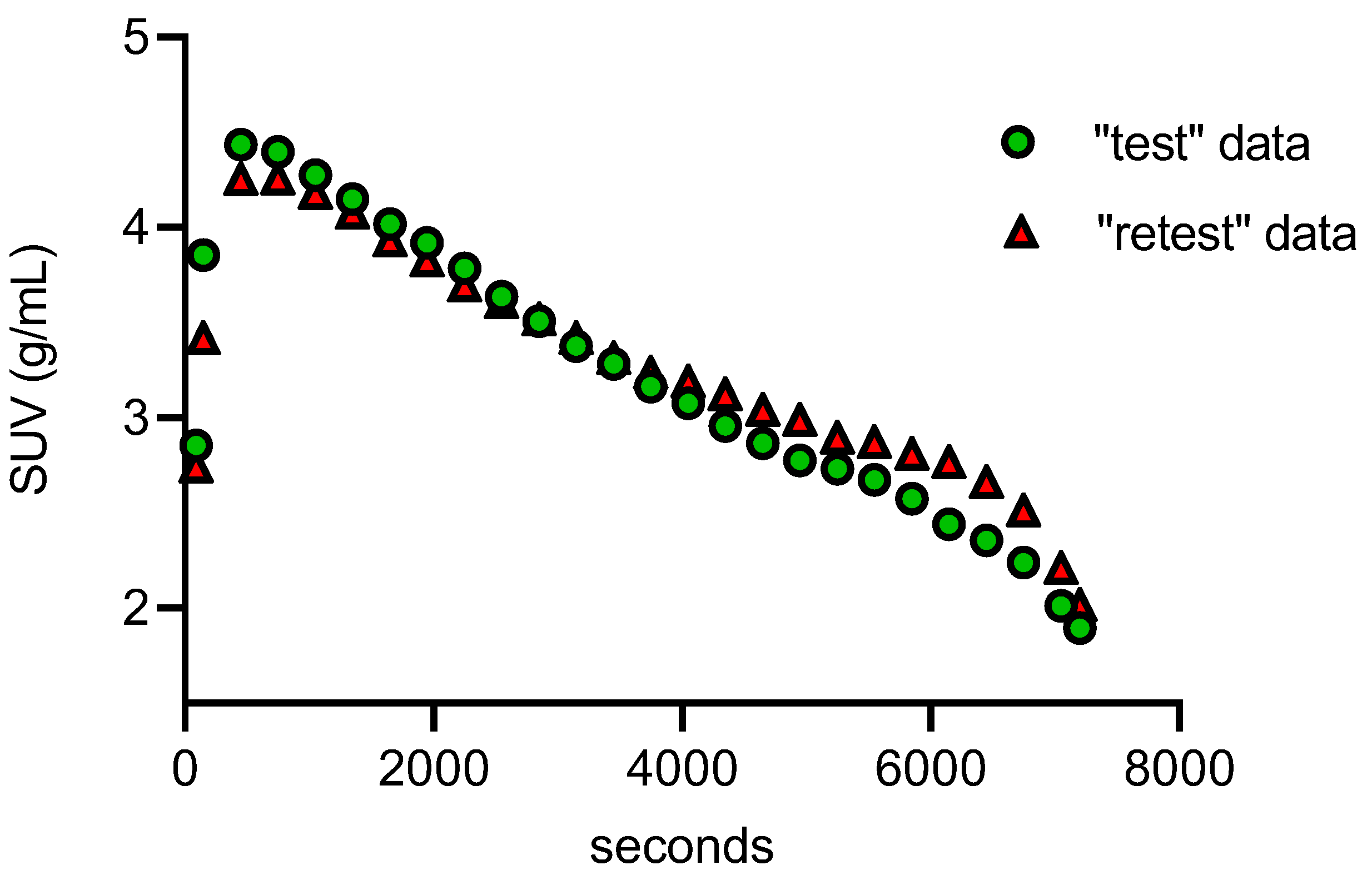
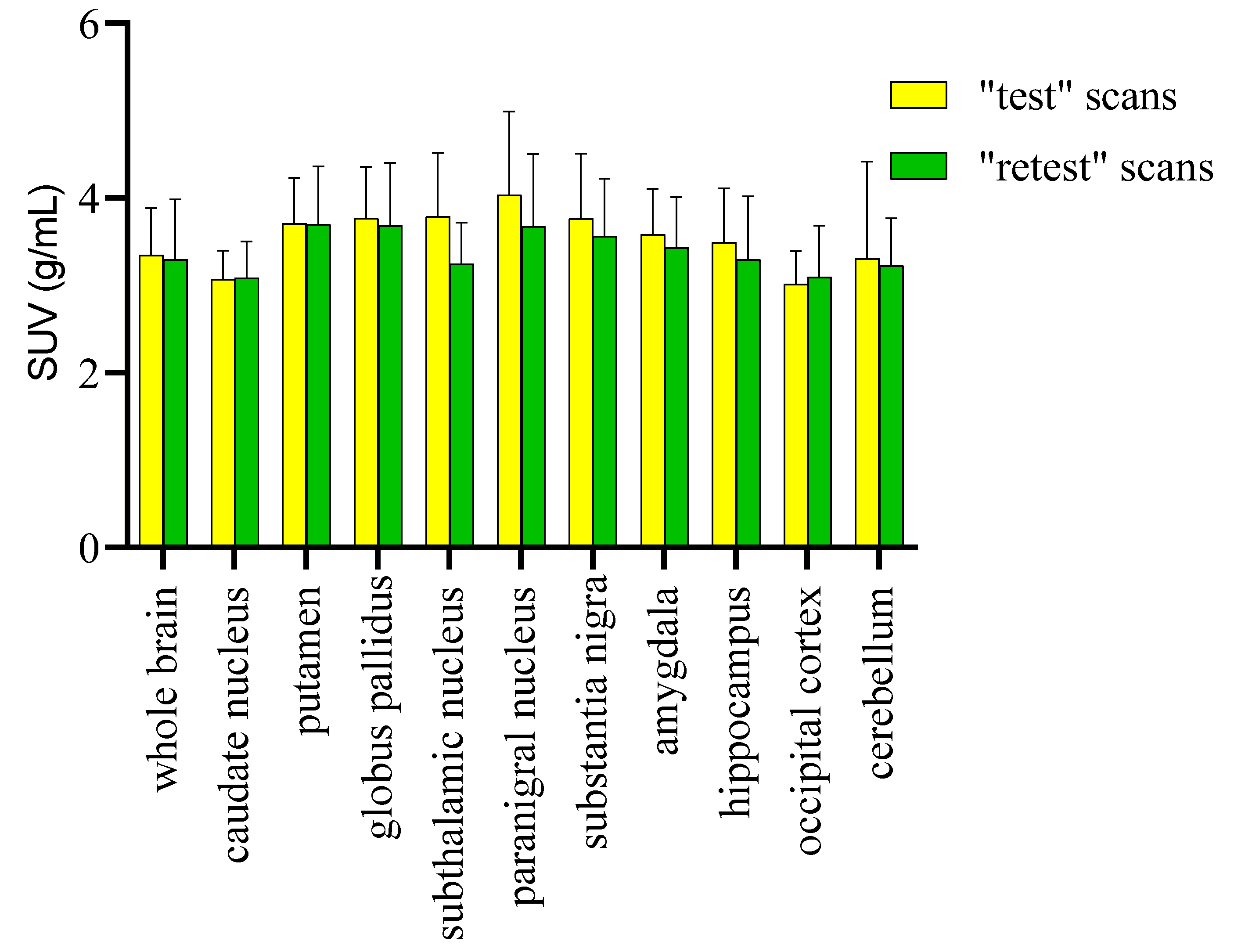
2. Results and Discussion
3. Material and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lasser, M.; Tiber, J.; Lowery, L.A. The Role of the Microtubule Cytoskeleton in Neurodevelopmental Disorders. Front. Cell. Neurosci. 2018, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- Brunden, K.R.; Lee, V.M.Y.; Smith, A.B.; Trojanowski, J.Q.; Ballatore, C. Altered microtubule dynamics in neurodegenerative disease: Therapeutic potential of microtubule-stabilizing drugs. Neurobiol. Dis. 2017, 105, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Kovalevich, J.; Cornec, A.-S.; Yao, Y.; James, M.; Crowe, A.; Lee, V.M.-Y.; Trojanowski, J.Q.; Smith, A.B.; Ballatore, C.; Brunden, K.R. Characterization of Brain-Penetrant Pyrimidine-Containing Molecules with Differential Microtubule-Stabilizing Activities Developed as Potential Therapeutic Agents for Alzheimer’s Disease and Related Tauopathies. J. Pharmacol. Exp. Ther. 2016, 357, 432–450. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Butler, T.R.; Prendergast, M.A. Ethanol impairs microtubule formation via interactions at a microtubule associated protein-sensitive site. Alcohol 2013, 47, 539–543. [Google Scholar] [CrossRef]
- Sau-chi, B.Y.; Hwang, S.; Rustan, T.D.; Frey, W.H. Human brain tubulin purification: Decrease in soluble tubulin with age. Neurochem. Res. 1985, 10, 1–18. [Google Scholar]
- Preedy, V.R. Neuropathology of Drug Addictions and Substance Misuse Volume 1: Foundations of Understanding, Tobacco, Alcohol, Cannabinoids and Opioids; Academic Press: London, UK, 2016. [Google Scholar]
- Verma, A.; Bennett, J.; Örme, A.M.; Polycarpou, E.; Rooney, B. Cocaine addicted to cytoskeletal change and a fibrosis high. Cytoskelet. 2019, 76, 177–185. [Google Scholar] [CrossRef]
- Solingapuram Sai, K.K.; Hurley, R.A.; Dodda, M.; Taber, K.H. Positron Emission Tomography: Updates on Imaging of Addiction. J. Neuropsychiatry Clin. Neurosci. 2019, 31, A6-288. [Google Scholar] [CrossRef]
- Reiter-Funk, C.K.; Dohrman, D.P. Chronic ethanol exposure increases microtubule content in PC12 cells. BMC Neurosci. 2005, 6, 16. [Google Scholar] [CrossRef]
- Spina, M.G.; Grecksch, G.; Kovar, K.-A.; Wolf, G.; Putzke, J. Microtubule-associated Protein 2 (MAP2) and c-fos Expression in the Rat Prefrontal Cortex following Subchronic Treatment with Substituted Amphetamines. Ann. N. Y. Acad. Sci. 2000, 914, 65–70. [Google Scholar] [CrossRef]
- Sloan, A.; Hussain, I.; Maqsood, M.; Eremin, O.; El-Sheemy, M. The effects of smoking on fracture healing. Surg. 2010, 8, 111–116. [Google Scholar] [CrossRef]
- Dent, E.W. Of microtubules and memory: Implications for microtubule dynamics in dendrites and spines. Mol. Biol. Cell 2017, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, G.; Casagrande, F.; Calogero, A.; De Gregorio, C.; Pezzoli, G.; Cartelli, D. Linking microtubules to Parkinson’s disease: The case of parkin. Biochem. Soc. Trans. 2015, 43, 292–296. [Google Scholar] [CrossRef]
- Clark, J.A.; Yeaman, E.J.; Blizzard, C.A.; Chuckowree, J.A.; Dickson, T.C. A Case for Microtubule Vulnerability in Amyotrophic Lateral Sclerosis: Altered Dynamics During Disease. Front. Cell. Neurosci. 2016, 10, 204. [Google Scholar] [CrossRef]
- Solingapuram Sai, K.K.; Milligan, C.; Rajagopal, S.A.; Prabhakaran, J.; Mann, J.J.; Mintz, A.; KUMAR, D. Initial in vivo evaluation of [11C]MPC-6827, a microtubule imaging agent in transgenic mice model of amyotrophic lateral sclerosis. J. Nucl. Med. 2019, 60 (Suppl. 1), 181. [Google Scholar]
- Zempel, H.; Mandelkow, E.-M. Tau missorting and spastin-induced microtubule disruption in neurodegeneration: Alzheimer Disease and Hereditary Spastic Paraplegia. Mol. Neurodegener. 2015, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Mastronardi, F.G.; Moscarello, M.A. Molecules affecting myelin stability: A novel hypothesis regarding the pathogenesis of multiple sclerosis. J. Neurosci. Res. 2005, 80, 301–308. [Google Scholar] [CrossRef]
- Paula-Barbosa, M.; Tavares, M.A.; Cadete-Leite, A. A quantitative study of frontal cortex dendritic microtubules in patients with Alzheimer’s disease. Brain. Res. 1987, 417, 139–142. [Google Scholar] [CrossRef]
- Kadavath, H.; Hofele, R.V.; Biernat, J.; Kumar, S.; Tepper, K.; Urlaub, H.; Mandelkow, E.; Zweckstetter, M. Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc. Natl. Acad. Sci. USA 2015, 112, 7501. [Google Scholar] [CrossRef]
- Romero, A.M.; Esteban-Pretel, G.; Marín, M.P.; Ponsoda, X.; Ballestín, R.; Canales, J.J.; Renau-Piqueras, J. Chronic Ethanol Exposure Alters the Levels, Assembly, and Cellular Organization of the Actin Cytoskeleton and Microtubules in Hippocampal Neurons in Primary Culture. Toxicol. Sci. 2010, 118, 602–612. [Google Scholar] [CrossRef]
- Mitchison, T.; Kirschner, M. Dynamic instability of microtubule growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef]
- Young, E.J.; Briggs, S.B.; Miller, C.A. The Actin Cytoskeleton as a Therapeutic Target for the Prevention of Relapse to Methamphetamine Use. CNS Neurol. Disord. Drug Targets 2015, 14, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.; Mach, R.H. Chapter 7-Development of brain PET imaging agents: Strategies for imaging neuroinflammation in Alzheimer’s disease. In Progress in Molecular Biology and Translational Science; Becker, J.T., Cohen, A.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 165, pp. 371–399. [Google Scholar]
- Nader, M.A.; Czoty, P.W.; Gould, R.W.; Riddick, N.V. Review. Positron emission tomography imaging studies of dopamine receptors in primate models of addiction. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 3223–3232. [Google Scholar] [CrossRef] [PubMed]
- Kasibhatla, S.; Baichwal, V.; Cai, S.X.; Roth, B.; Skvortsova, I.; Skvortsov, S.; Lukas, P.; English, N.M.; Sirisoma, N.; Drewe, J.; et al. MPC-6827: A Small-Molecule Inhibitor of Microtubule Formation That Is Not a Substrate for Multidrug Resistance Pumps. Cancer Res. 2007, 67, 5865. [Google Scholar] [CrossRef]
- Christos, D.K.; Pavel, D. Tubulins as Therapeutic Targets in Cancer: From Bench to Bedside. Curr. Pharm. Des. 2012, 18, 2778–2792. [Google Scholar]
- Kumar, J.S.D.; Solingapuram Sai, K.K.; Prabhakaran, J.; Oufkir, H.R.; Ramanathan, G.; Whitlow, C.T.; Dileep, H.; Mintz, A.; Mann, J.J. Radiosynthesis and in Vivo Evaluation of [11C]MPC-6827, the First Brain Penetrant Microtubule PET Ligand. J. Med. Chem. 2018, 61, 2118–2123. [Google Scholar] [CrossRef]
- Solingapuram Sai, K.K.; Whitlow, C.T.; Kumar, J.S.D.; Craft, S.; Mintz, A.; Macauley-Rambach, S. In vivo evaluations of microtubule-based pet radiotracer, [11C]MPC-6827 in murine models of Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2019, 15, P958–P959. [Google Scholar] [CrossRef]
- Solingapuram Sai, K.K.; Nader, M.; Zanderigo, F.; Whitlow, C.; Nader, S.; Rubin-Falcone, H.; Prabhakaran, J.; Martin, T.; Mintz, A.; Mann, J.J.; et al. Initial PET evaluation of [11C]MPC6827, and [11C]HD800 for microtubule imaging in nonhuman primate brain. J. Nucl. Med. 2019, 60, 1103. [Google Scholar]
- García-Varela, L.; Vállez García, D.; Rodríguez-Pérez, M.; van Waarde, A.; Sijbesma, J.W.A.; Schildt, A.; Kwizera, C.; Aguiar, P.; Sobrino, T.; Dierckx, R.A.J.O.; et al. Test–Retest Repeatability of [18F]MC225-PET in Rodents: A Tracer for Imaging of P-gp Function. ACS Chem. Neurosci. 2020, 11, 648–658. [Google Scholar] [CrossRef]
- Nader, M.A.; Morgan, D.; Gage, H.D.; Nader, S.H.; Calhoun, T.L.; Buchheimer, N.; Ehrenkaufer, R.; Mach, R.H. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat. Neurosci. 2006, 9, 1050–1056. [Google Scholar] [CrossRef]
- Solingapuram Sai, K.K.; Prabhakaran, J.; Ramanathan, G.; Rideout, S.; Whitlow, C.; Mintz, A.; Mann, J.J.; Kumar, J.S.D. Radiosynthesis and Evaluation of [11C]HD-800, a High Affinity Brain Penetrant PET Tracer for Imaging Microtubules. ACS Med. Chem. Lett. 2018, 9, 452–456. [Google Scholar] [CrossRef]
- Neth, B.J.; Mintz, A.; Whitlow, C.; Jung, Y.; Solingapuram Sai, K.; Register, T.C.; Kellar, D.; Lockhart, S.N.; Hoscheidt, S.; Maldjian, J.; et al. Modified ketogenic diet is associated with improved cerebrospinal fluid biomarker profile, cerebral perfusion, and cerebral ketone body uptake in older adults at risk for Alzheimer’s disease: A pilot study. Neurobiol. Aging 2020, 86, 54–63. [Google Scholar] [CrossRef]
- Bentourkia, M.h.; Tremblay, S.; Pifferi, F.; Rousseau, J.; Lecomte, R.; Cunnane, S. PET study of [11C]-acetoacetate kinetics in rat brain during dietary treatments affecting ketosis. Am. J. Physiol.-Endocrinol. Metab 2009, 296, E796–E801. [Google Scholar] [CrossRef]
- Nugent, S.; Tremblay, S.; Chen, K.W.; Ayutyanont, N.; Roontiva, A.; Castellano, C.-A.; Fortier, M.; Roy, M.; Courchesne-Loyer, A.; Bocti, C.; et al. Brain glucose and acetoacetate metabolism: A comparison of young and older adults. Neurobiol. Aging 2013, 35, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Nader, M.A. Chapter 1-Animal models for addiction medicine: From vulnerable phenotypes to addicted individuals. In Progress in Brain Research; Ekhtiari, H., Paulus, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 224, pp. 3–24. [Google Scholar]
- Craft, S.; Claxton, A.; Baker, L.D.; Hanson, A.J.; Cholerton, B.; Trittschuh, E.H.; Dahl, D.; Caulder, E.; Neth, B.; Montine, T.J.; et al. Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer’s Disease Biomarkers: A Pilot Clinical Trial. J. Alzheimers. Dis. 2017, 57, 1325–1334. [Google Scholar] [CrossRef]
- Latimer, C.S.; Shively, C.A.; Keene, C.D.; Jorgensen, M.J.; Andrews, R.N.; Register, T.C.; Montine, T.J.; Wilson, A.M.; Neth, B.J.; Mintz, A.; et al. A nonhuman primate model of early Alzheimer’s disease pathologic change: Implications for disease pathogenesis. Alzheimer’s Dement. 2019, 15, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Nader, M.A.; Czoty, P.W. PET Imaging of Dopamine D2 Receptors in Monkey Models of Cocaine Abuse: Genetic Predisposition Versus Environmental Modulation. Am. J. Psychiatry 2005, 162, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Flynn, S.M.; Epperly, P.M.; Davenport, A.T.; Cami-Kobeci, G.; Husbands, S.M.; Ko, M.-C.; Czoty, P.W. Effects of stimulation of mu opioid and nociceptin/orphanin FQ peptide (NOP) receptors on alcohol drinking in rhesus monkeys. Neuropsychopharmacology 2019, 44, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the standard MPC-6827 is available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damuka, N.; Czoty, P.W.; Davis, A.T.; Nader, M.A.; Nader, S.H.; Craft, S.; Macauley, S.L.; Galbo, L.K.; Epperly, P.M.; Whitlow, C.T.; et al. PET Imaging of [11C]MPC-6827, a Microtubule-Based Radiotracer in Non-Human Primate Brains. Molecules 2020, 25, 2289. https://doi.org/10.3390/molecules25102289
Damuka N, Czoty PW, Davis AT, Nader MA, Nader SH, Craft S, Macauley SL, Galbo LK, Epperly PM, Whitlow CT, et al. PET Imaging of [11C]MPC-6827, a Microtubule-Based Radiotracer in Non-Human Primate Brains. Molecules. 2020; 25(10):2289. https://doi.org/10.3390/molecules25102289
Chicago/Turabian StyleDamuka, Naresh, Paul W. Czoty, Ashley T. Davis, Michael A. Nader, Susan H. Nader, Suzanne Craft, Shannon L. Macauley, Lindsey K. Galbo, Phillip M. Epperly, Christopher T. Whitlow, and et al. 2020. "PET Imaging of [11C]MPC-6827, a Microtubule-Based Radiotracer in Non-Human Primate Brains" Molecules 25, no. 10: 2289. https://doi.org/10.3390/molecules25102289
APA StyleDamuka, N., Czoty, P. W., Davis, A. T., Nader, M. A., Nader, S. H., Craft, S., Macauley, S. L., Galbo, L. K., Epperly, P. M., Whitlow, C. T., Davenport, A. T., Martin, T. J., Daunais, J. B., Mintz, A., & Solingapuram Sai, K. K. (2020). PET Imaging of [11C]MPC-6827, a Microtubule-Based Radiotracer in Non-Human Primate Brains. Molecules, 25(10), 2289. https://doi.org/10.3390/molecules25102289





