Biogenesis of Prism-Like Silver Oxide Nanoparticles Using Nappa Cabbage Extract and Their p-Nitrophenol Sensing Activity
Abstract
1. Introduction
2. Results
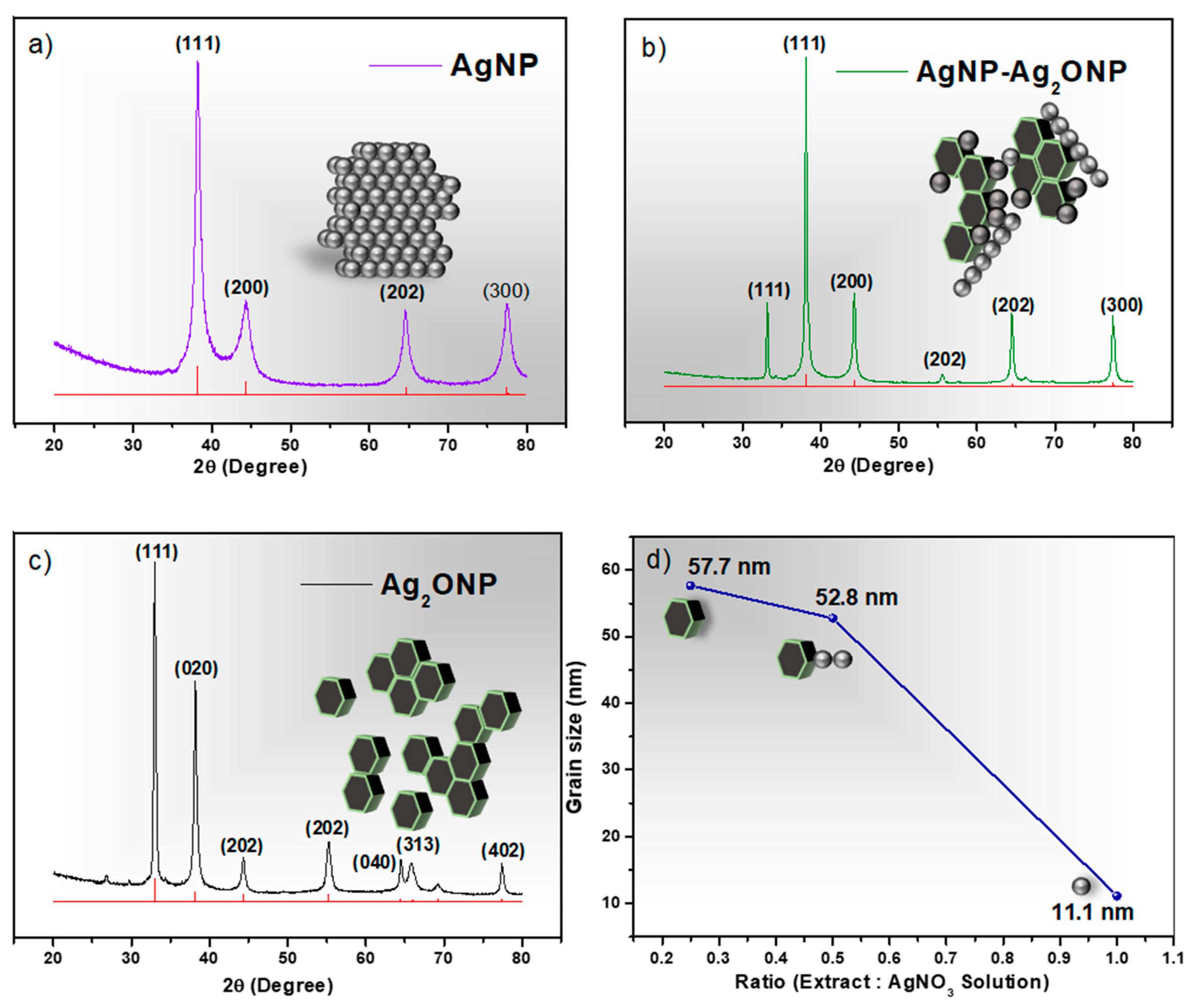
2.1. X-ray Diffraction (XRD) Analysis of Nappa Cabbage-Mediated Biosynthesis of Prism-Like Silver Oxide Nanoparticles (Ag2ONPs)
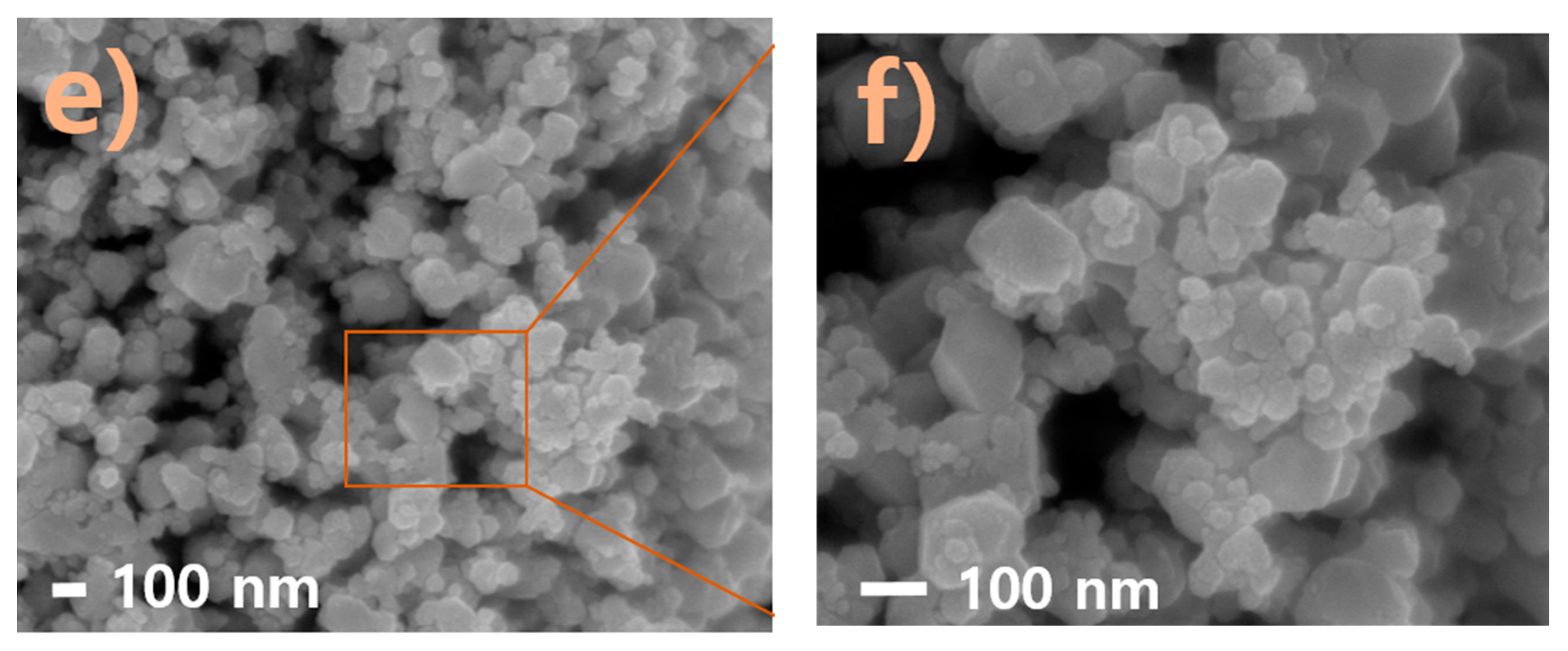
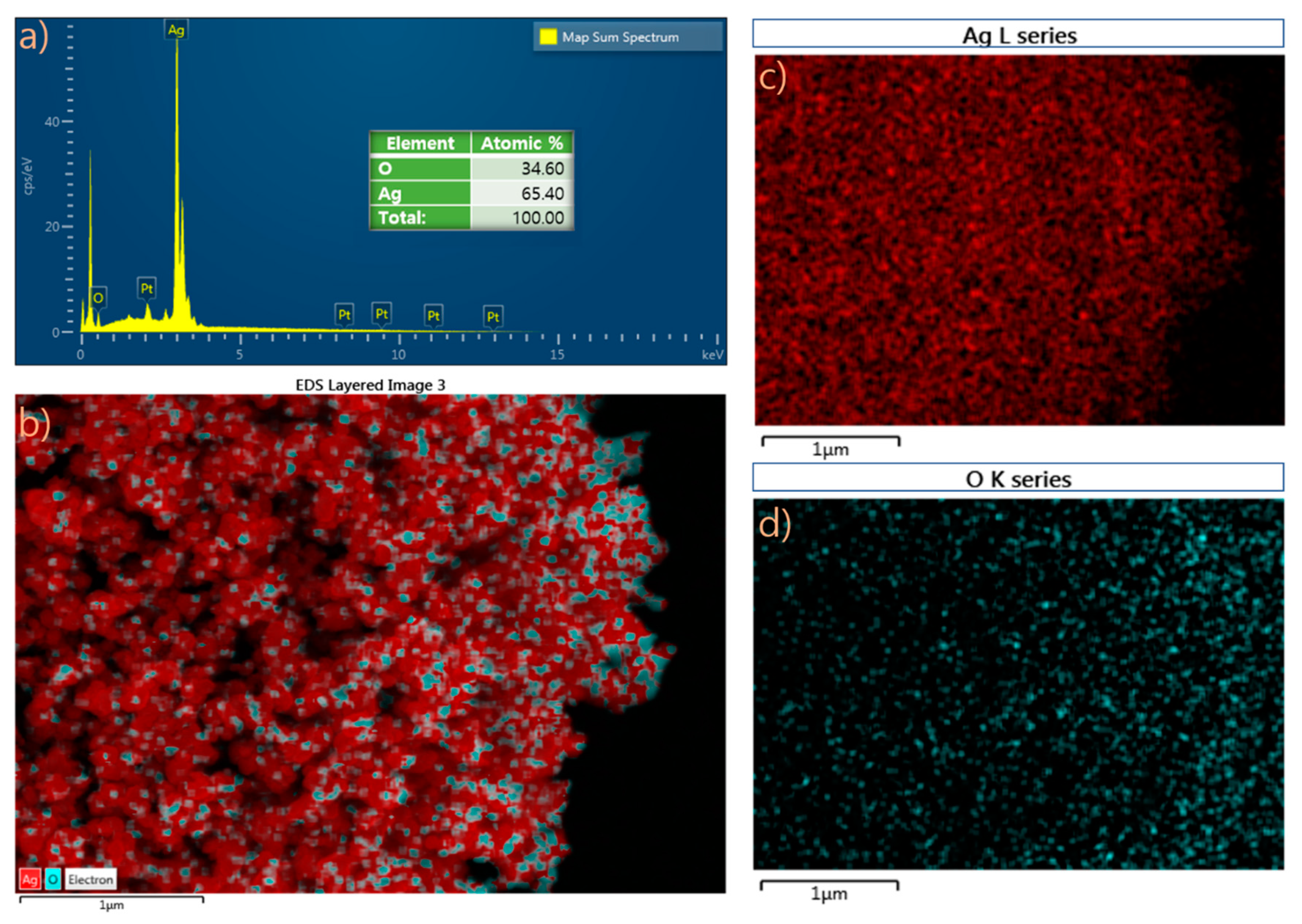
2.2. Field-Emission Scanning Spectroscopy (FESEM) and Energy-Dispersive Spectroscopy (EDS) Analyses of Nappa Cabbage-Mediated Biosynthesis of Prism-Like Silver Oxide Nanoparticles (Ag2ONPs)
2.3. Transmission Electron Microscopy (TEM) Analysis of Nappa Cabbage-Mediated Biosynthesis of Prism-Like Silver Oxide Nanoparticles (Ag2ONPs)
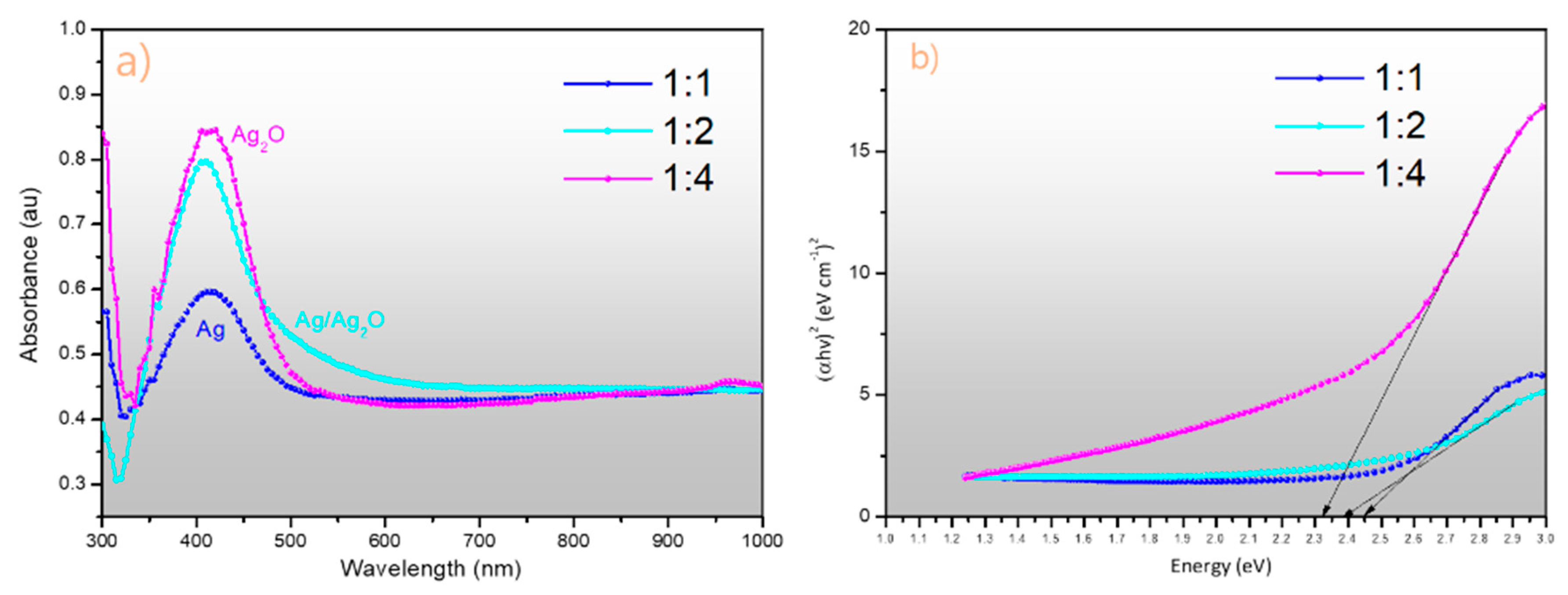
2.4. UV–Visible Light Spectral Analysis (UV–Vis) of Nappa Cabbage-Mediated Biosynthesis of Prism-Like Silver Oxide Nanoparticles (Ag2ONPs)
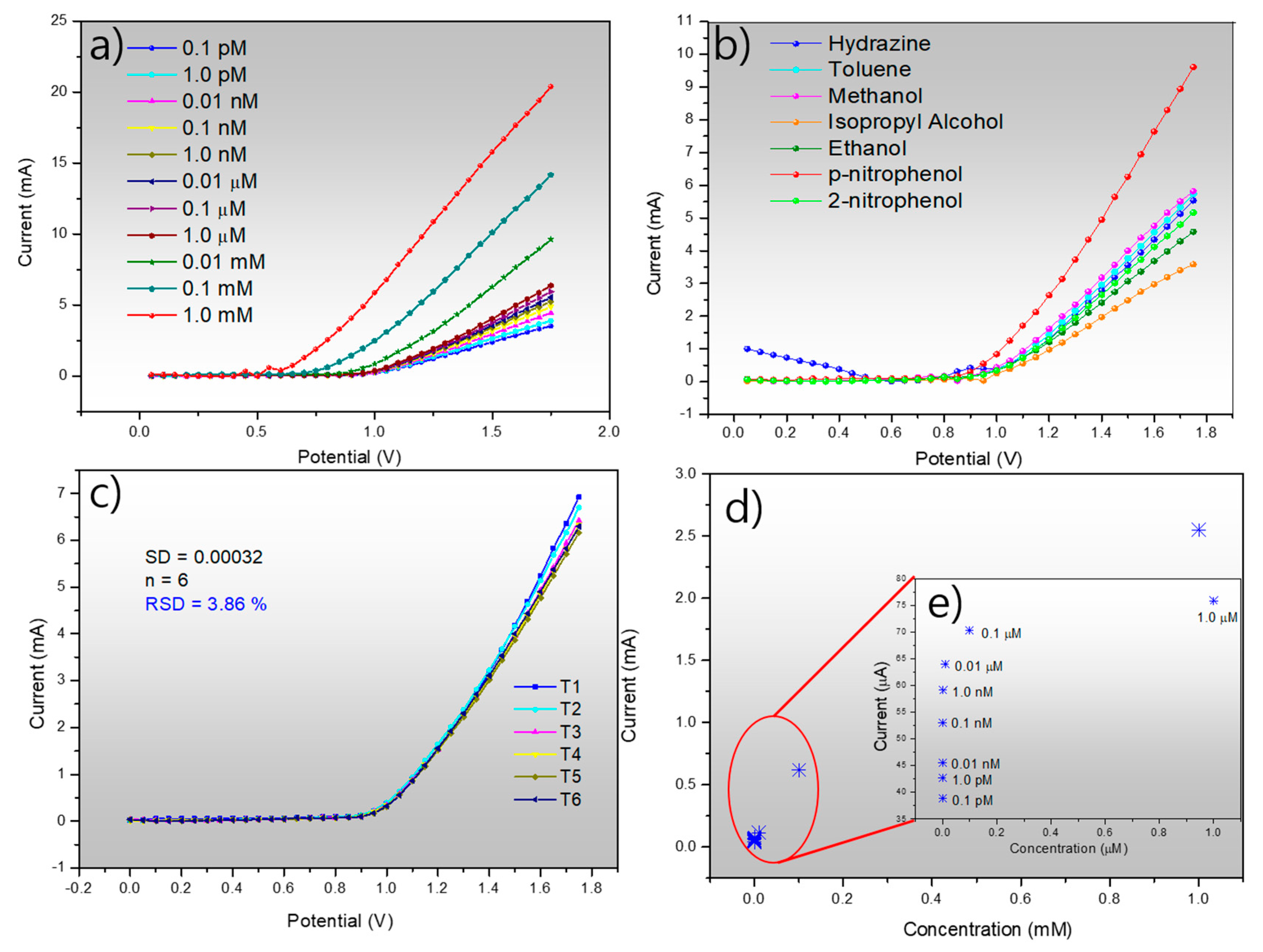
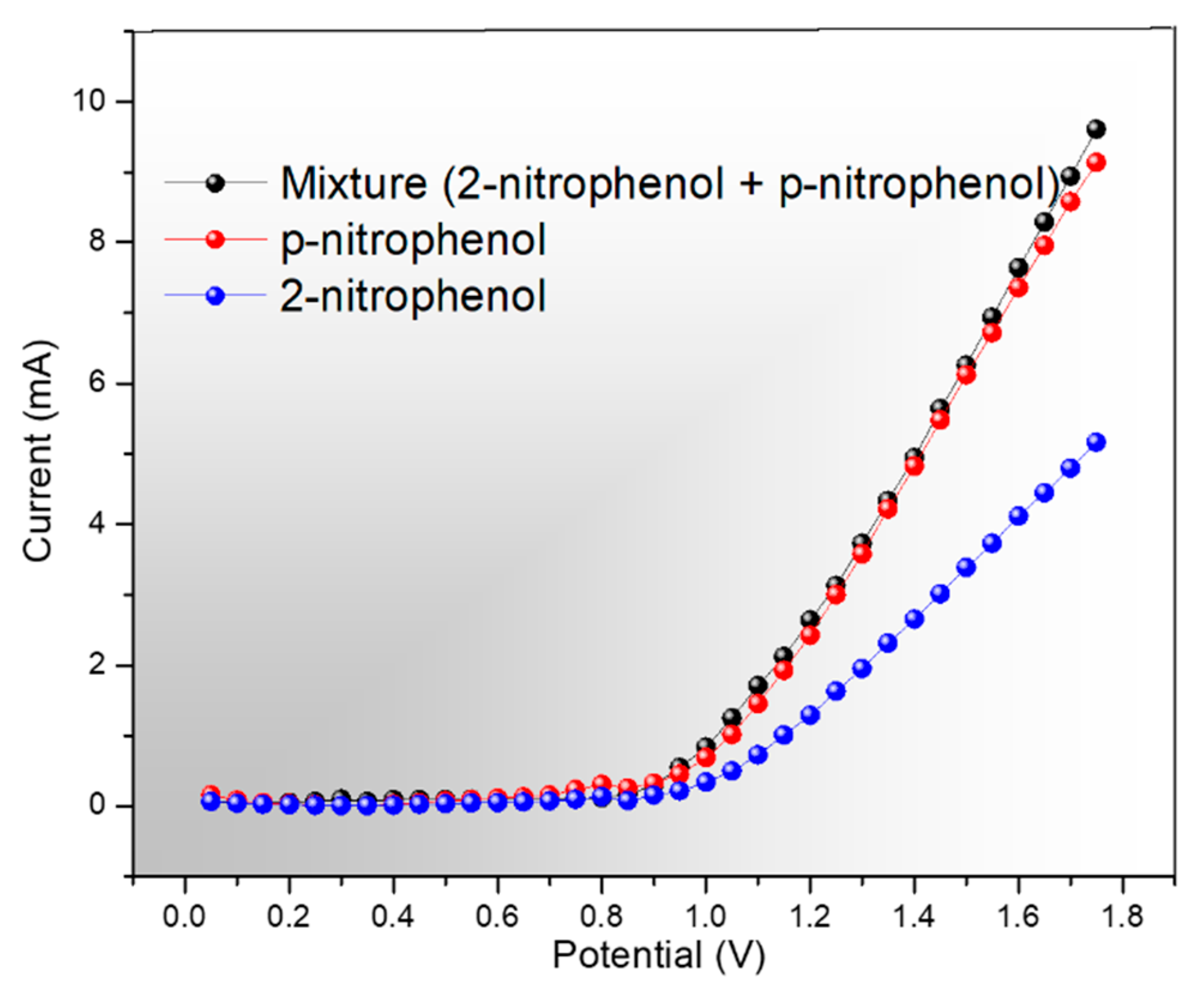
2.5. p-Nitrophenol Sensing Measurement
3. Materials and Methods
3.1. Materials
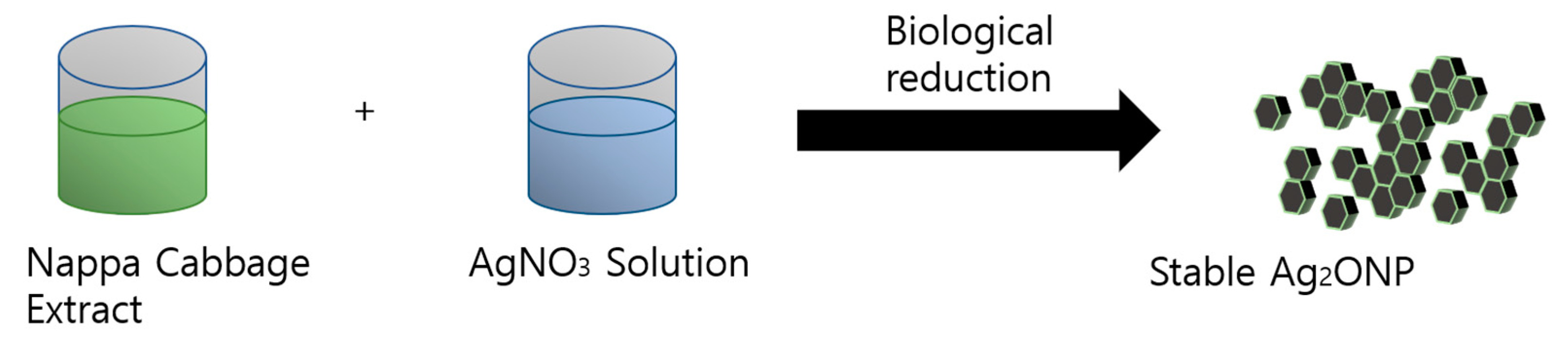
3.2. Preparation of Nappa Cabbage Extract
3.3. Synthesis of Prism-Like Silver Oxide Nanoparticles
3.4. X-ray Diffraction (XRD)
3.5. Field-Emission Scanning Spectroscopy (FESEM) and Energy-Dispersive Spectroscopy (EDS)
3.6. Transmission Electron Microscopy (TEM)
3.7. UV–Visible Light Spectral Analysis (UV–Vis)
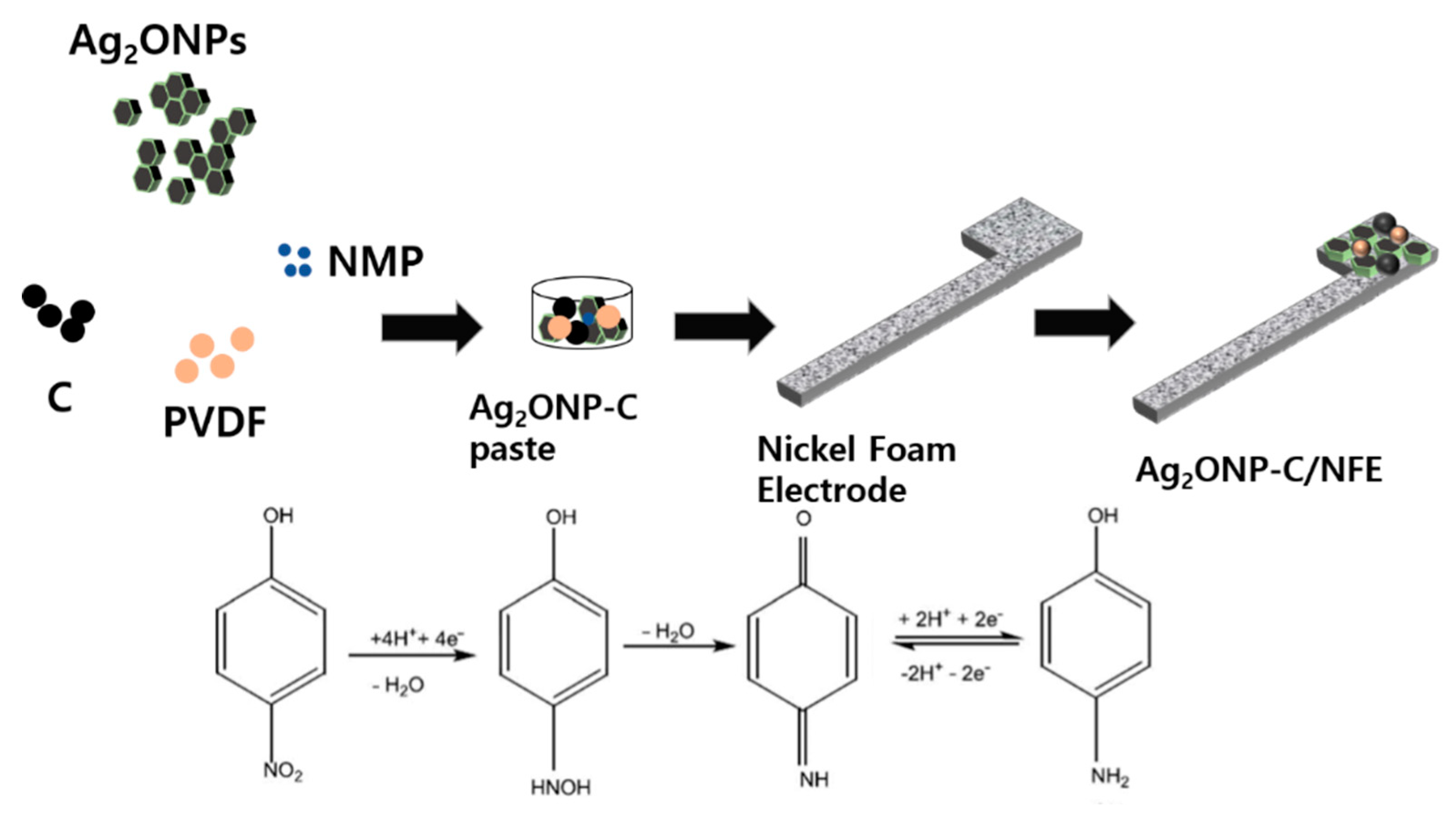
3.8. Fabrication of p-Nitrophenol Sensing Electrode
3.9. p-Nitrophenol Sensing Measurement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, Y.; Zhao, J.; Li, S.; Guo, M.; Fan, Z. An electrochemical sensor for the detection of p-nitrophenol based on a cyclodextrin-decorated gold nanoparticle-mesoporous carbon hybrid. Analyst 2019, 144, 4400–4406. [Google Scholar] [CrossRef] [PubMed]
- Mucci, M.; Douglas, G.; Lürling, M. Lanthanum modified bentonite behaviour and efficiency in adsorbing phosphate in saline waters. Chemosphere 2020, 249, 126131. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.S.; Jose, S.; Selvarajan, E.; Mathimani, T.; Pugazhendhi, A. Biosynthesized silver nanoparticles using Bacillus amyloliquefaciens; Application for cytotoxicity effect on A549 cell line and photocatalytic degradation of p-nitrophenol. J. Photochem. Photobiol. B Biol. 2020, 202, 111642. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yang, L.; Guo, Y.; Zheng, W.; Liu, H.; Wei, Y. Effect of p-nitrophenol degradation in aqueous dispersions of different crystallized goethites. J. Photochem. Photobiol. A Chem. 2018, 353, 337–343. [Google Scholar] [CrossRef]
- Vieira, G.B.; José, H.J.; Peterson, M.; Baldissarelli, V.Z.; Alvarez, P.; de Fátima Peralta Muniz Moreira, R. CeO2/TiO2 nanostructures enhance adsorption and photocatalytic degradation of organic compounds in aqueous suspension. J. Photochem. Photobiol. A Chem. 2018, 353, 325–336. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Yao, Y.-X.; Li, H.-B.; Liu, J.-Y.; Tan, X.-L.; Yu, J.-G.; Peng, Z.-G. Removal and Adsorption of p-Nitrophenol from Aqueous Solutions Using Carbon Nanotubes and Their Composites. J. Nanomater. 2014, 2014, 571745. [Google Scholar] [CrossRef]
- Hamidouche, S.; Bouras, O.; Zermane, F.; Cheknane, B.; Houari, M.; Debord, J.; Harel, M.; Bollinger, J.-C.; Baudu, M. Simultaneous sorption of 4-nitrophenol and 2-nitrophenol on a hybrid geocomposite based on surfactant-modified pillared-clay and activated carbon. Chem. Eng. J. 2015, 279, 964–972. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, X.; Zhong, H.; Wang, H.; Zeng, G.; Chen, X.; Wang, H.; Zhang, L.; Shao, J. Enhanced adsorptive removal of p-nitrophenol from water by aluminum metal-organic framework/reduced graphene oxide composite. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Kuang, S.; Le, Q.; Hu, J.; Wang, Y.; Yu, N.; Cao, X.; Zhang, M.; Sun, Y.; Gu, W.; Yang, Y.; et al. Effects of p-nitrophenol on enzyme activity, histology, and gene expression in Larimichthys crocea. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 228, 108638. [Google Scholar] [CrossRef]
- Liu, S.; Lai, C.; Li, B.; Zhang, C.; Zhang, M.; Huang, D.; Qin, L.; Yi, H.; Liu, X.; Huang, F.; et al. Role of radical and non-radical pathway in activating persulfate for degradation of p-nitrophenol by sulfur-doped ordered mesoporous carbon. Chem. Eng. J. 2020, 384, 123304. [Google Scholar] [CrossRef]
- Oueslati, M.H.; Ben Tahar, L.; Harrath, A.H. Synthesis of ultra-small gold nanoparticles by polyphenol extracted from Salvia officinalis and efficiency for catalytic reduction of p-nitrophenol and methylene blue. Green Chem. Lett. Rev. 2020, 13, 18–26. [Google Scholar] [CrossRef]
- Jing, Q.; Yi, Z.; Lin, D.; Zhu, L.; Yang, K. Enhanced sorption of naphthalene and p-nitrophenol by Nano-SiO2 modified with a cationic surfactant. Water Res. 2013, 47, 4006–4012. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Y.; Liu, G.; Dong, B.; Zhou, J.; Wang, A.; Wang, J.; Jin, R.; Lv, H.; Dou, Z.; Huang, W. Microbial synthesis of Pd/Fe3O4, Au/Fe3O4 and PdAu/Fe3O4 nanocomposites for catalytic reduction of nitroaromatic compounds. Sci. Rep. 2015, 5, 13515. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.S.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Removal of 2-nitrophenol by catalytic wet peroxide oxidation using carbon materials with different morphological and chemical properties. Appl. Catal. B Environ. 2013, 140–141, 356–362. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Gao, G.; Lv, B.; Xu, L.; Lu, Y.; Zhang, G. In-situ self-assembly of robust Fe (III)-carboxyl functionalized polyacrylonitrile polymeric bead catalyst for efficient photo-Fenton oxidation of p-nitrophenol. Sci. Total Environ. 2020, 702, 134910. [Google Scholar] [CrossRef]
- Brega, A.; Prandini, P.; Amaglio, C.; Pafumi, E. Determination of phenol, m-, o- and p-cresol, p-aminophenol and p-nitrophenol in urine by high-performance liquid chromatography. J. Chromatogr. A 1990, 535, 311–316. [Google Scholar] [CrossRef]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Hart, J.P. Voltammetric Behavior ofp-Nitrophenol and Its Trace Determination in Human Urine by Liquid Chromatography with a Dual Reductive Mode Electrochemical Detection System. Electroanalysis 2007, 19, 2176–2184. [Google Scholar] [CrossRef]
- Xiao, N.; Liu, S.G.; Mo, S.; Li, N.; Ju, Y.J.; Ling, Y.; Li, N.B.; Luo, H.Q. Highly selective detection of p-nitrophenol using fluorescence assay based on boron, nitrogen co-doped carbon dots. Talanta 2018, 184, 184–192. [Google Scholar] [CrossRef]
- Takayanagi, T.; Mine, M.; Mizuguchi, H. Capillary Electrophoresis/Dynamic Frontal Analysis for the Enzyme Assay of 4-Nitrophenyl Phosphate with Alkaline Phosphatase. Anal. Sci. 2020. [Google Scholar] [CrossRef]
- Vinoth, S.; Sampathkumar, P.; Giribabu, K.; Pandikumar, A. Ultrasonically assisted synthesis of barium stannate incorporated graphitic carbon nitride nanocomposite and its analytical performance in electrochemical sensing of 4-nitrophenol. Ultrason. Sonochem. 2019, 62, 104855. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Bai, S.; Huang, J.; Ma, Y.; Zeng, Q.; Wang, M.; Wang, L. Simultaneous detection of nitrophenol isomers using an easy-to-fabricate thiophene-based microporous polymer film modified electrode. Microchem. J. 2019, 153, 104465. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, C.; Mu, X.; Pang, H.; Chen, X.; Liu, D. Ultrasensitive SERS detection of rhodamine 6G and p-nitrophenol based on electrochemically roughened nano-Au film. Talanta 2020, 210, 120631. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Xu, Q.; Yang, Z.; Hu, X. A derivative photoelectrochemical sensing platform for 4-nitrophenolate contained organophosphates pesticide based on carboxylated perylene sensitized nano-TiO2. Anal. Chim. Acta 2013, 766, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Toberer, E.S.; Schladt, T.D.; Seshadri, R. Macroporous Manganese Oxides with Regenerative Mesopores. J. Am. Chem. Soc. 2006, 128, 1462–1463. [Google Scholar] [CrossRef]
- Kipke, A.; Hofmeister, H. Formation of silver nanoparticles in low-alkali borosilicate glass via silver oxide intermediates. Mater. Chem. Phys. 2008, 111, 254–259. [Google Scholar] [CrossRef]
- Reddy, P.N.; Reddy, M.H.P.; Pierson, J.F.; Uthanna, S. Characterization of Silver Oxide Films Formed by Reactive RF Sputtering at Different Substrate Temperatures. ISRN Opt. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Rahman, M.M.; Marwani, H.M.; Algethami, F.K.; Asiri, A.M.; Hameed, S.A.; Alhogbi, B. Ultra-sensitive p-nitrophenol sensing performances based on various Ag2O conjugated carbon material composites. Environ. Nanotechnol. Monit. Manag. 2017, 8, 73–82. [Google Scholar]
- Pierson, J.F.; Rousselot, C. Stability of reactively sputtered silver oxide films. Surf. Coatings Technol. 2005, 200, 276–279. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bahadar Khan, S.; Jamal, A.; Faisal, M.; Asiri, A.M. Fabrication of highly sensitive acetone sensor based on sonochemically prepared as-grown Ag2O nanostructures. Chem. Eng. J. 2012, 192, 122–128. [Google Scholar] [CrossRef]
- Balachandramohan, J.; Sivasankar, T.; Sivakumar, M. Facile sonochemical synthesis of Ag2O-guar gum nanocomposite as a visible light photocatalyst for the organic transformation reactions. J. Hazard. Mater. 2020, 385, 121621. [Google Scholar] [CrossRef] [PubMed]
- Karaca, E.; Gökcen, D.; Pekmez, N.Ö.; Pekmez, K. Galvanostatic synthesis of nanostructured Ag-Ag2O dispersed PPy composite on graphite electrode for supercapacitor applications. Int. J. Energy Res. 2020, 44, 158–170. [Google Scholar] [CrossRef]
- Skiba, M.I.; Vorobyova, V.I.; Pivovarov, A.; Makarshenko, N.P. Green Synthesis of Silver Nanoparticles in the Presence of Polysaccharide: Optimization and Characterization. J. Nanomater. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Goutam, S.P.; Saxena, G.; Roy, D.; Yadav, A.K.; Bharagava, R.N. Green Synthesis of Nanoparticles and Their Applications in Water and Wastewater Treatment. In Bioremediation of Industrial Waste for Environmental Safety; Springer: Singapore, 2020; pp. 349–379. [Google Scholar]
- Jeong, J.W.; Park, K.J.; Lim, J.H.; Sung, J.M. Analysis of hazard on fresh and salted baechus (nappa cabbage, Brassica rapa L. subsp. pekinensis) in Korea. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 69–76. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef]
- Johnson, I.; Prabu, H.J. Green synthesis and characterization of silver nanoparticles by leaf extracts of Cycas circinalis, Ficus amplissima, Commelina benghalensis and Lippia nodiflora. Int. Nano Lett. 2015, 5, 43–51. [Google Scholar] [CrossRef]
- Hussain, M.; Raja, N.I.; Iqbal, M.; Aslam, S. Applications of Plant Flavonoids in the Green Synthesis of Colloidal Silver Nanoparticles and Impacts on Human Health. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 1381–1392. [Google Scholar] [CrossRef]
- Kumar, P.P.N.V.; Shameem, U.; Kollu, P.; Kalyani, R.L.; Pammi, S.V.N. Green Synthesis of Copper Oxide Nanoparticles Using Aloe vera Leaf Extract and Its Antibacterial Activity Against Fish Bacterial Pathogens. Bionanoscience 2015, 5, 135–139. [Google Scholar] [CrossRef]
- Sougandhi, P.R.; Ramanaiah, S. Green synthesis and spectral characterization of silver nanoparticles from Psidium guajava leaf extract. Inorg. Nano-Metal Chem. 2020, 0, 1–5. [Google Scholar]
- Salari, S.; Jafari, S.M. The Influence of Ohmic Heating on Degradation of Food Bioactive Ingredients. Food Eng. Rev. 2020. [Google Scholar] [CrossRef]
- Utama, G.L.; Utba, F.; Irena, F.; Wira, D.W. Napa Cabbage (Brassica rapa subsp. pekinensis&) Wastes as Sources of Potential Ethanol-Fermenting Indigenous Yeasts with Stress Tolerance Ability. J. Jpn. Inst. Energy 2018, 97, 261–265. [Google Scholar]
- Vinay, S.P.; Udayabhanu; Nagaraju, G.; Chandrappa, C.P.; Chandrasekhar, N. Novel Gomutra (cow urine) mediated synthesis of silver oxide nanoparticles and their enhanced photocatalytic, photoluminescence and antibacterial studies. J. Sci. Adv. Mater. Devices 2019, 4, 392–399. [Google Scholar] [CrossRef]
- Tan, C.-S.; Chen, Y.-J.; Hsia, C.-F.; Huang, M.H. Facet-Dependent Electrical Conductivity Properties of Silver Oxide Crystals. Chem. Asian J. 2017, 12, 293–297. [Google Scholar] [CrossRef]
- Cao, G.; Wang, Y. Nanostructures and Nanomaterials; In World Scientific Series in Nanoscience and Nanotechnology; World Scientific: Singapore, 2011; Volume 2. [Google Scholar]
- Ribeiro, R.A.P.; Oliveira, M.C.; Bomio, M.R.D.; de Lazaro, S.R.; Andrés, J.; Longo, E. Connecting the surface structure, morphology and photocatalytic activity of Ag2O: An in depth and unified theoretical investigation. Appl. Surf. Sci. 2020, 509, 145321. [Google Scholar] [CrossRef]
- Laghrib, F.; Houcini, H.; Khalil, F.; Liba, A.; Bakasse, M.; Lahrich, S.; El Mhammedi, M.A. Synthesis of Silver Nanoparticles Using Chitosan as Stabilizer Agent: Application towards Electrocatalytical Reduction of p-Nitrophenol. ChemistrySelect 2020, 5, 1220–1227. [Google Scholar] [CrossRef]
- Chu, L.; Han, L.; Zhang, X. Electrochemical simultaneous determination of nitrophenol isomers at nano-gold modified glassy carbon electrode. J. Appl. Electrochem. 2011, 41, 687–694. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Unnikrishnan, B.; Chen, S.-M. Amperometric Determination of 4-Nitrophenol at Multi-Walled Carbon Nanotube-Poly(Diphenylamine) Composite Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2011, 6, 3902–3912. [Google Scholar]
- Karuppiah, C.; Palanisamy, S.; Chen, S.M.; Emmanuel, R.; Ali, M.A.; Muthukrishnan, P.; Prakash, P.; Al-Hemaid, F.M.A. Green biosynthesis of silver nanoparticles and nanomolar detection of p-nitrophenol. J. Solid State Electrochem. 2014, 18, 1847–1854. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, Y.; Ai, S.; Ma, Q.; Zhu, L.; Lu, L. Electrochemical oxidation determination and voltammetric behaviour of 4-nitrophenol based on Cu 2O nanoparticles modified glassy carbon electrode. Int. J. Environ. Anal. Chem. 2012, 92, 742–754. [Google Scholar] [CrossRef]
- Yin, H.; Ai, S.; Zhu, L.-S. Electrochemical oxidative determination of 4-nitrophenol based on a glassy carbon electrode modified with a hydroxyapatite nanopowder. Microchim. Acta 2010, 169, 87–92. [Google Scholar] [CrossRef]
- Arvinte, A.; Mahosenaho, M.; Pinteala, M.; Sesay, A.M.; Virtanen, V. Electrochemical oxidation of p-nitrophenol using graphene-modified electrodes, and a comparison to the performance of MWNT-based electrodes. Microchim. Acta 2011, 174, 337–343. [Google Scholar] [CrossRef]
- Madhu, R.; Karuppiah, C.; Chen, S.M.; Veerakumar, P.; Liu, S. Bin Electrochemical detection of 4-nitrophenol based on biomass derived activated carbons. Anal. Methods 2014, 6, 5274–5280. [Google Scholar] [CrossRef]
- Casella, I.G.; Contursi, M. The Electrochemical Reduction of Nitrophenols on Silver Globular Particles Electrodeposited under Pulsed Potential Conditions. J. Electrochem. Soc. 2007, 154, D697. [Google Scholar] [CrossRef]
- Ajitha, B.; Ashok Kumar Reddy, Y.; Reddy, P.S. Biogenic nano-scale silver particles by Tephrosia purpurea leaf extract and their inborn antimicrobial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 121, 164–172. [Google Scholar] [CrossRef]
- Hilal, M.; Han, J.I. Preparation of hierarchical flower-like nickel sulfide as hole transporting material for organic solar cells via a one-step solvothermal method. Sol. Energy 2019, 188, 403–413. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Electrode | Technique | Linear Range (μM) | Limit of Detection (μM) | Reference |
|---|---|---|---|---|
| β-1,4-p-DGA-AgNPs/GrCE | Differential pulse voltammetry | 1–100 | 0.6 | [48] |
| Au NPs/GCE | Semi-derivative voltammetry | 10–1000 | 5.81 | [49] |
| MWCNT/PDPA/GCE | Amperometry | 8.9–1500 | 0.632 | [50] |
| Ag NPs/GCE | Differential pulse voltammetry | 0.1–350 | 2.57 | [51] |
| HA-NP/GCE | Differential pulse voltammetry | 1–300 | 0.6 | [52] |
| Nano-Cu2O/GCE | Differential pulse voltammetry | 1–400 | 0.5 | [53] |
| Graphene/Nf/SPCE | Differential pulse voltammetry | 10–620 | 0.6 | [54] |
| AC/GCE | Linear sweep voltammetry | 1–500 | 5.81 | [55] |
| Ag particles/GCE | Differential pulse voltammetry | 1–400 | 0.5 | [56] |
| Ag2O-CNT-NCs/GCE | I–V method | 0.001–10 | 0.000091 ± 0.000002 | [29] |
| Ag2ONP–C/NFE | I–V method | 0.0000001–0.01 | 0.0000007 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banua, J.; Han, J.I. Biogenesis of Prism-Like Silver Oxide Nanoparticles Using Nappa Cabbage Extract and Their p-Nitrophenol Sensing Activity. Molecules 2020, 25, 2298. https://doi.org/10.3390/molecules25102298
Banua J, Han JI. Biogenesis of Prism-Like Silver Oxide Nanoparticles Using Nappa Cabbage Extract and Their p-Nitrophenol Sensing Activity. Molecules. 2020; 25(10):2298. https://doi.org/10.3390/molecules25102298
Chicago/Turabian StyleBanua, Jomaris, and Jeong In Han. 2020. "Biogenesis of Prism-Like Silver Oxide Nanoparticles Using Nappa Cabbage Extract and Their p-Nitrophenol Sensing Activity" Molecules 25, no. 10: 2298. https://doi.org/10.3390/molecules25102298
APA StyleBanua, J., & Han, J. I. (2020). Biogenesis of Prism-Like Silver Oxide Nanoparticles Using Nappa Cabbage Extract and Their p-Nitrophenol Sensing Activity. Molecules, 25(10), 2298. https://doi.org/10.3390/molecules25102298








