Effects of Structure and Constituent of Prussian Blue Analogs on Their Application in Oxygen Evolution Reaction
Abstract
1. Introduction
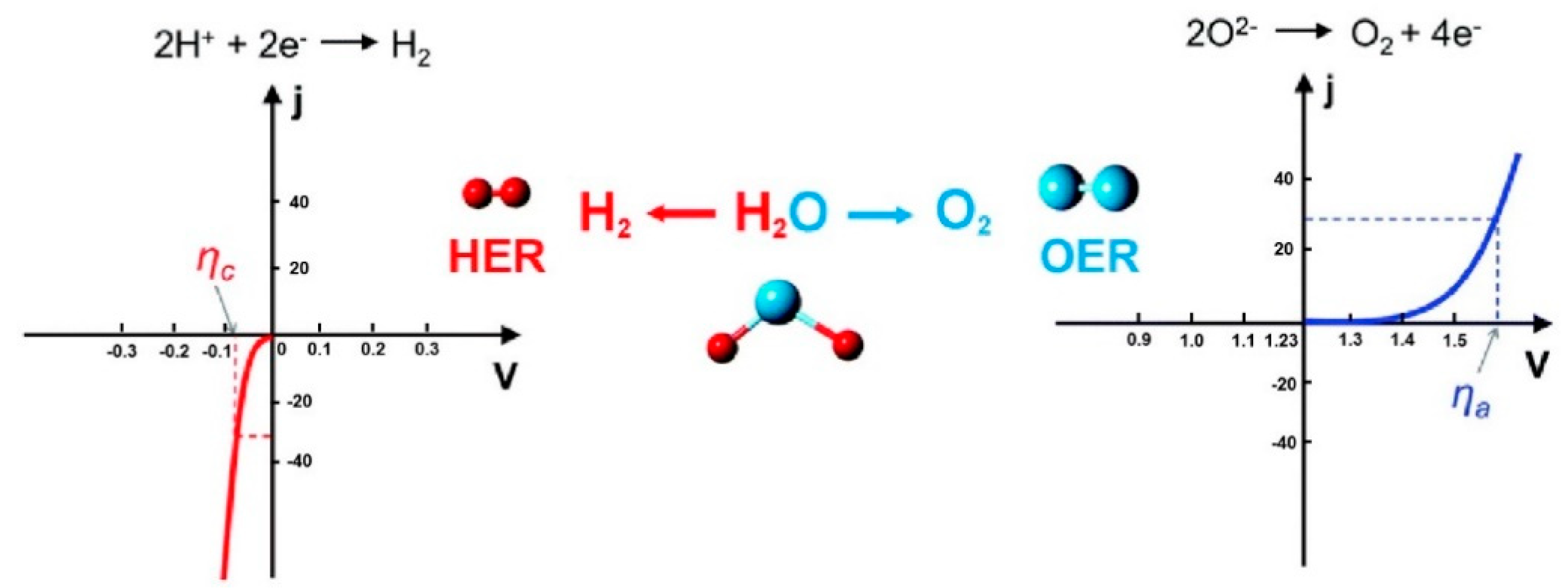
2. Oxygen Evolution Reaction
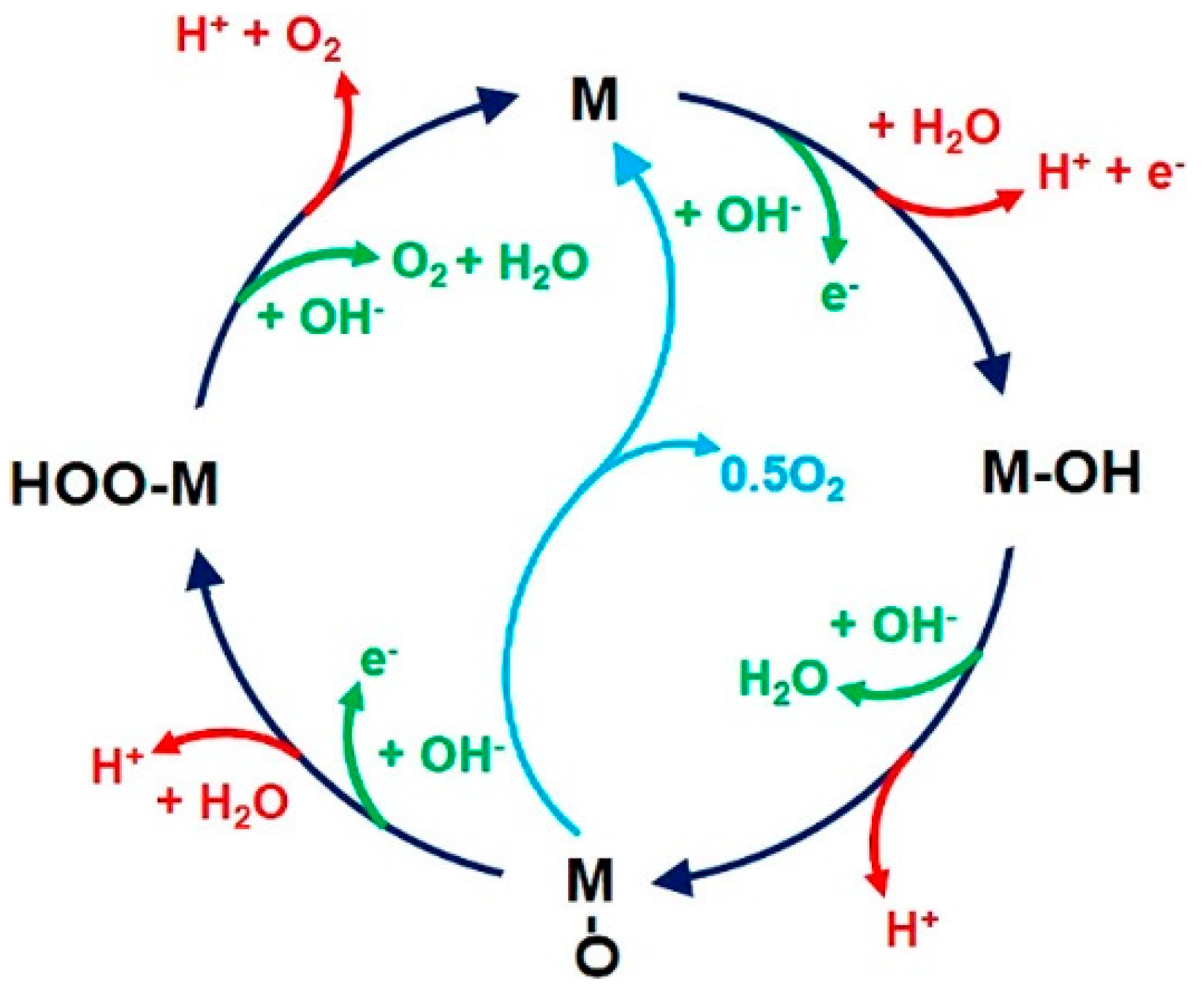
2.1. Reaction Mechanisms
2.2. Design Principles and Evaluation Criteria for the Catalysts
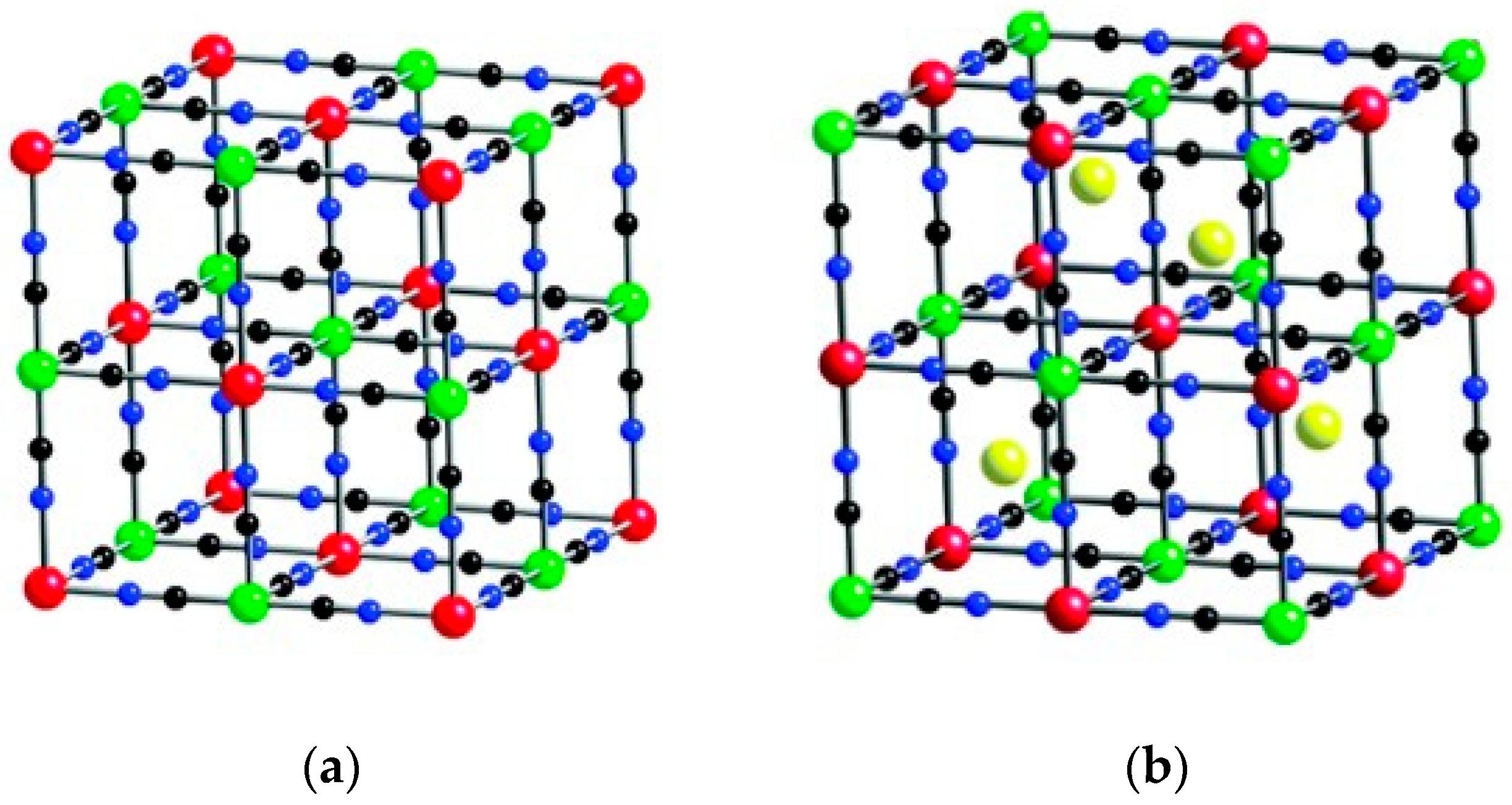
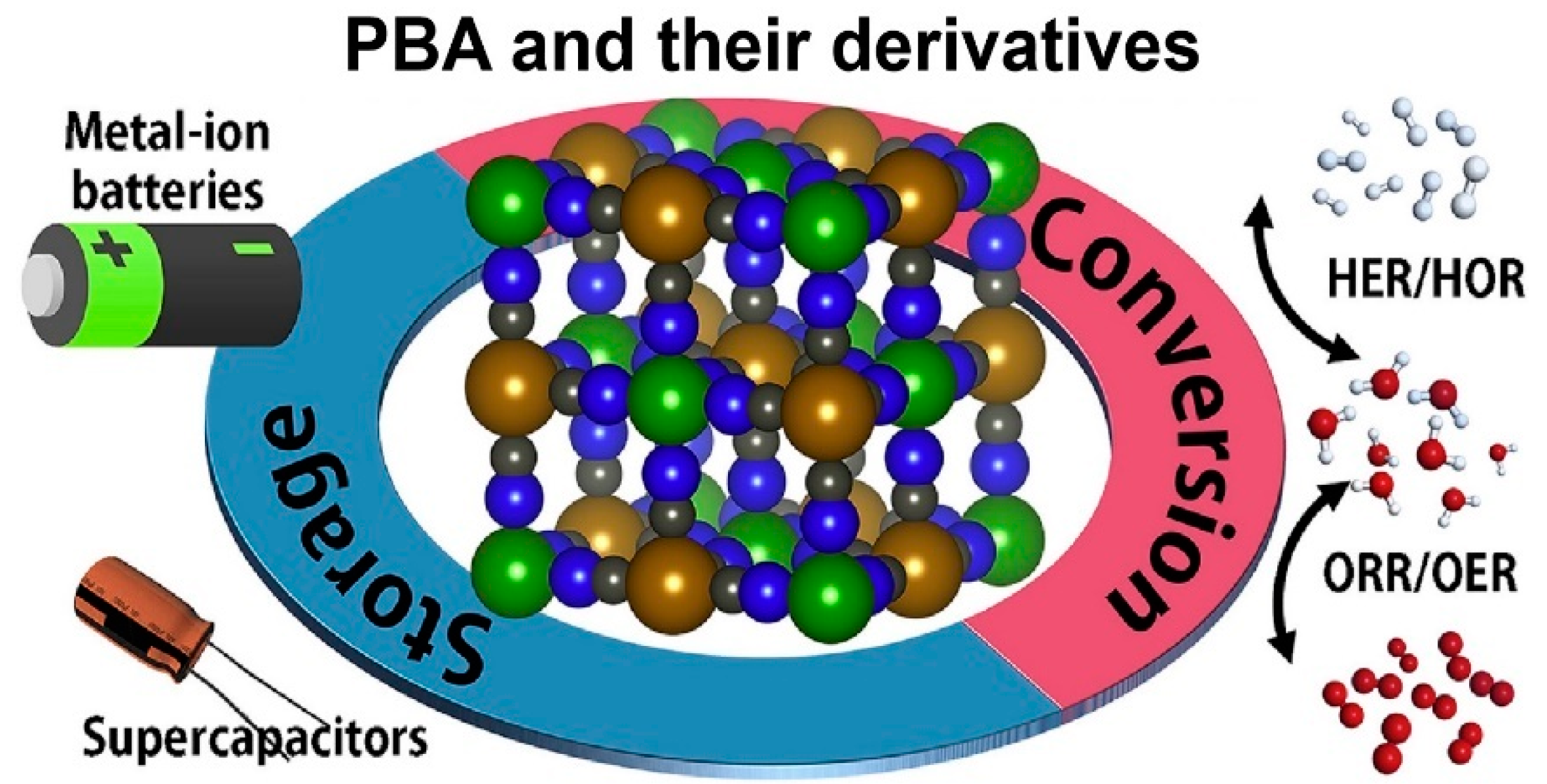
3. Prussian Blue Analogs
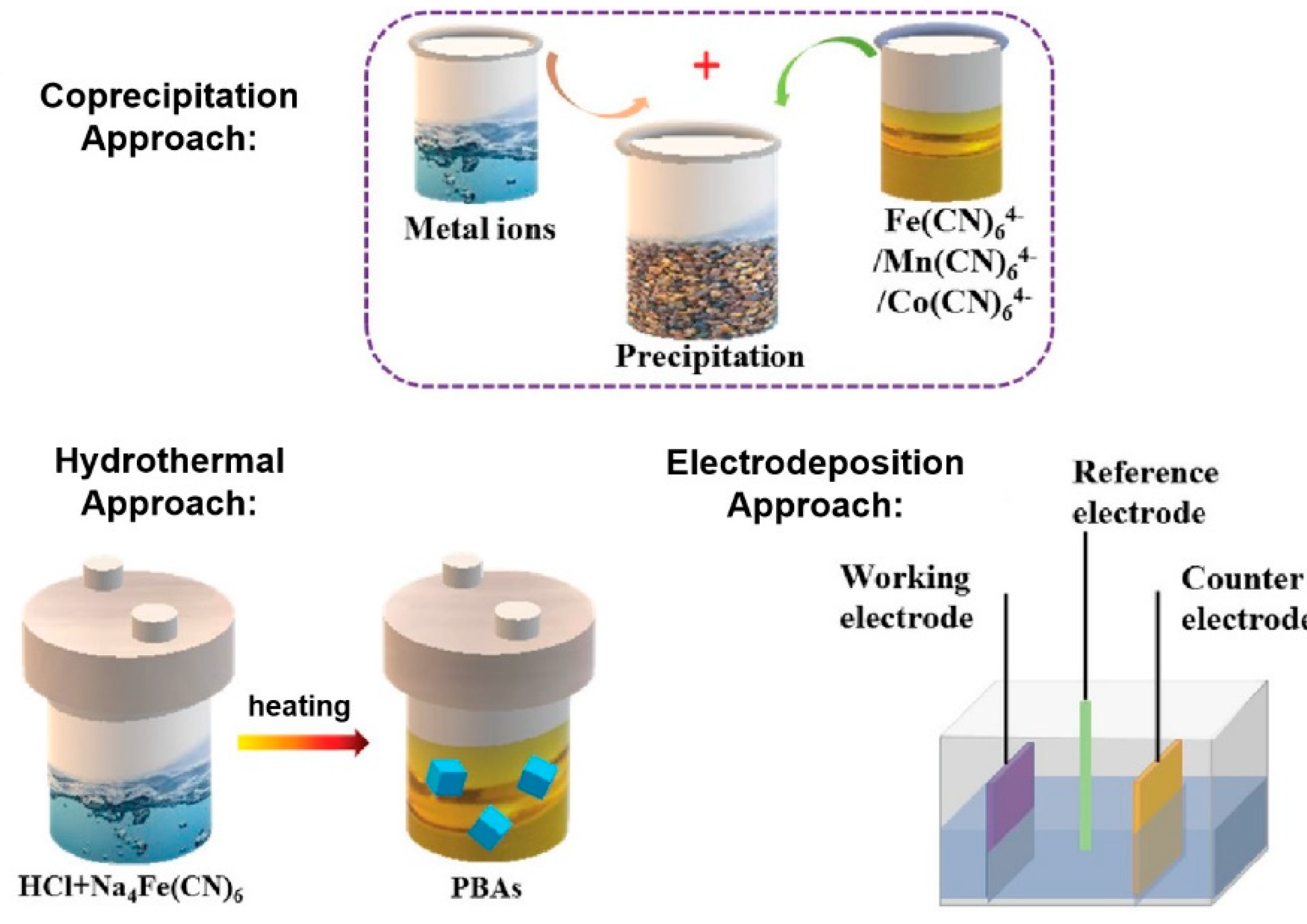
3.1. Methodologies for PBA Synthesis and Characterization
3.2. Applications
3.2.1. OER in Electrolysis of Water
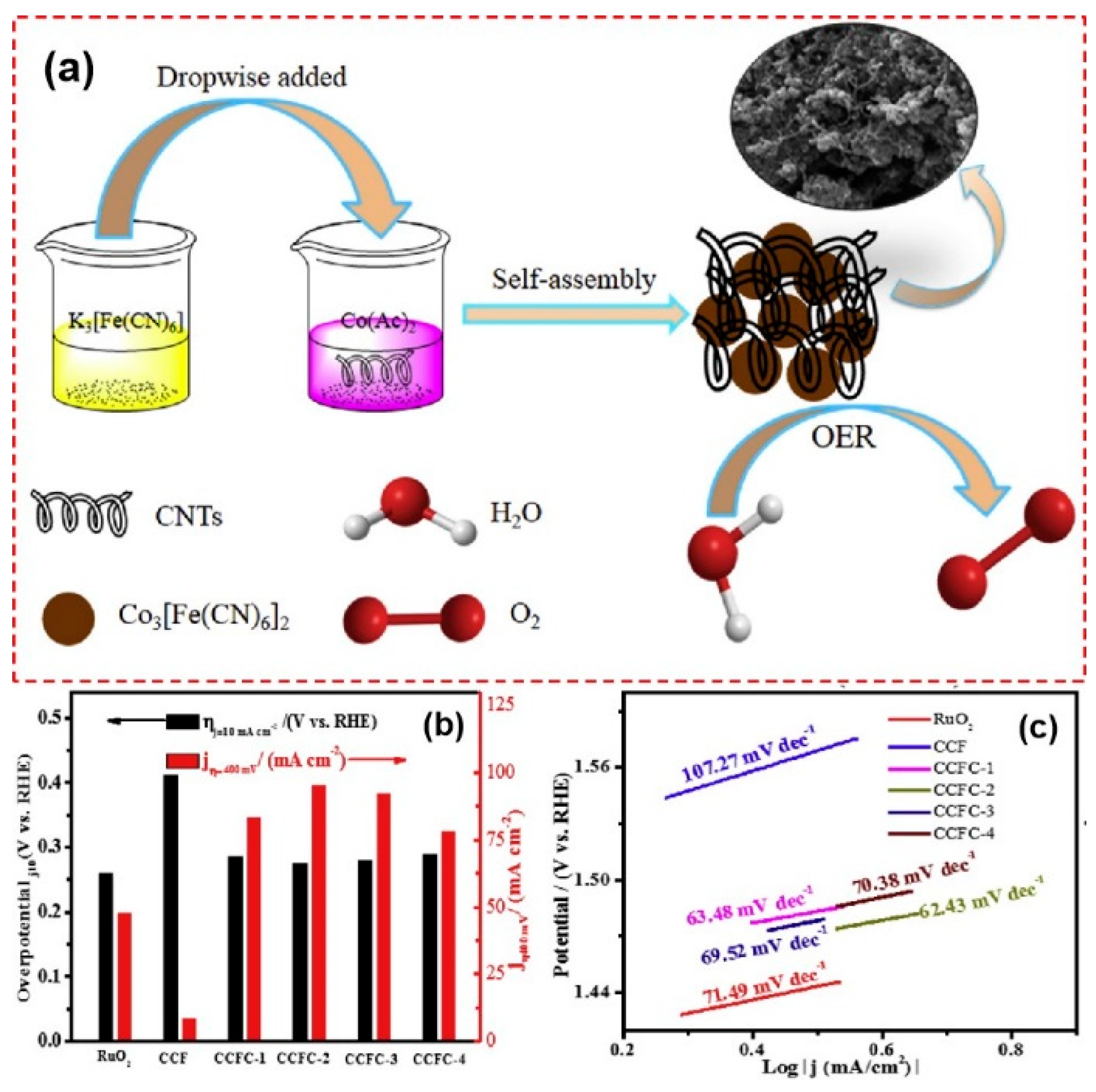
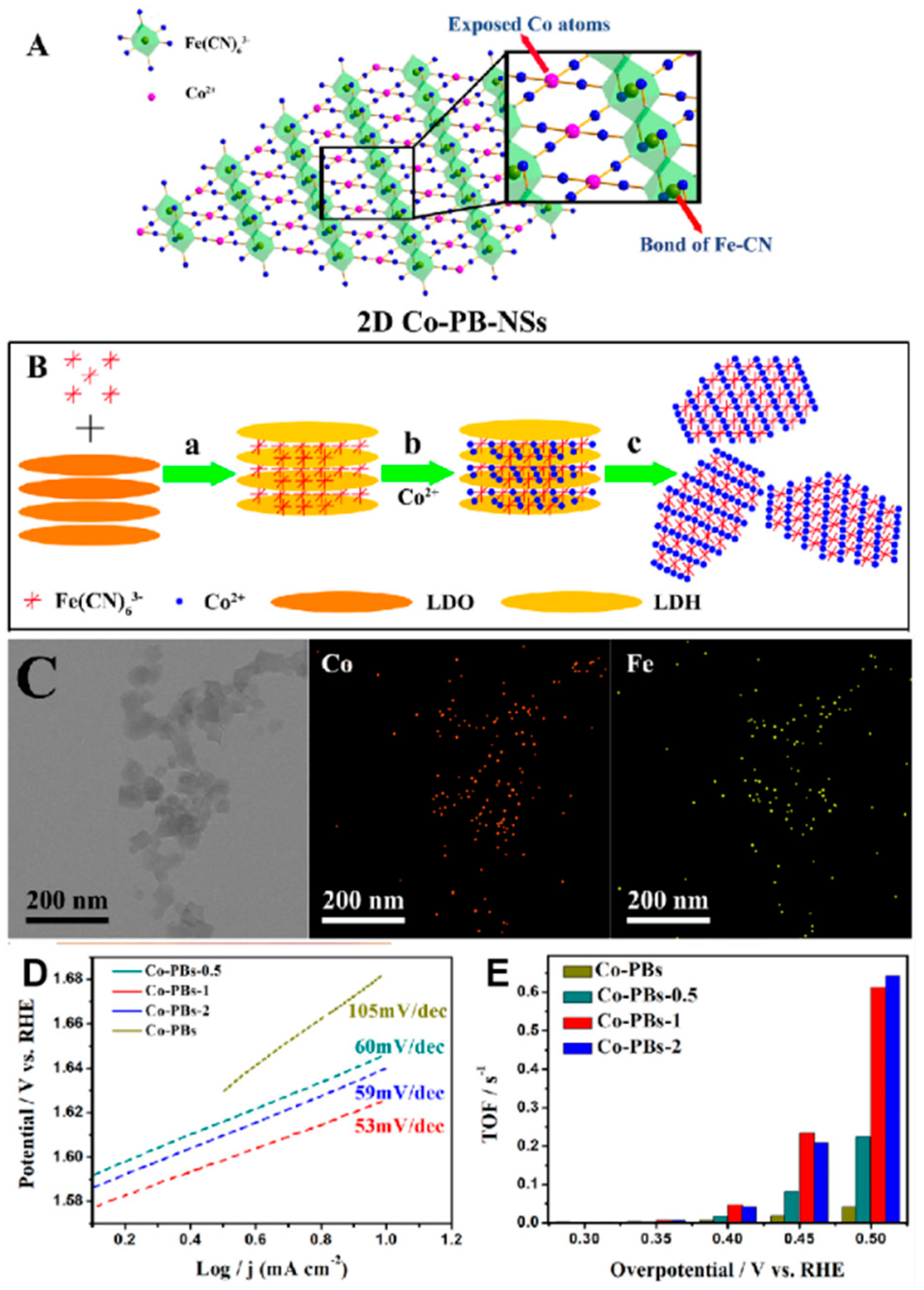
Modifying PBA
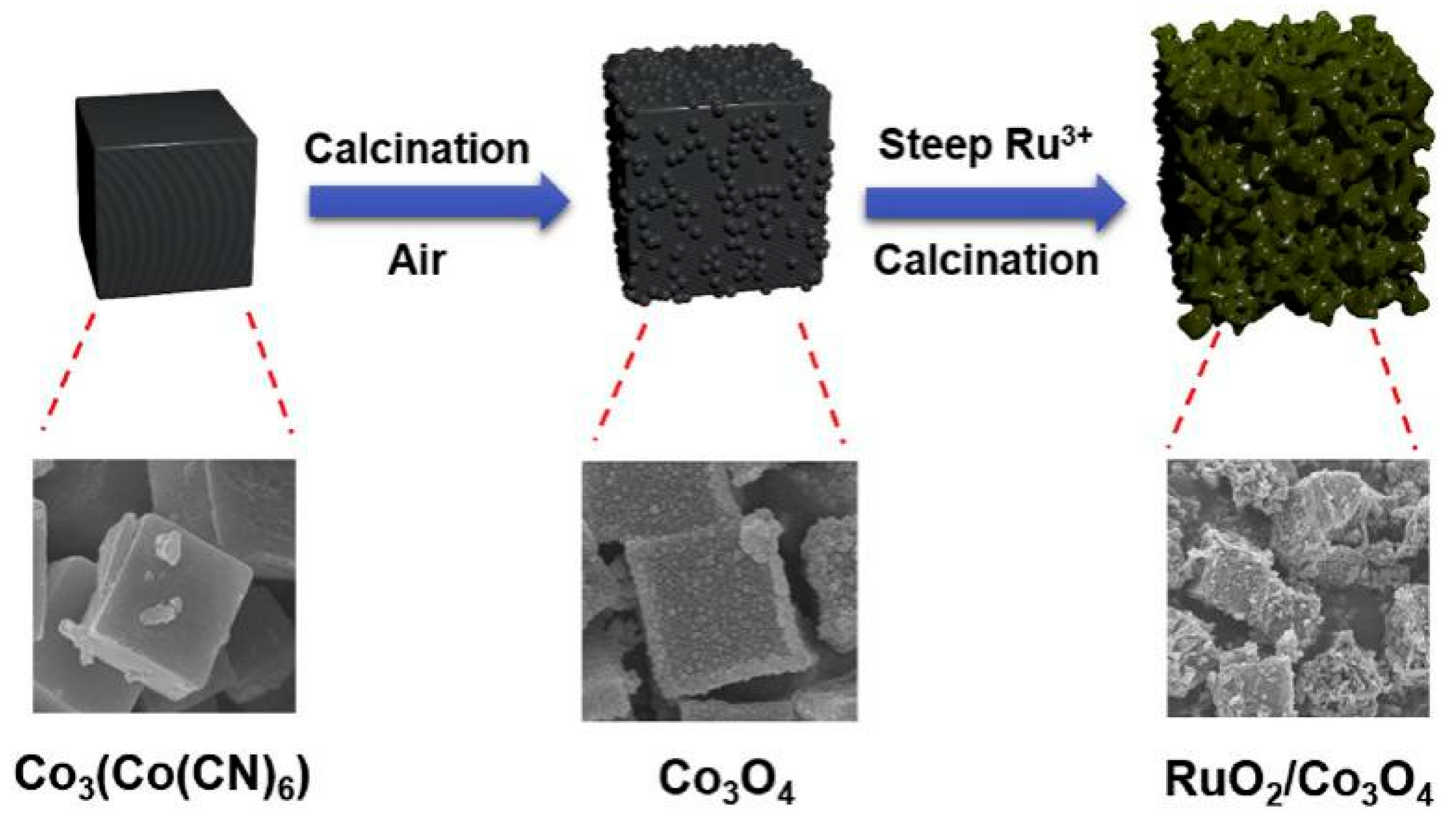
Using PBA as the Precursor
3.2.2. OER in Photoelectrolysis of Water
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Mahmood, A.; Guo, W.; Tabassum, H.; Zou, R. Metal-Organic Framework-Based Nanomaterials for Electrocatalysis. Adv. Energy Mater. 2016, 6, 1600423. [Google Scholar] [CrossRef]
- Tee, S.Y.; Win, K.Y.; Teo, W.S.; Koh, L.-D.; Liu, S.; Teng, C.P.; Han, M.-Y. Recent Progress in Energy-Driven Water Splitting. Adv. Sci. 2017, 4, 1600337. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Guo, C.; Zheng, Y.; Qiao, S. Surface and Interface Engineering of Noble-Metal-Free Electrocatalysts for Efficient Energy Conversion Processes. Acc. Chem. Res. 2017, 50, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, M.; Gao, M.; Jin, J.; Van Spronsen, M.A.; Salmeron, M.; Yang, P. High-Performance Pt–Co Nanoframes for Fuel-Cell Electrocatalysis. Nano Lett. 2020, 20, 1974–1979. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, N.; Guo, C.; Vasileff, A.; Qiao, S. Design Strategies toward Advanced MOF-Derived Electrocatalysts for Energy-Conversion Reactions. Adv. Energy Mater. 2017, 7, 1700518. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; Xiao, X.; Zhang, F.; Song, S.; Chen, S.; Ren, Z. Recent Advances in Self-Supported Layered Double Hydroxides for Oxygen Evolution Reaction. Research 2020, 2020, 3976278. [Google Scholar] [CrossRef]
- Li, W.; Han, C.; Cheng, G.; Chou, S.; Liu, H.; Dou, S. Chemical Properties, Structural Properties, and Energy Storage Applications of Prussian Blue Analogues. Small 2019, 15, e1900470. [Google Scholar] [CrossRef]
- Allam, R.J. Improved oxygen production technologies. Energy Procedia 2009, 1, 461–470. [Google Scholar] [CrossRef]
- Chung, D.Y.; Lopes, P.P.; Martins, P.F.B.D.; He, H.; Kawaguchi, T.; Zapol, P.; You, H.; Tripkovic, D.; Strmcnik, D.; Zhu, Y.; et al. Dynamic stability of active sites in hydr(oxy)oxides for the oxygen evolution reaction. Nat. Energy 2020, 5, 222–230. [Google Scholar] [CrossRef]
- Man, I.C.; Su, H.-Y.; Calle-Vallejo, F.; Hansen, H.A.; Martínez, J.I.; Inoglu, N.G.; Kitchin, J.R.; Jaramillo, T.; Nørskov, J.K.; Rossmeisl, J. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.-M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef]
- Abbott, D.F.; Lebedev, D.; Waltar, K.; Povia, M.; Nachtegaal, M.; Fabbri, E.; Copéret, C.; Schmidt, T.J. Iridium Oxide for the Oxygen Evolution Reaction: Correlation between Particle Size, Morphology, and the Surface Hydroxo Layer from Operando XAS. Chem. Mater. 2016, 28, 6591–6604. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; Liu, S.; Xu, W.; Wu, L.; Hsieh, Y.-C.; Liu, P.; Zhu, Y.; Sasaki, K.; Renner, J.N.; et al. Reaction mechanism for oxygen evolution on RuO2, IrO2, and RuO2@IrO2 core-shell nanocatalysts. J. Electroanal. Chem. 2018, 819, 296–305. [Google Scholar] [CrossRef]
- Patra, C.R. Prussian blue nanoparticles and their analogues for application to cancer theranostics. Nanomedicine 2016, 11, 569–572. [Google Scholar] [CrossRef]
- Yue, Y.; Binder, A.; Guo, B.; Zhang, Z.; Qiao, Z.-A.; Tian, C.; Dai, S. Mesoporous Prussian Blue Analogues: Template-Free Synthesis and Sodium-Ion Battery Applications. Angew. Chem. Int. Ed. 2014, 53, 3134–3137. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, W.; Hu, M. Prussian Blue Analogue Mesoframes for Enhanced Aqueous Sodium-ion Storage. Crystals 2018, 8, 23. [Google Scholar] [CrossRef]
- Cao, L.-M.; Lu, D.; Zhong, D.-C.; Lu, T.-B. Prussian blue analogues and their derived nanomaterials for electrocatalytic water splitting. Coord. Chem. Rev. 2020, 407, 213156. [Google Scholar] [CrossRef]
- Singh, B.; Indra, A. Designing Self-Supported Metal Organic Framework Derived Catalysts for Electrochemical Water Splitting. Chem. Asian J. 2020, 15, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Nai, J.; Lou, X.W. Hollow Structures Based on Prussian Blue and Its Analogs for Electrochemical Energy Storage and Conversion. Adv. Mater. 2018, 31, 1706825. [Google Scholar] [CrossRef] [PubMed]
- Dau, H.; Limberg, C.; Reier, T.; Risch, M.; Roggan, S.; Strasser, P. The Mechanism of Water Oxidation: From Electrolysis via Homogeneous to Biological Catalysis. ChemCatChem 2010, 2, 724–761. [Google Scholar] [CrossRef]
- Sultan, S.; Ha, M.; Kim, D.Y.; Tiwari, J.N.; Myung, C.W.; Meena, A.; Shin, T.J.; Chae, K.H.; Kim, K.S. Superb water splitting activity of the electrocatalyst Fe3Co(PO4)4 designed with computation aid. Nat. Commun. 2019, 10, 5195. [Google Scholar] [CrossRef]
- Wu, Z.; Lu, X.F.; Zang, S.; Lou, X.W. Non-Noble-Metal-Based Electrocatalysts toward the Oxygen Evolution Reaction. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Song, J.; Du, Y.; Xi, S.; Dou, S.; Nsanzimana, J.M.V.; Wang, C.; Xu, Z.J.; Wang, X. Chemical and structural origin of lattice oxygen oxidation in Co–Zn oxyhydroxide oxygen evolution electrocatalysts. Nat. Energy 2019, 4, 329–338. [Google Scholar] [CrossRef]
- Shi, X.; Siahrostami, S.; Li, G.L.; Zhang, Y.; Chakthranont, P.; Studt, F.; Jaramillo, T.; Zheng, X.; Nørskov, J. Understanding activity trends in electrochemical water oxidation to form hydrogen peroxide. Nat. Commun. 2017, 8, 701. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, H.; Kong, D.; Yan, K.; Hsu, P.-C.; Zheng, G.; Yao, H.; Liang, Z.; Sun, X.; Cui, Y. Electrochemical tuning of layered lithium transition metal oxides for improvement of oxygen evolution reaction. Nat. Commun. 2014, 5, 4345. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef]
- Craig, M.J.; Coulter, G.; Dolan, E.; Soriano-López, J.; Mates-Torres, E.; Schmitt, W.; Garcia-Melchor, M. Universal scaling relations for the rational design of molecular water oxidation catalysts with near-zero overpotential. Nat. Commun. 2019, 10, 4993–4999. [Google Scholar] [CrossRef]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S. Emerging Two-Dimensional Nanomaterials for Electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.F.; Xia, B.Y.; Zang, S.-Q.; Lou, X.W. Metal–Organic Frameworks Based Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2020, 59, 4634–4650. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Zang, S.; Lou, X.W. Hierarchical Hollow Heterostructures for Photocatalytic CO2 Reduction and Water Splitting. Small Methods 2019, 4. [Google Scholar] [CrossRef]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 3. [Google Scholar] [CrossRef]
- Short, G.D.; Bishop, E. Concentration Overpotentials on Antimony Electrodes in Differential Electrolytic Potentiometry. Anal. Chem. 1965, 37, 962–967. [Google Scholar] [CrossRef]
- Burstein, G. A hundred years of Tafel’s Equation: 1905–2005. Corros. Sci. 2005, 47, 2858–2870. [Google Scholar] [CrossRef]
- Nicholson, R.S. Theory and Application of Cyclic Voltammetry for Measurement of Electrode Reaction Kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Nicholson, R.S.; Shain, I. Theory of Stationary Electrode Polarography. Single Scan and Cyclic Methods Applied to Reversible, Irreversible, and Kinetic Systems. Anal. Chem. 1964, 36, 706–723. [Google Scholar] [CrossRef]
- Geiger, W.E. Reflections on Future Directions in Organometallic Electrochemistry†. Organometallics 2011, 30, 28–31. [Google Scholar] [CrossRef]
- Benavente, J. Electrochemical Impedance Spectroscopy as a Tool for Electrical and Structural Characterizations of Membranes in Contact with Electrolyte Solutions. In Recent Advances in Multidisciplinary Applied Physics, Proceedings of the First International Meeting on Applied Physics, Badajoz, Spain, 13–18 October 2003; Elsevier: Amsterdam, The Netherlands, 2005; pp. 463–471. [Google Scholar] [CrossRef]
- Costentin, C.; Drouet, S.; Robert, M.; Savéant, J.-M. Turnover Numbers, Turnover Frequencies, and Overpotential in Molecular Catalysis of Electrochemical Reactions. Cyclic Voltammetry and Preparative-Scale Electrolysis. J. Am. Chem. Soc. 2012, 134, 11235–11242. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Recent Trends and Perspectives in Electrochemical Water Splitting with an Emphasis on Sulfide, Selenide, and Phosphide Catalysts of Fe, Co, and Ni: A Review. ACS Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Jones, J.E.; Hansen, L.D.; Jones, S.E.; Shelton, D.S.; Thorne, J.M. Faradaic Efficiencies Less Than 100% during Electrolysis of Water Can Account for Reports of Excess Heat in “Cold Fusion” Cells. J. Phys. Chem. 1995, 99, 6973–6979. [Google Scholar] [CrossRef]
- Kraft, A. On the discovery and history of Prussian blue. Bull. Hist. Chem. 2008, 33, 61–67. [Google Scholar]
- Buser, H.J.; Schwarzenbach, D.; Petter, W.; Ludi, A. The crystal structure of Prussian Blue: Fe4[Fe(CN)6]3·xH2O. Inorg. Chem. 1977, 16, 2704–2710. [Google Scholar] [CrossRef]
- Grandjean, F.; Samain, L.; Long, G.J. Characterization and utilization of Prussian blue and its pigments. Dalton Trans. 2016, 45, 18018–18044. [Google Scholar] [CrossRef]
- Aguilà, D.; Prado, Y.; Koumousi, E.S.; Mathonière, C.; Clerac, R. Switchable Fe/Co Prussian blue networks and molecular analogues. Chem. Soc. Rev. 2016, 45, 203–224. [Google Scholar] [CrossRef]
- Pasta, M.; Wang, R.; Ruffo, R.; Qiao, R.; Lee, H.-W.; Shyam, B.; Guo, M.; Wang, Y.; Wray, L.A.; Yang, W.; et al. Manganese–cobalt hexacyanoferrate cathodes for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 4211–4223. [Google Scholar] [CrossRef]
- Pasta, M.; Wessells, C.D.; Huggins, R.A.; Cui, Y. A high-rate and long cycle life aqueous electrolyte battery for grid-scale energy storage. Nat. Commun. 2012, 3, 1149. [Google Scholar] [CrossRef]
- Bie, X.; Kubota, K.; Hosaka, T.; Chihara, K.; Komaba, S. Synthesis and electrochemical properties of Na-rich Prussian blue analogues containing Mn, Fe, Co, and Fe for Na-ion batteries. J. Power Sources 2018, 378, 322–330. [Google Scholar] [CrossRef]
- Wessells, C.D.; Huggins, R.A.; Cui, Y. Copper hexacyanoferrate battery electrodes with long cycle life and high power. Nat. Commun. 2011, 2, 550. [Google Scholar] [CrossRef]
- Salunkhe, R.; Kaneti, Y.V.; Kim, J.; Kim, J.H.; Yamauchi, Y. Nanoarchitectures for Metal–Organic Framework-Derived Nanoporous Carbons toward Supercapacitor Applications. Acc. Chem. Res. 2016, 49, 2796–2806. [Google Scholar] [CrossRef]
- Zhang, G.; Yao, H.; Zhang, F.; Gao, Z.; Li, Q.; Yang, Y.; Lu, X. A high over-potential binder-free electrode constructed of Prussian blue and MnO2 for high performance aqueous supercapacitors. Nano Res. 2019, 12, 1061–1069. [Google Scholar] [CrossRef]
- Krap, C.P.; Balmaseda, J.; Del Castillo, L.F.; Zamora, B.; Reguera, E. Hydrogen Storage in Prussian Blue Analogues: H2 Interaction with the Metal Found at the Cavity Surface. Energy Fuels 2010, 24, 581–589. [Google Scholar] [CrossRef]
- Karyakin, A.A. Advances of Prussian blue and its analogues in (bio)sensors. Curr. Opin. Electrochem. 2017, 5, 92–98. [Google Scholar] [CrossRef]
- Han, L.; Yu, X.-Y.; Lou, X.W. Formation of Prussian-Blue-Analog Nanocages via a Direct Etching Method and their Conversion into Ni-Co-Mixed Oxide for Enhanced Oxygen Evolution. Adv. Mater. 2016, 28, 4601–4605. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.B.; Chikyow, T. Recent advances in Prussian blue and Prussian blue analogues: Synthesis and thermal treatments. Coord. Chem. Rev. 2017, 352, 328–345. [Google Scholar] [CrossRef]
- Wessells, C.D.; Peddada, S.V.; McDowell, M.T.; Huggins, R.A.; Cui, Y. The Effect of Insertion Species on Nanostructured Open Framework Hexacyanoferrate Battery Electrodes. J. Electrochem. Soc. 2011, 159, A98–A103. [Google Scholar] [CrossRef]
- Ming, H.; Torad, N.; Chiang, Y.-D.; Wu, K.C.W.; Yamauchi, Y. Size- and shape-controlled synthesis of Prussian Blue nanoparticles by a polyvinylpyrrolidone-assisted crystallization process. CrystEngComm 2012, 14, 3387–3396. [Google Scholar] [CrossRef]
- Zakaria, M.B.; Hu, M.; Tsujimoto, Y.; Sakka, Y.; Suzuki, N.; Kamachi, Y.; Imura, M.; Ishihara, S.; Ariga, K.; Yamauchi, Y. Controlled Crystallization of Cyano-Bridged Cu-Pt Coordination Polymers with Two-Dimensional Morphology. Chem. Asian J. 2014, 9, 1511–1514. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, G.; Ma, M.; Qiao, Y. Role of Acid in Tailoring Prussian Blue as Cathode for High-Performance Sodium-Ion Battery. Chem. Eur. J. 2017, 23, 15991–15996. [Google Scholar] [CrossRef]
- Neff, V.D. Electrochemical Oxidation and Reduction of Thin Films of Prussian Blue. J. Electrochem. Soc. 1978, 125, 6. [Google Scholar] [CrossRef]
- Isfahani, V.B.; Dizaji, H.R.; Memarian, N.; Arab, A. Electrodeposition of prussian blue films: Study of deposition time effect on electrochemical properties. Mater. Res. Express 2019, 6, 096449. [Google Scholar] [CrossRef]
- Li, Y.; Wang, K.; Zhou, W.; Li, Y.; Vila, R.; Huang, W.; Wang, H.; Chen, G.; Wu, G.-H.; Tsao, Y.; et al. Cryo-EM Structures of Atomic Surfaces and Host-Guest Chemistry in Metal-Organic Frameworks. Phys. B Condens. Matter 2019, 1, 428–438. [Google Scholar] [CrossRef]
- Zhao, Y.; Mavrokefalos, C.K.; Zhang, P.; Erni, R.; Li, J.; Triana, C.A.; Patzke, G.R. Self-Templating Strategies for Transition Metal Sulfide Nanoboxes as Robust Bifunctional Electrocatalysts. Chem. Mater. 2020, 32, 1371–1383. [Google Scholar] [CrossRef]
- Li, X.; Zhu, K.; Pang, J.; Tian, M.; Liu, J.; Rykov, A.I.; Zheng, M.; Wang, X.; Zhu, X.; Huang, Y.; et al. Unique role of Mössbauer spectroscopy in assessing structural features of heterogeneous catalysts. Appl. Catal. B Environ. 2018, 224, 518–532. [Google Scholar] [CrossRef]
- Simonov, A.; De Baerdemaeker, T.; Boström, H.L.B.; Gómez, M.L.R.; Gray, H.J.; Chernyshov, D.; Bosak, A.; Bürgi, H.-B.; Goodwin, A. Hidden diversity of vacancy networks in Prussian blue analogues. Nature 2020, 578, 256–260. [Google Scholar] [CrossRef]
- Chen, J.; Wei, L.; Mahmood, A.; Pei, Z.; Zhou, Z.; Chen, X.; Chen, Y. Prussian blue, its analogues and their derived materials for electrochemical energy storage and conversion. Energy Storage Mater. 2020, 25, 585–612. [Google Scholar] [CrossRef]
- Tang, S.; Li, L.; Ren, H.; Lv, Q.; Lv, R. Sodium-ion electrochemical tuning of Prussian blue analog as an efficient oxygen evolution catalyst. Mater. Today Chem. 2019, 12, 71–77. [Google Scholar] [CrossRef]
- Han, L.; Galán-Mascarós, J.R. The Positive Effect of Iron Doping in the Electrocatalytic Activity of Cobalt Hexacyanoferrate. Catalysts 2020, 10, 130. [Google Scholar] [CrossRef]
- Elayappan, V.; Shanmugam, R.; Chinnusamy, S.; Yoo, D.J.; Mayakrishnan, G.; Kim, K.; Noh, H.S.; Kim, M.K.; Yoo, D.J.; Vijayakumar, E.; et al. Three-dimensional bimetal TMO supported carbon based electrocatalyst developed via dry synthesis for hydrogen and oxygen evolution. Appl. Surf. Sci. 2020, 505, 144642. [Google Scholar] [CrossRef]
- Ghasemi, S.; Hosseini, S.R.; Asen, P. Preparation of graphene/nickel-iron hexacyanoferrate coordination polymer nanocomposite for electrochemical energy storage. Electrochim. Acta 2015, 160, 337–346. [Google Scholar] [CrossRef]
- Ramos, M.K.; Zarbin, A.J.G. Graphene/copper oxide nanoparticles thin films as precursor for graphene/copper hexacyanoferrate nanocomposites. Appl. Surf. Sci. 2020, 515, 146000. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, B.; Wang, X.; Yang, D.; Chen, Y. Self-assembled globular clusters-like cobalt hexacyanoferrate/carbon nanotubes hybrid as efficient nonprecious electrocatalyst for oxygen evolution reaction. J. Power Sources 2019, 434, 126670. [Google Scholar] [CrossRef]
- Husmann, S.; Booth, S.G.; Zarbin, A.; Dryfe, R. Electrodeposition of Prussian Blue/Carbon Nanotube Composites at a Liquid-Liquid Interface. J. Braz. Chem. Soc. 2018, 29, 1130–1139. [Google Scholar] [CrossRef]
- Han, L.; Tang, P.; Carmona, A.R.; Rodriguez-Garcia, B.; Torrens, M.; Morante, J.R.; Arbiol, J.; Galán-Mascarós, J.R. Enhanced Activity and Acid pH Stability of Prussian Blue-type Oxygen Evolution Electrocatalysts Processed by Chemical Etching. J. Am. Chem. Soc. 2016, 138, 16037–16045. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, M.; Nune, S.V.K.; Karadas, F. A Novel Synthetic Route for the Preparation of an Amorphous Co/Fe Prussian Blue Coordination Compound with High Electrocatalytic Water Oxidation Activity. Inorg. Chem. 2016, 55, 4301–4307. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Duan, Y.; Liu, J.-D.; Chen, Y.; Liu, X.-K.; Liu, W.; Ma, T.; Li, Y.; Zheng, X.-S.; Yao, T.; et al. Unconventional CN vacancies suppress iron-leaching in Prussian blue analogue pre-catalyst for boosted oxygen evolution catalysis. Nat. Commun. 2019, 10, 2799. [Google Scholar] [CrossRef]
- Bui, H.T.; Ahn, D.Y.; Shrestha, N.K.; Sung, M.M.; Lee, J.K.; Han, S.-H. Self-assembly of cobalt hexacyanoferrate crystals in 1-D array using ion exchange transformation route for enhanced electrocatalytic oxidation of alkaline and neutral water. J. Mater. Chem. A 2016, 4, 9781–9788. [Google Scholar] [CrossRef]
- Bui, H.T.; Shrestha, N.K.; Khadtare, S.; Bathula, C.D.; Giebeler, L.; Noh, Y.Y.; Han, S.-H. Anodically Grown Binder-Free Nickel Hexacyanoferrate Film: Toward Efficient Water Reduction and Hexacyanoferrate Film Based Full Device for Overall Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 18015–18021. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, G.X.; Xu, Y.X.; Zhang, H.L.; Guo, X.T.; Liu, Y.; Pang, H. A new strategy for the controllable growth of MOF@PBA architectures. J. Mater. Chem. A 2019, 7, 17266–17271. [Google Scholar] [CrossRef]
- Huang, H.; Xue, Q.; Zhang, Y.; Chen, Y. Two-dimensional cobalt prussian blue nanosheets: Template-directed synthesis and electrocatalytic oxygen evolution property. Electrochim. Acta 2020, 333, 135544. [Google Scholar] [CrossRef]
- Yu, L.; Hu, H.; Bin Wu, H.; Lou, X.W. Complex Hollow Nanostructures: Synthesis and Energy-Related Applications. Adv. Mater. 2017, 29, 1604563. [Google Scholar] [CrossRef] [PubMed]
- Xuan, C.; Peng, Z.; Xia, K.; Wang, J.; Xiao, W.; Lei, W.; Gong, M.; Huang, T.; Wang, D. Self-supported ternary Ni-Fe-P nanosheets derived from metal-organic frameworks as efficient overall water splitting electrocatalysts. Electrochim. Acta 2017, 258, 423–432. [Google Scholar] [CrossRef]
- Sivanantham, A.; Ganesan, P.; Shanmugam, S. Hierarchical NiCo2S4 Nanowire Arrays Supported on Ni Foam: An Efficient and Durable Bifunctional Electrocatalyst for Oxygen and Hydrogen Evolution Reactions. Adv. Funct. Mater. 2016, 26, 4661–4672. [Google Scholar] [CrossRef]
- Xi, W.; Yan, G.; Lang, Z.; Ma, Y.; Tan, H.-Q.; Zhu, H.; Wang, Y.; Li, Y. Oxygen-Doped Nickel Iron Phosphide Nanocube Arrays Grown on Ni Foam for Oxygen Evolution Electrocatalysis. Small 2018, 14, e1802204. [Google Scholar] [CrossRef]
- Guo, P.; Wang, Z.; Ge, S.; Chen, H.; Zhang, J.; Wang, H.; Liu, S.; Wei, S.; Lu, X. In Situ Coupling Reconstruction of Cobalt–Iron Oxide on a Cobalt Phosphate Nanoarray with Interfacial Electronic Features for Highly Enhanced Water Oxidation Catalysis. ACS Sustain. Chem. Eng. 2020, 8, 4773–4780. [Google Scholar] [CrossRef]
- Yuan, B.; Li, C.; Guan, L.; Li, K.; Lin, Y. Prussian blue analog nanocubes tuning synthesis of coral-like Ni3S2@MIL-53(NiFeCo) core-shell nanowires array and boosting oxygen evolution reaction. J. Power Sources 2020, 451, 227295. [Google Scholar] [CrossRef]
- Zamel, N.; Li, X.; Shen, J. Numerical estimation of the effective electrical conductivity in carbon paper diffusion media. Appl. Energy 2012, 93, 39–44. [Google Scholar] [CrossRef]
- Ishizaki, M.; Fujii, H.; Toshima, K.; Tanno, H.; Sutoh, H.; Kurihara, M. Preparation of Co-Fe oxides immobilized on carbon paper using water-dispersible Prussian-blue analog nanoparticles and their oxygen evolution reaction (OER) catalytic activities. Inorg. Chim. Acta 2020, 502, 119345. [Google Scholar] [CrossRef]
- Guo, B.-Y.; Zhang, X.-Y.; Ma, X.; Chen, T.-S.; Chen, Y.; Wen, M.-L.; Qin, J.-F.; Nan, J.; Chai, Y.-M.; Dong, B. RuO2/Co3O4 Nanocubes based on Ru ions impregnation into prussian blue precursor for oxygen evolution. Int. J. Hydrog. Energy 2020, 45, 9575–9582. [Google Scholar] [CrossRef]
- Ahn, I.-K.; Joo, W.; Lee, J.-H.; Kim, H.G.; Lee, S.-Y.; Jung, Y.; Kim, J.-Y.; Lee, G.-B.; Kim, M.; Joo, Y.-C. Metal-organic Framework-driven Porous Cobalt Disulfide Nanoparticles Fabricated by Gaseous Sulfurization as Bifunctional Electrocatalysts for Overall Water Splitting. Sci. Rep. 2019, 9, 19539. [Google Scholar] [CrossRef]
- Ma, Q.; Dong, R.; Liu, H.; Zhu, A.; Qiao, L.; Ma, Y.; Wang, J.; Xie, J.; Pan, J. Prussian blue analogue-derived Mn–Fe oxide nanocubes with controllable crystal structure and crystallinity as highly efficient OER electrocatalysts. J. Alloys Compd. 2020, 820. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.B.; Madhavi, S.; Hng, H.H.; Lou, X.W. Formation of Fe2O3 Microboxes with Hierarchical Shell Structures from Metal–Organic Frameworks and Their Lithium Storage Properties. J. Am. Chem. Soc. 2012, 134, 17388–17391. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-Y.; Yu, L.; Bin Wu, H.; Lou, X.W. Formation of Nickel Sulfide Nanoframes from Metal-Organic Frameworks with Enhanced Pseudocapacitive and Electrocatalytic Properties. Angew. Chem. Int. Ed. 2015, 54, 5331–5335. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, P.; Gao, T.; Huangfu, J.; Wang, X.-J.; Liu, S.; Zhang, Y.; Song, B. Controlled Synthesis of Hollow Bimetallic Prussian Blue Analog for Conversion into Efficient Oxygen Evolution Electrocatalyst. ACS Sustain. Chem. Eng. 2019, 8, 1319–1328. [Google Scholar] [CrossRef]
- Xie, J.-Y.; Liu, Z.-Z.; Li, J.; Feng, L.; Yang, M.; Ma, Y.; Liu, D.-P.; Wang, L.; Chai, Y.-M.; Dong, B. Fe-doped CoP core–shell structure with open cages as efficient electrocatalyst for oxygen evolution. J. Energy Chem. 2020, 48, 328–333. [Google Scholar] [CrossRef]
- Kahnamouei, M.H.; Shahrokhian, S. Mesoporous Nanostructured Composite Derived from Thermal Treatment CoFe Prussian Blue Analogue Cages and Electrodeposited NiCo-S as an Efficient Electrocatalyst for an Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2020, 12, 16250–16263. [Google Scholar] [CrossRef]
- Liao, H.; Guo, X.; Hou, Y.; Liang, H.; Zhou, Z.; Yang, H. Construction of Defect-Rich Ni-Fe-Doped K0.23 MnO2 Cubic Nanoflowers via Etching Prussian Blue Analogue for Efficient Overall Water Splitting. Small 2020, 16, e1905223. [Google Scholar] [CrossRef]
- Chen, S.; Ma, L.; Wu, S.; Wang, S.; Li, Z.; Emmanuel, A.A.; Huqe, R.; Zhi, C.; Zapien, J.A. Uniform Virus-Like Co–N–Cs Electrocatalyst Derived from Prussian Blue Analog for Stretchable Fiber-Shaped Zn–Air Batteries. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Inoue, H.; Ichiroku, N.; Torimoto, T.; Sakata, T.; Mori, H.; Yoneyama, H. Photoinduced Electron Transfer from Zinc Sulfide Microcrystals Modified with Various Alkanethiols to Methyl Viologen. Langmuir 1994, 10, 4517–4522. [Google Scholar] [CrossRef]
- Bak, T.; Nowotny, J.; Rekas, M.; Sorrell, C. Photo-electrochemical hydrogen generation from water using solar energy. Materials-related aspects. Int. J. Hydrog. Energy 2002, 27, 991–1022. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. New Non-Oxide Photocatalysts Designed for Overall Water Splitting under Visible Light. J. Phys. Chem. C 2007, 111, 7851–7861. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef] [PubMed]
- Ager, J.W.; Shaner, M.R.; Walczak, K.A.; Sharp, I.D.; Ardo, S. Experimental demonstrations of spontaneous, solar-driven photoelectrochemical water splitting. Energy Environ. Sci. 2015, 8, 2811–2824. [Google Scholar] [CrossRef]
- Pihosh, Y.; Turkevych, I.; Mawatari, K.; Uemura, J.; Kazoe, Y.; Kosar, S.; Makita, K.; Sugaya, T.; Matsui, T.; Fujita, D.; et al. Photocatalytic generation of hydrogen by core-shell WO3/BiVO4 nanorods with ultimate water splitting efficiency. Sci. Rep. 2015, 5, 11141. [Google Scholar] [CrossRef] [PubMed]
- Gerken, J.B.; McAlpin, J.G.; Chen, J.Y.C.; Rigsby, M.L.; Casey, W.H.; Britt, R.D.; Stahl, S.S. Electrochemical Water Oxidation with Cobalt-Based Electrocatalysts from pH 0–14: The Thermodynamic Basis for Catalyst Structure, Stability, and Activity. J. Am. Chem. Soc. 2011, 133, 14431–14442. [Google Scholar] [CrossRef]
- Chen, J.Y.C.; Dang, L.; Liang, H.; Bi, W.; Gerken, J.B.; Jin, S.; Alp, E.E.; Stahl, S.S. Operando Analysis of NiFe and Fe Oxyhydroxide Electrocatalysts for Water Oxidation: Detection of Fe4+ by Mössbauer Spectroscopy. J. Am. Chem. Soc. 2015, 137, 15090–15093. [Google Scholar] [CrossRef]
- Galán-Mascarós, J.R. Water Oxidation at Electrodes Modified with Earth-Abundant Transition-Metal Catalysts. ChemElectroChem 2014, 2, 37–50. [Google Scholar] [CrossRef]
- Xie, J.; Xie, Y. Structural Engineering of Electrocatalysts for the Hydrogen Evolution Reaction: Order or Disorder? ChemCatChem 2015, 7, 2568–2580. [Google Scholar] [CrossRef]
- Görlin, M.; Chernev, P.; De Araújo, J.F.; Reier, T.; Dresp, S.; Paul, B.; Krähnert, R.; Dau, H.; Strasser, P.; Goerlin, M.; et al. Oxygen Evolution Reaction Dynamics, Faradaic Charge Efficiency, and the Active Metal Redox States of Ni–Fe Oxide Water Splitting Electrocatalysts. J. Am. Chem. Soc. 2016, 138, 5603–5614. [Google Scholar] [CrossRef] [PubMed]
- Moss, B.; Hegner, F.S.; Corby, S.; Selim, S.; Francàs, L.; Lopez, N.; Gimenez, S.; Galán-Mascarós, J.R.; Durrant, J.R.; Francas-Forcada, L. Unraveling Charge Transfer in CoFe Prussian Blue Modified BiVO4 Photoanodes. ACS Energy Lett. 2018, 4, 337–342. [Google Scholar] [CrossRef]
- Selim, S.; Pastor, E.; García-Tecedor, M.; Morris, M.R.; Francas, L.; Sachs, M.; Moss, B.; Corby, S.; Mesa, C.A.; Gimenez, S.; et al. Impact of Oxygen Vacancy Occupancy on Charge Carrier Dynamics in BiVO4 Photoanodes. J. Am. Chem. Soc. 2019, 141, 18791–18798. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, T.G.U.; Ghobadi, A.; Soydan, M.C.; Vishlaghi, M.B.; Kaya, S.; Karadas, F.; Ozbay, E. Strong Light-matter Interaction in Au Plasmonic Nanoantennas Coupled with Prussian Blue Catalyst on BiVO4 for Photoelectrochemical Water Splitting. ChemSusChem 2020. [Google Scholar] [CrossRef] [PubMed]
- Hegner, F.S.; Herraiz-Cardona, I.; Cardenas-Morcoso, D.; Lopez, N.; Galán-Mascarós, J.R.; Gimenez, S. Cobalt Hexacyanoferrate on BiVO4 Photoanodes for Robust Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 37671–37681. [Google Scholar] [CrossRef]
- Barroso, M.; Cowan, A.J.; Pendlebury, S.R.; Grätzel, M.; Klug, D.R.; Durrant, J.R. The Role of Cobalt Phosphate in Enhancing the Photocatalytic Activity of α-Fe2O3toward Water Oxidation. J. Am. Chem. Soc. 2011, 133, 14868–14871. [Google Scholar] [CrossRef]
- Klahr, B.; Gimenez, S.; Fabregat-Santiago, F.; Bisquert, J.; Hamann, T.W. Photoelectrochemical and Impedance Spectroscopic Investigation of Water Oxidation with “Co–Pi”-Coated Hematite Electrodes. J. Am. Chem. Soc. 2012, 134, 16693–16700. [Google Scholar] [CrossRef]
- Badia-Bou, L.; Mas, E.; Rodenas, P.; Barea, E.; Fabregat-Santiago, F.; Gimenez, S.; Peris, E.V.; Bisquert, J. Water Oxidation at Hematite Photoelectrodes with an Iridium-Based Catalyst. J. Phys. Chem. C 2013, 117, 3826–3833. [Google Scholar] [CrossRef]
- Hegner, F.S.; Cardenas-Morcoso, D.; Giménez, S.; Lopez, N.; Galán-Mascarós, J.R. Level Alignment as Descriptor for Semiconductor/Catalyst Systems in Water Splitting: The Case of Hematite/Cobalt Hexacyanoferrate Photoanodes. ChemSusChem 2017, 10, 4552–4560. [Google Scholar] [CrossRef]
- Shaddad, M.; Arunachalam, P.; Labis, J.; Hezam, M.; Al-Mayouf, A.M. Fabrication of robust nanostructured (Zr)BiVO4/nickel hexacyanoferrate core/shell photoanodes for solar water splitting. Appl. Catal. B Environ. 2019, 244, 863–870. [Google Scholar] [CrossRef]
- Sathiskumar, C.; Ramakrishnan, S.; Vinothkannan, M.; Kim, A.R.; Karthikeyan, S.; Yoo, D.J. Nitrogen-Doped Porous Carbon Derived from Biomass Used as Trifunctional Electrocatalyst toward Oxygen Reduction, Oxygen Evolution and Hydrogen Evolution Reactions. Nanomaterials 2019, 10, 76. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Lu, Y.; Ma, D. Effects of Structure and Constituent of Prussian Blue Analogs on Their Application in Oxygen Evolution Reaction. Molecules 2020, 25, 2304. https://doi.org/10.3390/molecules25102304
Zhao D, Lu Y, Ma D. Effects of Structure and Constituent of Prussian Blue Analogs on Their Application in Oxygen Evolution Reaction. Molecules. 2020; 25(10):2304. https://doi.org/10.3390/molecules25102304
Chicago/Turabian StyleZhao, Dongni, Yuezhen Lu, and Dongge Ma. 2020. "Effects of Structure and Constituent of Prussian Blue Analogs on Their Application in Oxygen Evolution Reaction" Molecules 25, no. 10: 2304. https://doi.org/10.3390/molecules25102304
APA StyleZhao, D., Lu, Y., & Ma, D. (2020). Effects of Structure and Constituent of Prussian Blue Analogs on Their Application in Oxygen Evolution Reaction. Molecules, 25(10), 2304. https://doi.org/10.3390/molecules25102304






