Last Decade of Unconventional Methodologies for the Synthesis of Substituted Benzofurans
Abstract
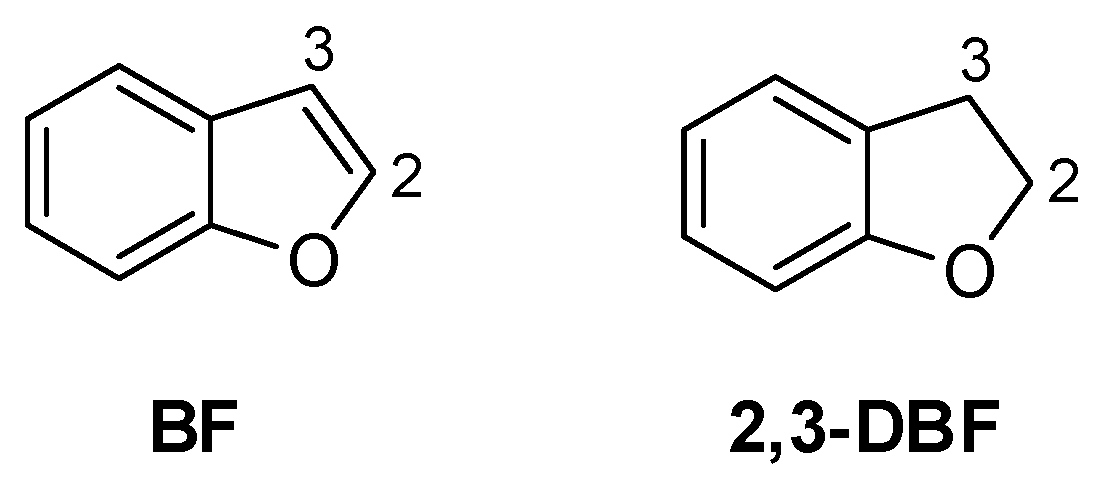
1. Introduction
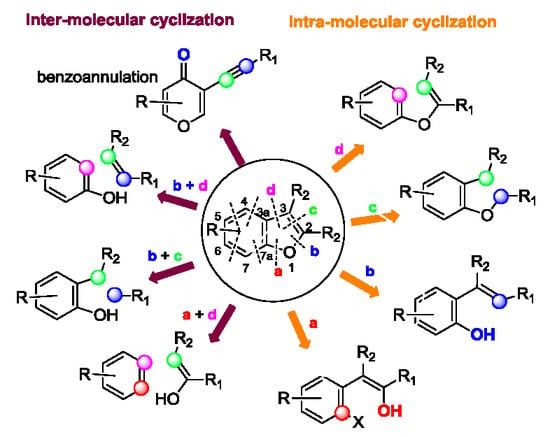
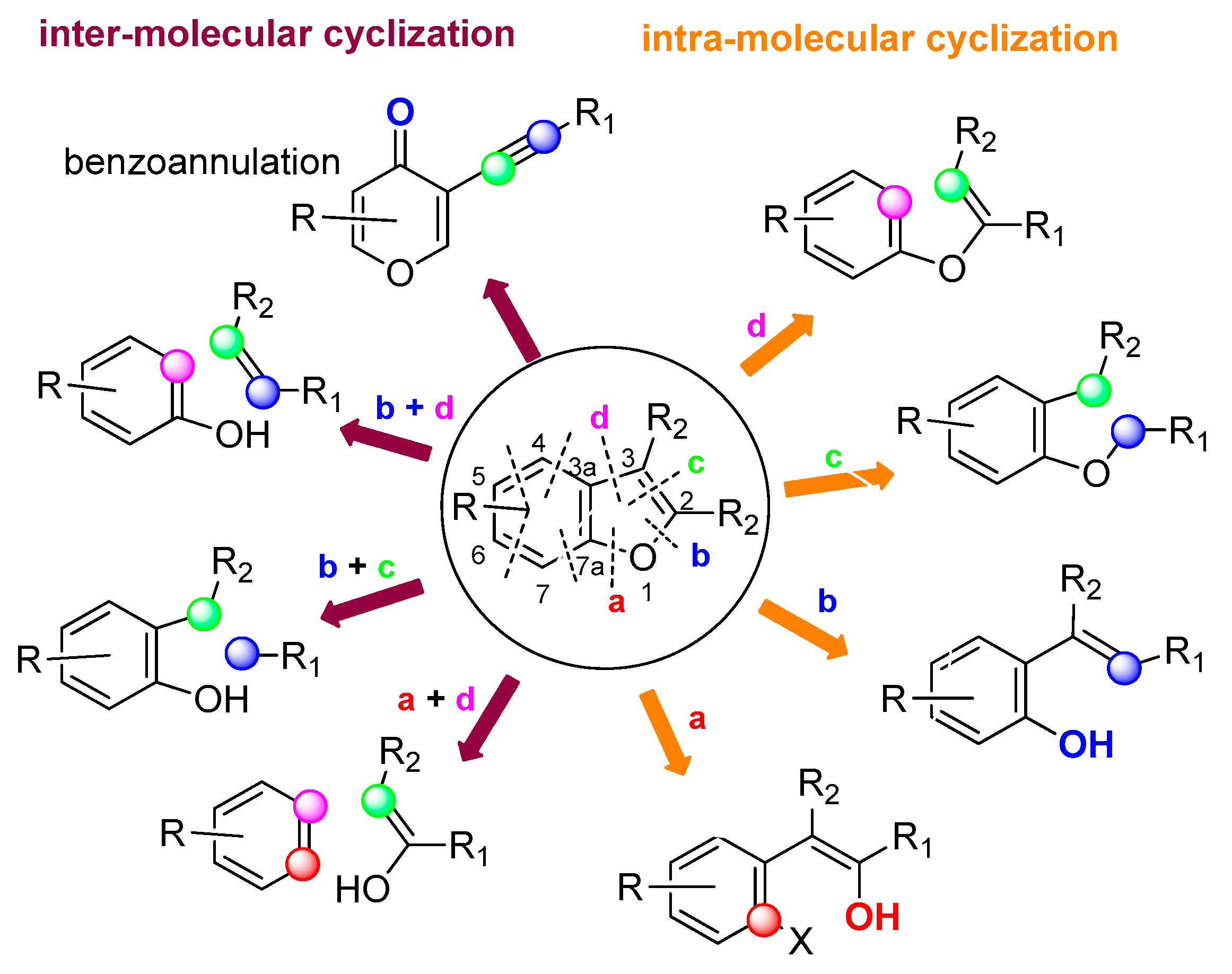
2. Ring Generation via Intra-Molecular Cyclization
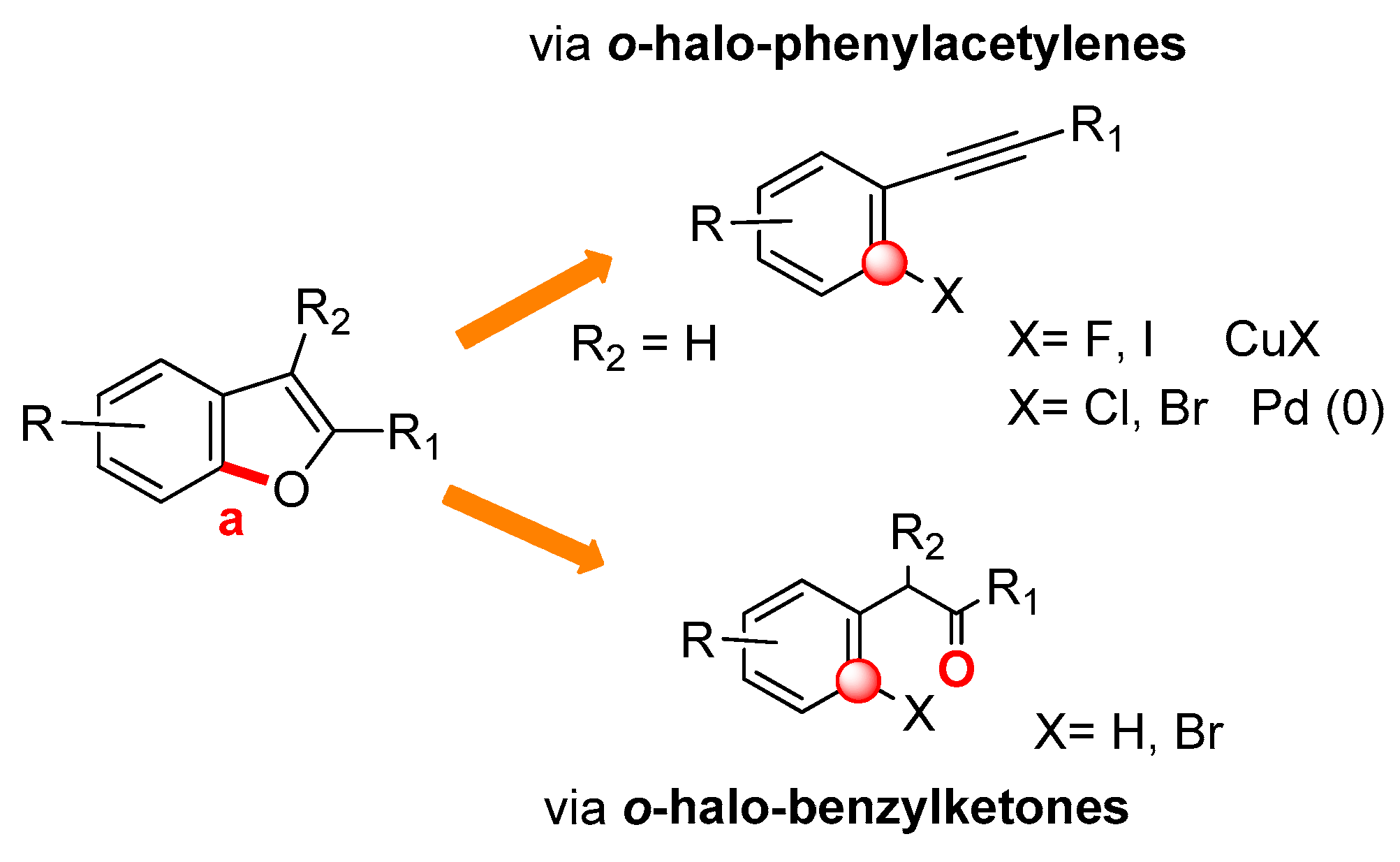
2.1. C7a–O Bond Formation: (Route a)
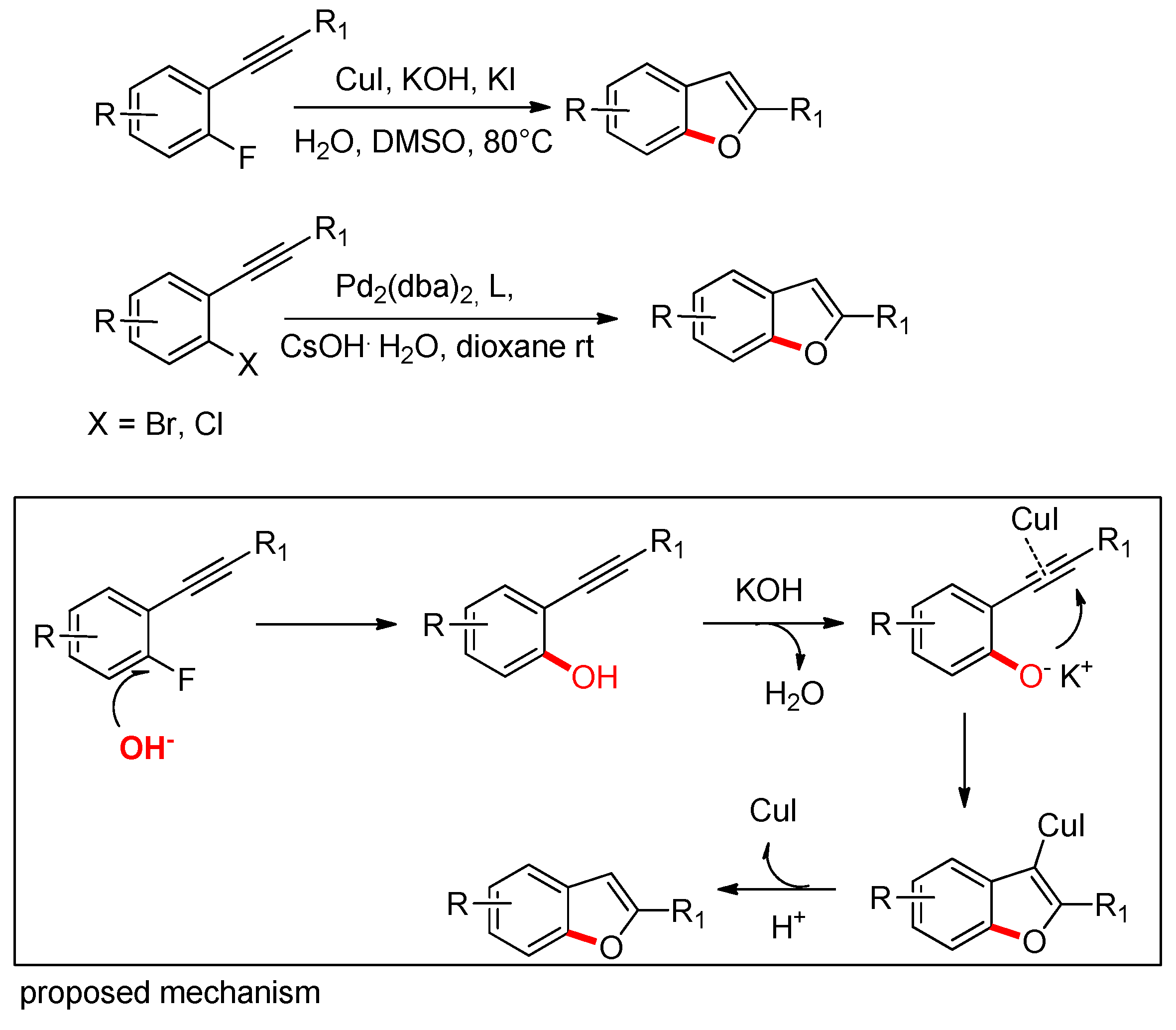
2.1.1. From o-Halophenylacetylenes
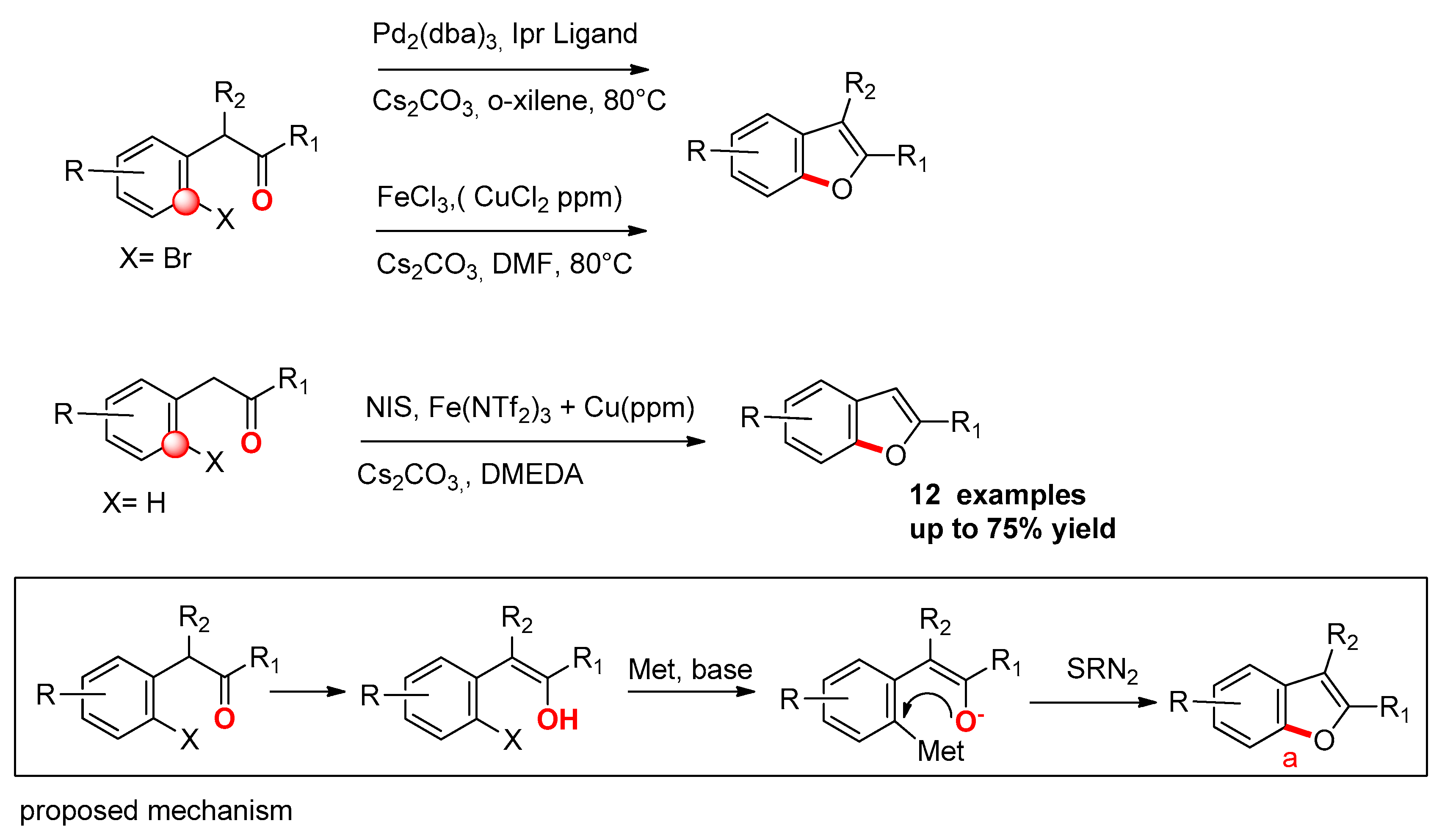
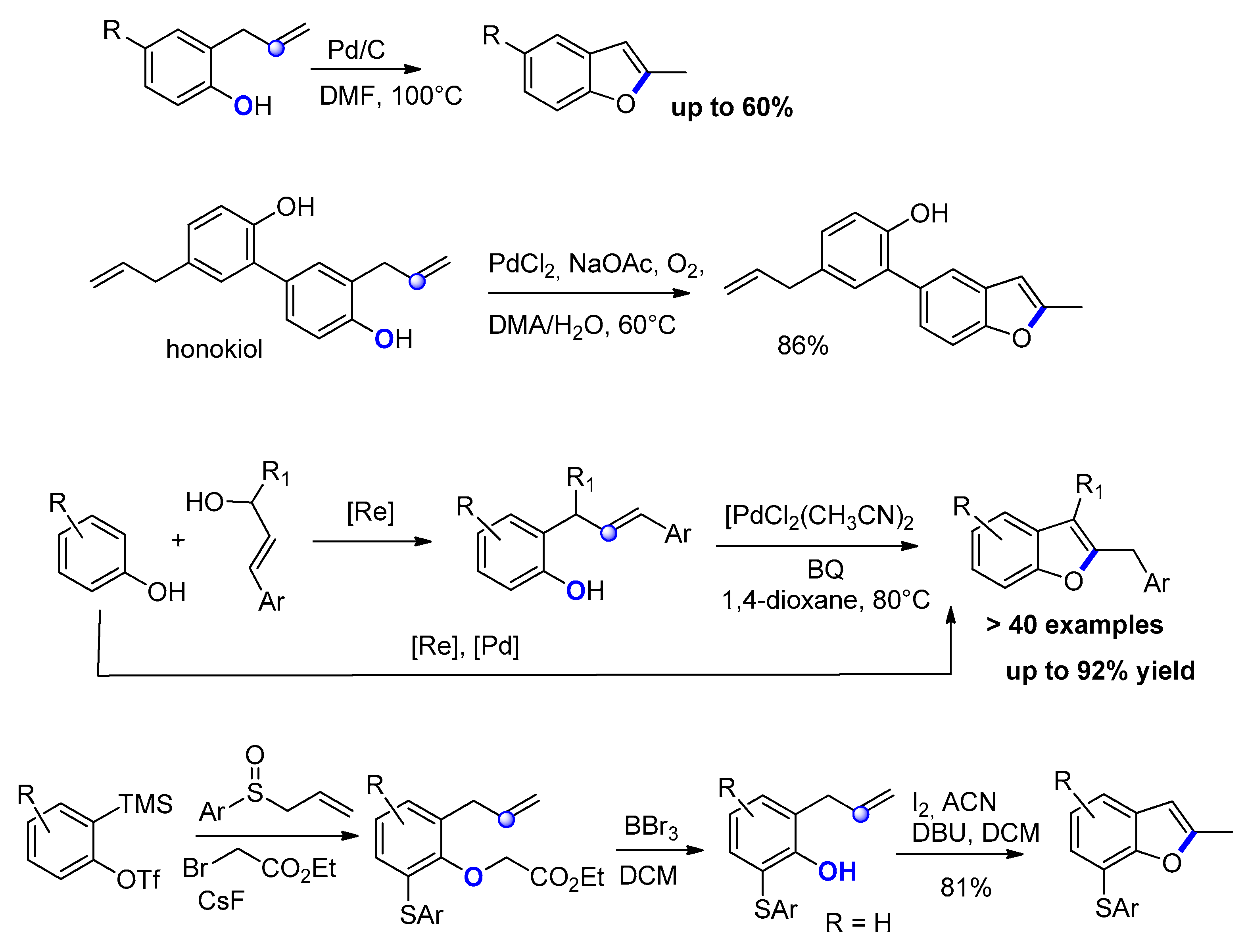
2.1.2. From o-Halo-Benzylketones
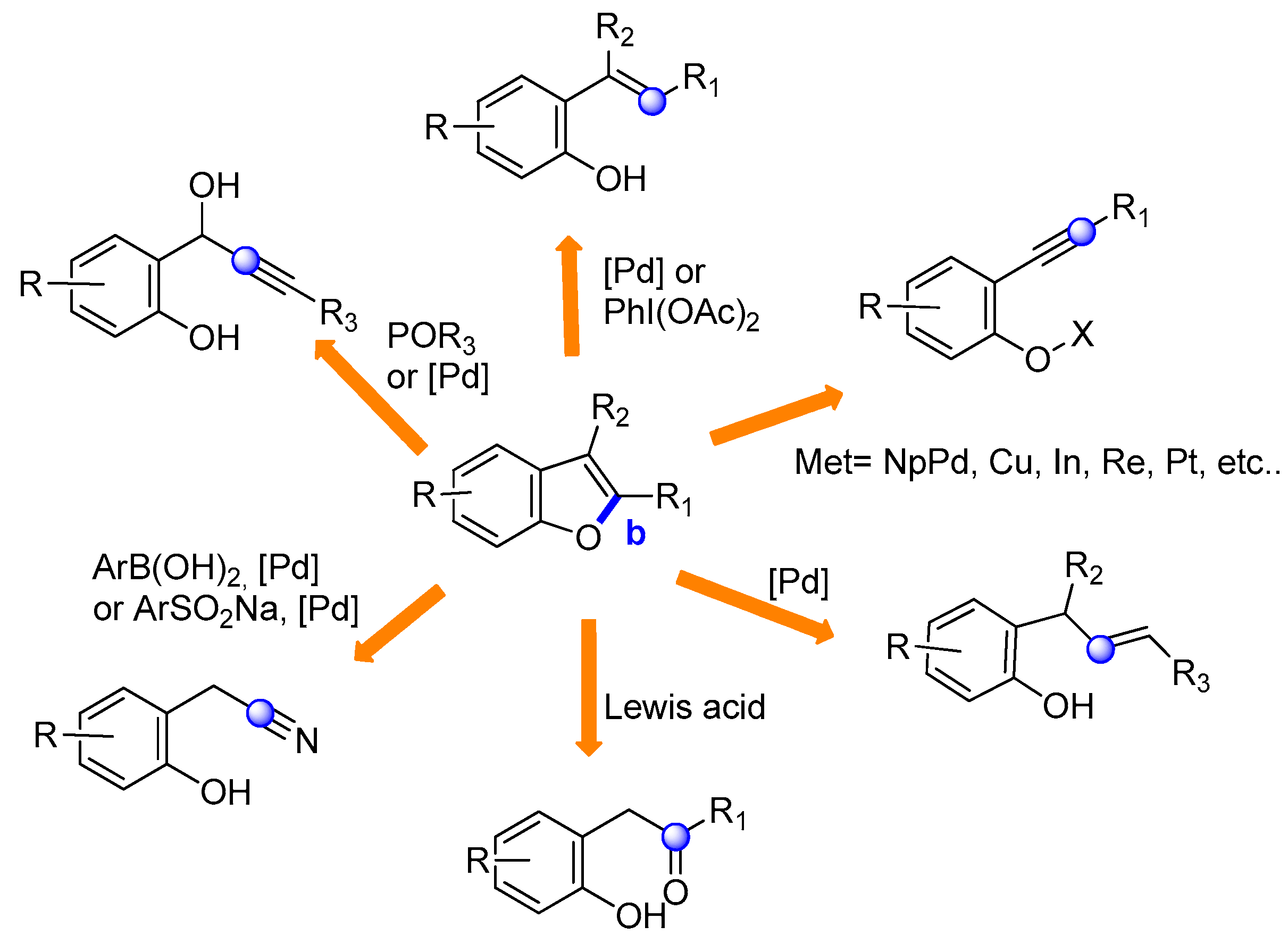
2.2. O–C2 Bond Formation: (Route b)
2.2.1. Via C-H Activation of o-Alkenylphenols
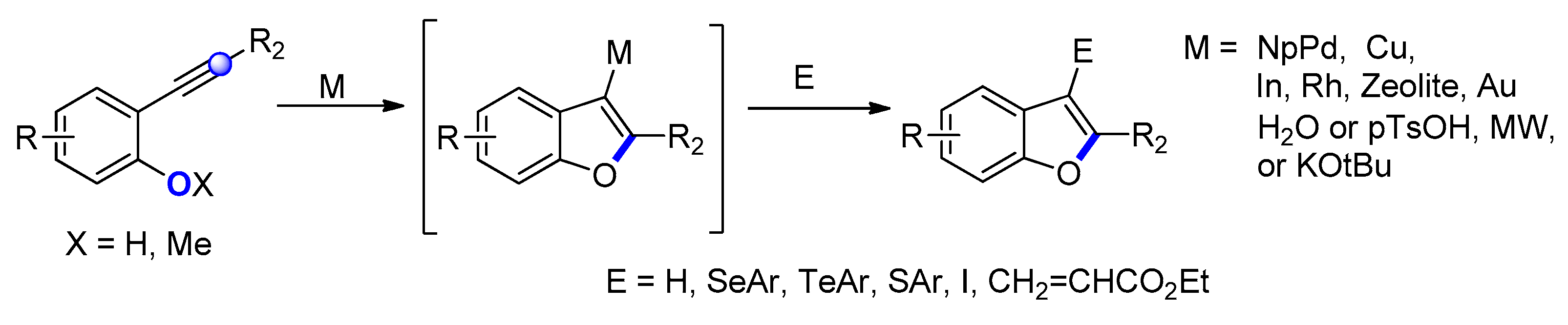
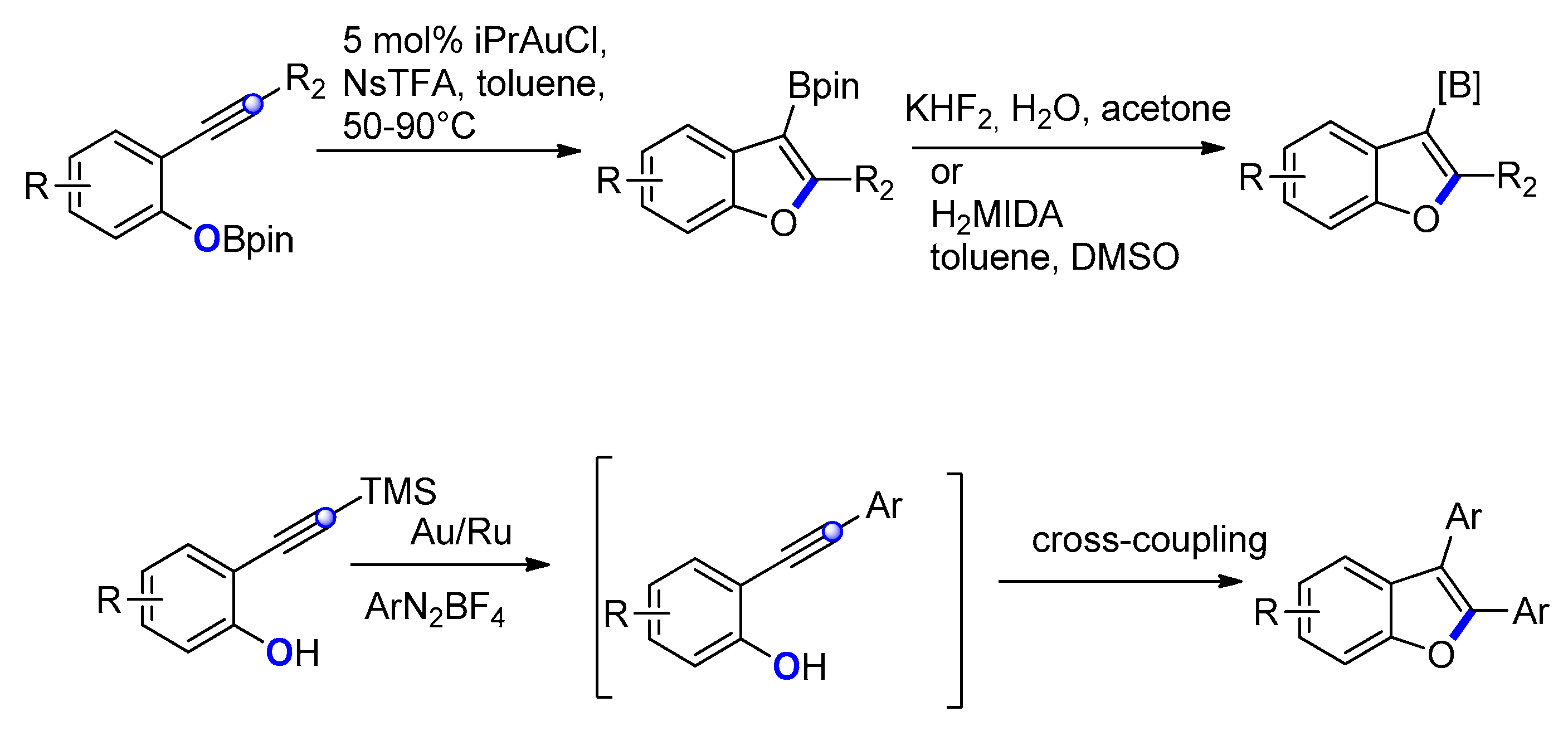
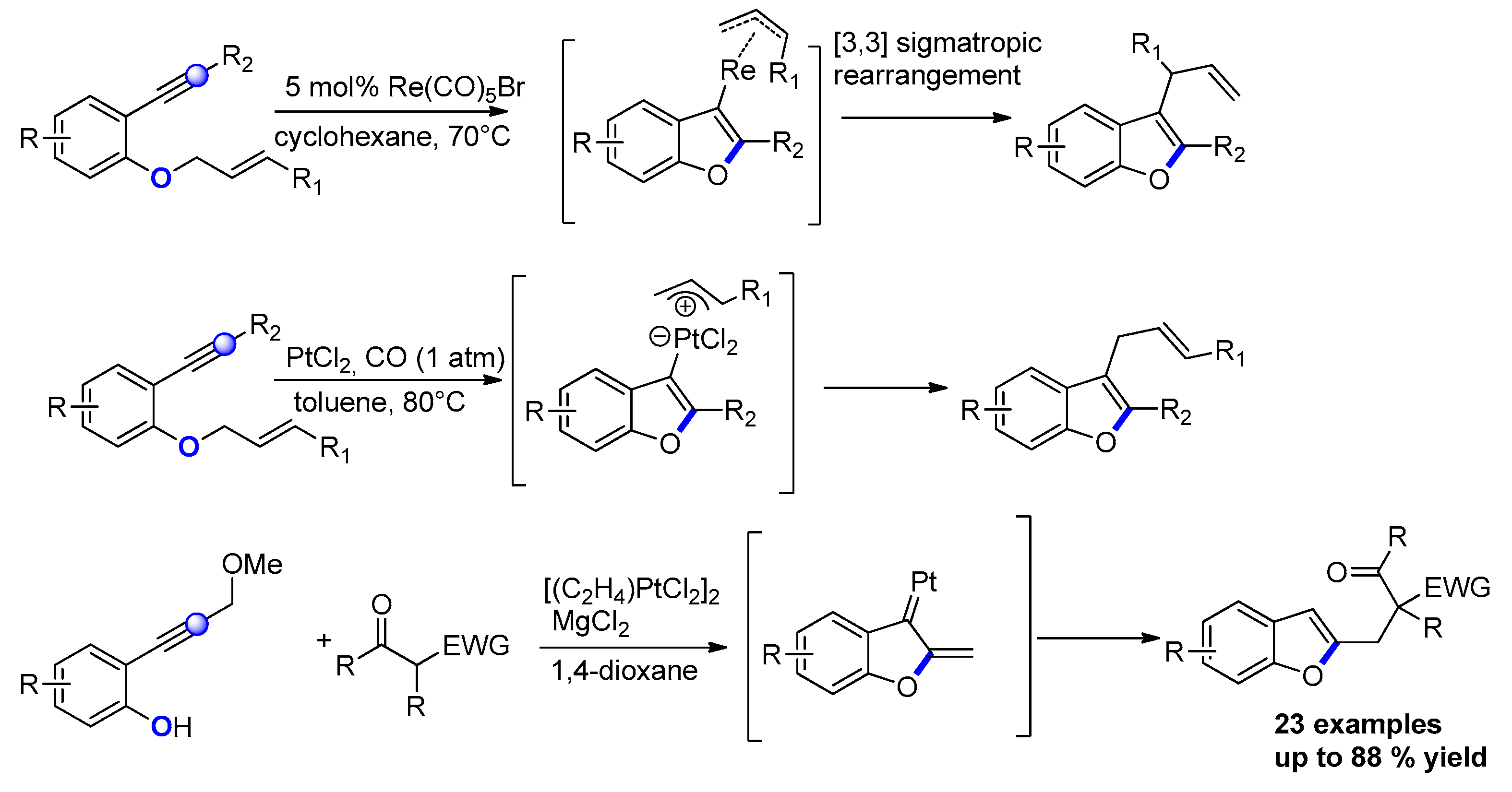
2.2.2. From o-Alkynylphenols
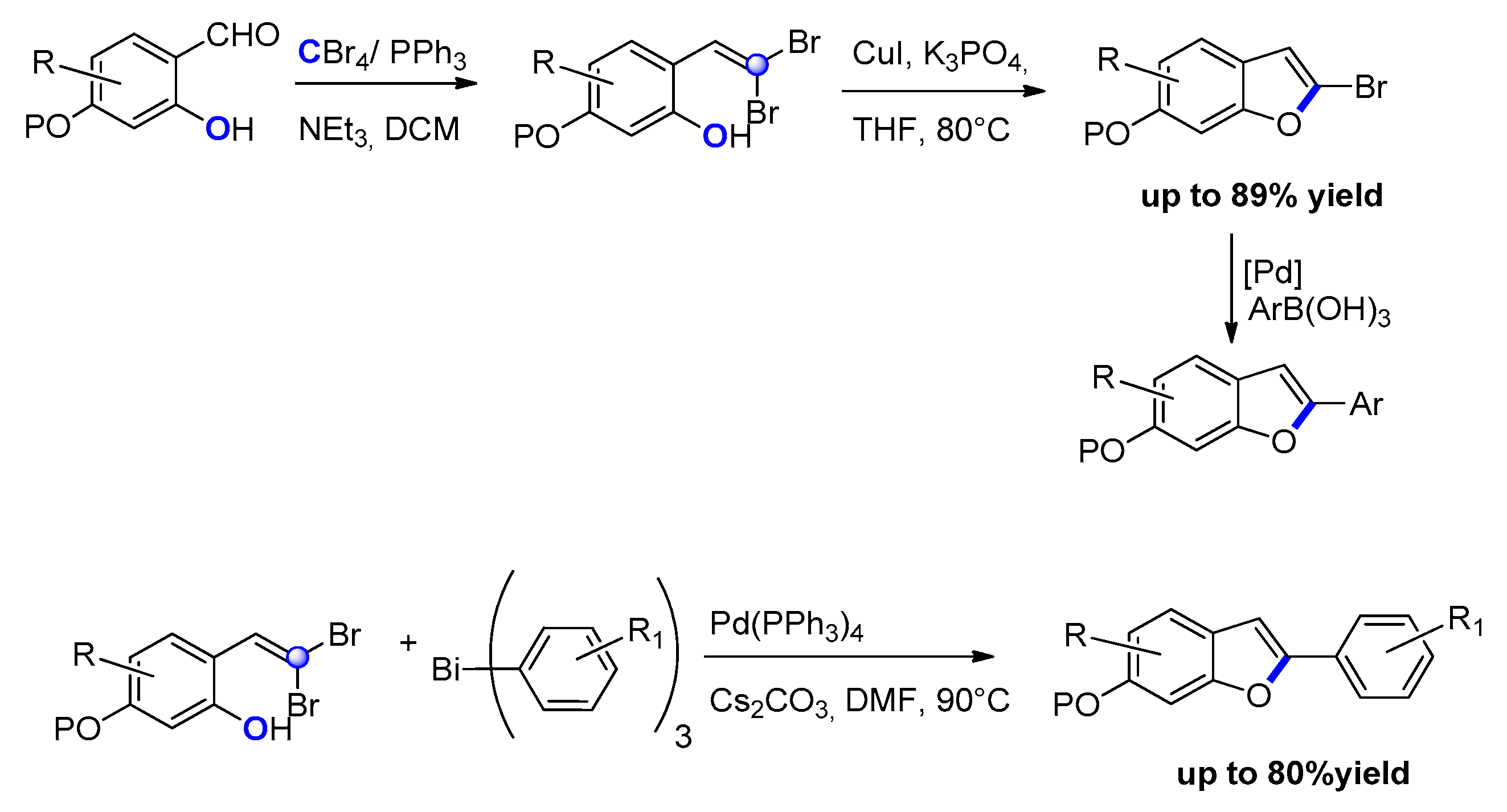
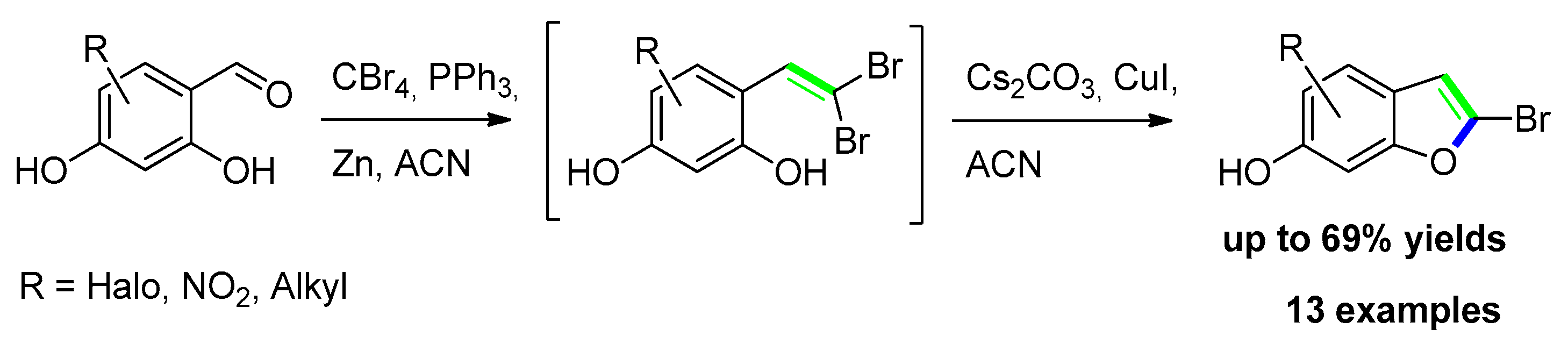
2.2.3. From o-Gem-Dibromoalkenyl Phenols
2.2.4. From o-Allylphenols
2.2.5. From o-Hydroxybenzyl Ketones
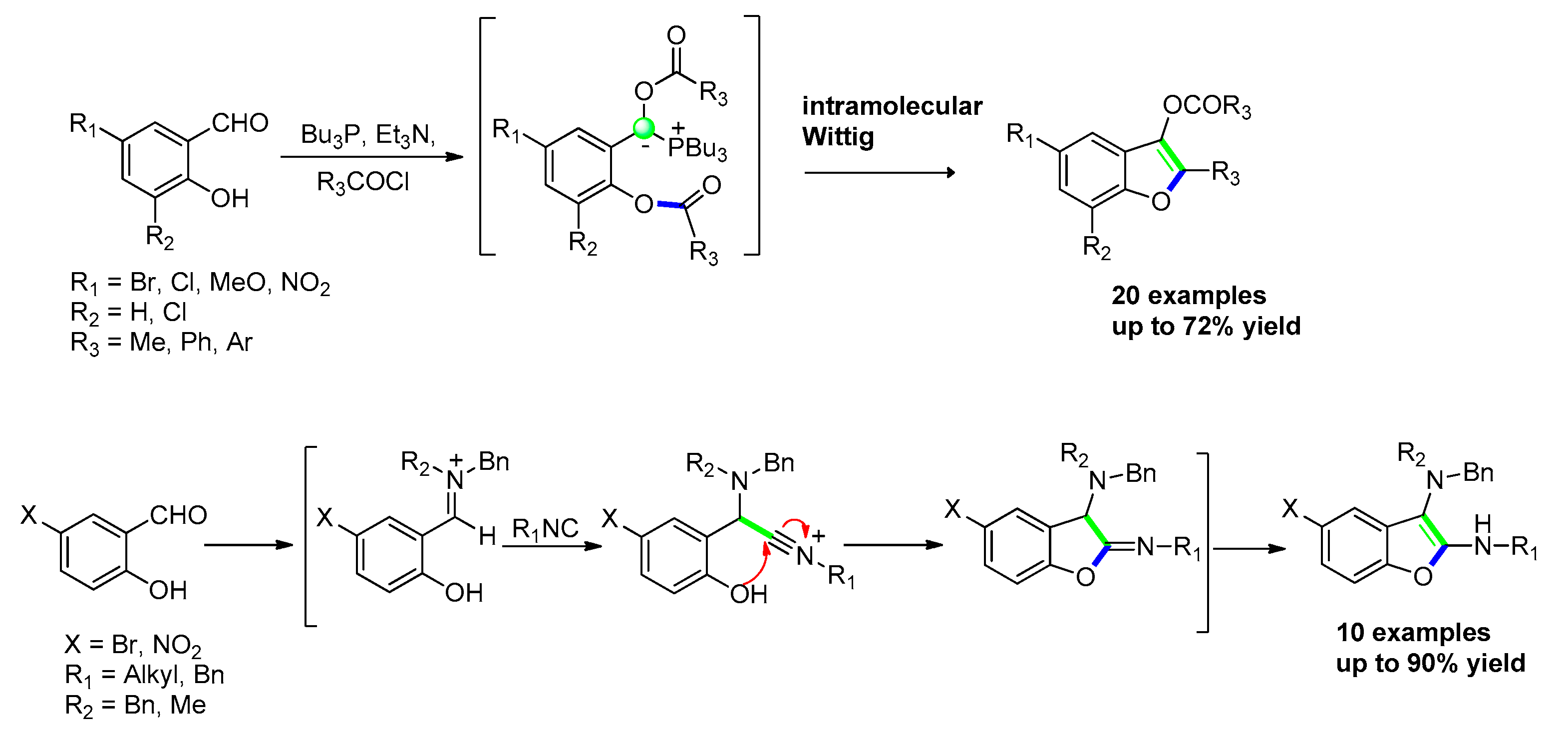
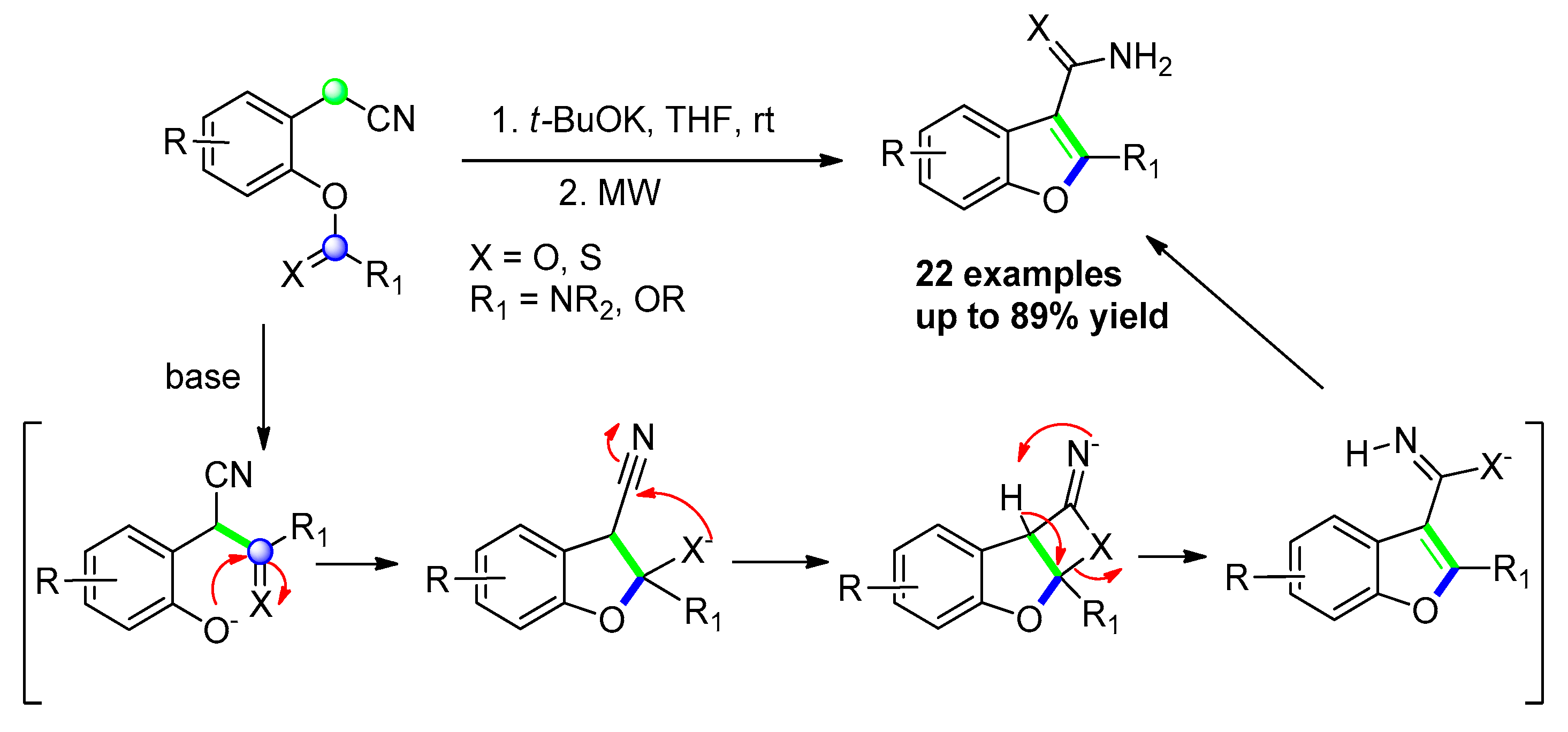
2.2.6. From o-(Cyanomethyl) Phenols
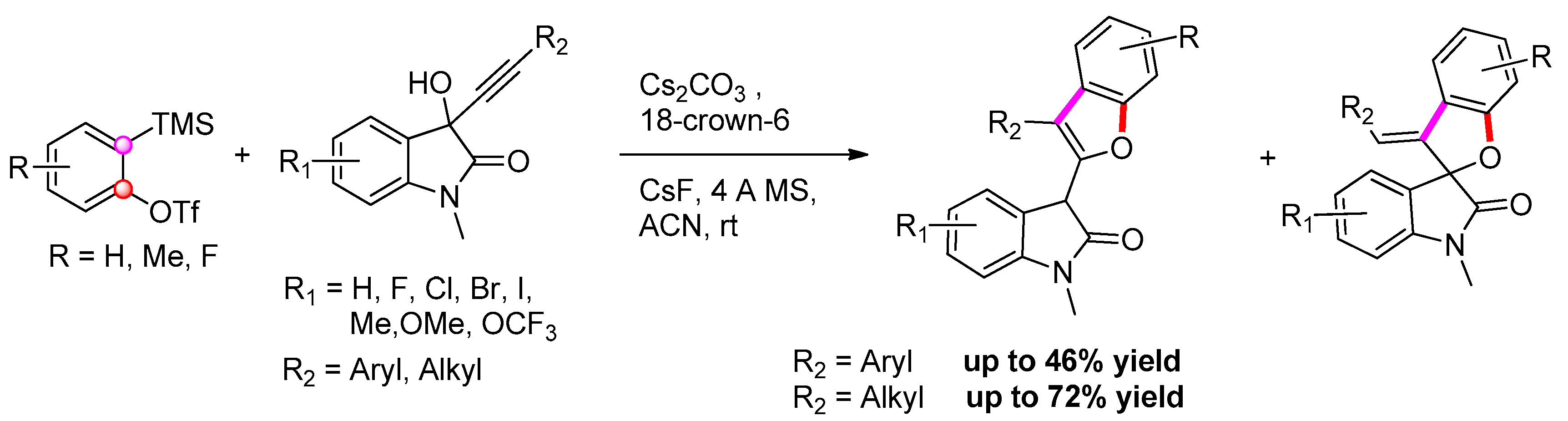
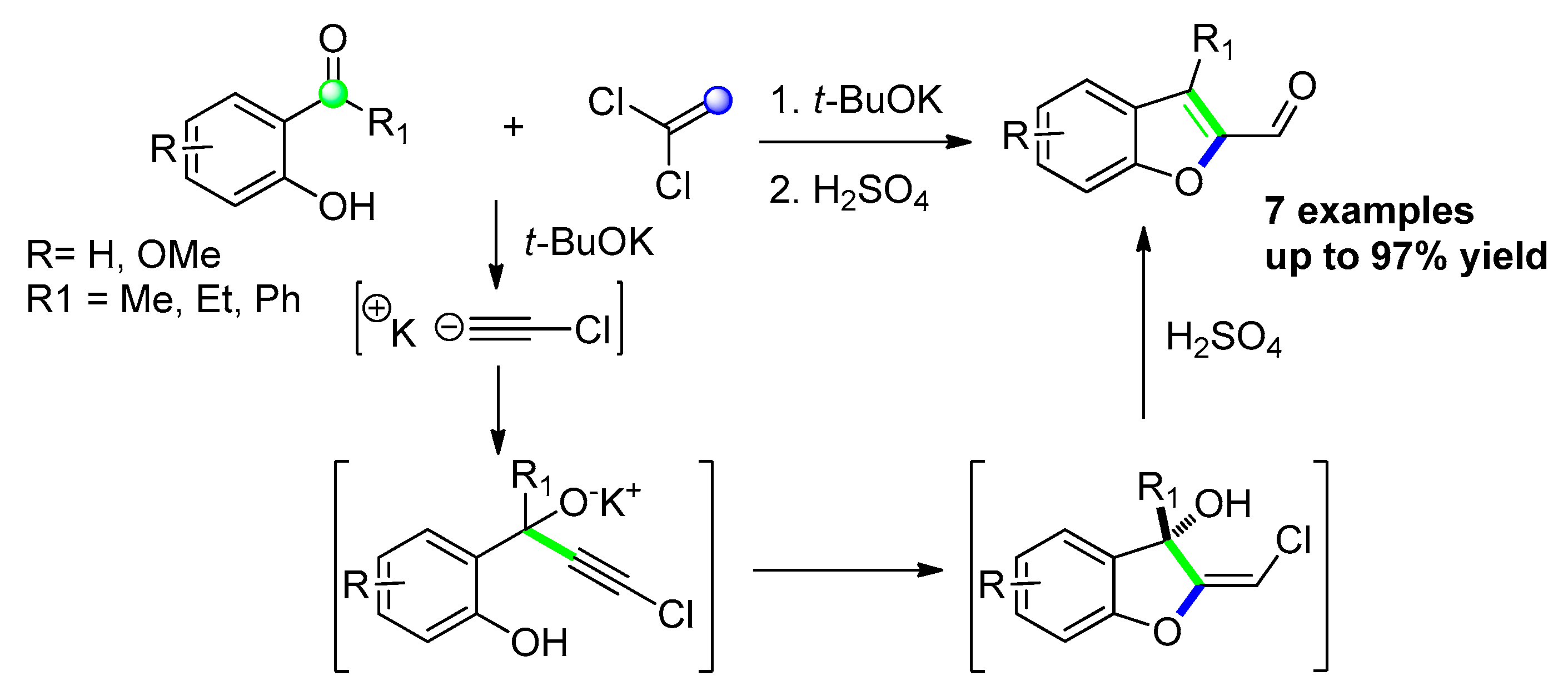
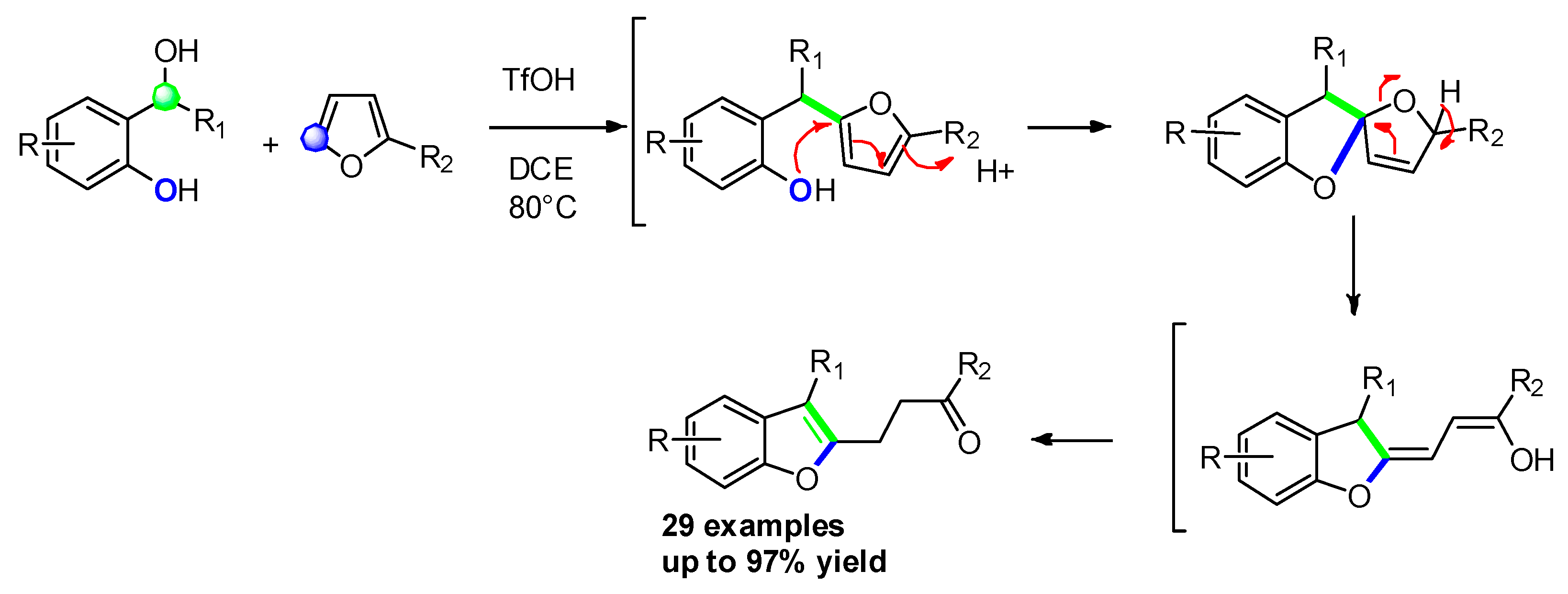
2.2.7. From 1-(2-hydroxyphenyl) Propargyl Alcohol Derivatives
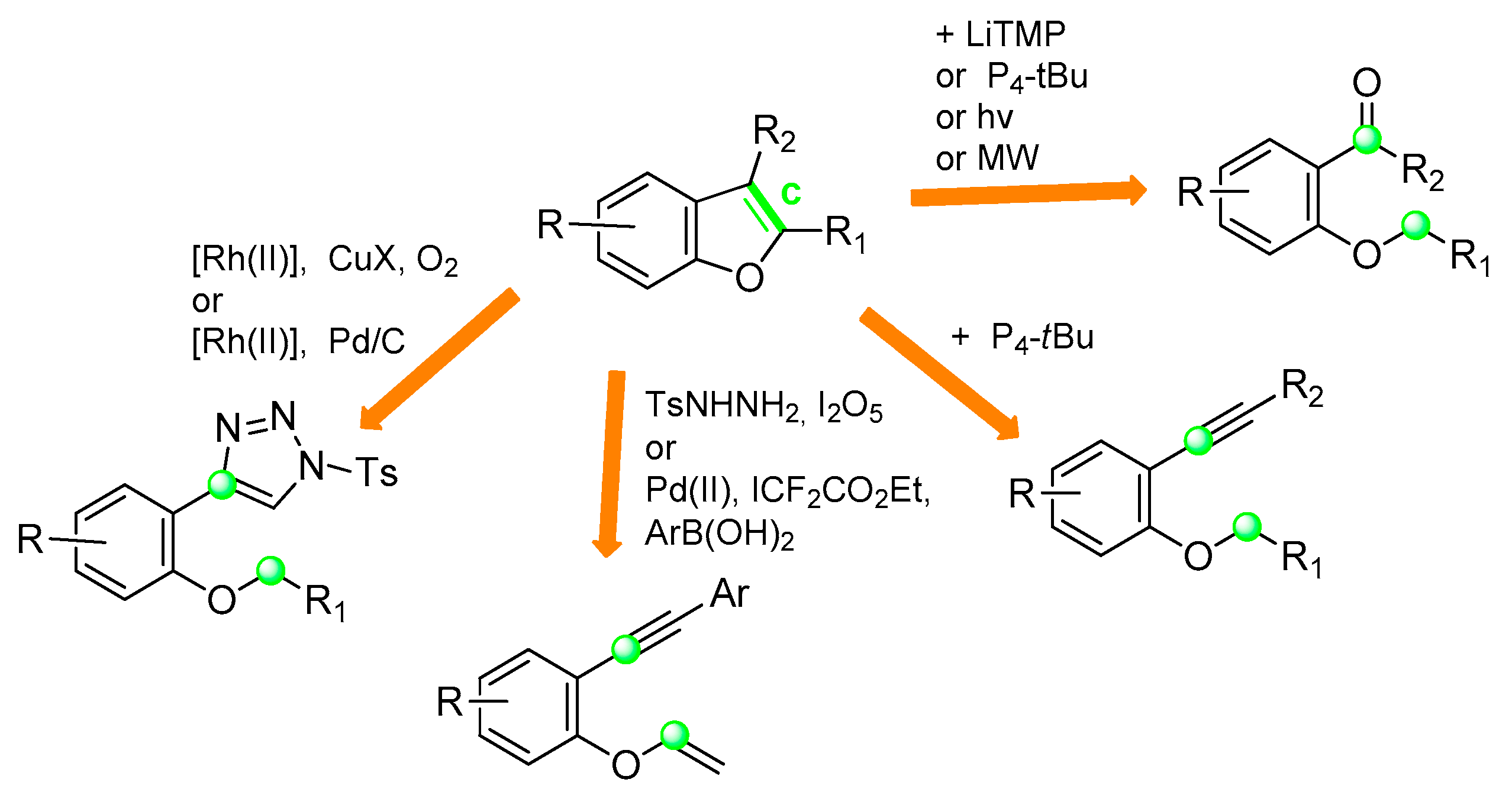
2.3. C2–C3 Bond Formation: (Route c)
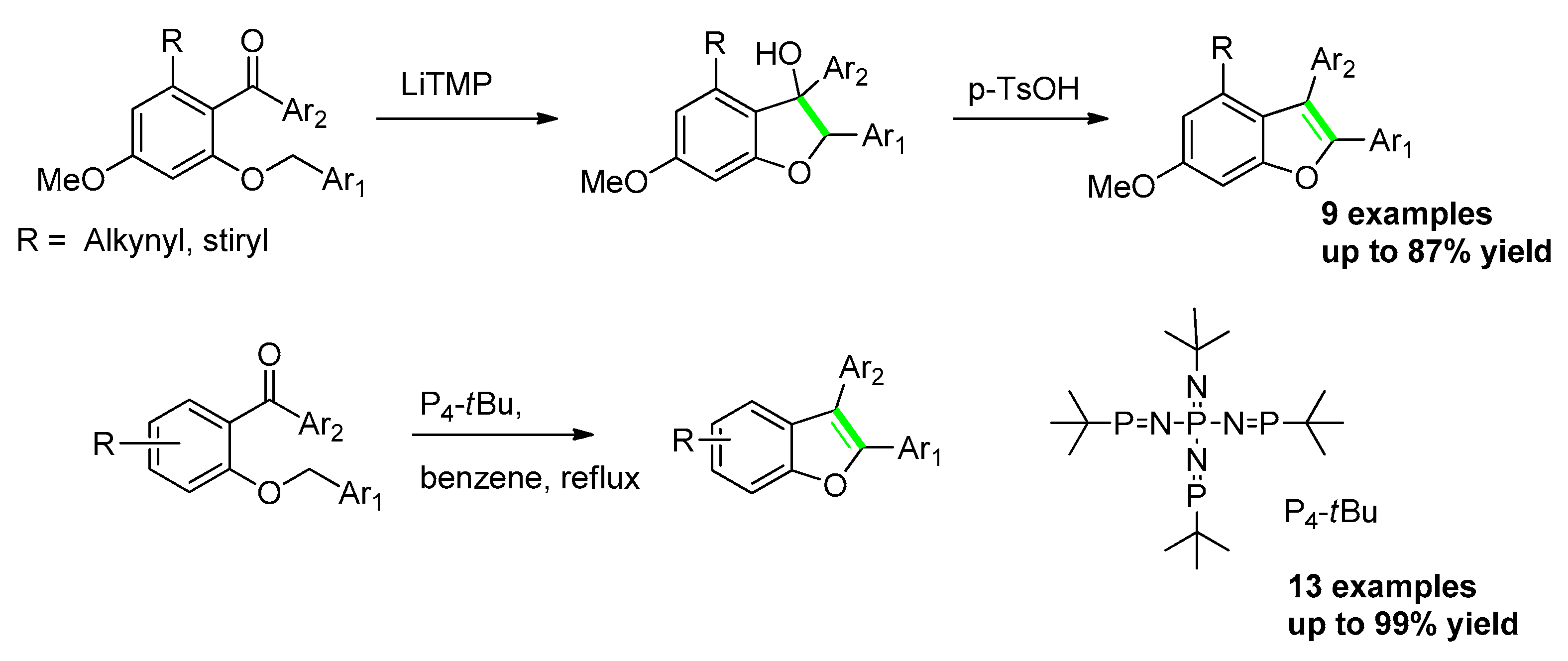
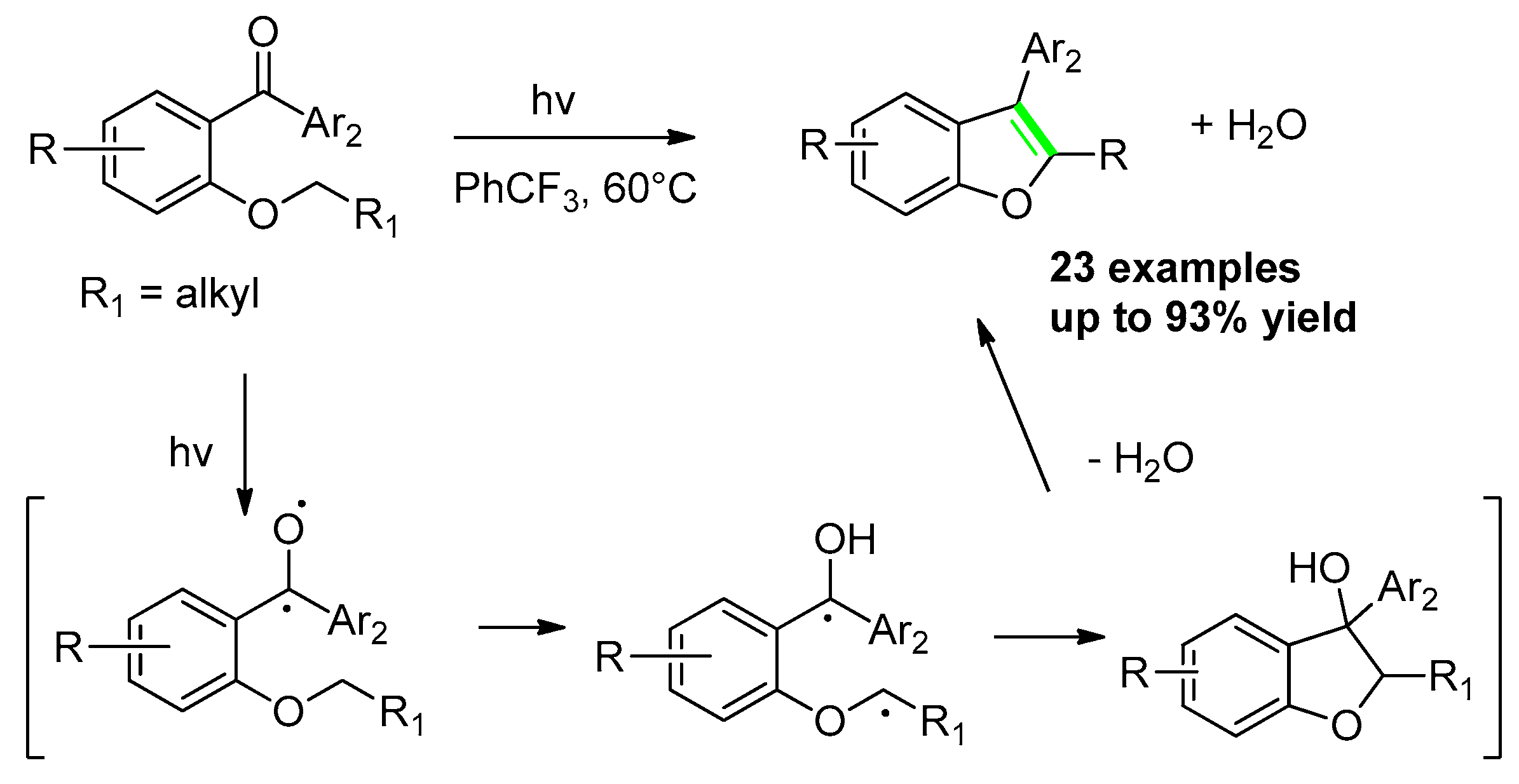
2.3.1. From o-(Alkoxy)Phenyl Arylketones
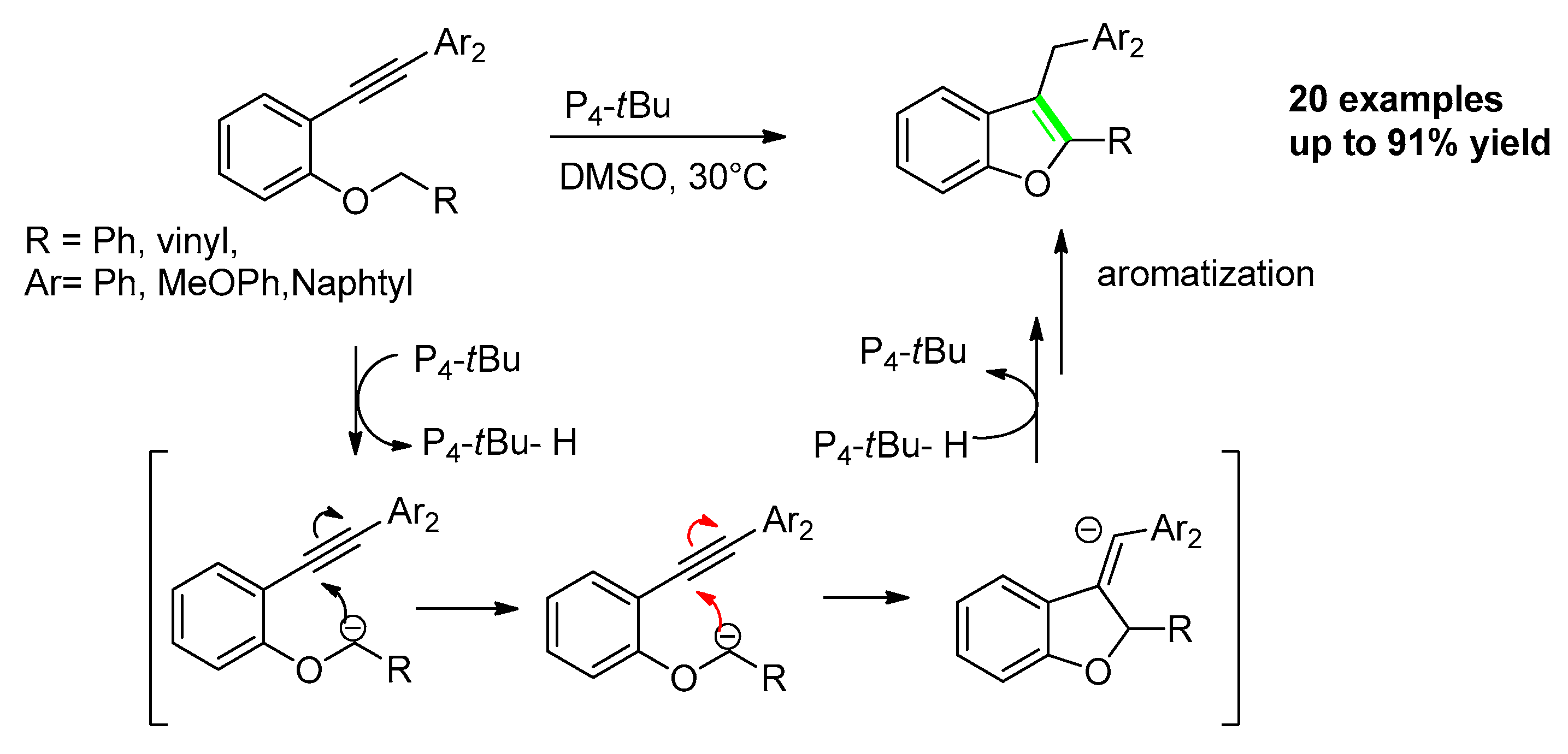
2.3.2. From o-Alkynylphenyl benzyl (or Allyl)Ethers
2.3.3. From o-Alkynylphenyl Vinylethers
2.3.4. From o-Triazole-Phenyl Benzylethers
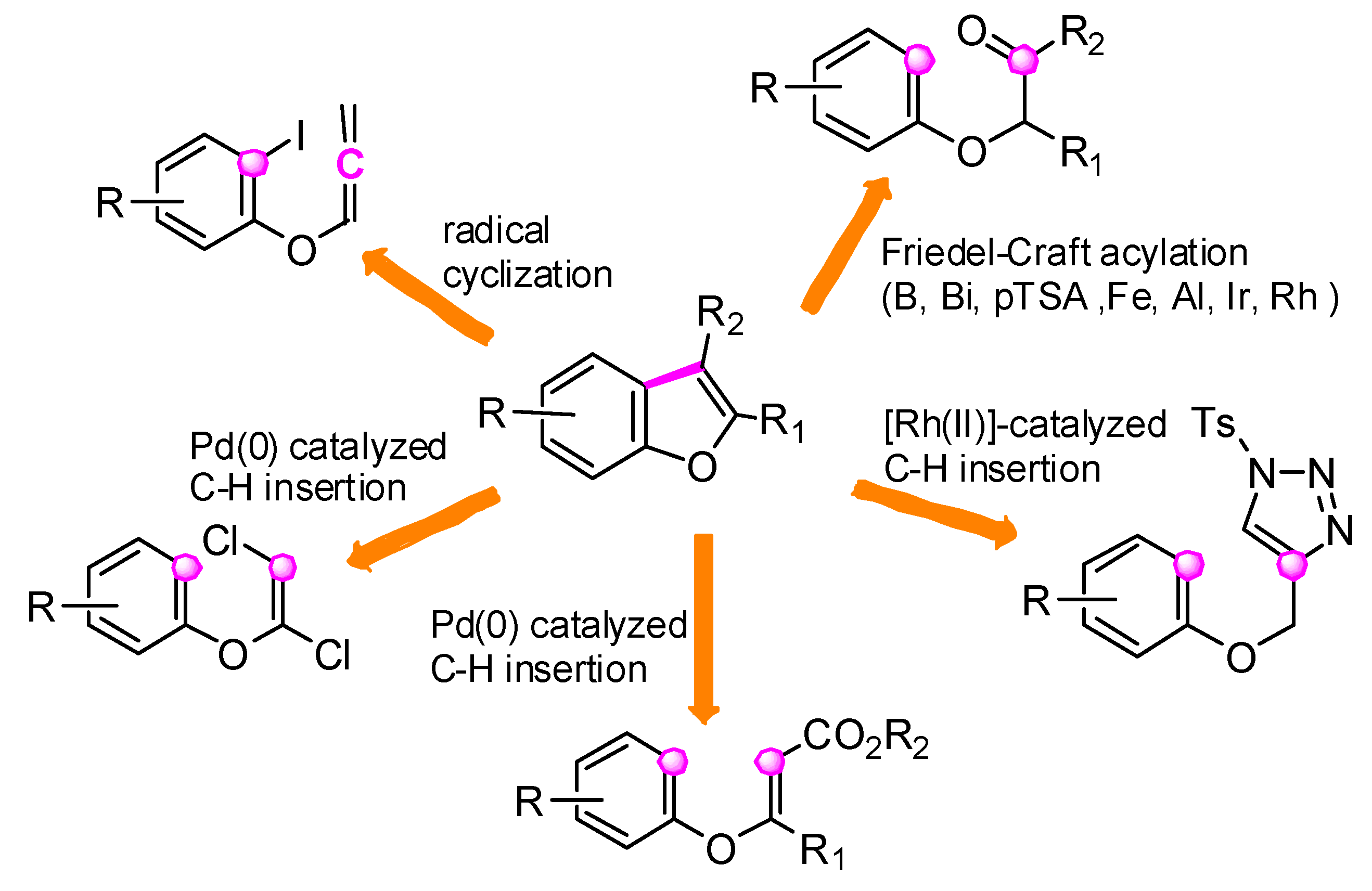
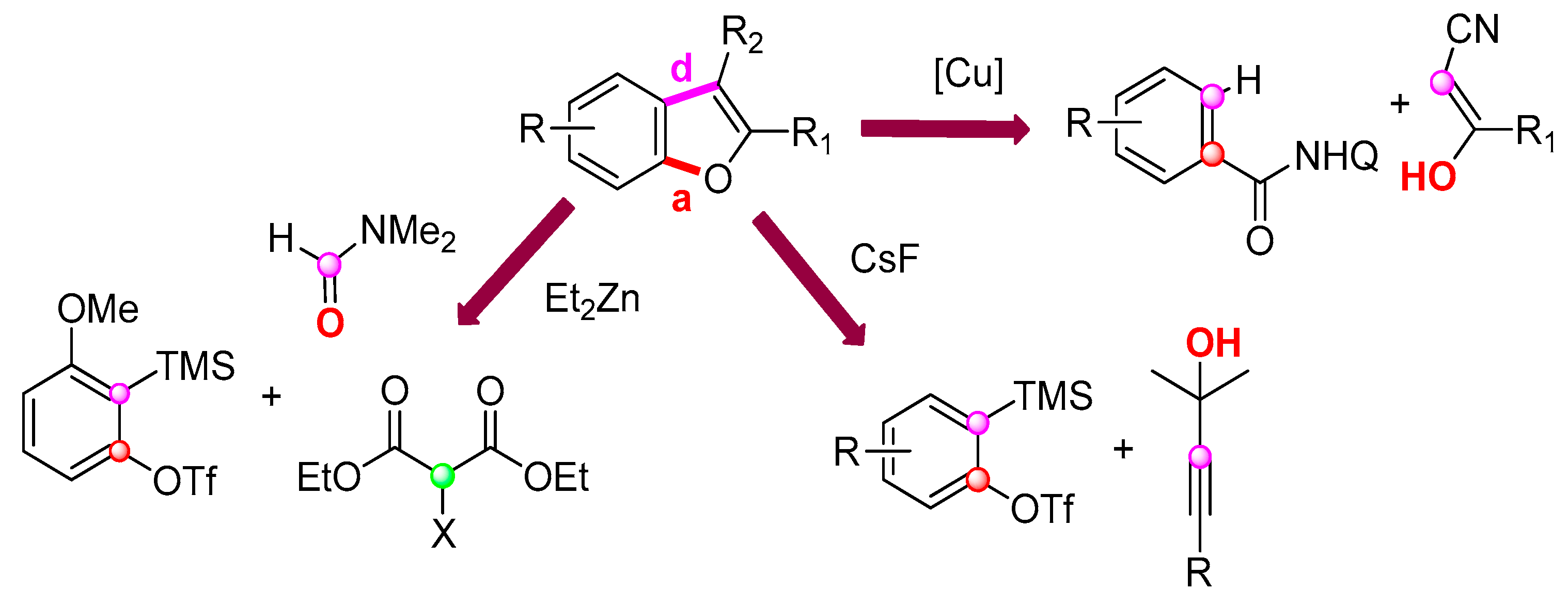
2.4. C3–C3a Bond Formation: (Route d)
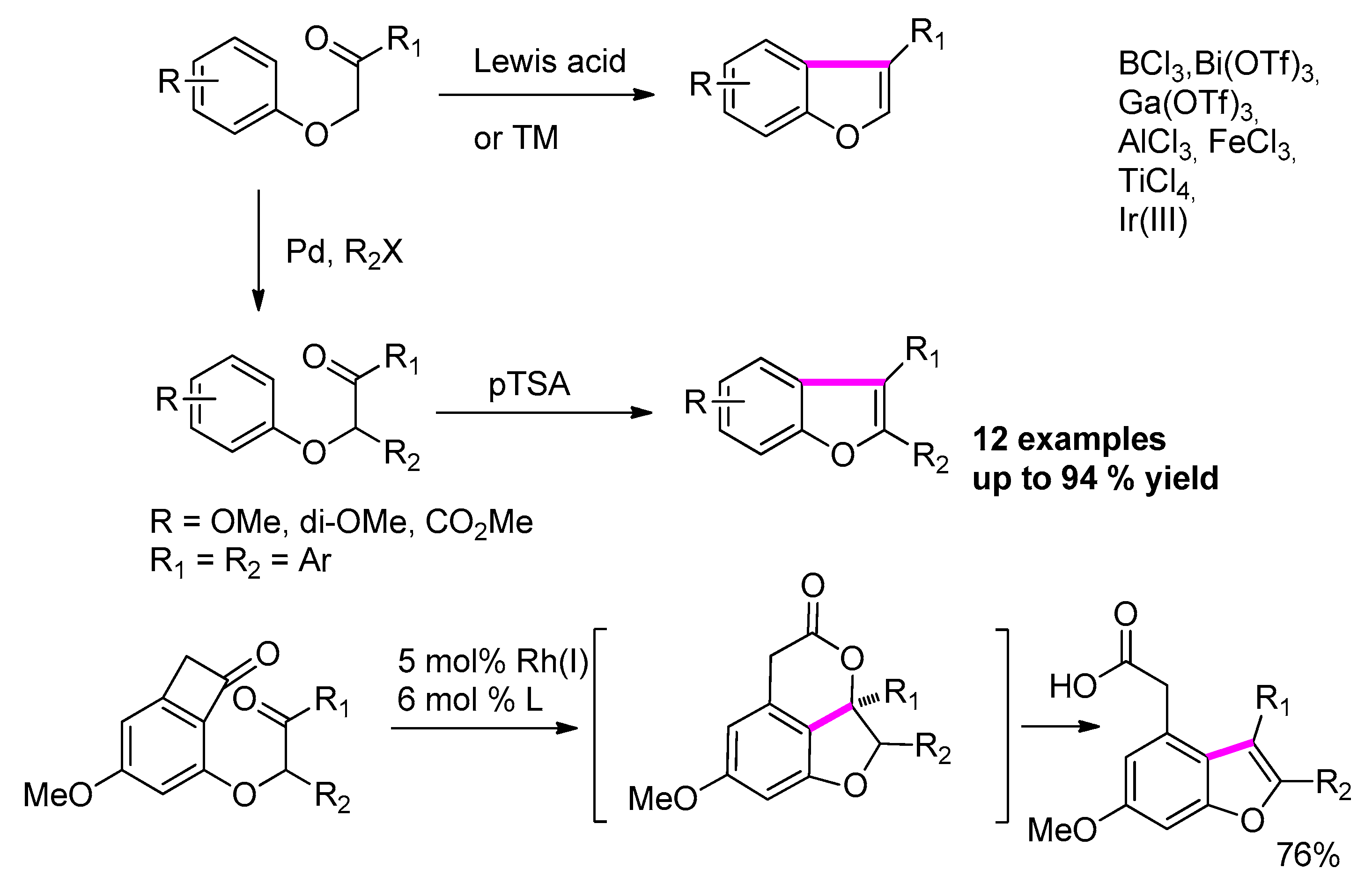
2.4.1. Via Friedel–Crafts Acylation
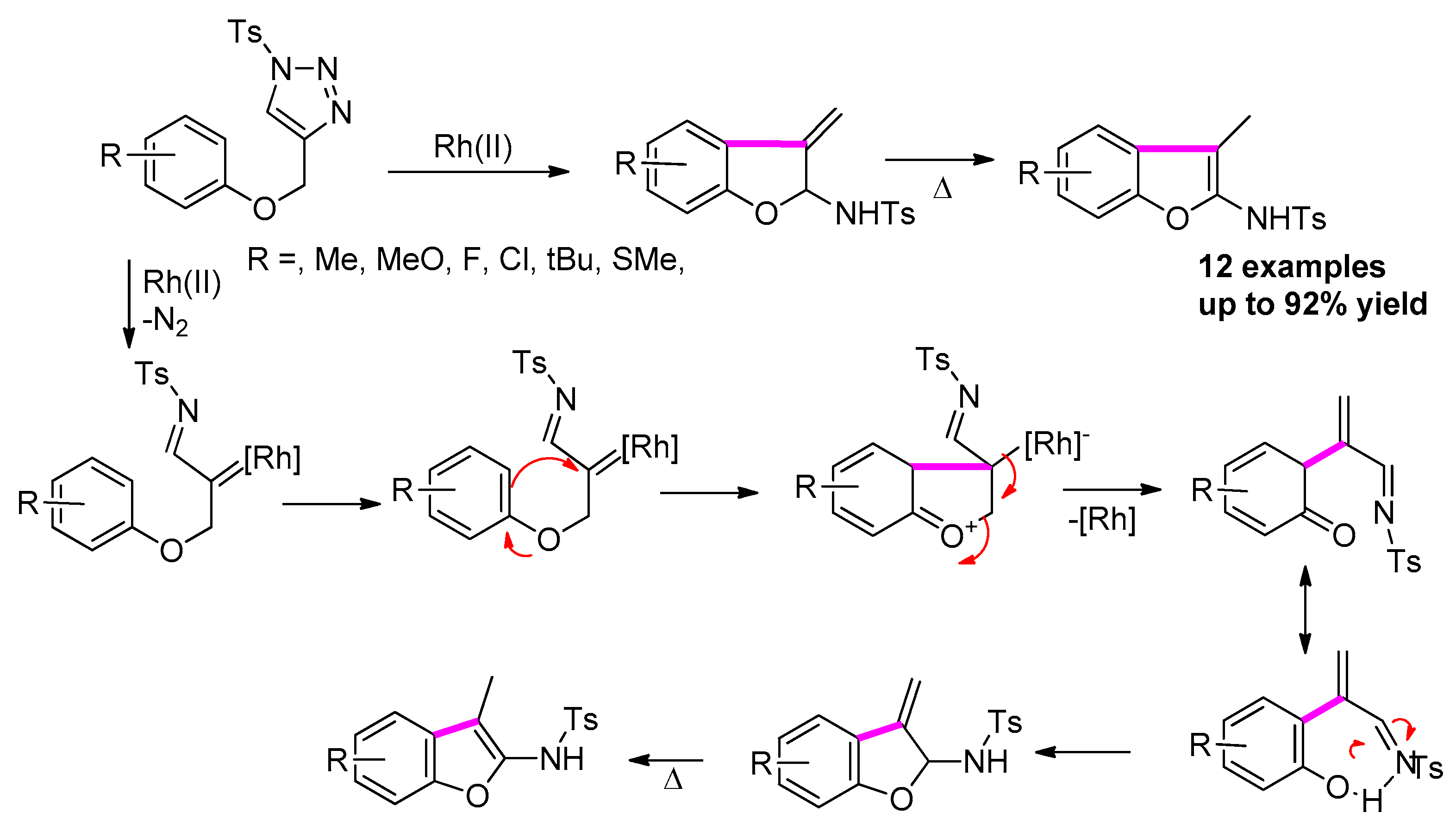
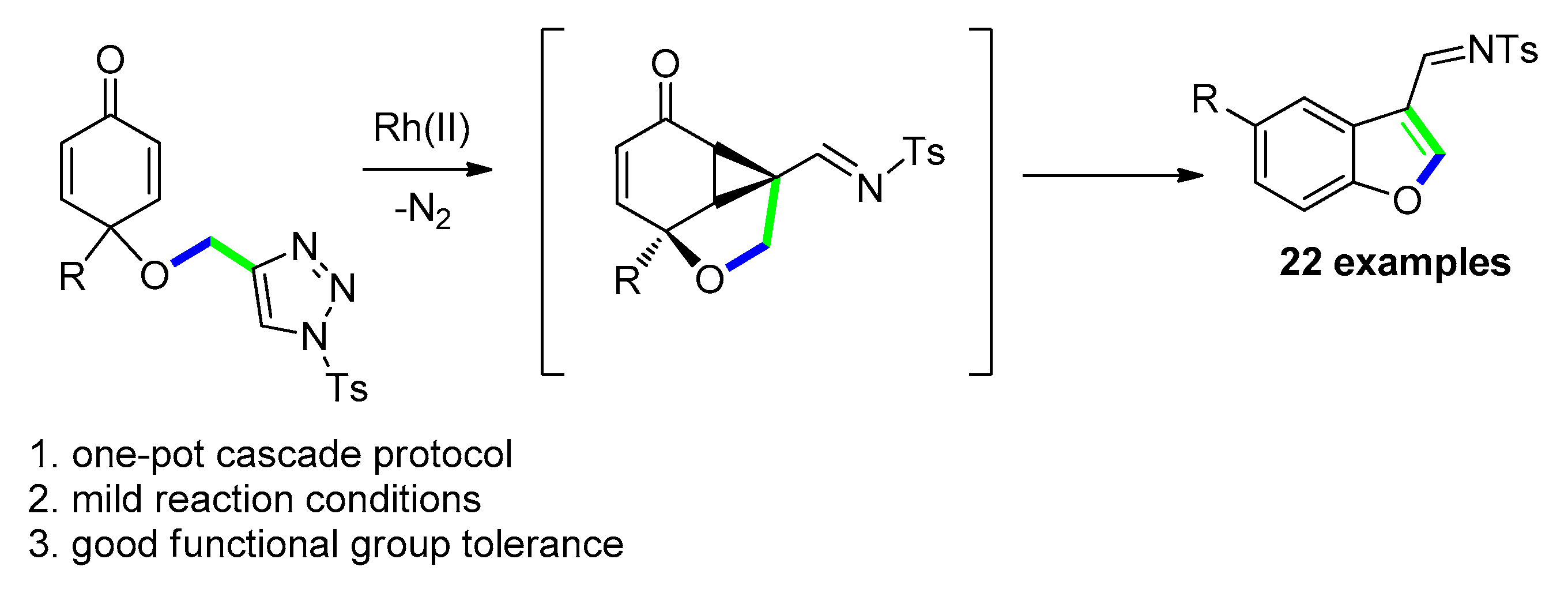
2.4.2. Via [Ru(II)] C-H Insertion of N-Sulfonyl-1,2,3-Triazole Derivatives
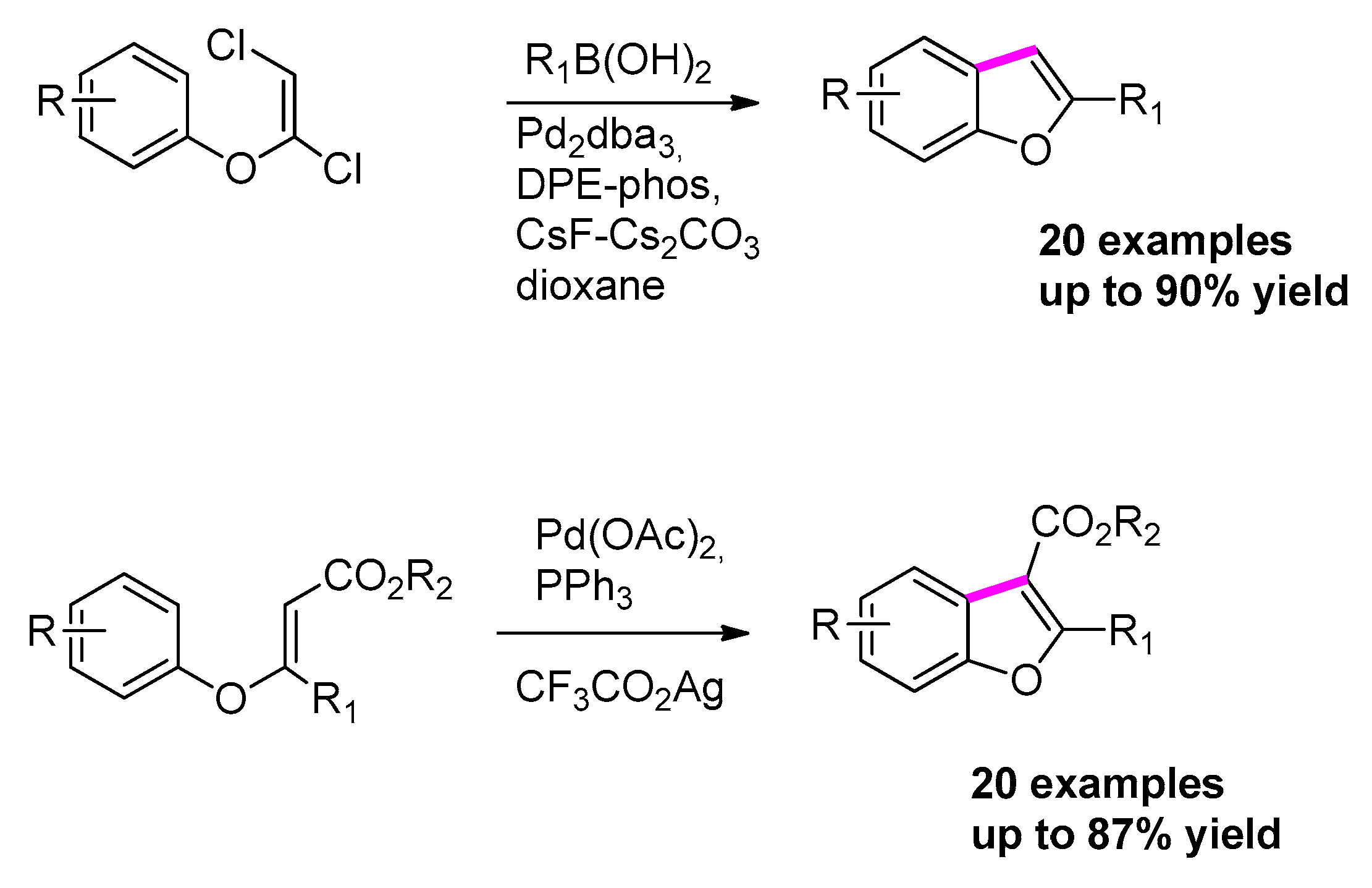
2.4.3. Via [Pd(0)] C–H Insertion
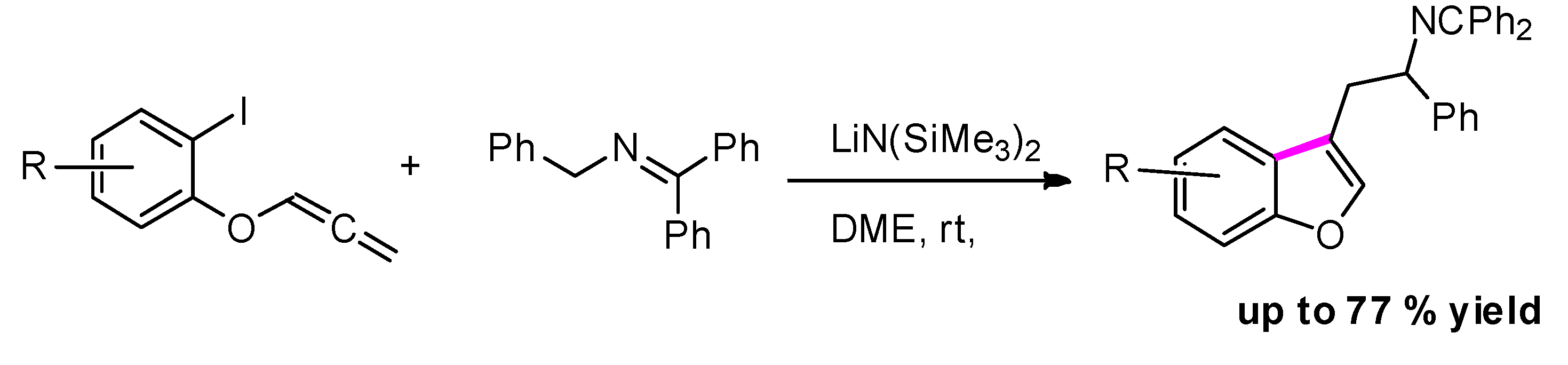
2.4.4. Via Radical Cyclization of o-Iodophenyl Allenyl Ethers
3. Ring Generation via Intermolecular Cyclization
3.1. C7a–O and C3–C3a Bond Formation: (Route a + d)
3.1.1. Via o-C-H alkylation/Decarboxylation
3.1.2. Via Propargyl Claisen Rearrangement/Cycloaddition
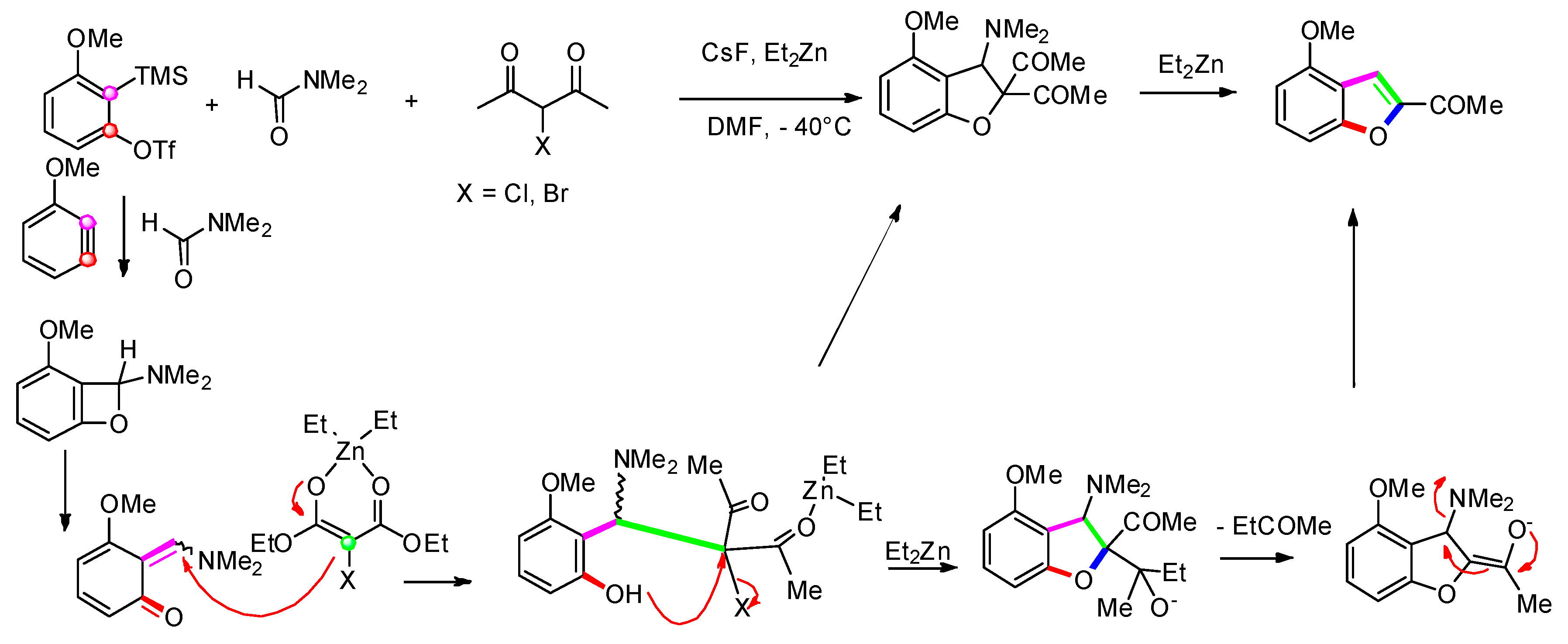
3.1.3. Via Addition of Zinc-Enolate to Methines
3.2. O–C2 and C2–C3 Bond Formation: (Route b + c)
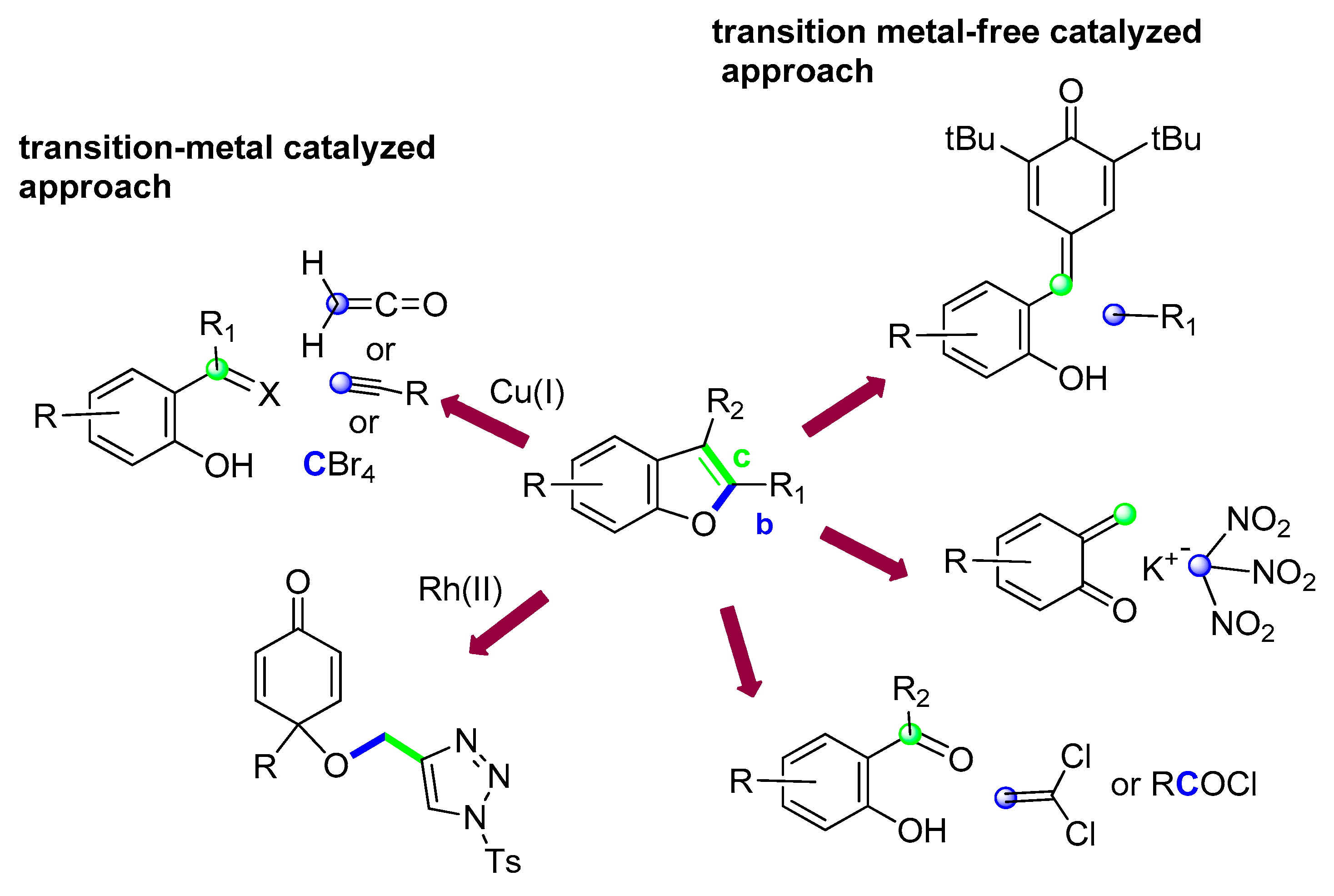
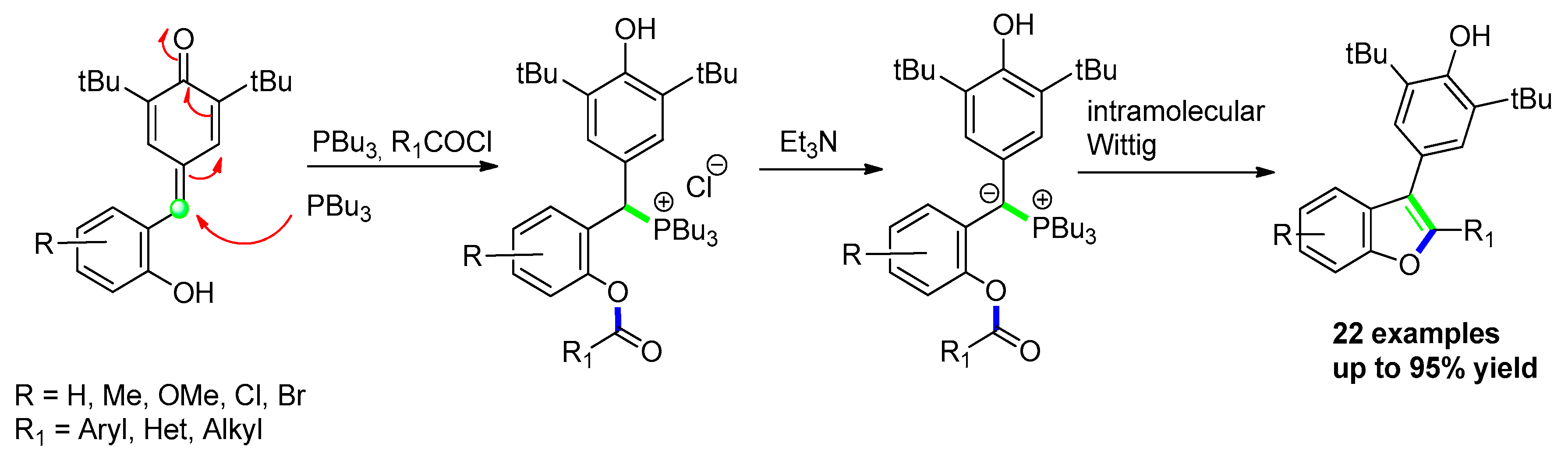
3.2.1. Via Transition-Metal-Free Catalyzed Approaches: p-Quinone Methides
3.2.2. Via transition-Metal-Free Catalyzed Approaches: o-Quinone Methides
3.2.3. Via Transition-Metal-Free Catalyzed Approaches: o-Hydroxyphenone or Salicylaldehydes
3.2.4. Transition-Metal-Catalyzed Approaches: [Rh(II)] Catalyzed Addition of N-Sulfonyl-1,2,3-Triazole
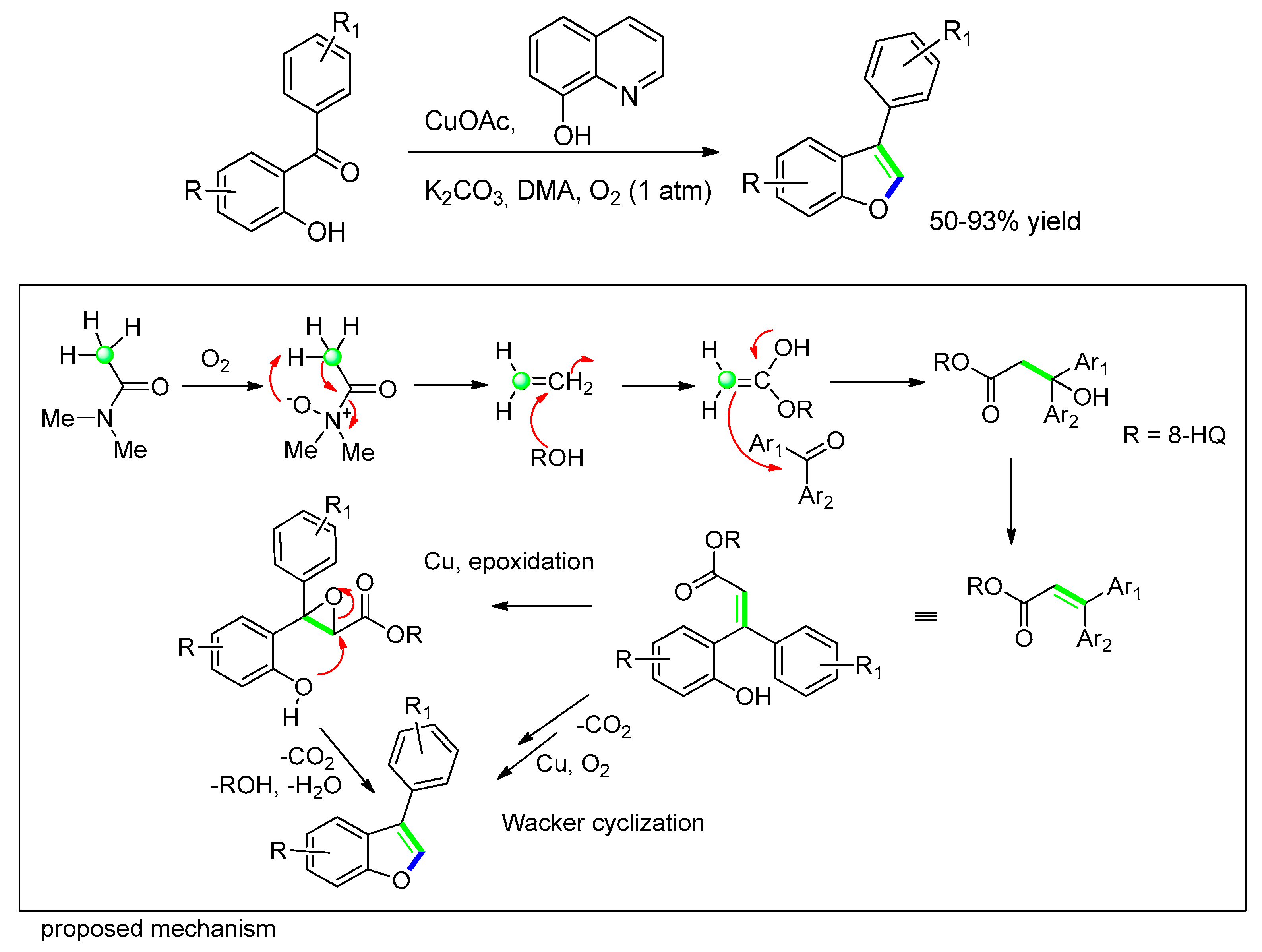
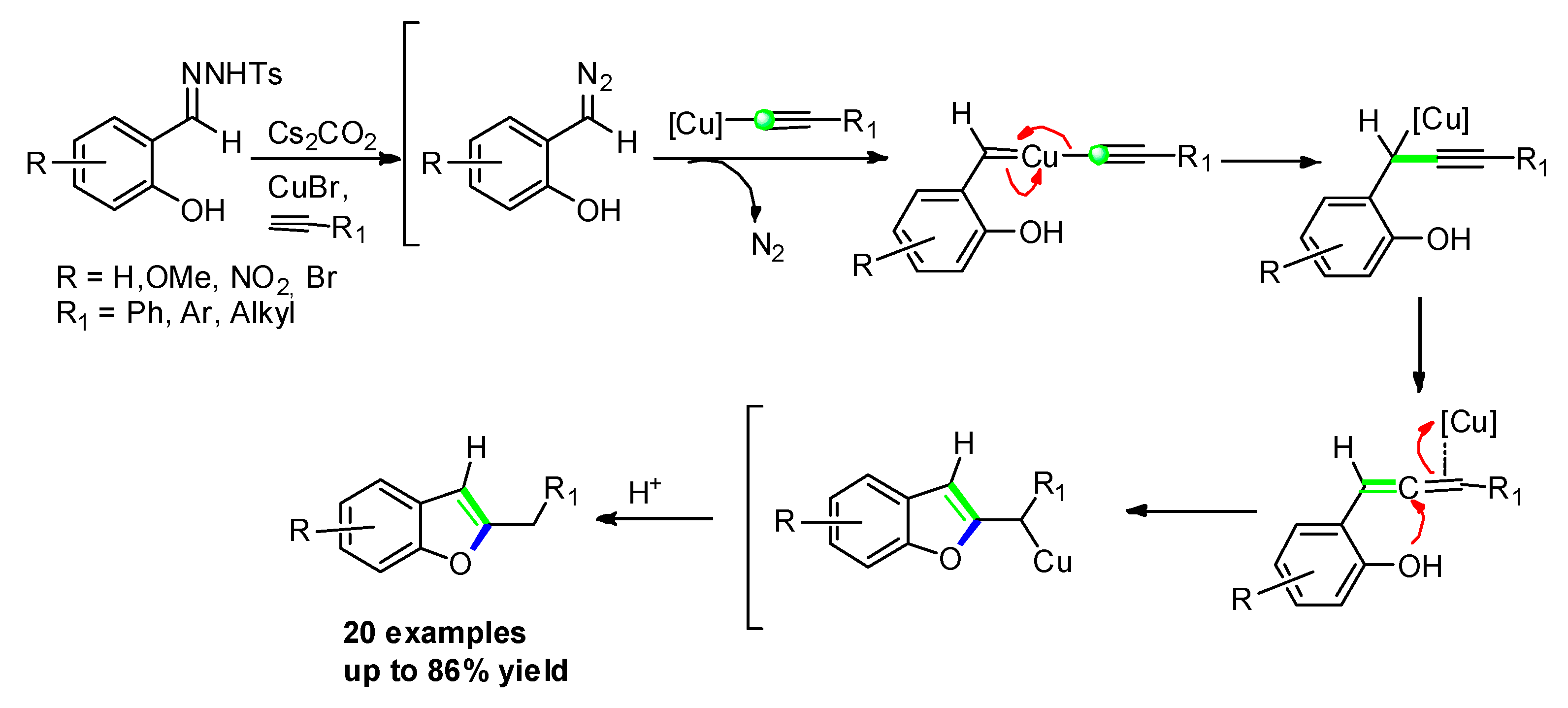
3.2.5. Transition–Metal Catalyzed Approaches: [Cu(I)]-Catalyzed Addition to o-Hydroxybenzophenones/Salicylaldehydes
3.2.6. Miscellaneous
3.3. O–C2 and C3–C3a Bond Formation: (Route b + d)
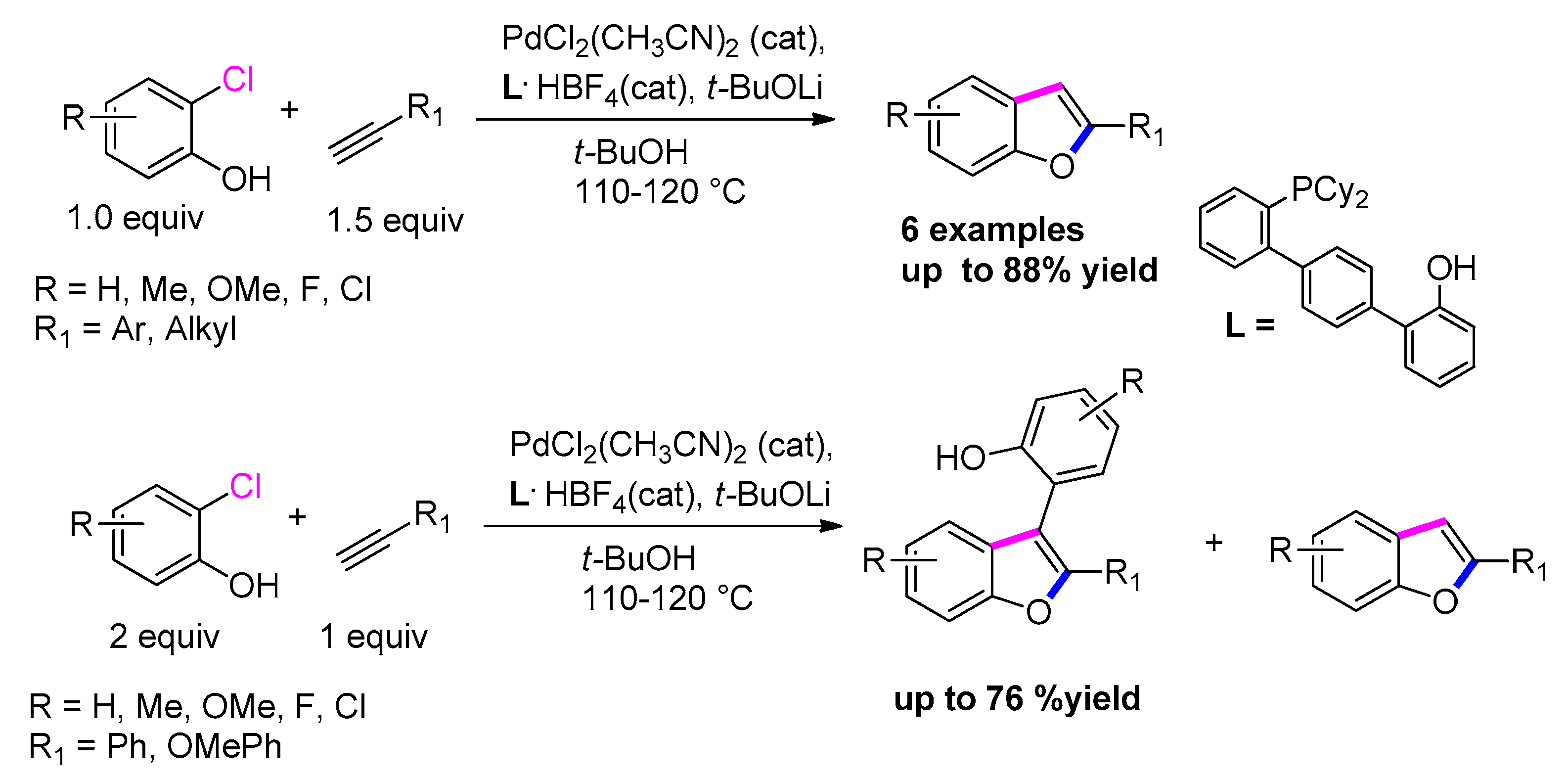
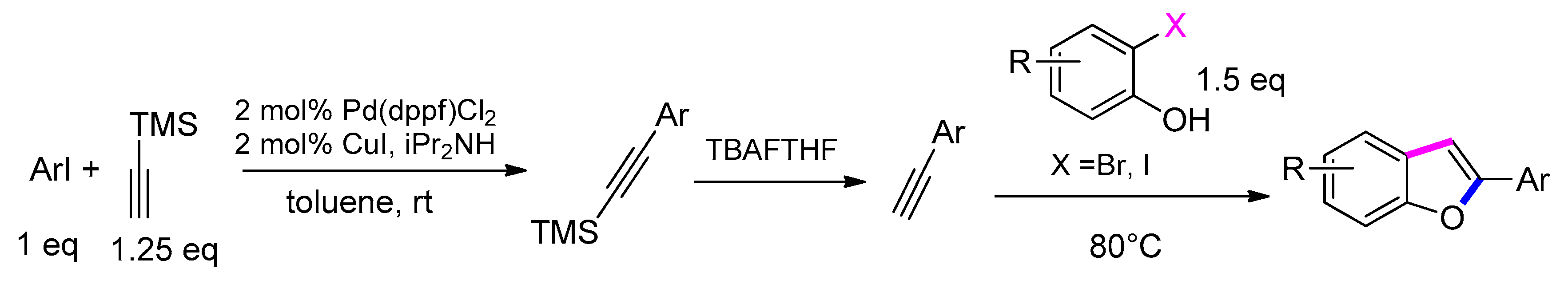
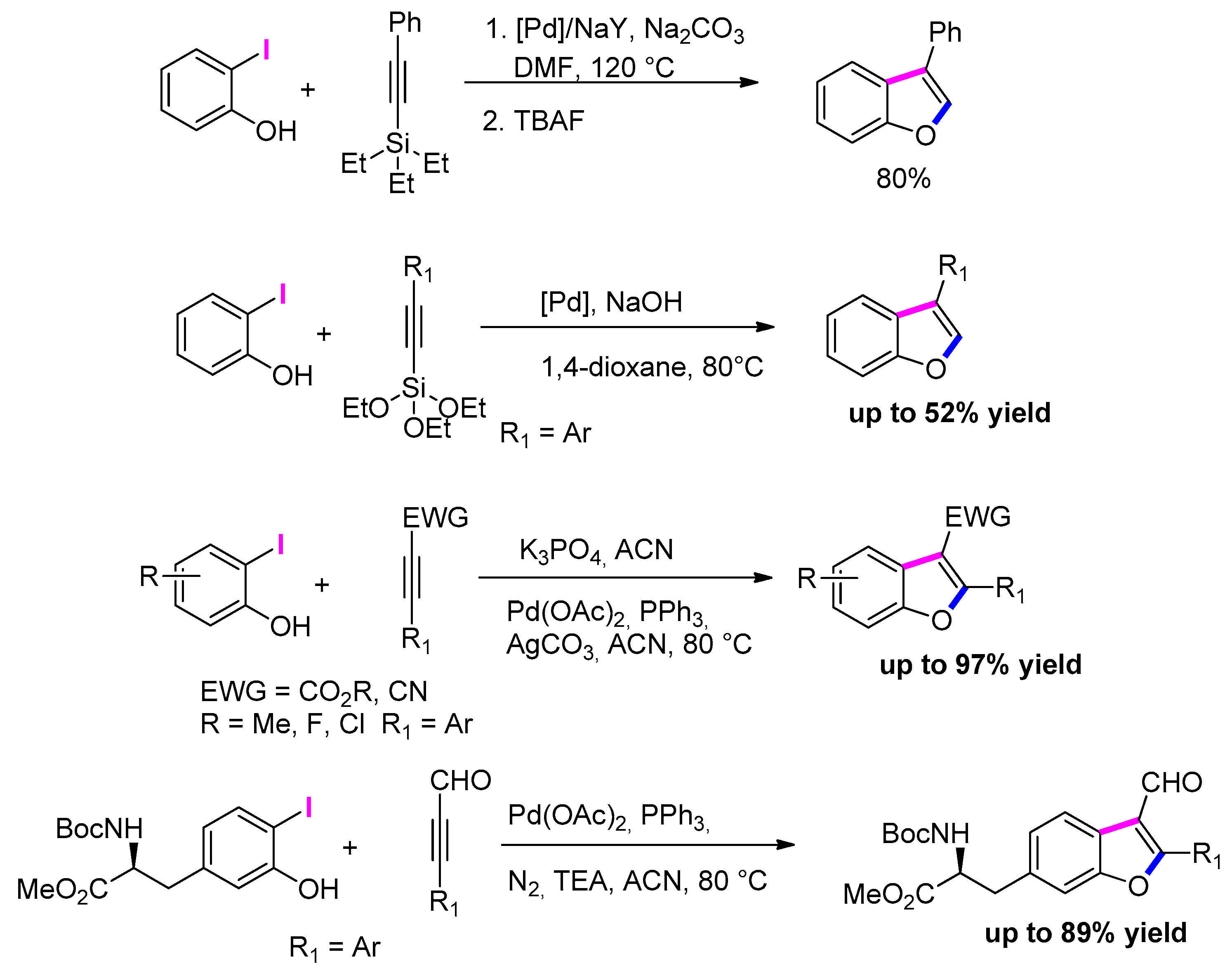
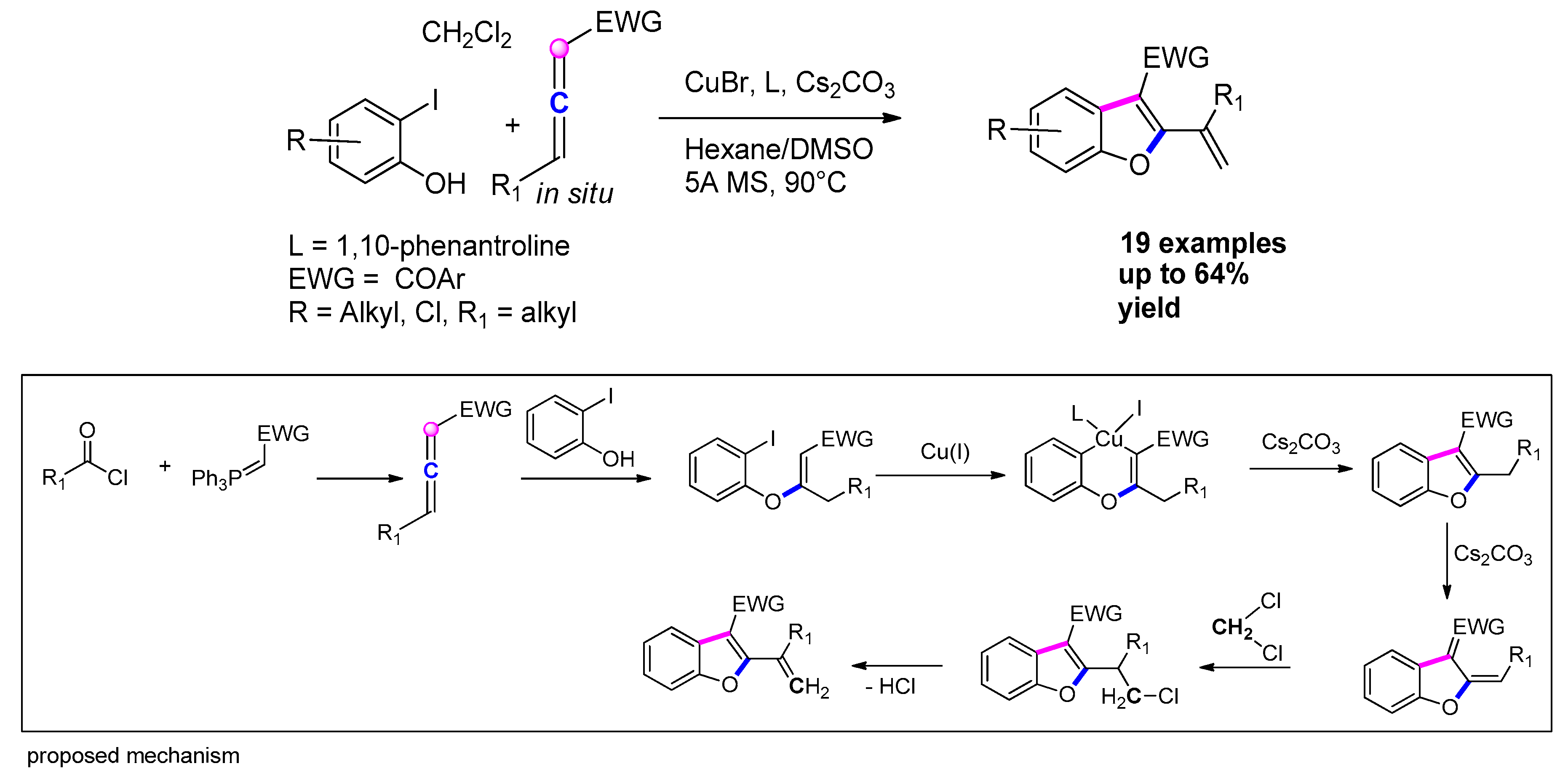
3.3.1. From o-Halophenols and Terminal Alkynes
3.3.2. From o-Halophenols and Internal Alkynes
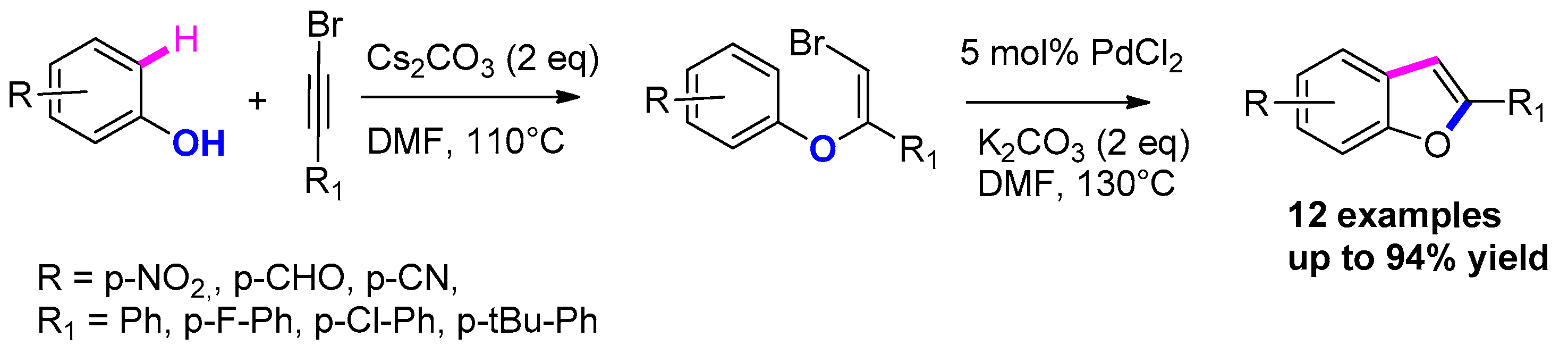
3.3.3. From o-Halophenols and Allenes
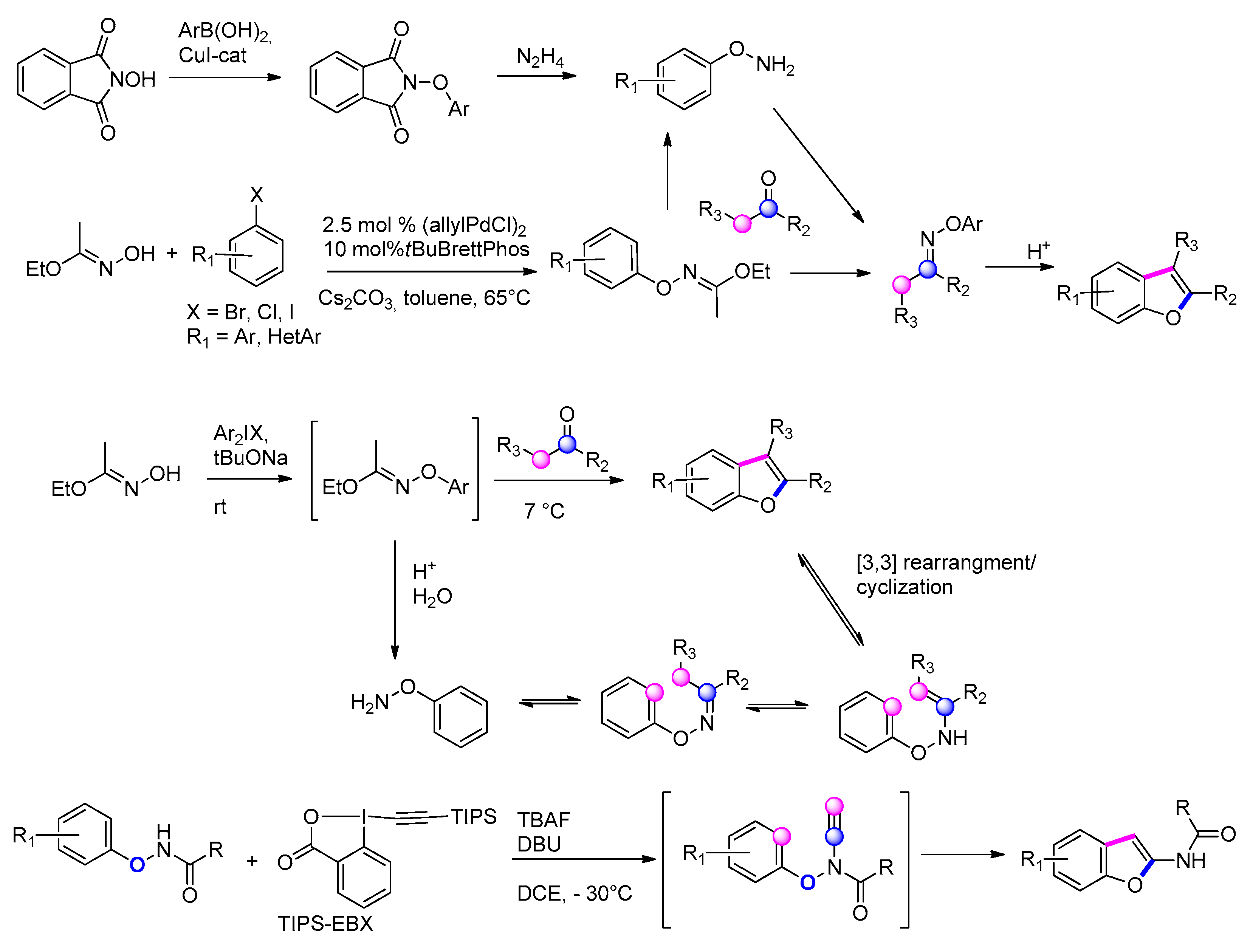
3.3.4. From Phenols: O-aryloxime/[3,3]-Sigmatropic Rearrangement/Cyclization
3.3.5. Via Transition-Metal-Catalyzed Annulation of N-Aryloxyacetamides and Propargyl Alcohols
3.3.6. Metal-Free [3 + 2] Annulation of Phenols with Acetylenes
3.3.7. [Pd]-Catalyzed [3 + 2] Annulation of Phenols with Internal Alkynes
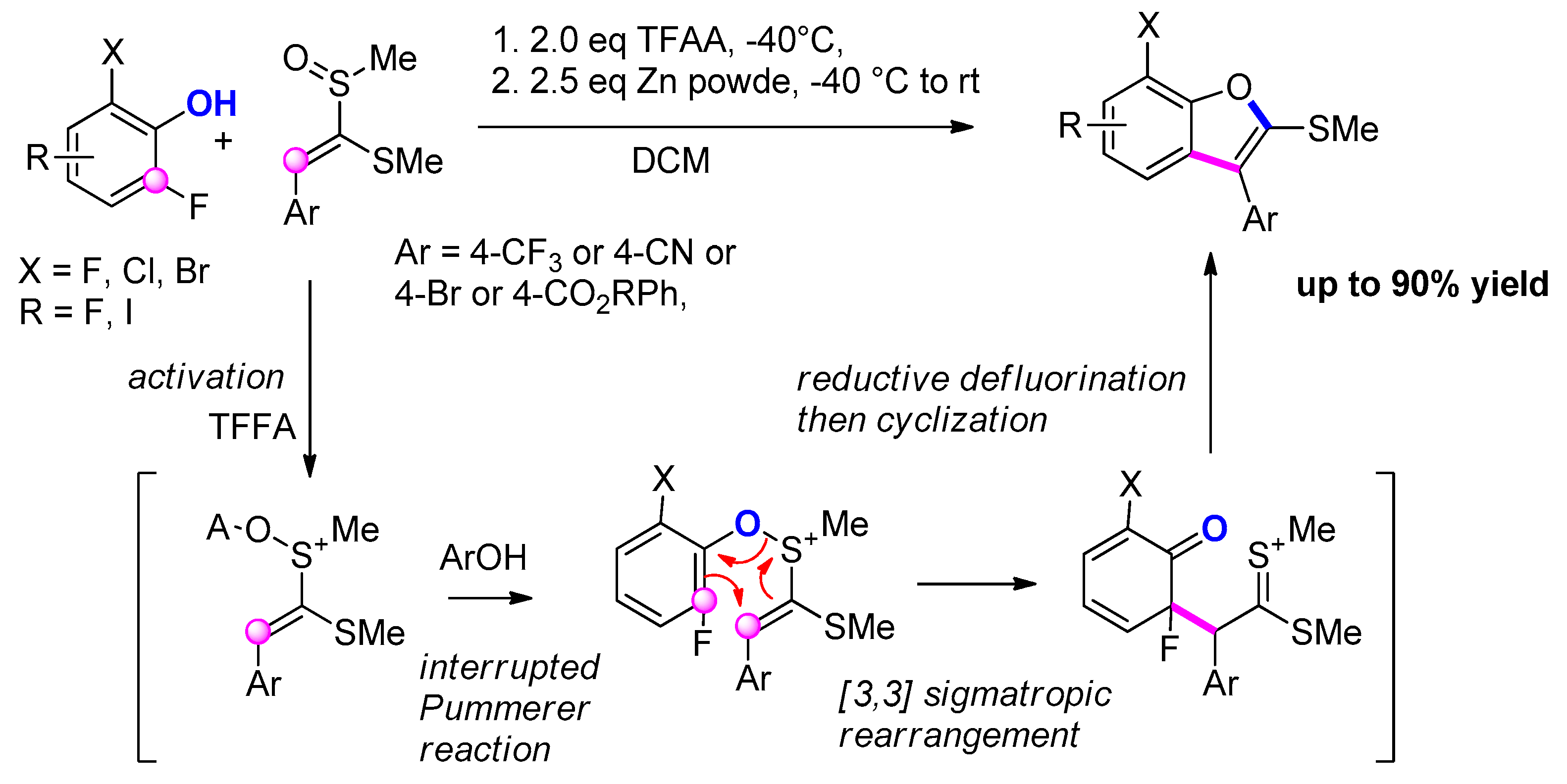
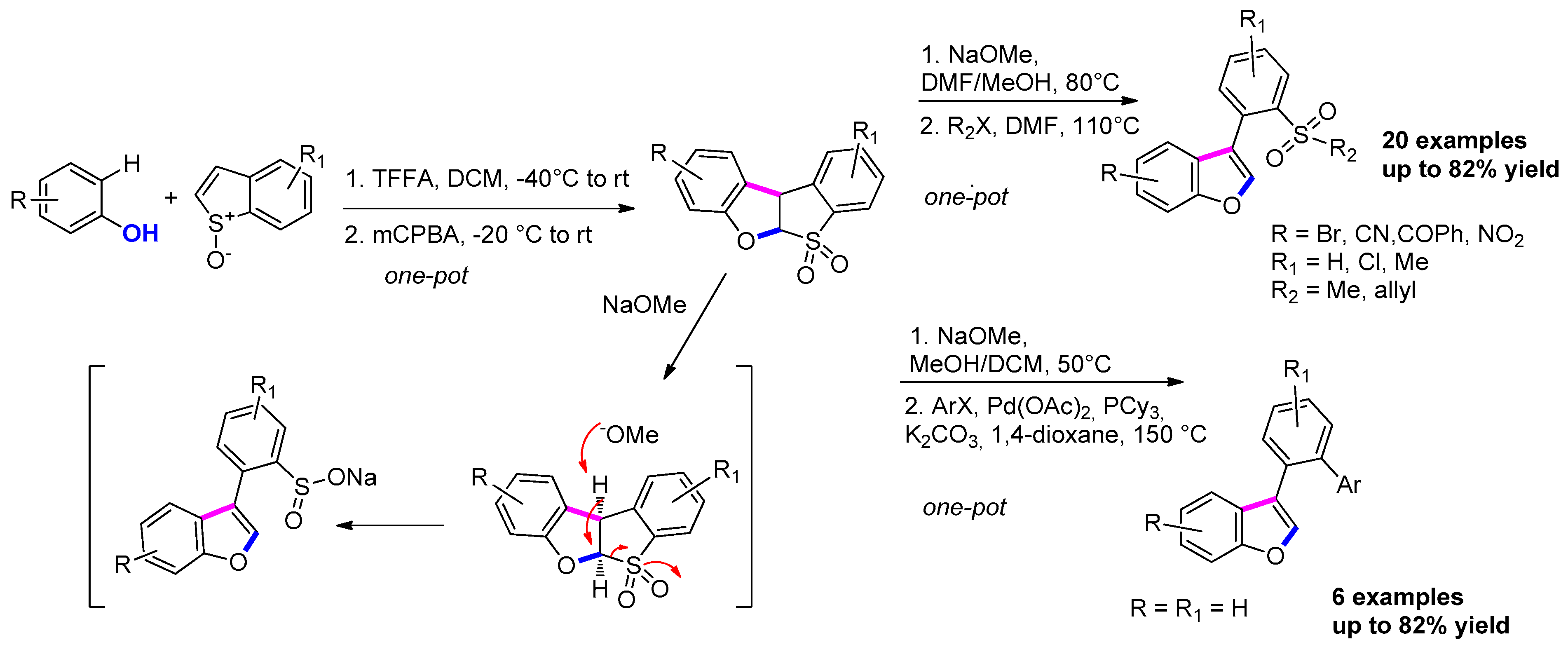
3.3.8. Via Interrupted Pummerer Reaction/[3,3] Sigmatropic Rearrangement/Cyclization
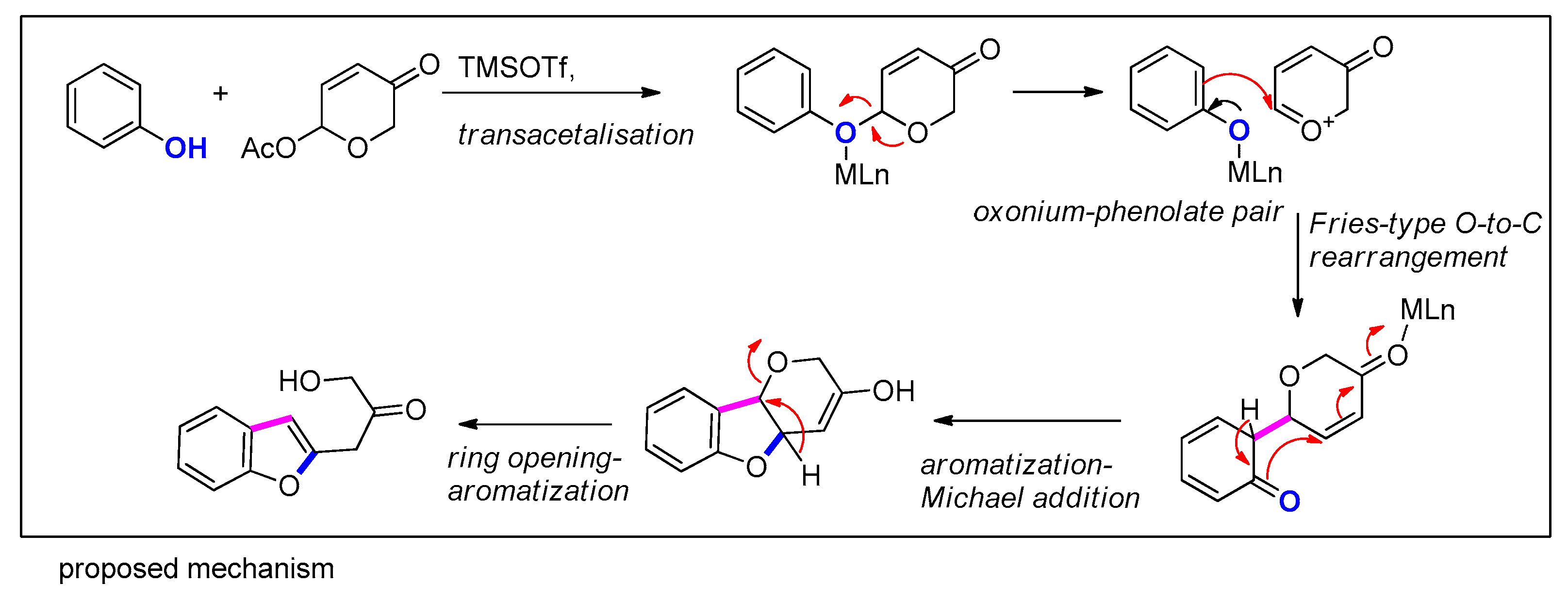
3.3.9. Via Fries-type O-C Rearrangement/Michael Addition of Phenols
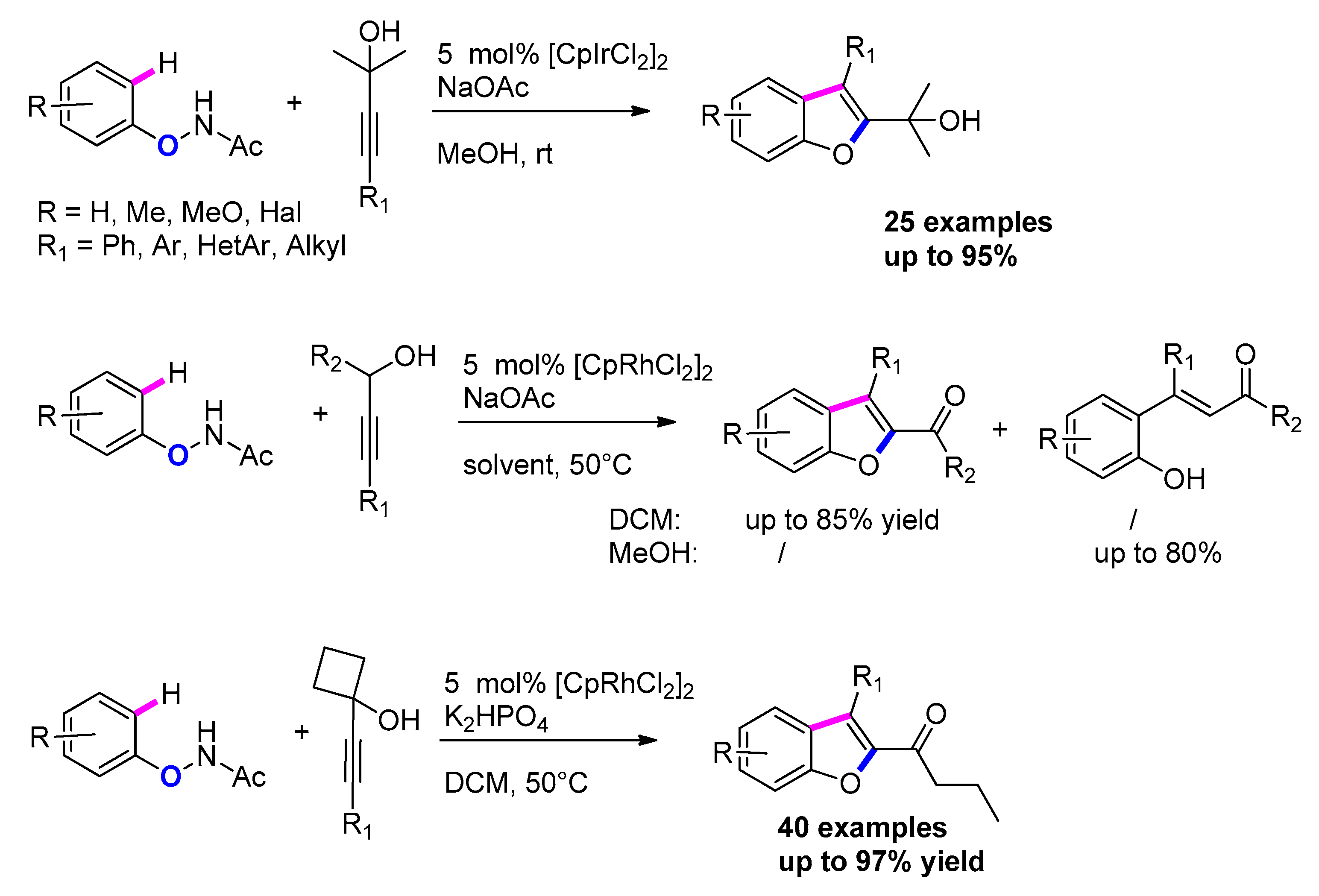
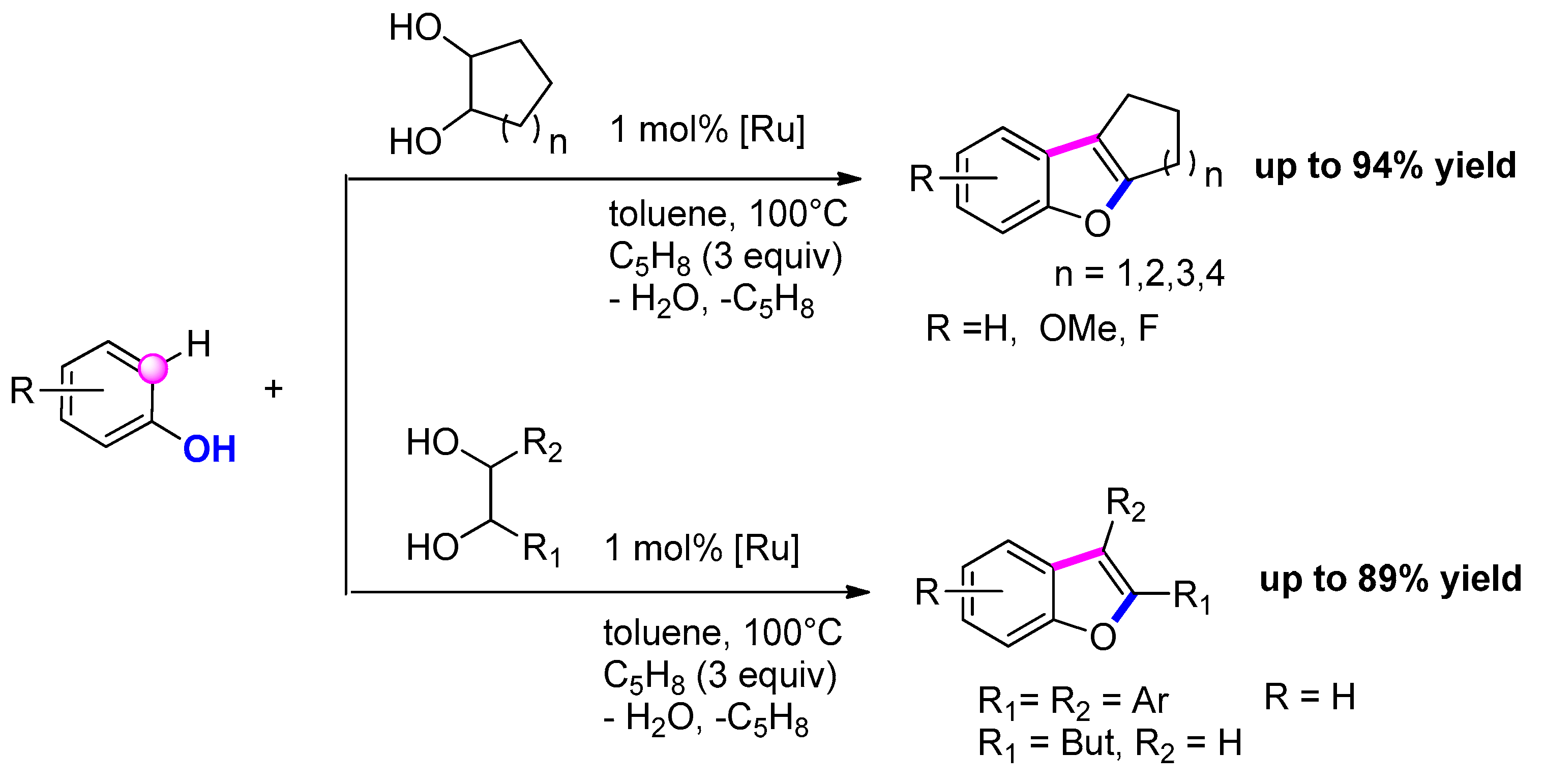
3.3.10. Via [Ru]-Catalyzed C–H Alkylation of Phenols with 1,2-Diols
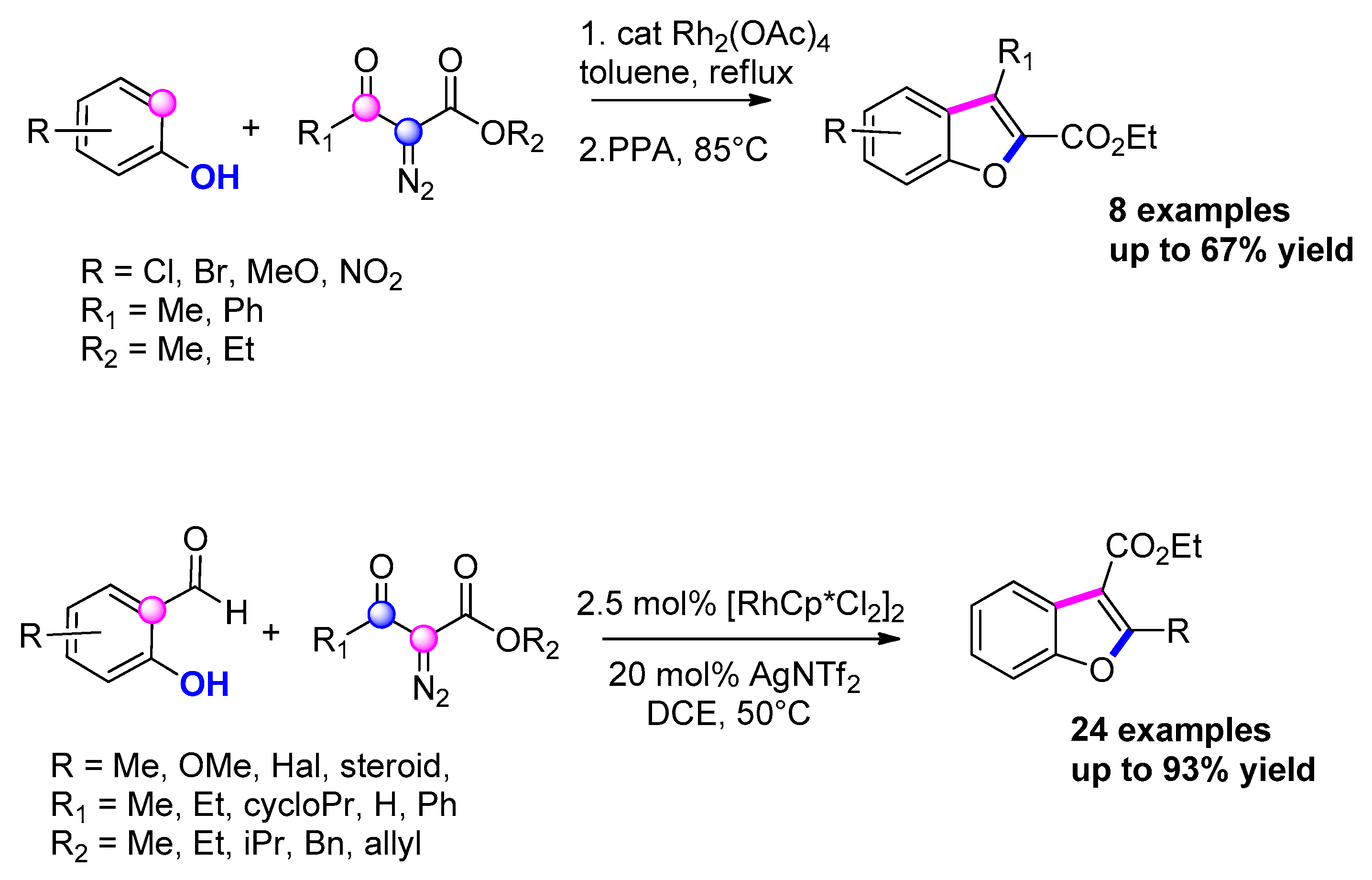
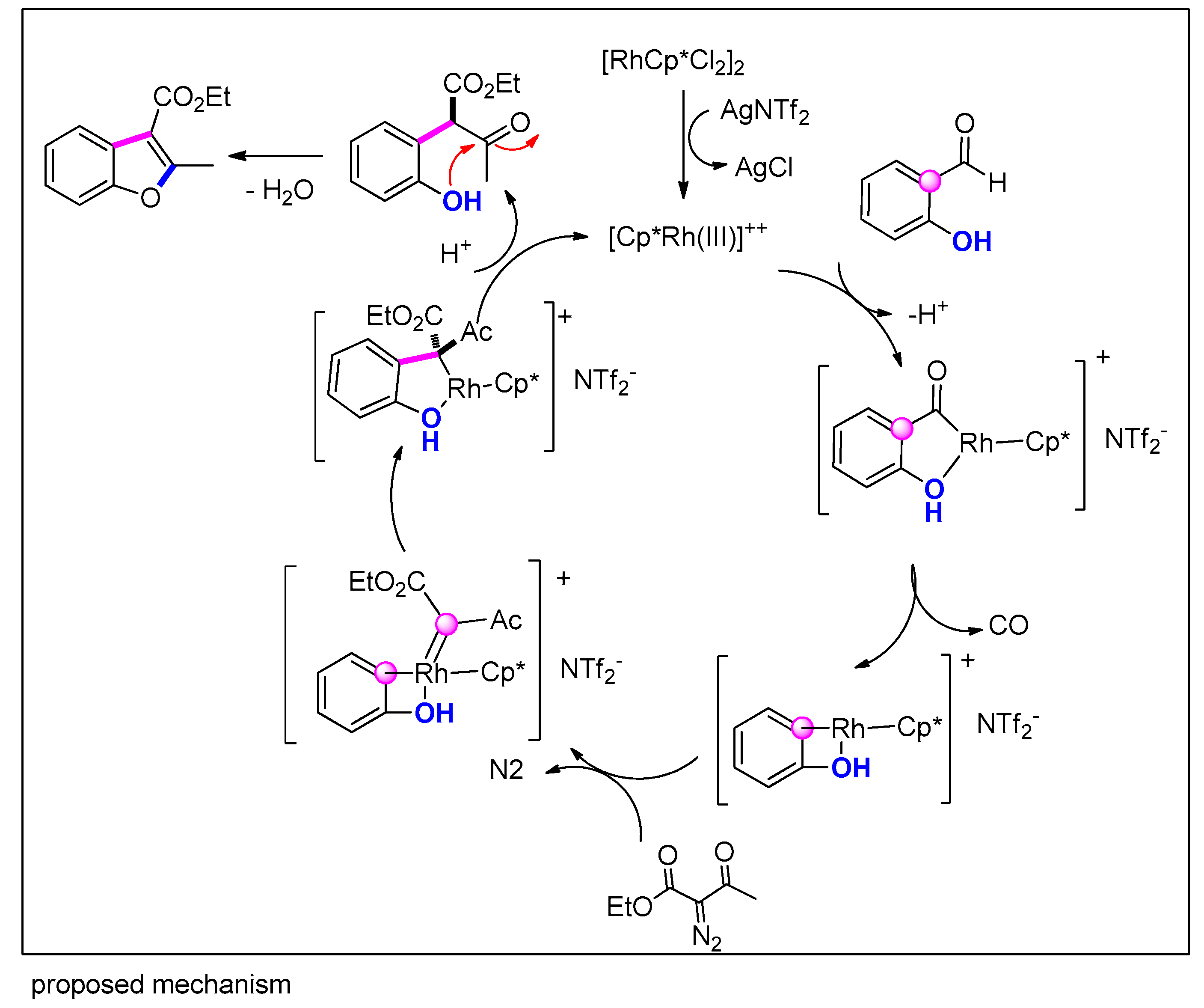
3.3.11. Via [Rh]-Catalyzed Carbene Insertion with Phenols/Salicylaldehydes
3.3.12. Via Michael Addition/Cyclization of Nucleophiles on Benzoquinones
3.3.13. Via FeCl3-Catalyzed Allenic Claisen Rearrangement/ Dehydrogenative Cyclization
4. Benzoannulation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Radadiya, A.; Shah, A. Bioactive benzofuran derivatives: An insight on lead developments, radioligands and advances of the last decade. Eur. J. Med. Chem. 2015, 97, 356–376. [Google Scholar] [CrossRef]
- Shamsuzzaman, H.K. Bioactive Benzofuran derivatives: A review. Eur. J. Med. Chem. 2015, 97, 483–504. [Google Scholar] [CrossRef]
- Nevagi, R.J.; Dighe, S.N.; Dighe, S.N. Biological and medicinal significance of benzofuran. Eur. J. Med. Chem. 2015, 97, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Laurita, T.; D’Orsi, R.; Chiummiento, L.; Funicello, M.; Lupattelli, P. Recent Advances in Synthetic Strategies to 2,3-Dihydrobenzofurans. Synthesis 2020, 52, 1452–1477. [Google Scholar] [CrossRef]
- Li, Y.T.; Yao, C.S.; Bai, J.Y.; Lin, M.; Cheng, G.F. Anti-inflammatory effect of amurensin H on asthma-like reaction induced by allergen in sensitized mice. Acta Pharmacol. Sin. 2006, 27, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Brkljača, R.; White, J.M.; Urban, S. Phytochemical Investigation of the Constituents Derived from the Australian Plant Macropidia fuliginosa. J. Nat. Prod. 2015, 78, 1600–1608. [Google Scholar] [CrossRef]
- Vo, D.D.; Elofsson, M. Total Synthesis of Viniferifuran, Resveratrol-Piceatannol Hybrid, Anigopreissin A and Analogues–Investigation of Demethylation Strategies. Adv. Synth. Catal. 2016, 358, 4085–4092. [Google Scholar] [CrossRef]
- Chiummiento, L.; Funicello, M.; Lopardo, M.T.; Lupattelli, P.; Choppin, S.; Colobert, F. Concise total synthesis of permethylated anigopreissin A, a novel benzofuryl resveratrol dimer. Eur. J. Org. Chem. 2012, 188–192. [Google Scholar] [CrossRef]
- Convertini, P.; Tramutola, F.; Iacobazzi, V.; Lupattelli, P.; Chiummiento, L.; Infantino, V. Permethylated Anigopreissin A inhibits human hepatoma cell proliferation by mitochondria-induced apoptosis. Chem. Biol. Interact. 2015, 237, 1–8. [Google Scholar] [CrossRef]
- Gaisina, I.N.; Gallier, F.; Ougolkov, A.V.; Kim, K.H.; Kurome, T.; Guo, S.; Holzle, D.; Luchini, D.N.; Blond, S.Y.; Billadeau, D.D.; et al. From a natural product lead to the identification of potent and selective benzofuran-3-yl- (indol-3-yl)maleimides as glycogen synthase kinase 3b inhibitors that suppress proliferation and survival of pancreatic cancer cells. J. Med. Chem. 2009, 52, 1853–1863. [Google Scholar] [CrossRef]
- Naik, R.; Harmalkar, D.S.; Xu, X.Z.; Jang, K.; Lee, K. Bioactive benzofuran derivatives: Moracins A-Z in medicinal chemistry. Eur. J. Med. Chem. 2015, 90, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Peng, E.; Huang, N.; Huang, Q.; Quq, A.; Lau, M.; Colonno, R.; Li, L. Discovery of novel potent HCV NS5B polymerase non-nucleoside inhibitors bearing a fused benzofuran scaffold. Bioorg. Med. Chem. Lett. 2018, 28, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Galal, S.A.; El-All, A.S.A.; Abdallah, M.M.; El-Diwani, H.I. Synthesis of potent antitumor and antiviral benzofuran derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Hiremathad, A.; Chand, K.; Tolayan, L.; Rajeshwari; Keri, R.S.; Esteves, A.R.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Hydroxypyridinone-benzofuran hybrids with potential protective roles for Alzheimer’s disease therapy. J. Inorg. Biochem. 2018, 179, 82–96. [Google Scholar] [CrossRef]
- Deepti, G.; Amandeep, K.; Bhupesh, G. Benzofuran and indole: Promising Scaffolds for drug development in Alzheimer’s disease. Chem. Med. Chem. 2018, 13, 1275–1299. [Google Scholar] [CrossRef]
- Marion, T.; Sylviane, T.; Philippe, G.; Joelle, D. Synthesis of polysubstituted benzofuran derivatives as novel inhibitors of parasitic growth. Bioorg. Med. Chem. 2013, 21, 4885–4892. [Google Scholar] [CrossRef]
- Sangeeta, B.; Deepti, R. Synthetic routes and biological activities of benzofuran and its derivatives: A review. Lett. Org. Chem. 2017, 14, 381–402. [Google Scholar] [CrossRef]
- Hiremathad, A.; Patil, M.R.; Chand, K.; Santos, M.A.; Keri, R.S. Benzofuran: An emerging scaffold for antimicrobial agents. RSC Adv. 2015, 5, 96809–96828. [Google Scholar] [CrossRef]
- Simonetti, S.O.; Larghi, E.L.; Bracca, A.B.J.; Kaufman, T.S. Angular tricyclic benzofurans and related natural products of fungal origin. Isolation, biological activity and synthesis. Nat. Prod. Rep. 2013, 30, 941–969. [Google Scholar] [CrossRef]
- Shimazu, S.; Takahata, K.; Katsuki, H.; Tsunekawa, H.; Tanigawa, A.; Yoneda, F.; Knoll, J.; Akaike, A. (−)-1-(Benzofuran-2-yl)-2-propylaminopentane enhances locomotor activity in rats due to its ability to induce dopamine release. Eur. J. Pharmacol. 2001, 421, 181–189. [Google Scholar] [CrossRef]
- Lavranos, T.C.; Leske, A.F.; Inglis, D.J.; Brown, C.K.; Bibby, D.C.; Kremmidiotis, G. Abstract 2774: Anti-cancer activity of the tumor-selective, hypoxia-inducing, agent BNC105 in platinum resistant ovarian cancer. Cancer Res. 2012, 72, 2774. [Google Scholar] [CrossRef]
- Joule, J.A.; Mills, K. Heterocyclic Chemistry, 5th ed.; Wiley-Blackwell: New York, NY, USA, 2010; pp. 437–443. [Google Scholar]
- De Luca, L.; Nieddu, G.; Porcheddu, A.; Giacomelli, G. Some Recent Approaches to the Synthesis of 2-Substituted Benzofurans. Curr. Med. Chem. 2009, 16, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hashem, A.A.; Hussein, H.A.R.; Aly, A.S.; Gouda, M.A. Synthesis of benzofuran derivatives via different methods. Synth. Commun. 2014, 44, 2285–2312. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V. The recent advances in the synthesis of benzo[b]furans. Adv. Heterocycl. Chem. 2015, 117, 261–376. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V.; Dehghani, M. Reactivity of Benzo[b]furans: A Full Perspective. Curr. Org. Chem. 2016, 20, 1069–1134. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V. Recent Advances in the Synthesis of Biologically Active Compounds Containing Benzo[b]Furans as a Framework. Curr. Org. Synth. 2016, 13, 780–833. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V.; Hamidi, H.; Hajiabbas, P.; Amiri, T. Total synthesis of natural products containing benzofuran rings. RSC Adv. 2017, 7, 24470–24521. [Google Scholar] [CrossRef]
- More, K.R. Review on Synthetic Routes for Synthesis of Benzofuran-Based Compounds. J. Chem. Pharm. Res. 2017, 9, 210–220. [Google Scholar]
- Miao, Y.-h.; Hu, Y.-h.; Yang, J.; Liu, T.; Sun, J.; Wang, X.-j. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019, 9, 27510–27540. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Ji, Y.; Fang, Y.; Prakash, I. Novel Syntheses of 2,3-Disubstituted Benzofurans. J. Org. Chem. 2001, 66, 5613–5615. [Google Scholar] [CrossRef]
- Carrër, A.; Florent, J.-C.; Auvrouin, E.; Rousselle, P.; Bertounesque, E. Synthesis of 3-Aryl-2-arylamidobenzofurans Based on the Curtius Rearrangement. J. Org. Chem. 2011, 76, 2502–2520. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.; Beletskaya, I.P.; Yus, M. Transition-Metal-Catalyzed Addition of Heteroatom−Hydrogen Bonds to Alkynes. Chem. Rev. 2004, 104, 3079–3160. [Google Scholar] [CrossRef] [PubMed]
- Zeni, G.; Larock, R.C. Synthesis of Heterocycles via Palladium-Catalyzed Oxidative Addition. Chem. Rev. 2006, 106, 4644–4680. [Google Scholar] [CrossRef] [PubMed]
- Cacchi, S.; Fabrizi, G.; Goggiamani, A. The palladium-catalyzed assembly and functionalization of benzo [b] furans. Curr. Org. Chem. 2006, 10, 1423–1455. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G.; Goggiamani, A. Copper catalysis in the construction of indole and benzo[b]furan rings. Org. Biomol. Chem. 2011, 9, 641–652. [Google Scholar] [CrossRef]
- Sadig, J.E.R.; Willis, M.C. Palladium- and Copper-Catalyzed Aryl Halide Amination, Etherification and Thioetherification Reactions in the Synthesis of Aromatic Heterocycles. Synthesis 2011, 1–22. [Google Scholar] [CrossRef]
- Agasti, S.; Dey, A.; Maiti, D. Palladium-catalyzed benzofuran and indole synthesis by multiple C–H functionalizations. Chem. Commun. 2017, 53, 6544–6566. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, L.; Liu, X.; Li, B.; Sun, N. Copper-promoted hydration and annulation of 2-fluorophenylacetylene derivatives: From alkynes to benzo[b]furans and benzo[b]thiophenes. Beilstein J. Org. Chem. 2014, 10, 2886–2891. [Google Scholar] [CrossRef]
- Lavery, C.B.; Rotta-Loria, N.L.; McDonald, R.; Stradiotto, M. Pd2dba3/Bippyphos: A Robust Catalyst System for the Hydroxylation of Aryl Halides with Broad Substrate Scope. Adv. Synth. Catal. 2013, 355, 981–987. [Google Scholar] [CrossRef]
- Faragó, J.; Kotschy, A. Synthesis of Benzo[b]furans by Palladium–NHC Catalyzed Ring Closure of o-Bromobenzyl Ketones. Synthesis 2009, 85–90. [Google Scholar] [CrossRef]
- Bonnamour, J.; Piedrafita, M.; Bolm, C. Iron and cupper salts in the synthesis of benzo[b]furan. Adv. Synth. Catal. 2010, 352, 1577–1581. [Google Scholar] [CrossRef]
- Henry, M.C.; Sutherland, A. Synthesis of Benzo[b]furans by Intramolecular C−O Bond Formation Using Iron and Copper Catalysis. Org. Lett. 2020, 22, 2766–2770. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Hou, W.; Du, Y.; Zhang, Y.; Pan, Y.; Mao, D.; Zhao, K. Oxidative Aromatic C–O Bond Formation: Synthesis of 3-Functionalized Benzo[b]furans by FeCl3-Mediated Ring Closure of -Aryl Ketones. Org. Lett. 2009, 11, 4978–4981. [Google Scholar] [CrossRef]
- Lyons, T.W.; Sanford, M.S. Palladium-Catalyzed Ligand-Directed C-H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-J.; Shi, Z.-J. From C(sp2)–H to C(sp3)–H: Systematic studies on transition metal-catalyzed oxidative C–C formation. Chem. Soc. Rev. 2012, 41, 5588–5598. [Google Scholar] [CrossRef] [PubMed]
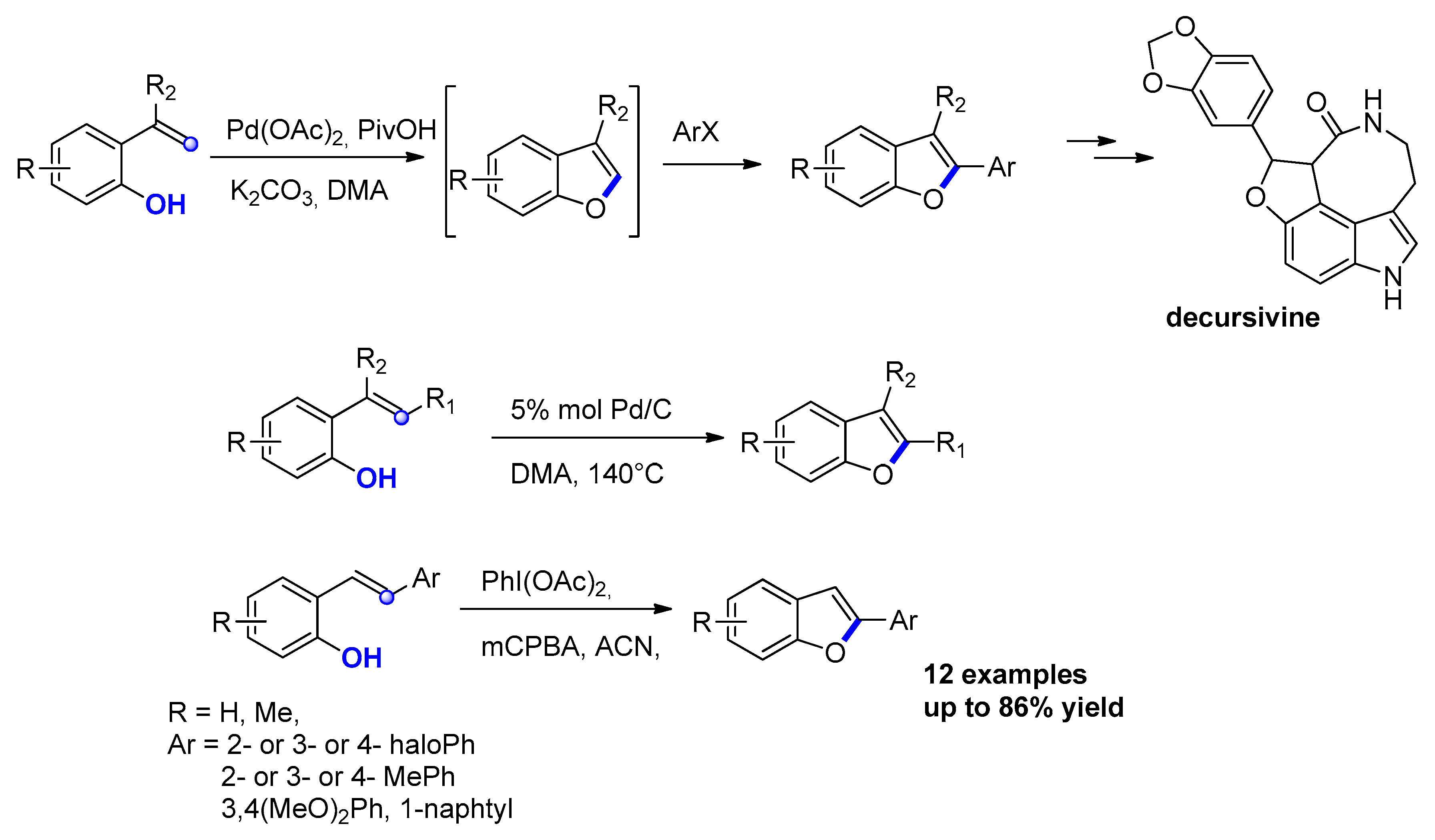
- Guo, L.; Zhang, F.; Hu, W.; Li, L.; Jia, Y. Palladium-catalyzed synthesis of benzofurans via C–H activation/oxidation tandem reaction and its application to the synthesis of decursivine and serotobenine. Chem. Commun. 2014, 50, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhu, Y.; Yang, N.; Jiang, Q.; Liua, R. One-Step Synthesis of Substituted Benzofurans from ortho-Alkenylphenols via Palladium-Catalyzed C-H functionalization. Adv. Synth. Catal. 2016, 358, 1731–1735. [Google Scholar] [CrossRef]
- Singh, F.V.; Mangaonkar, S.R. Hypervalent iodine(III)-Catalyzed Synthesis of 2-Arylbenzofurans. Synthesis 2018, 50, 4940–4948. [Google Scholar] [CrossRef]
- Huang, W.; Hung-Chang Liu, J.; Alayoglu, P.; Li, Y.; Witham, C.A.; Tsung, C.-K.; Toste, F.D.; Somorjai, G.A. Highly active heterogeneous palladium nanoparticle catalysts for homogeneous electrophilic reactions in solution and the utilization of a continuous flow reactor. J. Am.Chem. Soc. 2010, 132, 16771–16773. [Google Scholar] [CrossRef]
- Maranon, L.A.; Martínez, M.M.; Sarandeses, L.A.; Gomez-Bengoa, E.; Sestel, J.P. Indium(III)-Catalyzed Synthesis of Benzo[b]furans by Intramolecular Hydroalkoxylation of ortho-Alkynylphenols: Scope and Mechanistic Insights. J. Org. Chem. 2018, 83, 7970–7980. [Google Scholar] [CrossRef]
- Rong, Z.; Gao, K.; Zhou, L.; Lin, J.; Qian, G. Facile synthesis of 2-substituted benzo[b]furans and indoles by copper-catalyzed intramolecular cyclization of 2-alkynyl phenols and tosylanilines. RSC Adv. 2019, 9, 17975–17978. [Google Scholar] [CrossRef]
- Boyer, A.; Isono, N.; Lackner, S.; Lautens, M. Domino rhodium(I)-catalysed reactions for the efficient synthesis of substituted benzofurans and indoles. Tetrahedron 2010, 66, 6468–6482. [Google Scholar] [CrossRef]
- Sarbajna, A.; Pandey, P.; Rahaman, S.M.W.; Singh, K.; Tyagi, A.; Dixneuf, P.H.; Bera, J.K. A Triflamide-Tethered N-Heterocyclic Carbene–Rhodium(I)Catalyst for Hydroalkoxylation Reactions: Ligand-Promoted Nucleophilic Activation of Alcohols. ChemCatChem 2017, 9, 1397–1401. [Google Scholar] [CrossRef]
- Rubio-Marqus, P.; Rivero-Crespo, M.A.; Leyva-Pérez, A.; Corma, A. Well-Defined Noble Metal Single Sites in Zeolites as an Alternative to Catalysis by Insoluble Metal Salts. J. Am. Chem. Soc. 2015, 137, 11832–11837. [Google Scholar] [CrossRef] [PubMed]
- Morozov, O.S.; Lunchev, A.V.; Bush, A.A.; Tukov, A.A.; Asachenko, A.F.; Khrustalev, V.N.; Zalesskiy, S.S.; Ananikov, V.P.; Nechaev, M.S. Expanded-Ring N-Heterocyclic Carbenes Efficiently Stabilized Gold(I) Cations, Leading to High Activity in p-Acid-Catalyzed Cyclizations. Chem. Eur. J. 2014, 20, 6162–6170. [Google Scholar] [CrossRef]
- Jacubert, M.; Hamze, A.; Provot, O.; Peyrat, J.-F.; Brion, J.-D.; Alami, M. p-Toluenesulfonic acid-mediated cyclization of o-(1-alkynyl)anisoles or thioanisoles: Synthesis of 2-arylsubstituted benzofurans and benzothiophenes. Tetrahedron Lett. 2009, 50, 3588–3592. [Google Scholar] [CrossRef]
- Sun, S.-X.; Wang, J.-J.; Xu, Z.-J.; Cao, L.-Y.; Shi, Z.-F.; Zhang, H.-L. Highly efficient heterogeneous synthesis of benzofurans under aqueous condition. Tetrahedron 2014, 70, 3798–3806. [Google Scholar] [CrossRef]
- Zhou, H.; Niu, J.-J.; Xu, J.-W.; Hu, S.-J. Novel Route to 2-Trifluoromethylated Benzofurans. Synth. Commun. 2009, 39, 716–732. [Google Scholar] [CrossRef]
- Hirner, J.J.; Faizi, D.J.; Blum, S.A. Alkoxyboration: Ring-Closing Addition of B−O σ Bonds across Alkynes. J. Am. Chem. Soc. 2014, 136, 4740–4745. [Google Scholar] [CrossRef]
- Issaian, A.; Tu, K.N.; Blum, S.A. Boron–Heteroatom Addition Reactions via Borylative Heterocyclization: Oxyboration, Aminoboration, and Thioboration. Acc. Chem. Res. 2017, 50, 2598–2609. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P.; Busto, E.; Lázaro-Milla, C. Photoinduced Gold-Catalyzed Domino C(sp) Arylation/Oxyarylation of TMS-Terminated Alkynols with Arenediazonium Salts. J. Org. Chem. 2017, 82, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Rong, M.-G.; Qin, T.-Z.; Zi, W. Rhenium-Catalyzed Intramolecular Carboalkoxylation and Carboamination of Alkynes for the Synthesis of C3-Substituted Benzofurans and Indoles. Org. Lett. 2019, 21, 5421–5425. [Google Scholar] [CrossRef] [PubMed]
- Furstner, A.; Davies, P.W. Heterocycles by PtCl2-Catalyzed Intramolecular Carboalkoxylation or Carboamination of Alkynes. J. Am. Chem. Soc. 2005, 127, 15024–15025. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, P.A.; Huynh, K.; Ozumerzifon, T.J.; Ferreira, E.M. Lewis Acid Mediated Vinylogous Additions of Enol Nucleophiles into an α,β-Unsaturated Platinum Carbene. Org. Lett. 2016, 18, 64–67. [Google Scholar] [CrossRef]
- Newman, S.G.; Aureggi, V.; Bryan, C.S.; Lautens, M. Intramolecular cross-coupling of gem-dibromoolefins: A mild approach to 2-bromo benzofused heterocycles. Chem. Commun. 2009, 5236–5238. [Google Scholar] [CrossRef]
- Kim, C.G.; Jun, J.G. An Efficient Total Synthesis of Mulberrofuran B and L. Bull. Korean Chem. Soc. 2015, 36, 2278–2283. [Google Scholar] [CrossRef]
- Damodar, K.; Kim, J.K.; Jun, J.G. Unified syntheses of gramniphenols F and G, cicerfuran, morunigrol C and its derivative. Tetrahedron Lett. 2016, 57, 1183–1186. [Google Scholar] [CrossRef]
- Rao, M.L.N.; Murty, V.N. Rapid Access to Benzofuran-Based Natural Products through a Concise Synthetic Strategy. Eur. J. Org. Chem. 2016, 2177–2186. [Google Scholar] [CrossRef]
- Rao, M.N.; Murty, V.N.; Nanda, S. Functional group manoeuvring for tuning stability and reactivity: Synthesis of cicerfuran, moracins (D, E, M) and chromene-fused benzofuran-based natural products. Org. Biomol. Chem. 2017, 15, 9415–9423. [Google Scholar] [CrossRef]
- Liu, X.; Astruc, D. Development of the Applications of Palladium on Charcoal in Organic Synthesis. Adv. Synth. Catal. 2018, 360, 3426–3459. [Google Scholar] [CrossRef]
- Savvidou, A.; Tzaras, D.I.; Koutoulogenis, G.S.; Theodorou, A.; Kokotos, C.G. Synthesis of Benzofuran and Indole Derivatives Catalyzed by Palladium on Carbon. Eur. J. Org. Chem. 2019, 3890–3897. [Google Scholar] [CrossRef]
- Lin, D.; Wang, L.; Yan, Z.Z.; Ye, J.; Hu, A.X.; Liao, H.D.; Liu, J.; Peng, J.M. Semi-synthesis, structural modification and biological evaluation of 5-arylbenzofuran neolignans. RSC Adv. 2018, 8, 34331–34342. [Google Scholar] [CrossRef]
- Rehan, M.; Nallagonda, R.; Das, B.G.; Meena, T.; Ghorai, P. Synthesis of Functionalized Benzo[b]furans via Oxidative Cyclization of o-Cinnamyl Phenols. J. Org. Chem. 2017, 82, 3411–3424. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiu, D.; Gu, R.; Wang, J.; Shi, J.; Li, Y. Aryne 1,2,3-Trifunctionalization with Aryl Allyl Sulfoxides. J. Am. Chem. Soc. 2016, 138, 10814–10817. [Google Scholar] [CrossRef]
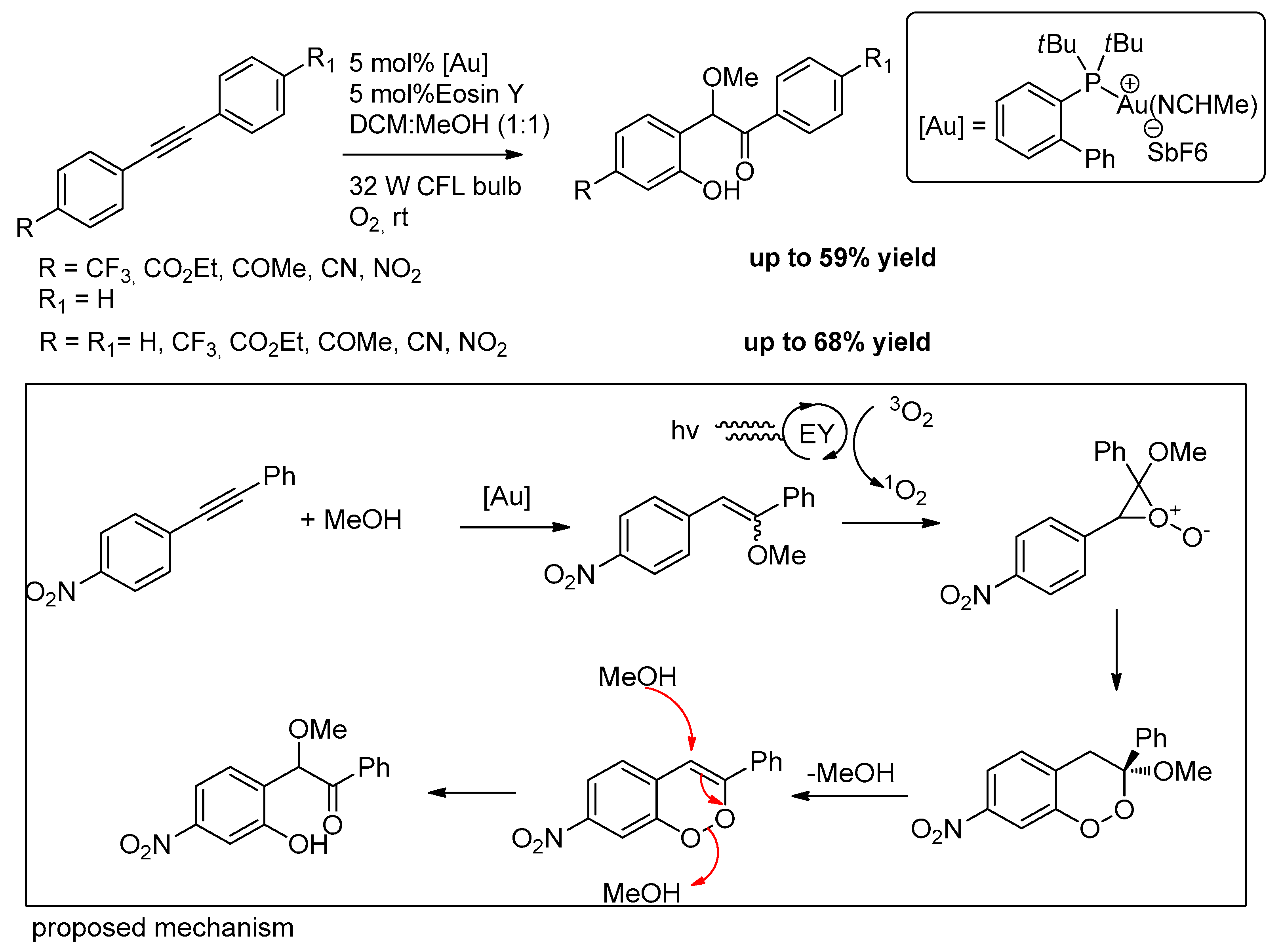
- Sancheti, S.P.; Akram, M.O.; Roy, R.; Bedi, V.; Kundu, S.; Patil, N.T. ortho-Oxygenative 1,2-Difunctionalization of Diarylalkynes under Merged Gold/Organophotoredox Relay Catalysis. Chem. Asian J. 2019, 14, 4601–4606. [Google Scholar] [CrossRef]
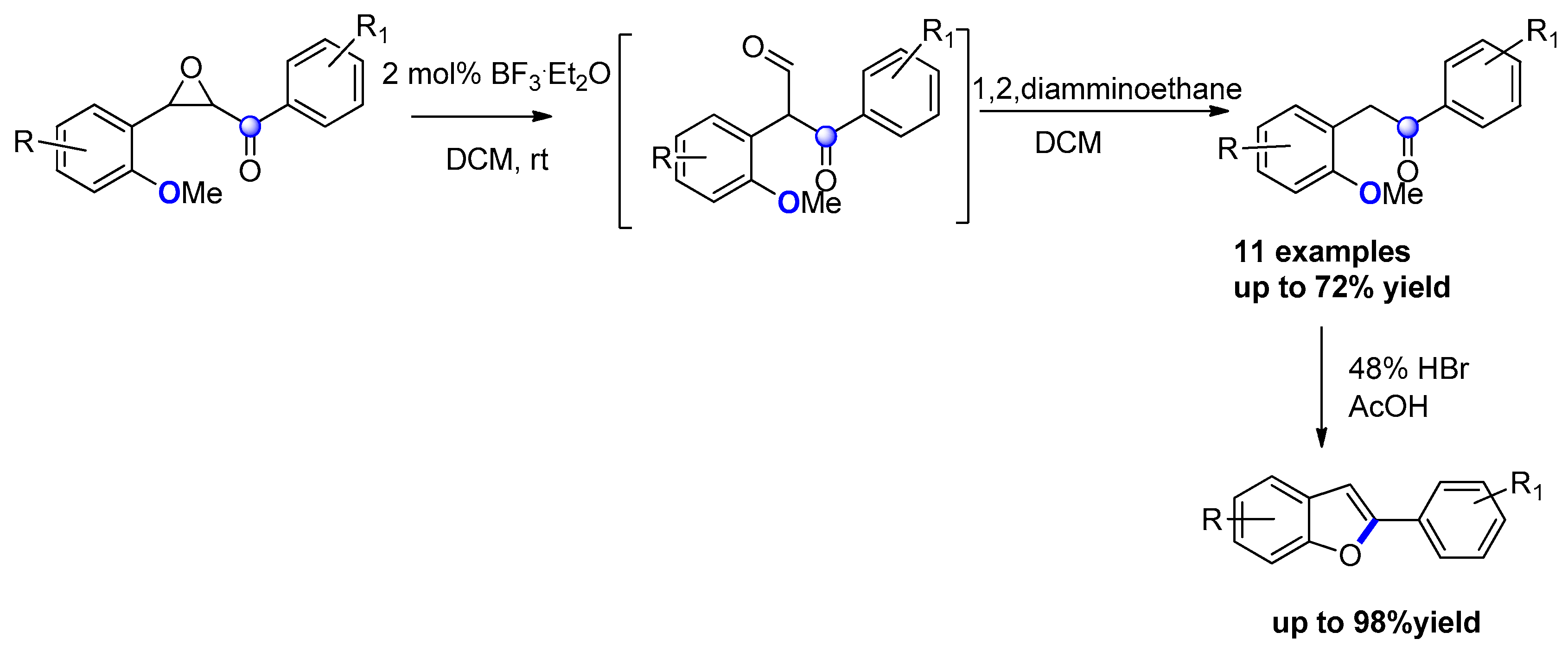
- Ruan, L.; Shi, M.; Mao, S.; Yu, L.; Yang, F.; Tang, J. An efficient approach to construct 2-arylbenzo[b]furans from 2-methoxychalcone epoxides. Tetrahedron 2014, 70, 1065–1070. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Xu, L.; Wang, Q.; Chen, J.; Ding, J.; Wu, H. Palladium-Catalyzed Addition of Potassium Aryltrifluoroborates to Aliphatic Nitriles: Synthesis of Alkyl Aryl Ketones, Diketone Compounds, and 2-Arylbenzo[b]furans. J. Org. Chem. 2013, 78, 5273–5281. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Liu, M.; Ding, J.; Chen, J.; Wu, H. Palladium-Catalyzed Reaction of Arylboronic Acids with Aliphatic Nitriles: Synthesis of Alkyl Aryl Ketones and 2-Arylbenzofurans. Synthesis 2013, 45, 2241–2244. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Su, W. Palladium-catalyzed tandem reaction of 2-hydroxyarylacetonitriles with sodium sulfinates: One-pot synthesis of 2-arylbenzofurans. Org. Biomol. Chem. 2014, 12, 4078–4083. [Google Scholar] [CrossRef]
- Skillinghaug, B.; Skold, C.; Rydfjord, J.; Svensson, F.; Behrends, M.; Savmarker, J.; Sjoberg, P.J.; Larhed, M. Palladium(II)-Catalyzed Desulfitative Synthesis of Aryl Ketones from Sodium Arylsulfinates and Nitriles: Scope, Limitations, and Mechanistic Studies. J. Org. Chem. 2014, 79, 12018–12032. [Google Scholar] [CrossRef]
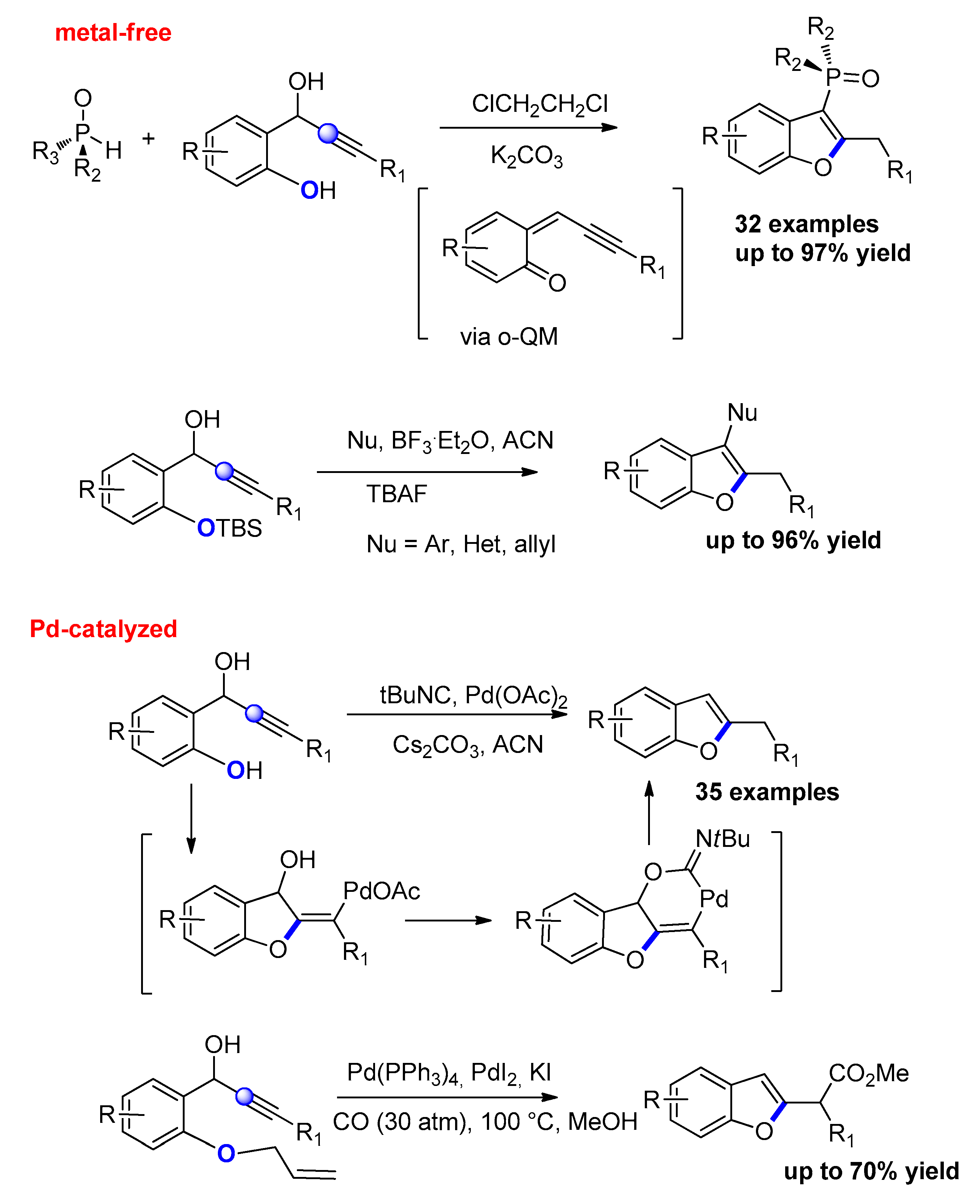
- Du, J.-Y.; Ma, Y.-H.; Yuan, R.-Q.; Xin, N.; Nie, S.-Z.; Ma, C.-L.; Li, C.-Z.; Zhao, C.-Q. Metal-Free One-Pot Synthesis of 3-Phosphinoylbenzofurans via Phospha-Michael Addition/Cyclization of H-Phosphine Oxides and in Situ Generated ortho-Quinone Methides. Org. Lett. 2018, 20, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.R.; Krishna, G.; Kavitha, N.; Latha, B.; Shin, D.-S. Access to 2,3-Disubstituted Benzofurans through One-Pot Acid-Catalyzed Nucleophilic Substitution/TBAF-Mediated Oxacycloisomerization. Eur. J. Org. Chem. 2012, 5381–5388. [Google Scholar] [CrossRef]
- Mancuso, R.; Gabriele, B. The sequential homobimetallic catalysis concept applied to the synthesis of benzofuran. Chem. Heterocycl. Compd. 2014, 50, 160–170. [Google Scholar] [CrossRef]
- Rajesh, M.; Thirupathi, N.; Reddy, T.J.; Kanojiya, S.; Reddy, M.S. Pd-Catalyzed Isocyanide Assisted Reductive Cyclization of 1-(2-Hydroxyphenyl)-propargyl Alcohols for 2-Alkyl/Benzyl Benzofurans and Their Useful Oxidative Derivatization. J. Org. Chem. 2015, 80, 12311–12320. [Google Scholar] [CrossRef]
- Chen, D.Y.-K.; Kang, Q.; Wu, T.R. Modular Synthesis of Polyphenolic Benzofurans, and Application in the Total Synthesis of Malibatol A and Shoreaphenol. Molecules 2010, 15, 5909–5927. [Google Scholar] [CrossRef]
- Kraus, G.A.; Gupta, V. A new synthetic strategy for the synthesis of bioactive stilbene dimers. A direct synthesis of amurensin H. Tetrahedron Lett. 2009, 50, 7180–7183. [Google Scholar] [CrossRef]
- Liu, W.; Chen, N.; Yang, X.; Li, L.; Li, C.-J. Dehydrative condensation of carbonyls with non-acidic ethylenes enabled by light: Synthesis of benzofurans. Chem. Commun. 2016, 52, 13120–13123. [Google Scholar] [CrossRef]
- Kanazawa, C.; Goto, K.; Terada, M. Phosphazene base-catalyzed intramolecular cyclization for efficient synthesis of benzofurans via carbon–carbon bond formation. Chem. Commun. 2009, 5248–5250. [Google Scholar] [CrossRef]
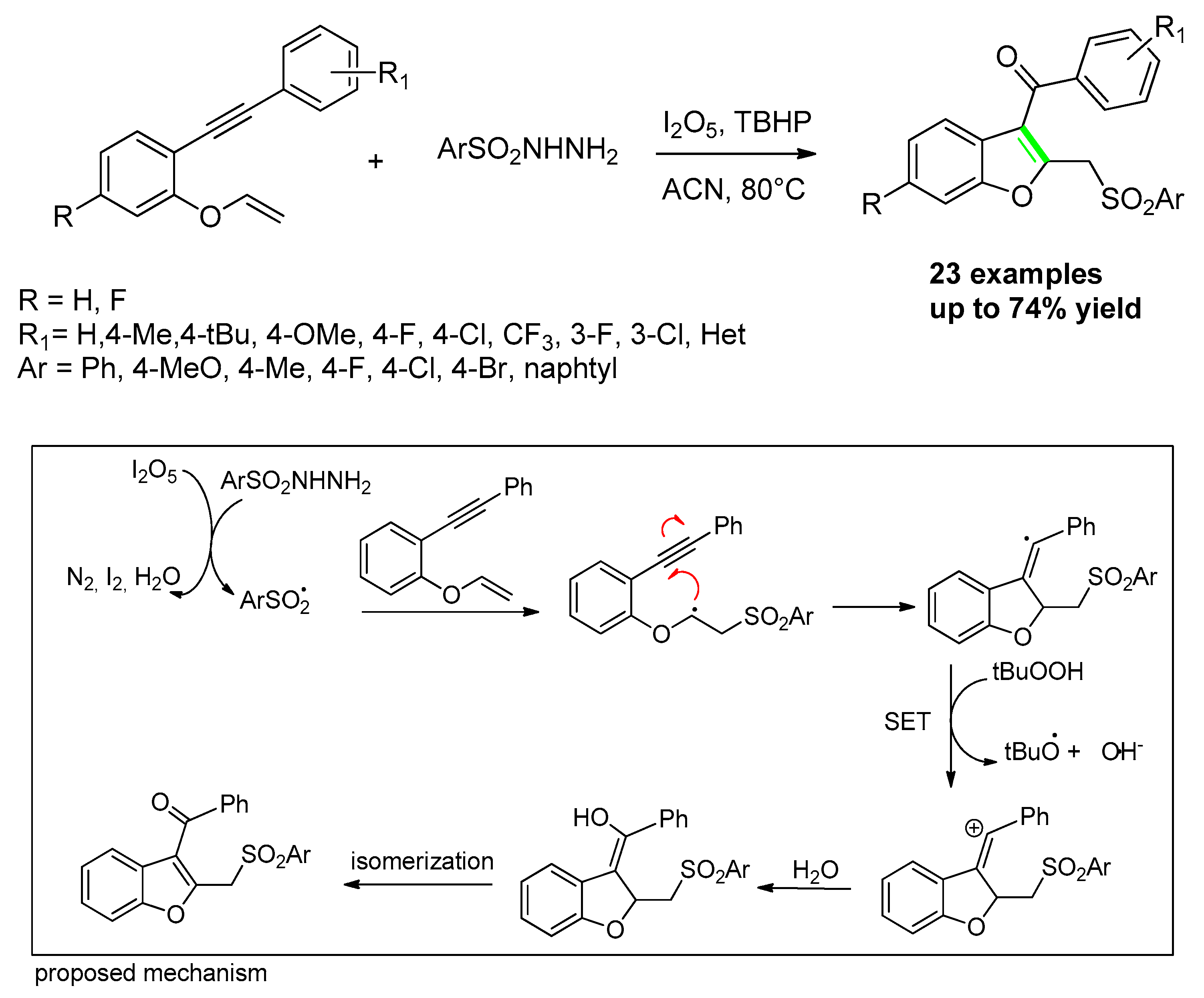
- Wang, L.; Zhang, Y.; Zhang, M.; Bao, P.; Lv, X.; Liu, H.-G.; Zhao, X.; Li, J.-S.; Luo, Z.; Wei, W. Metal-free I2O5-mediated oxidative synthesis of sulfonylated benzofurans through cyclization reaction of 1,6-enynes and arylsulfonylhydrazides. Tetrahedron. Lett. 2019, 60, 1845–1848. [Google Scholar] [CrossRef]
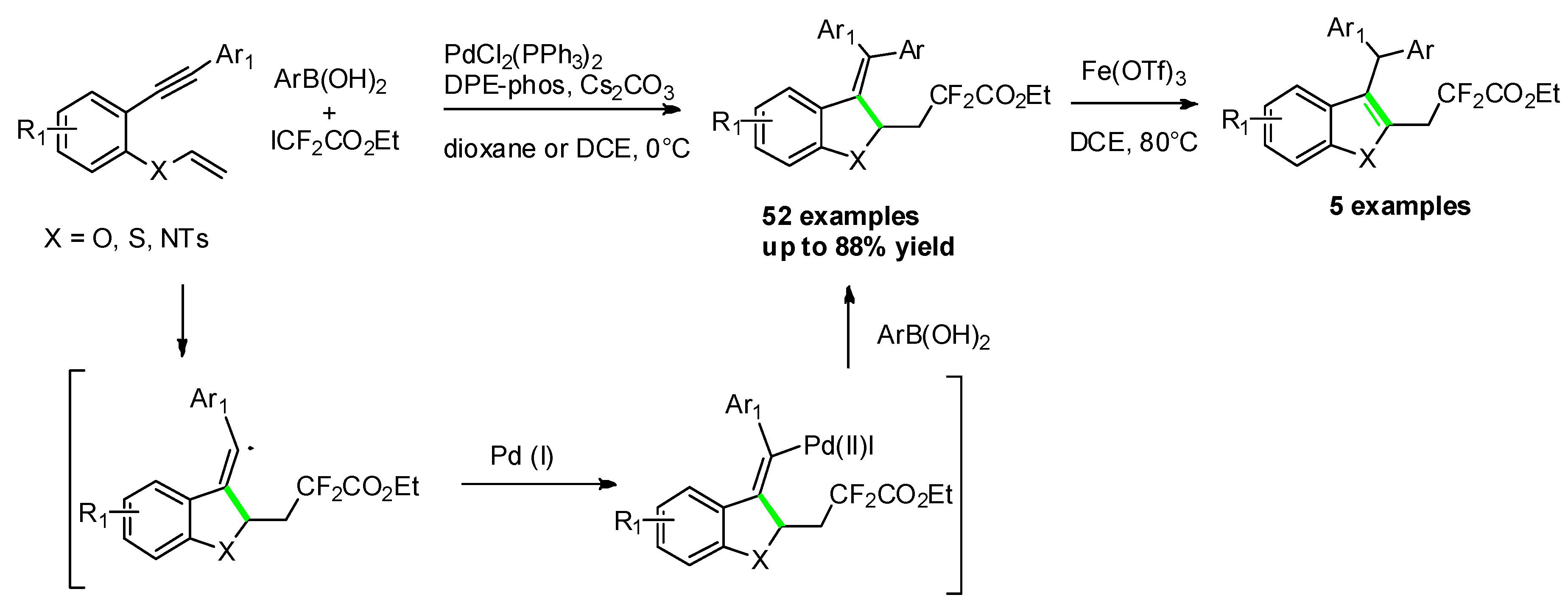
- Zhang, P.; Wang, C.; Cui, M.; Du, M.; Li, W.; Jia, Z.; Zhao, Q. Synthesis of Difluoroalkylated Benzofuran, Benzothiophene, and Indole Derivatives via Palladium-Catalyzed Cascade Difluoroalkylation and Arylation of 1,6-Enynes. Org. Lett. 2020, 22, 1149–1154. [Google Scholar] [CrossRef]
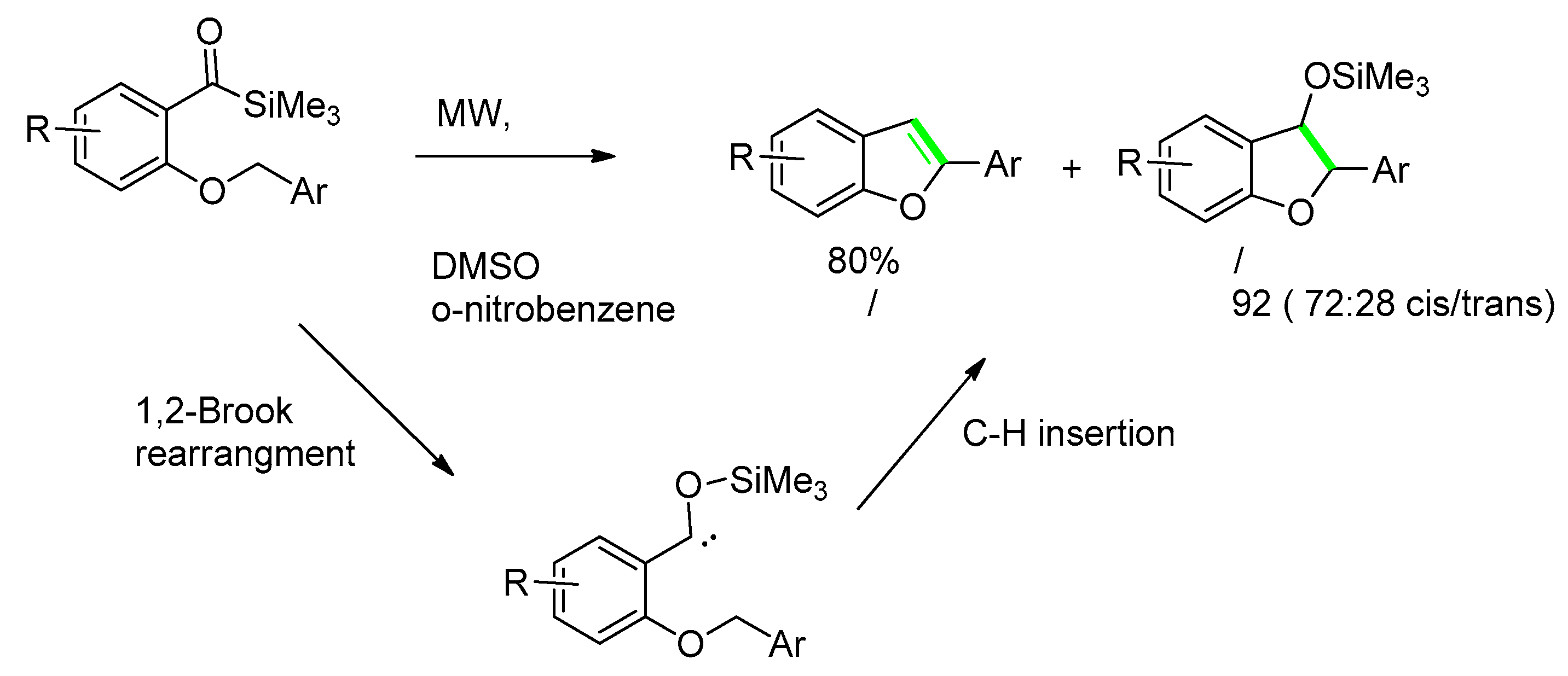
- Shen, Z.; Dong, V.M. Benzofurans Prepared by C-H Bond Functionalization with Acylsilanes. Angew. Chem. Int. Ed. 2009, 48, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Brook, A.G. Molecular rearrangements of organosilicon compounds. Acc. Chem. Res. 1974, 7, 77–84. [Google Scholar] [CrossRef]
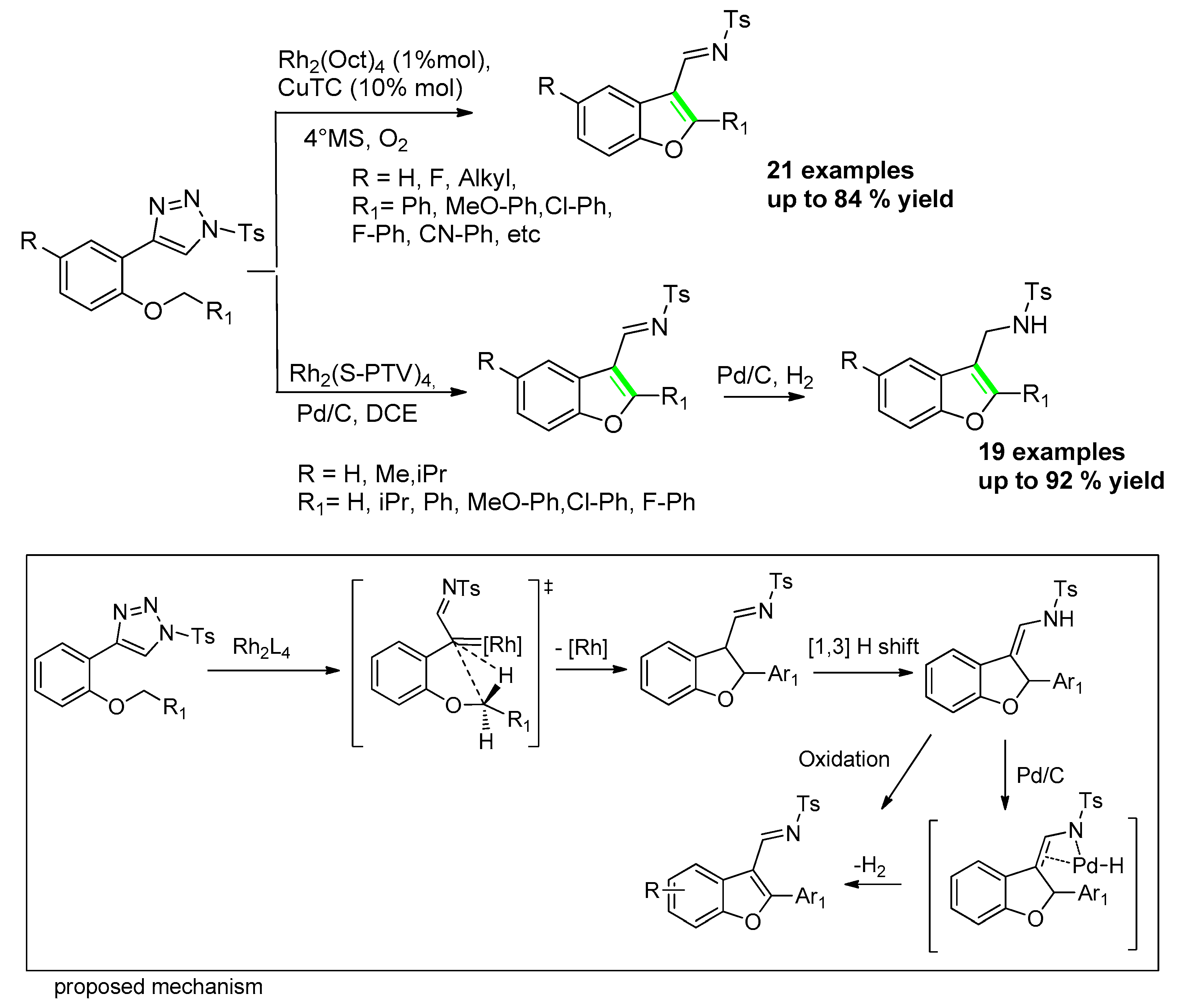
- Davies, H.M.L.; Alford, J.S. Reactions of metallocarbenes derived from N-sulfonyl-1, 2, 3-triazoles. Chem. Soc. Rev. 2014, 41, 5151–5162. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xia, X.-H.; Wang, Y.; Bora, P.P.; Kang, Q. Synthesis of Benzofurans via Tandem Rhodium-Catalyzed C(sp3)-H Insertion and Copper-Catalyzed Dehydrogenation. Adv. Synth. Catal. 2015, 357, 2089–2097. [Google Scholar] [CrossRef]
- Ma, X.; Wu, F.; Yi, X.; Wang, H.; Chen, W. One-pot synthesis of 2,3-disubstituted dihydrobenzofurans and benzofurans via rhodium-catalyzed intramolecular C–H insertion reaction. Chem. Commun. 2015, 51, 6862–6865. [Google Scholar] [CrossRef]
- Kim, K.; Kim, I. Total Synthesis of Diptoindonesin G via a Highly Efficient Domino Cyclodehydration/Intramolecular Friedel-Crafts Acylation/Regioselective Demethylation Sequence. Org. Lett. 2010, 12, 5314–5317. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, M.; Kim, I. Palladium-Catalyzed α-Arylation of Aryloxyketones for the Synthesis of 2,3-Disubstituted Benzofurans. J. Org. Chem. 2014, 79, 6153–6163. [Google Scholar] [CrossRef]
- Kim, I.; Choi, J. versatile approach to oligostilbenoid natural products synthesis of permethylated analogues of viniferifuran, malibatol A, and shoreaphenol. Org. Biomol. Chem. 2009, 7, 2788–2795. [Google Scholar] [CrossRef]
- Wang, H.-S.; Chan, C.-K.; Chang, M.-Y. Ga(OTf)3-mediated synthesis of substituted benzofurans. Tetrahedron 2016, 72, 5132–5141. [Google Scholar] [CrossRef]
- Umareddy, P.; Arava, V.R. Facile synthesis of 3-aryl benzofurans, 3-aryl benzothiophenes, 2-aryl indoles and their dimers. Synth. Commun. 2019, 49, 2156–2167. [Google Scholar] [CrossRef]
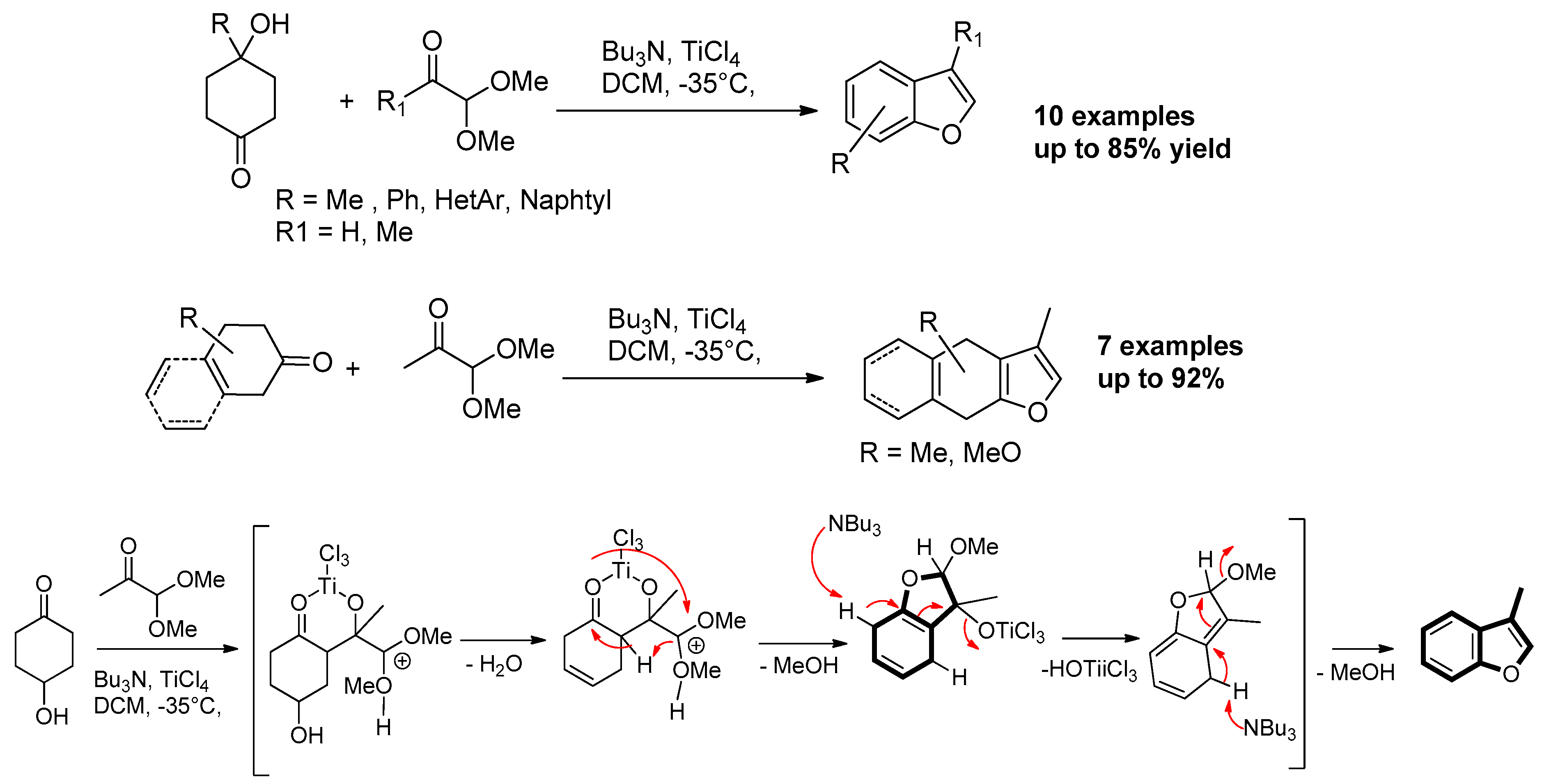
- Zhang, Q.; Luo, J.; Wang, B.; Xiao, X.; Gan, Z.; Tang, Q. Titanium tetrachloride promoted cyclodehydration of aryloxyketones: Facile synthesis of benzofurans and naphthofurans with high regioselectivity. Tetrahedron Lett. 2019, 60, 1337–1340. [Google Scholar] [CrossRef]
- Shibata, T.; Hashimoto, Y.-K.; Otsuka, M.; Tsuchikama, K.; Endo, K. Ir(III)-Catalyzed Room-Temperature Synthesis of Multisubstituted Benzofurans Initiated by C–H Activation of α-Aryloxy Ketones. Synlett 2011, 14, 2075–2079. [Google Scholar] [CrossRef]
- Qin, Y.; Zhan, J.-L.; Shan, T.-t.; Xu, T. Total synthesis of penta-Me amurensin H and diptoindonesin G featuring a Rh-catalyzed carboacylation/aromatization cascade enabled by C-C activation. Tetrahedron Lett. 2019, 60, 925–927. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Chen, K.-L.; Tyan, Y.-C.; Liang, C.-F.; Lin, P.-C. Synthesis of substituted 3-methylene-2,3-dihydrobenzofurans and 3-methylbenzofurans by rhodium (II)-catalyzed annulation. Tetrahedron 2015, 71, 6210–6218. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Zhang, Y.-S.; He, L.; Wei, Y.; Shi, M. Intramolecular Annulation of Aromatic Rings with N-Sulfonyl 1,2,3-Triazoles: Divergent Synthesis of 3-Methylene-2,3-Dihydrobenzofurans and 3-Methylene-2,3-Dihydroindoles. Chem. Commun. 2015, 51, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Geary, L.M.; Hultin, P.G. Modular Construction of 2-Substituted Benzo[b]furans from 1,2-Dichlorovinyl Ethers. Org. Lett. 2009, 11, 5478–5481. [Google Scholar] [CrossRef]
- Geary, L.M.; Hultin, P.G. 2-Substituted Benzo[b]furans from (E)-1,2-Dichlorovinyl Ethers and Organoboron Reagents: Scope and Mechanistic Investigations into the One-Pot Suzuki Coupling/Direct Arylation. Eur. J. Org. Chem. 2010, 5563–5573. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Li, P.; Wang, L. Palladium-Catalyzed Oxidative Cyclization of 3-Phenoxyacrylates: An Approach To Construct Substituted Benzofurans from Phenols. J. Org. Chem. 2011, 76, 4692–4696. [Google Scholar] [CrossRef]
- Deng, G.; Li, M.; Yu, K.; Liu, C.; Liu, Z.; Duan, S.; Chen, W.; Yang, X.; Zhang, H.; Walsh, P.J. Synthesis of Benzofuran Derivatives through Cascade Radical Cyclization/Intermolecular Coupling of 2-Azaallyls. Angew. Chem. Int. Ed. 2019, 58, 2826–2830. [Google Scholar] [CrossRef]
- Quan, Y.; Xie, Z. Iridium Catalyzed Regioselective Cage Boron Alkenylation of o-Carboranes via Direct Cage B–H Activation. J. Am. Chem. Soc. 2014, 136, 15513–15516. [Google Scholar] [CrossRef]
- Kumar, N.Y.P.; Bechtoldt, A.; Raghuvanshi, K.; Ackermann, L. Ruthenium(II)-Catalyzed Decarboxylative C−H Activation: Versatile Routes to meta-Alkenylated Arenes. Angew. Chem. Int. Ed. 2016, 55, 6929–6932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shrestha, R.; Hartwig, J.F.; Zhao, P. A decarboxylative approach for regioselective hydroarylation of alkynes. Nat. Chem. 2016, 8, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Sahoo, H.; Dana, S.; Baidya, M. Ruthenium(II)-Catalyzed Hydroarylation of Maleimides Using Carboxylic Acids as a Traceless Directing Group. Org. Lett. 2017, 19, 4138–4141. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Vasu, D.; Im, H.; Hong, S. Palladium(II)-Catalyzed Tandem Synthesis of Acenes Using Carboxylic Acids as Traceless Directing Groups. Angew. Chem. Int. Ed. 2016, 55, 8652–8655. [Google Scholar] [CrossRef] [PubMed]
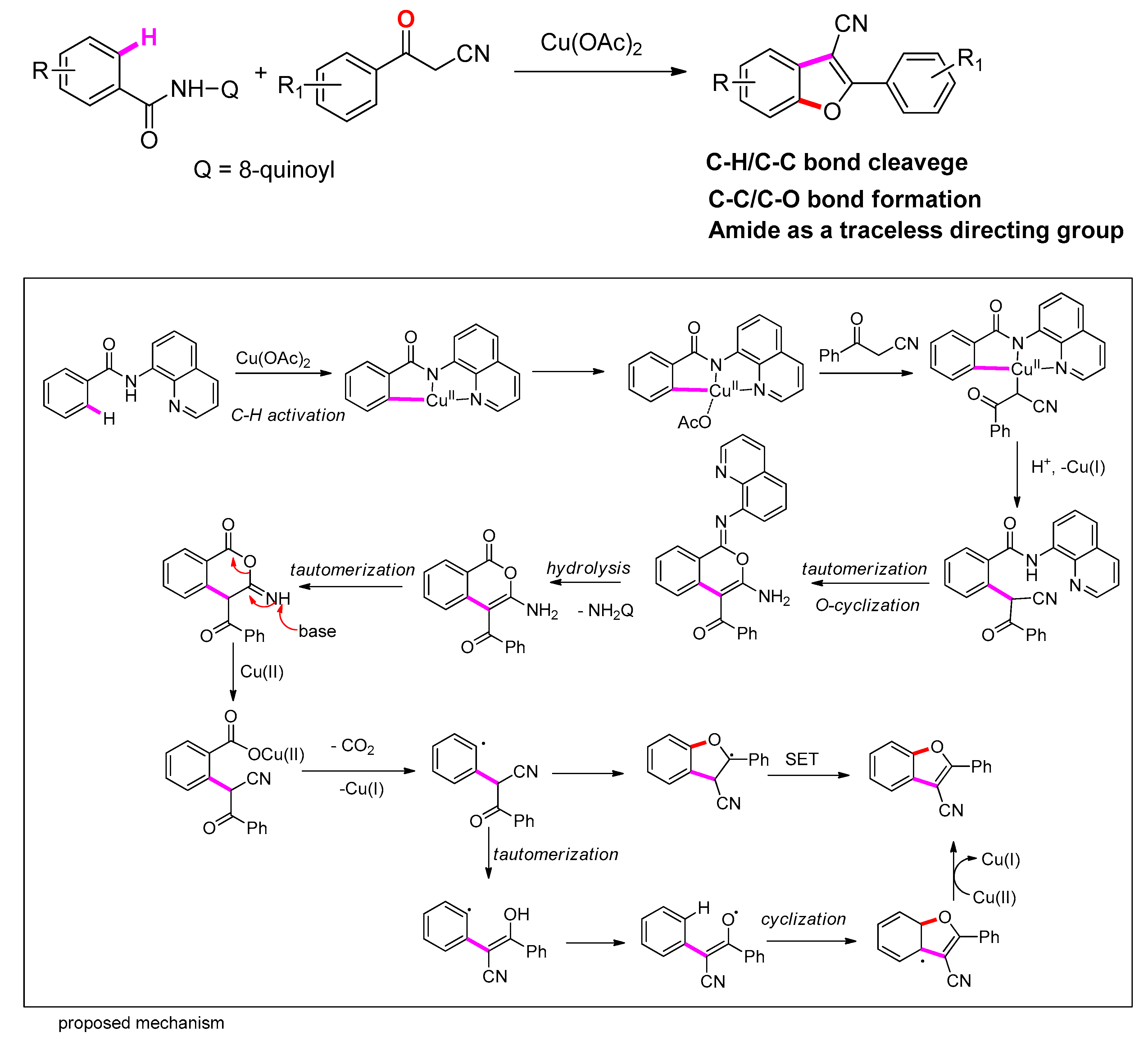
- Yu, S.; Lv, N.; Liu, Z.; Zhang, Y. Cu(II)-Mediated C-C/C-O Bond Formation via C-H/C-C Bond Cleavage: Access to Benzofurans Using Amide as a Traceless Directing Group. Adv. Synth. Catal. 2020, 362, 118–125. [Google Scholar] [CrossRef]
- Kalvacherla, B.; Batthula, S.; Balasubramanian, S.; Palakodety, R.K. Transition-Metal-Free Cyclization of Propargylic Alcohols with Aryne: Synthesis of 3-Benzofuranyl-2-oxindole and 3-Spirooxindole Benzofuran Derivatives. Org. Lett. 2018, 20, 3824–3828. [Google Scholar] [CrossRef]
- Yoshioka, E.; Tanaka, H.; Kohtani, S.; Miyabe, H. Straightforward Synthesis of Dihydrobenzofurans and Benzofurans from Arynes. Org. Lett. 2013, 15, 3938–3941. [Google Scholar] [CrossRef]
- Singh, G.; Goswami, P.; Sharma, S.; Anand, R.V. A One-Pot Approach to 2,3-Diarylbenzo[b]furans through N-Heterocyclic Carbene-Catalyzed 1,6-Conjugate Addition Followed by Acid Mediated Dehydrative Annulation. J. Org. Chem. 2018, 83, 10546–10554. [Google Scholar] [CrossRef]
- Liou, Y.-C.; Karanam, P.; Jang, Y.-J.; Lin, W. Synthesis of Functionalized Benzofurans from para-Quinone Methides via Phospha-1,6-Addition/O-Acylation/Wittig Pathway. Org. Lett. 2019, 21, 8008–8012. [Google Scholar] [CrossRef]
- Mattson, A.E.; Scheid, K.A. Nucleophilic Acylation of o-Quinone Methides: An Umpolung Strategy for the Synthesis of α-Aryl Ketones and Benzofurans. J. Am. Chem. Soc. 2007, 129, 4508–4509. [Google Scholar] [CrossRef]
- Rokita, S.E. Quinone Methides; Wiley: Hoboken, NJ, USA, 2009; p. 464. [Google Scholar]
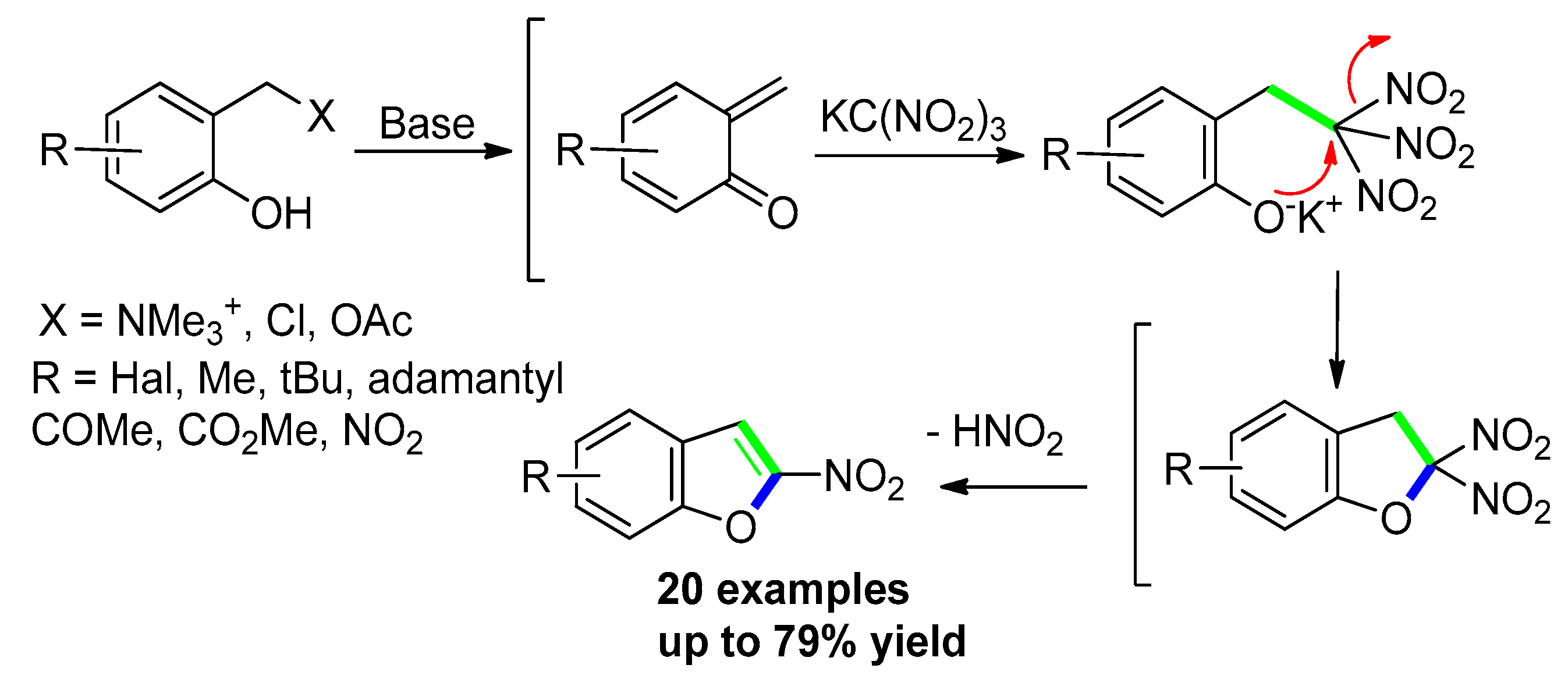
- Osyanin, V.A.; Osipov, D.V.; Demidov, M.R.; Klimochkin, Y.N. Potassium Trinitromethanide as a 1,1-Ambiphilic Synthon Equivalent: Access to 2-Nitroarenofurans. J. Org. Chem. 2014, 79, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Schevenels, F.; Marko, I.E. Efficient and Connective Assembly of Highly Functionalized Benzofurans Using o-Hydroxyphenones and Dichloroethylene. Org. Lett. 2012, 14, 1298–1301. [Google Scholar] [CrossRef] [PubMed]
- Syu, S.; Lee, Y.-T.; Jang, Y.-J.; Lin, W. Preparation of Functional Benzofurans, Benzothiophenes, and Indoles Using Ester, Thioester, and Amide via Intramolecular Wittig Reactions. Org. Lett. 2011, 13, 2971–2973. [Google Scholar] [CrossRef] [PubMed]
- Ramazani, A.; Mahyari, A.T.; Rouhani, M.; Rezaei, A. A novel three-component reaction of a secondary amine and a 2-hydroxybenzaldehyde derivative with an isocyanide in the presence of silica gel: An efficient one-pot synthesis of benzo[b]furan derivatives. Tetrahedron Lett. 2009, 50, 5625–5627. [Google Scholar] [CrossRef]
- Sontakke, G.S.; Pal, K.; Volla, C.M.R. Rh(II)-Catalyzed Denitrogenative Transannulation of N-Sulfonyl-1,2,3-triazolyl Cyclohexadienones for the Synthesis of Benzofurans and Cyclopropa[cd]indole-carbaldehydes. J. Org. Chem. 2019, 84, 12198–12208. [Google Scholar] [CrossRef] [PubMed]
- Moure, M.; SanMartin, J.R.; Dominguez, E. Benzofurans from Benzophenones and Dimethylacetamide: Copper-Promoted Cascade Formation of Furan O1-C2 and C2–C3 Bonds Under Oxidative Conditions. Angew. Chem. Int. Ed. 2012, 51, 3220–3224. [Google Scholar] [CrossRef]
- Yang, F.; Rauch, K.; Kettelhoit, K.; Ackermann, L. Aldehyde-Assisted Ruthenium(II)-Catalyzed C-H Oxygenations. Angew. Chem. Int. Ed. 2014, 53, 11285–11288. [Google Scholar] [CrossRef]
- Zhou, L.; Shi, Y.; Xiao, Q.; Liu, Y.; Ye, F.; Zhang, Y.; Wang, J. CuBr-Catalyzed Coupling of N-Tosylhydrazones and Terminal Alkynes: Synthesis of Benzofurans and Indoles. Org. Lett. 2011, 13, 968–971. [Google Scholar] [CrossRef]
- Xiao, Q.; Xia, Y.; Li, H.; Zhang, Y.; Wang, J. Coupling of N-Tosylhydrazones with Terminal Alkynes Catalyzed by Copper(I): Synthesis of Trisubstituted Allenes. Angew. Chem. Int. Ed. 2011, 50, 1114–1117. [Google Scholar] [CrossRef]
- Sivaraman, A.; Harmalkar, D.S.; Kang, J.; Choi, Y.; Lee, K. A protecting group-free divergent synthesis of natural benzofurans via one-pot synthesis of 2-bromo-6-hydroxybenzofurans. Org. Biomol. Chem. 2019, 17, 2153–2161. [Google Scholar] [CrossRef]
- Makarov, A.S.; Kekhvaeva, A.E.; Chalikidi, P.N.; Abaev, V.T.; Trushkov, I.V.; Uchuskin, M.G. A Simple Synthesis of Densely Substituted Benzofurans by Domino Reaction of 2-Hydroxybenzyl Alcohols with 2-Substituted Furans. Synthesis 2019, 51, 3747–3757. [Google Scholar] [CrossRef]
- Kim, H.J.; Seo, J.W.; Lee, M.H.; Shin, D.S.; Kim, H.B.; Cho, H.; Lee, B.S.; Chi, D.Y. New synthetic method for benzofurans from 2-(cyanomethyl)phenyl derivatives. Tetrahedron 2012, 68, 3942–3947. [Google Scholar] [CrossRef]
- Murai, M.; Okamoto, K.; Miki, K.; Ohe, K. Palladium-catalyzed three-component coupling reactions of 2-(cyanomethyl)phenol, aryl halides, and carbon monoxide. Tetrahedron 2015, 71, 4432–4437. [Google Scholar] [CrossRef]
- Shea, K.M. Palladium in Heterocyclic Chemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2007; p. 303. [Google Scholar]
- Heravi, M.M.; Sadjadi, S. Recent advances in the application of the Sonogashira method in the synthesis of heterocyclic compounds. Tetrahedron 2009, 65, 7761–7775. [Google Scholar] [CrossRef]
- Wang, J.-R.; Manabe, K. Hydroxyterphenylphoshine-Palladium Catalyst for Benzo[b]furan Synthesis from 2-Chlorophenols. Bifunctional Ligand Strategy for Cross-Coupling of Chloroarenes. J. Org. Chem. 2010, 75, 5340–5353. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ozawa, H.; Katsumata, H.; Akiyama, T.; Manabe, K. One-pot synthesis of 2,3-disubstituted benzofurans from 2-chlorophenols using palladium–dihydroxyterphenylphosphine catalyst. Tetrahedron Lett. 2018, 59, 3175–3178. [Google Scholar] [CrossRef]
- Bosiak, M.J. A Convenient Synthesis of 2-Arylbenzo[b]furans from Aryl Halides and 2-Halophenols by Catalytic One-Pot Cascade Method. ACS Catal. 2016, 6, 2429–2434. [Google Scholar] [CrossRef]
- Tréguier, B.; Rasolofonjatovo, E.; Hamze, A.; Provot, O.; Wdzieczak-Bakala, J.; Dubois, J.; Brion, J.-D.; Alami, M. Synthesis of 2-(1-phenylvinyl)benzofurans and 2-(1-phenylvinyl)indoles as antimitotic agents by a tandem palladium-assisted coupling-cyclization reaction between 1-phenylvinyl iodides and ortho-substituted arylalkynes. Eur. J. Org. Chem. 2011, 4868–4876. [Google Scholar] [CrossRef]
- Byers, P.M.; Rashid, J.I.; Mohamed, R.K.; Alabugin, I.V. Polyaromatic Ribbon/Benzofuran Fusion via Consecutive Endo Cyclizations of Enediynes. Org. Lett. 2012, 14, 6032–6035. [Google Scholar] [CrossRef]
- Song, T.; Yang, Y. Metal Nanoparticles Supported on Biomass-Derived Hierarchical Porous Heteroatom-Doped Carbon from Bamboo Shoots Design, Synthesis and Applications. Chem. Rec. 2019, 19, 1283–1301. [Google Scholar] [CrossRef]
- Ji, G.; Duan, Y.; Zhang, S.; Yang, Y. Synthesis of benzofurans from terminal alkynes and iodophenols catalyzed by recyclable palladium nanoparticles supported on N,O-dual doped hierarchical porous carbon under copper- and ligand-free conditions. Catalysis Today 2019, 330, 101–108. [Google Scholar] [CrossRef]
- Saha, D.; Dey, R.; Ranu, B.C. A Simple and Efficient One-Pot Synthesis of Substituted Benzo[b]furans by Sonogashira Coupling–5-endo-dig Cyclization Catalyzed by Palladium Nanoparticles in Water Under Ligand- and Copper-Free Aerobic Conditions. Eur. J. Org. Chem. 2010, 6067–6071. [Google Scholar] [CrossRef]
- Markina, N.A.; Chen, Y.; Larock, R.C. Efficient microwave-assisted one-pot three-component synthesis of 2,3-disubstituted benzofurans under Sonogashira conditions. Tetrahedron 2013, 69, 2701–2713. [Google Scholar] [CrossRef] [PubMed]
- Vo, D.D.; Elofsson, M. Synthesis of 4-Formyl-2-arylbenzofuran Derivatives by PdCl(C3H5)dppb-Catalyzed Tandem Sonogashira Coupling-Cyclization under Microwave Irradiation- Application to the Synthesis of Viniferifuran Analogues. Chem. Sel. 2017, 2, 6245–6248. [Google Scholar] [CrossRef]
- Chang, C.-W.; Chein, R.-J. Absolute Configuration of Anti-HIV-1 Agent (-)-Concentricolide: Total Synthesis of (+)-(R)-Concentricolide. J. Org. Chem. 2011, 76, 4154–4157. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.M.; Asha, S.; Menon, R.; Anilkumar, G. One-Pot Synthesis of Benzofurans via Cu–Catalyzed Tandem Sonogashira coupling-Cyclization Reactions. Chemistry Select 2019, 4, 5544–5547. [Google Scholar] [CrossRef]
- Mertens, M.A.S.; Thomas, F.; Noth, M.; Moegling, J.; El-Awaad, I.; Sauer, D.F.; Dhoke, G.V.; Xu, W.; Pich, A.; Herres-Pawlis, S.; et al. One-Pot Two-Step Chemoenzymatic Cascade for the Synthesis of a Bis-benzofuran Derivative. Eur. J. Org. Chem. 2019, 6341–6346. [Google Scholar] [CrossRef]
- Batail, N.; Bendjeriou, A.; Lomberget, T.; Barret, R.; Dufaud, V.; Djakovitch, L. First Heterogeneous Ligand-and Salt-Free Larock Indole Synthesis. Adv. Synth. Catal. 2009, 351, 2055–2062. [Google Scholar] [CrossRef]
- Singh, C.; Prakasham, A.P.; Ghosh, P. Palladium Acyclic Diaminocarbene (ADC) Triflate Complexes as Effective Precatalysts for the Hiyama Alkynylation/Cyclization Reaction Yielding Benzofuran Compounds: Probing the Influence of the Triflate Co-Ligand in the One-Pot Tandem Reaction. Chem. Sel. 2019, 4, 329–336. [Google Scholar] [CrossRef]
- Yuan, H.; Bi, K.-J.; Li, B.; Yue, R.-C.; Ye, J.; Shen, Y.-H.; Shan, L.; Jin, H.-Z.; Sun, Q.-Y.; Zhang, W.-D. Construction of 2-Substituted-3-Functionalized Benzofurans via Intramolecular Heck Coupling: Application to Enantioselective Total Synthesis of Daphnodorin B. Org. Lett. 2013, 4742–4745. [Google Scholar] [CrossRef]
- Vasconcelos, S.N.S.; de Oliveira, I.M.; Shamim, A.; Zukerman-Schpector, J.; Pimenta, D.C.; Stefani, H.A. Stereoselective Oxa-Michael Addition of Tyrosine to Propargyl Aldehyde/Esters: Formation of Benzofurans and Flavones. Adv. Synth. Catal. 2019, 361, 4243–4254. [Google Scholar] [CrossRef]
- Wan, J.-P.; Wang, H.; Liu, Y.; Ding, H. Synthesis of 2-Vinylbenzofurans via the Copper-Catalyzed Multicomponent Reactions Involving an Oxa-Michael/Arylation/Vinylation Cascade. Org. Lett. 2014, 16, 5160–5163. [Google Scholar] [CrossRef] [PubMed]
- Tabolin, A.A.; Ioffe, S.L. Rearrangement of N-Oxyenamines and Related Reactions. Chem. Rev. 2014, 114, 5426–5476. [Google Scholar] [CrossRef] [PubMed]
- Contiero, F.; Jones, K.M.; Matts, E.A.; Porzelle, A.; Tomkinson, N.C.O. Direct Preparation of Benzofurans from O-Arylhydroxylamines. Synlett 2009, 3003–3006. [Google Scholar] [CrossRef]
- Maimone, T.J.; Buchwald, S.L. Pd-Catalyzed O-Arylation of Ethyl Acetohydroximate: Synthesis of O-Arylhydroxylamines and Substituted Benzofurans. J. Am. Chem. Soc. 2010, 132, 9990–9991. [Google Scholar] [CrossRef]
- Ghosh, R.; Stridfeldt, E.; Olofsson, B. Metal-Free One-Pot Synthesis of Benzofurans. Chem. Eur. J. 2014, 20, 8888–8892. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.-H.; Li, W.; Lin, C.-D.; Zhang, L.-B.; Wen, L.-R. N-Phenoxyamides as Multitasking Reagents: Base-Controlled Selective Construction of Benzofurans or Dihydrobenzofuro[2,3-d]oxazoles. J. Org. Chem. 2019, 84, 8523–8530. [Google Scholar] [CrossRef]
- Yan, D.; Jiang, H.; Sun, W.; Wei, W.; Zhao, J.; Zhang, X.; Wu, Y.-D. Synthesis of Benzofurans and Benzoxazoles through a [3,3]- Sigmatropic Rearrangement: O−NHAc as a Multitasking Functional Group. Org. Process Res. Dev. 2019, 23, 1646–1653. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P.; Quirs, M.T.; Lopez, R.; Menndez, M.I.; Sochacka-C’wikła, A. Unveiling the Reactivity of Propargylic Hydroperoxides under Gold Catalysis. J. Am. Chem. Soc. 2013, 135, 898–905. [Google Scholar] [CrossRef]
- Chen, W.; Liu, F.-X.; Gong, W.; Zhou, Z.; Gao, H.; Shi, J.; Wu, B.; Yi, W. Hydroxyl Group-Prompted and Iridium(III)-Catalyzed Regioselective C−H Annulation of N-phenoxyacetamides with Propargyl Alcohols. Adv. Synth. Catal. 2018, 360, 2470–2475. [Google Scholar] [CrossRef]
- Yi, W.; Chen, W.; Liu, F.-X.; Zhong, Y.; Wu, D.; Zhou, Z.; Gao, H. Rh(III)-Catalyzed and Solvent-Controlled Chemoselective Synthesis of Chalcone and Benzofuran Frameworks via Synergistic Dual Directing Groups Enabled Regioselective C−H Functionalization: A Combined Experimental and Computational Study. ACS Catal. 2018, 8, 9508–9519. [Google Scholar] [CrossRef]
- Pan, J.-L.; Liu, C.; Chen, C.; Liu, T.-Q.; Wang, M.; Sun, Z.; Zhang, S.-Y. Dual Directing-Groups-Assisted Redox-Neutral Annulation and Ring Opening of N-Aryloxyacetamides with 1-Alkynylcyclobutanols via Rhodium(III)-Catalyzed C−H/C−C Activations. Org. Lett. 2019, 21, 2823–2827. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-T.; Nan, W.-H.; Luo, Q.-L. Metal-free sequential reaction via a propargylation, annulation and isomerization sequence for the one-pot synthesis of 2,3-disubstituted benzofurans. RSC Adv. 2014, 4, 34774–34779. [Google Scholar] [CrossRef]
- Baire, B.; Tharra, P. Regioselective, Cascade [3 + 2] Annulation of β-Naphthols (Resorcinols) with Z-enoate Propargylic alcohols: A Novel entry into Complex Naphtho(benzo)furans. Chem. Comm. 2016, 52, 14290–14293. [Google Scholar] [CrossRef]
- Zhang, D.; Man, J.; Chen, Y.; Yin, L.; Zhong, J.; Zhang, Q.-F. Synthesis of poly-functionalized benzofurans via one-pot domino oxidation/[3 + 2] cyclization reactions of a hydroquinone ester and ynamides. RSC Adv. 2019, 9, 12567. [Google Scholar] [CrossRef]
- Kuram, M.R.; Bhanuchandra, M.; Sahoo, A.K. Direct Access to Benzo[b]furans through Palladium-Catalyzed Oxidative Annulation of Phenols and Unactivated Internal Alkynes. Angew. Chem. Int. Ed. 2013, 125, 4705–4710. [Google Scholar] [CrossRef]
- Wang, S.; Li, P.; Yu, L.; Wang, L. Sequential and one-pot reactions of phenols with bromoalkynes for the synthesis of (Z)-2-bromovinyl phenylethers and benzo[b]furans. Org. Lett. 2011, 13, 5968–5971. [Google Scholar] [CrossRef]
- Maji, A.; Reddi, Y.; Sunoj, R.B.; Maiti, D. Mechanistic Insights on Orthogonal Selectivity in Heterocycle Synthesis. ACS Catal. 2018, 8, 10111–10118. [Google Scholar] [CrossRef]
- Agasti, S.; Maity, S.; Szabo, K.J.; Maiti, D. Palladium-Catalyzed Synthesis of 2,3-Disubstituted Benzofurans: An Approach Towards the Synthesis of Deuterium Labeled Compounds. Adv. Synth. Catal. 2015, 357, 2331–2338. [Google Scholar] [CrossRef]
- Agasti, S.; Sharma, U.; Naveen, T.; Maiti, D. Orthogonal selectivity with cinnamic acids in3-substituted benzofuran synthesis through C–H olefination of phenols. Chem. Commun. 2015, 51, 5375–5378. [Google Scholar] [CrossRef]
- Yorimitsu, H. Cascades of Interrupted Pummerer Reaction-Sigmatropic Rearrangement. Chem. Rec. 2017, 17, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Kobatake, T.; Fujino, D.; Yoshida, S.; Yorimitsu, H.; Oshima, K. Synthesis of 3-Trifluoromethylbenzo[b]furans from Phenols via Direct Ortho Functionalization by Extended Pummerer Reaction. J. Am. Chem. Soc. 2010, 132, 11838–11840. [Google Scholar] [CrossRef] [PubMed]
- Ookubo, Y.; Wakamiya, A.; Yorimitsu, H.; Osuka, A. Synthesis of a Library of Fluorescent 2-Aryl-3-trifluoromethylnaphthofurans from Naphthols by Using a Sequential Pummerer-Annulation/Cross-Coupling Strategy and their Photophysical Properties. Chem. Eur. J. 2012, 18, 12690–12697. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Yorimitsu, H.; Osuka, A. Two-Step, Practical, and Diversity-Oriented Synthesis of Multisubstituted Benzofurans from Phenols through Pummerer Annulation Followed by Cross-coupling. Bull. Chem. Soc. Jpn. 2014, 87, 1349–1366. [Google Scholar] [CrossRef]
- Murakami, K.; Yorimitsu, H.; Osuka, A. Practical, Modular, and General Synthesis of Benzofurans through Extended Pummerer Annulation/Cross-Coupling Strategy. Angew. Chem. Int. Ed. 2014, 53, 7510–7513. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Hori, M.; Yanagi, T.; Murakami, K.; Nogi, K.; Yorimitsu, H. Sigmatropic Dearomatization/Defluorination Strategy for C-F Transformation: Synthesis of Fluorinated Benzofurans from Polyfluorophenols. Angew. Chem. Int. Ed. 2018, 57, 14230–14234. [Google Scholar] [CrossRef]
- Yang, K.; Pulis, A.P.; Perry, G.J.P.; Procter, D.J. Transition-Metal-Free Synthesis of C3-Arylated Benzofurans from Benzothiophenes and Phenols. Org. Lett. 2018, 20, 7498–7503. [Google Scholar] [CrossRef]
- Tramutola, F.; Chiummiento, L.; Funicello, M.; Lupattelli, P. Practical and efficient ipso-iodination of arylboronic acids via KF/I2 System. Tetrahedron Lett. 2015, 56, 1122–1123. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Q.; Luo, J.; Gan, Z.; Jiang, W.; Tang, Q. One-Step Regioselective Synthesis of Benzofurans from Phenols and α-Haloketones. Molecules 2019, 24, 2187. [Google Scholar] [CrossRef]
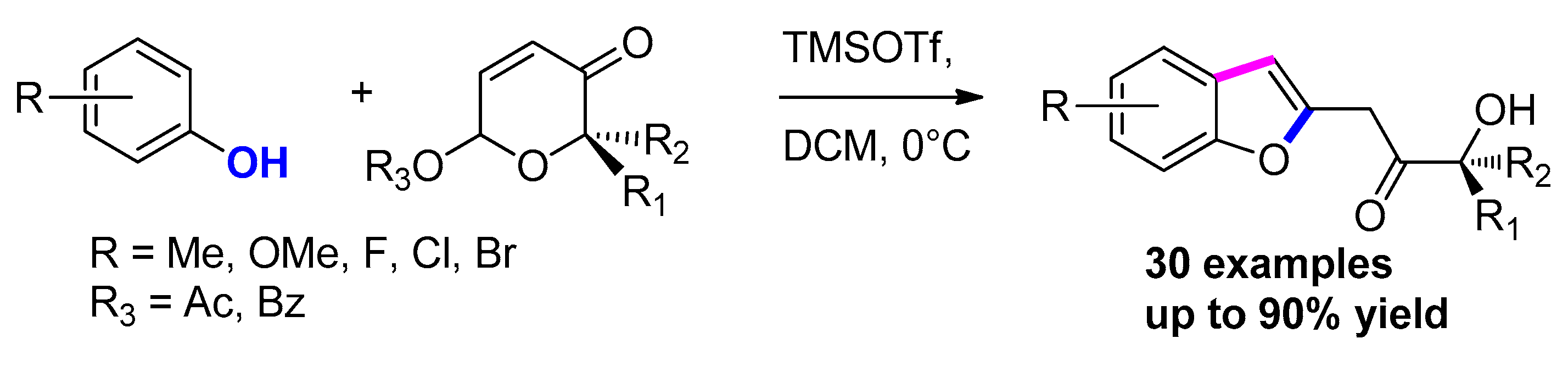
- Bankar, S.K.; Mathew, J.; Ramasastry, S.S.V. Synthesis of benzofurans via an acid catalyzed transacetalisation/Fries-type O–C rearrangement/Michael addition/ring-opening aromatisation cascade of β-pyrones. Chem. Commun. 2016, 52, 5569–5572. [Google Scholar] [CrossRef]
- Porcu, S.; Demuro, S.; Luridiana, A.; Cocco, A.; Frongia, A.; Aitken, D.J.; Charnay-Pouget, F.; Guillot, R.; Sarais, G.; Secci, F. Brønsted Acid Mediated Cascade Reaction To Access 3-(2-Bromoethyl)benzofurans. Org. Lett. 2018, 20, 7699–7702. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Kwon, K.-H.; Yi, C.S. Dehydrative C−H Alkylation and Alkenylation of Phenols with Alcohols: Expedient Synthesis for Substituted Phenols and Benzofurans. J. Am. Chem. Soc. 2012, 134, 7325–7328. [Google Scholar] [CrossRef] [PubMed]
- Honey, M.A.; Blake, A.J.; Campbell, I.B.; Judkins, B.D.; Moody, C.J. One-pot synthesis of N-methylindoles from N-methylanilines and of benzofurans from phenols using transition-metal carbene X–H insertion reactions. Tetrahedron 2009, 65, 8995–9001. [Google Scholar] [CrossRef]
- Sun, P.; Gao, S.; Yang, C.; Guo, S.; Lin, A.; Yao, H. Controllable Rh(III)-Catalyzed Annulation between Salicylaldehydes and Diazo Compounds: Divergent Synthesis of Chromones and Benzofurans. Org. Lett. 2016, 18, 6464–6467. [Google Scholar] [CrossRef] [PubMed]
- Sakhabutdinova, G.N.; Raskil’dina, G.Z.; Zlotskii, S.S.; Sultanova, R.M. Rhodium(II)-Catalyzed Reaction of Salicylaldehyde and Its Derivatives with Diazocarbonyl Compounds. Russ. J. Organic Chem. 2018, 54, 1772–1776. [Google Scholar] [CrossRef]
- Wu, F.; Bai, R.; Gu, Y. Synthesis of Benzofurans from Ketones and 1,4-Benzoquinones. Adv. Synth. Catal. 2016, 358, 2307–2316. [Google Scholar] [CrossRef]
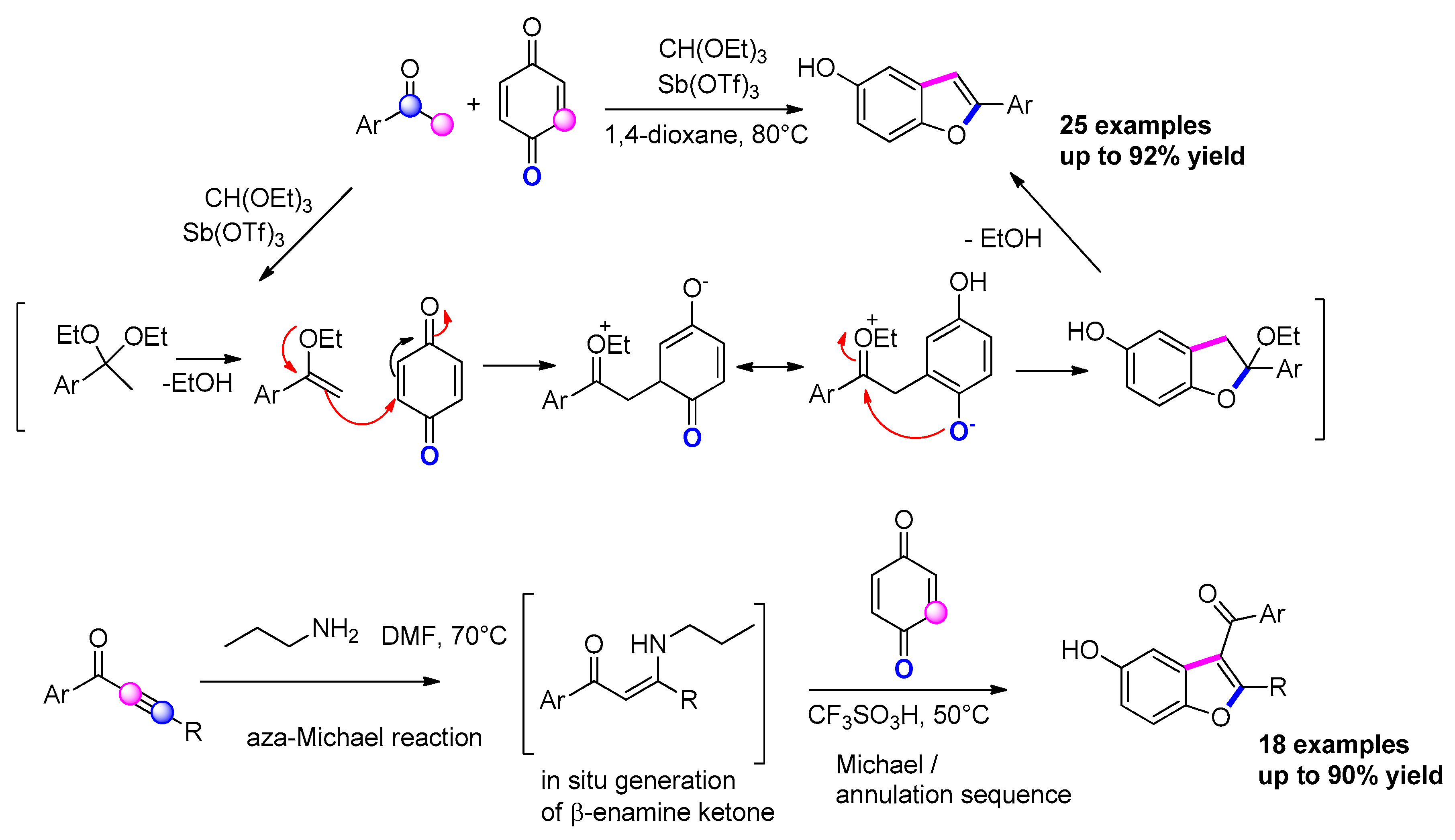
- Cui, H.-L.; Deng, H.-Q.; Lei, J.-J. Metal-free one-pot synthesis of benzofurans with ynones and quinones through aza-Michael/Michael/annulation sequence. Tetrahedron 2017, 73, 7282–7290. [Google Scholar] [CrossRef]
- Maeno, Z.; Yamamoto, M.; Mitsudome, T.; Mizugaki, T.; Jitsukawa, K. Efficient Synthesis of Benzofurans via Cross-Coupling of Catechols with Hydroxycoumarins Using O2 as an Oxidant Catalyzed by AlPO4-Supported Rh Nanoparticle. Chem. Sel. 2019, 4, 11394–11397. [Google Scholar] [CrossRef]
- Zhang, C.; Zhen, L.; Yao, Z.; Jiang, L. Iron(III)-Catalyzed Domino Claisen Rearrangement/Regio- and Chemoselective Aerobic Dehydrogenative Cyclization of β-Naphthyl-Substituted-Allenylmethyl Ether. Org. Lett. 2019, 21, 955–959. [Google Scholar] [CrossRef]
- Tanabe, Y.; Mitarai, K.; Higashi, T.; Misaki, T.; Nishii, Y. Efficient one-step synthesis of trialkylsubstituted 2(5H)-furanones utilizing direct Ti-crossed aldol condensation and its application to the straightforward synthesis of (R)-mintlactone and (R)-menthofuran. Chem. Commun. 2002, 2542–2543. [Google Scholar] [CrossRef]
- Rashid, S.; Bhat, B.A.; Mehta, G. A vicarious, one-pot synthesis of benzo- and naphthofurans: Applications to the syntheses of stereumene B and paeoveitols. Tetrahedron Lett. 2019, 60, 1122–1125. [Google Scholar] [CrossRef]
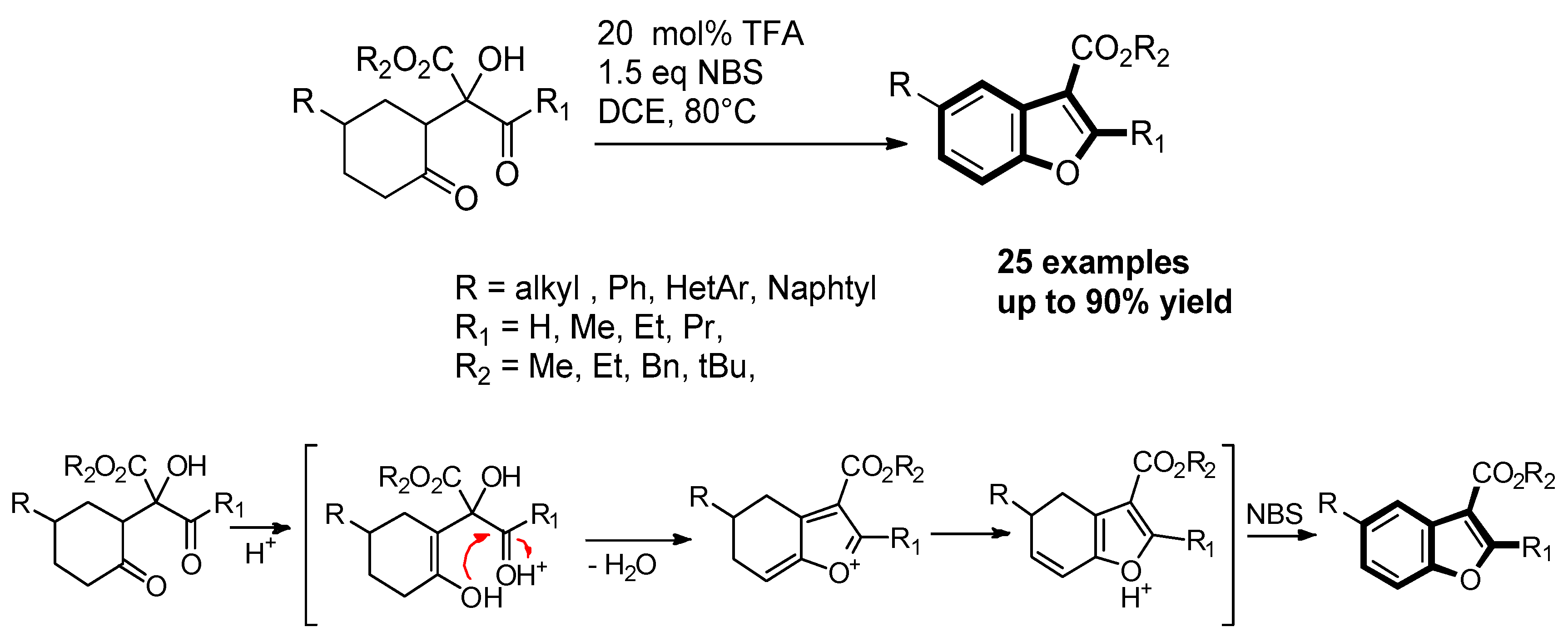
- Qiang, S.; Haixuan, L. De novo synthesis of benzofurans via trifluoroacetic acid catalyzed cyclization/oxidative aromatization cascade reaction of 2-hydroxy-1,4-diones. Org. Biomol. Chem. 2019, 17, 7547–7551. [Google Scholar] [CrossRef]
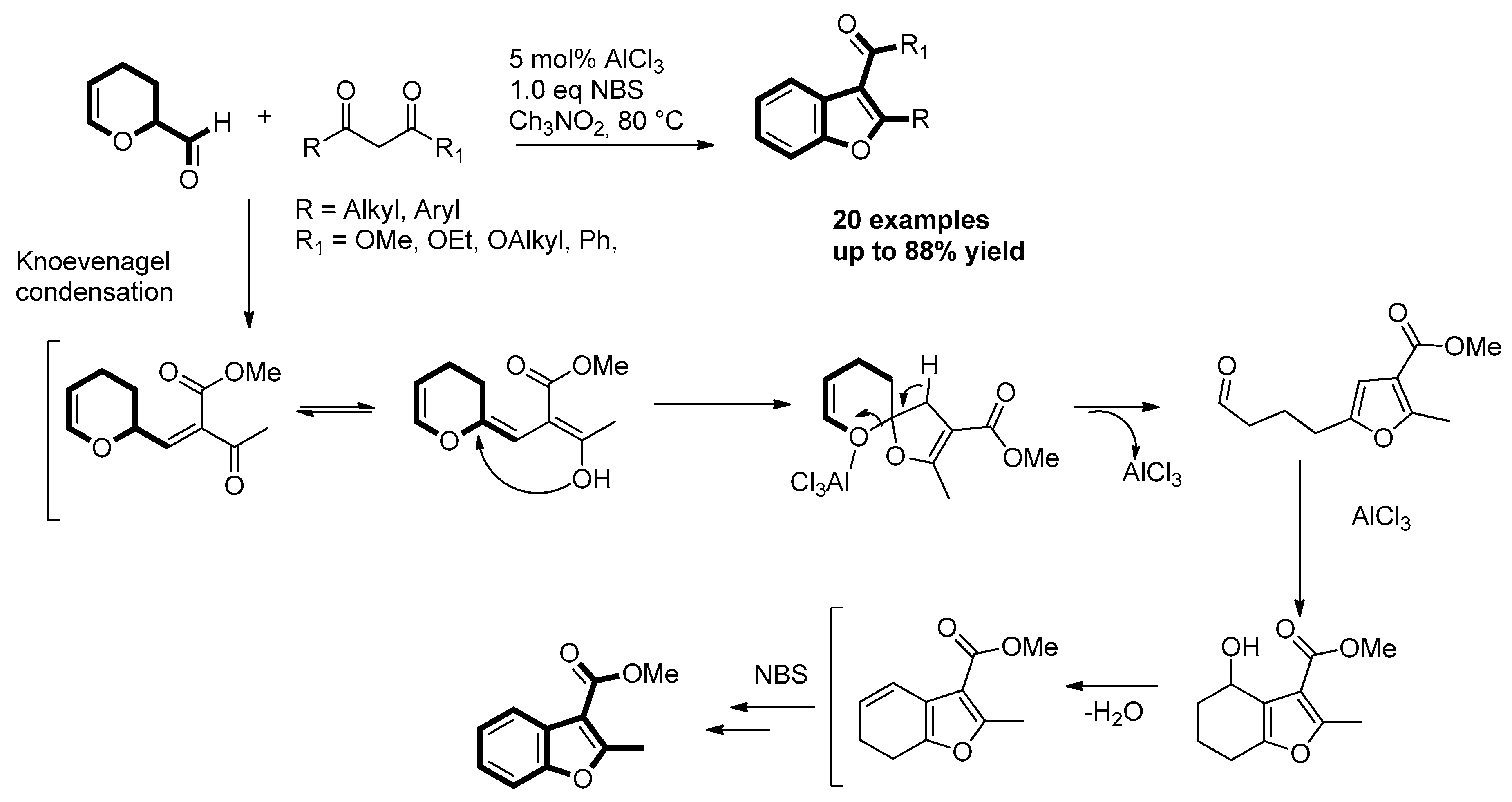
- Huang, W.; Xu, J.; Liu, C.; Chen, Z.; Gu, Y. Lewis Acid-Catalyzed Synthesis of Benzofurans and 4,5,6,7-Tetrahydrobenzofurans from Acrolein Dimer and 1,3-Dicarbonyl Compounds. J. Org. Chem. 2019, 84, 2941–2950. [Google Scholar] [CrossRef] [PubMed]
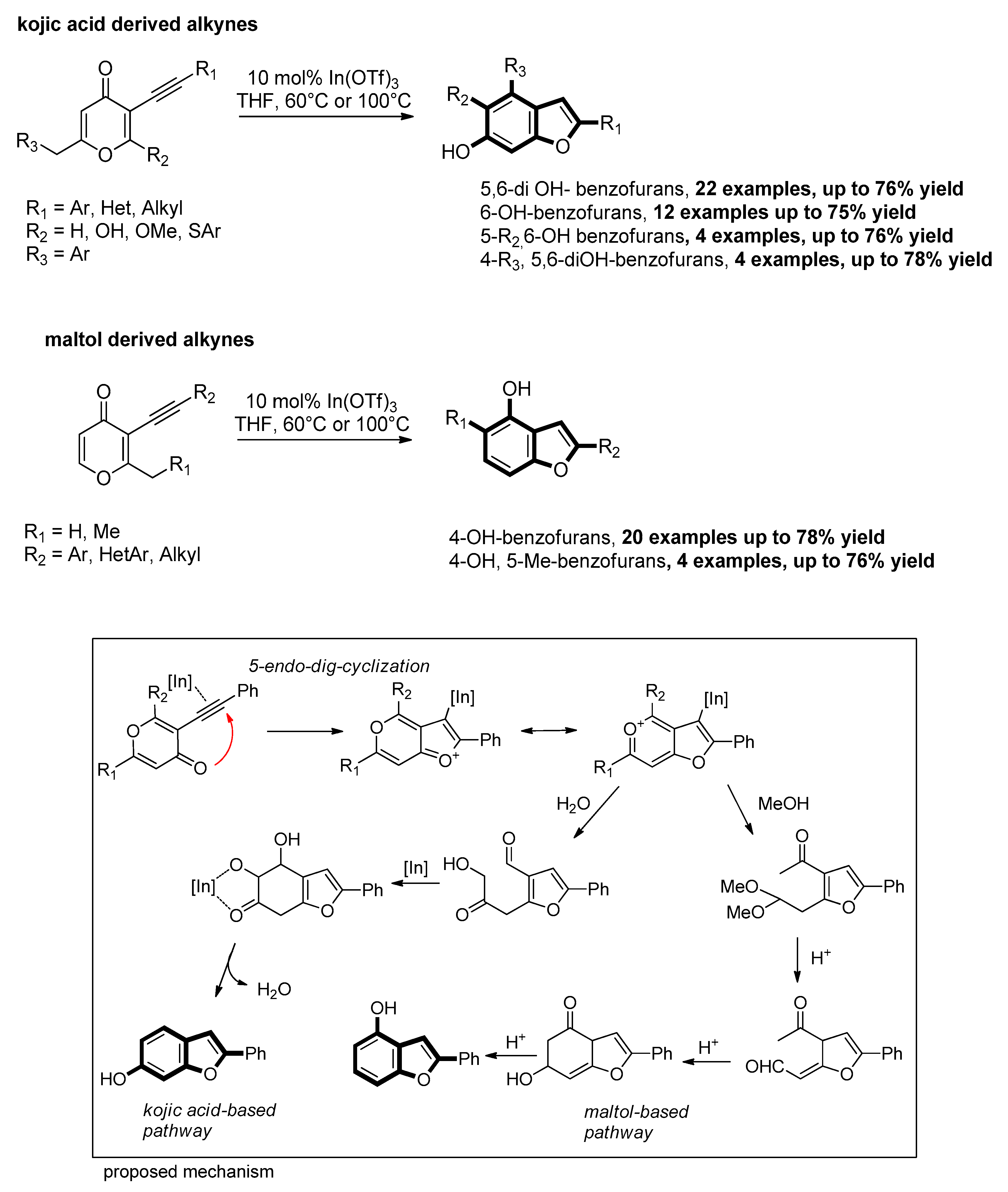
- Zhang, L.; Cao, T.; Jiang, H.; Zhu, S. Deconstructive Reorganization: De Novo Synthesis of Hydroxylated Benzofuran. Angew. Chem. Int. Ed. 2020, 59, 4670–4677. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiummiento, L.; D’Orsi, R.; Funicello, M.; Lupattelli, P. Last Decade of Unconventional Methodologies for the Synthesis of Substituted Benzofurans. Molecules 2020, 25, 2327. https://doi.org/10.3390/molecules25102327
Chiummiento L, D’Orsi R, Funicello M, Lupattelli P. Last Decade of Unconventional Methodologies for the Synthesis of Substituted Benzofurans. Molecules. 2020; 25(10):2327. https://doi.org/10.3390/molecules25102327
Chicago/Turabian StyleChiummiento, Lucia, Rosarita D’Orsi, Maria Funicello, and Paolo Lupattelli. 2020. "Last Decade of Unconventional Methodologies for the Synthesis of Substituted Benzofurans" Molecules 25, no. 10: 2327. https://doi.org/10.3390/molecules25102327
APA StyleChiummiento, L., D’Orsi, R., Funicello, M., & Lupattelli, P. (2020). Last Decade of Unconventional Methodologies for the Synthesis of Substituted Benzofurans. Molecules, 25(10), 2327. https://doi.org/10.3390/molecules25102327