Chemical and Biological Aspects of Montanine-Type Alkaloids Isolated from Plants of the Amaryllidaceae Family
Abstract
:1. Introduction
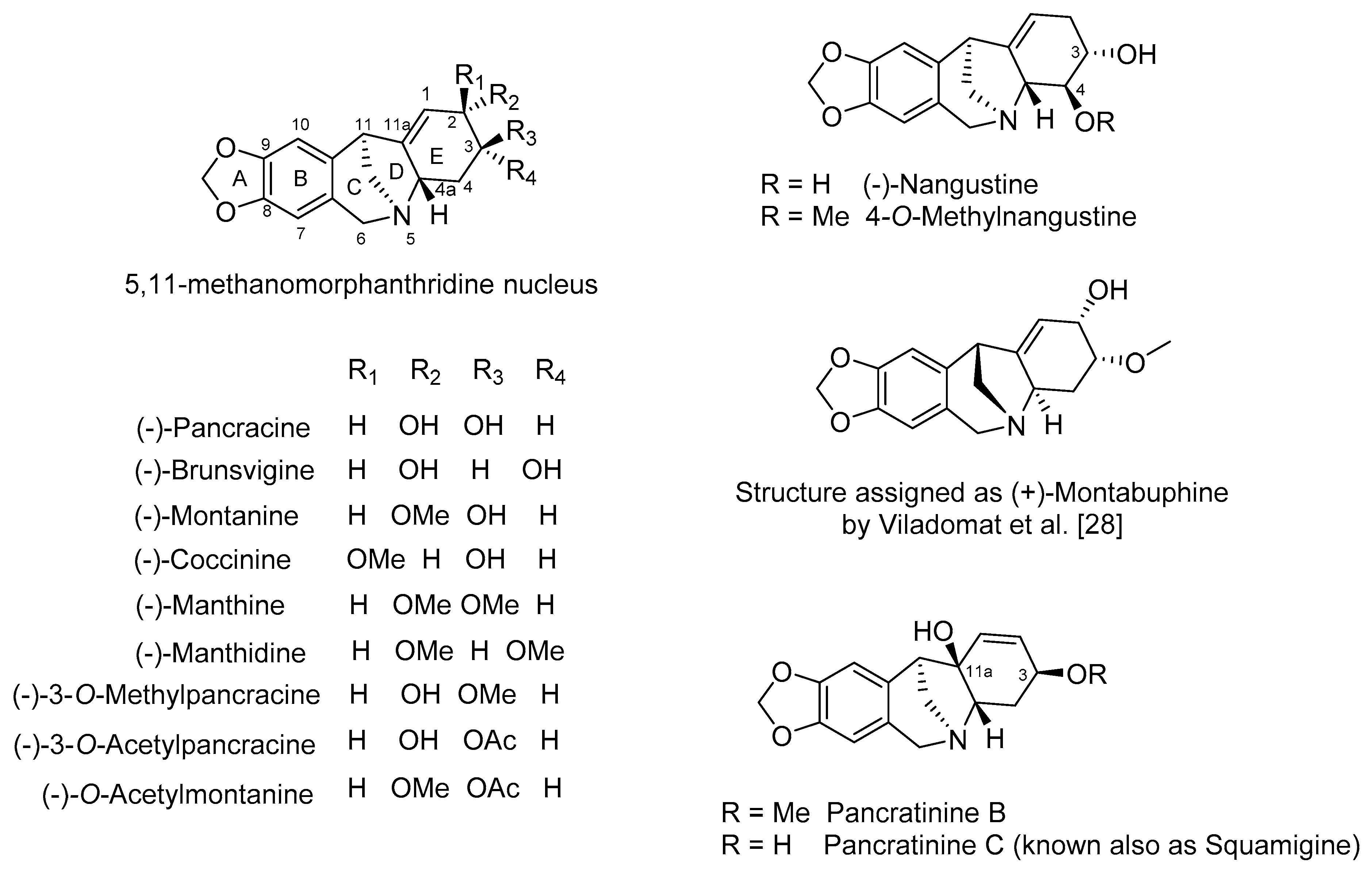
2. Biosynthesis, Phytochemistry and Occurrence of Montanine-Type Alkaloids
3. Biological Activity of Montanine-Type Amaryllidaceae Alkaloids
3.1. Anticancer Potential of Montanine-Type Amaryllidaceae Alkaloids
3.2. Other Biological Activities of Montanine-Type Amaryllidaceae Alkaloids
4. Preparation of Montanine-Type Alkaloids by Rearrangement of Haemanthamine-Type Ring System
5. Preparation and Structure Activity Relationship Studies on Synthetic Analogues of Montanine Type Alkaloids
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dahlgren, R.M.T.; Clifford, H.T.; Yeo, P.F. The Families of the Monocotyledons. Structure, Evolution and Taxonomy, 1st ed.; Springer: New York, NY, USA, 1985; pp. 1–520. [Google Scholar]
- Elgorashi, E.E.; van Staden, J. Bioactivity and bioactive compounds from African Amaryllidaceae. In African Natural Plant Products: New Discoveries and Challenges in Chemistry and Quality; ACS Symposium Series; Juliani, H.R., Simon, J.E., Ho, C.T., Eds.; American Chemical Society: Washington, DC, USA, 2009; Volume 1021, pp. 151–170. [Google Scholar]
- Nair, J.J.; Bastida, J.; van Staden, J. In vivo cytotoxicity studies of Amaryllidaceae alkaloids. Nat. Prod. Commun. 2016, 11, 121–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalecká, M.; Havelek, R.; Královec, K.; Brůčková, L.; Cahlíková, L. Amaryllidaceae family alkaloids as potential drugs for cancer treatment. Chem. Listy 2013, 107, 701–708. [Google Scholar]
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2016, 33, 1318–1343. [Google Scholar] [CrossRef] [PubMed]
- Havelek, R.; Muthna, D.; Tomsik, P.; Kralovec, K.; Seifrtova, M.; Cahlikova, L.; Hostalkova, A.; Safratova, M.; Perwein, M.; Cermakova, E.; et al. Anticancer potential of Amaryllidaceae alkaloids evaluated by screening with a panel of human cells, real-time cellular analysis and Ehrlich tumor-bearing mice. Chem. Biol. Interact. 2017, 275, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Fennell, C.W.; van Staden, J. Crinum species in traditional and modern medicine. J. Ethnopharmacol. 2001, 78, 15–26. [Google Scholar] [CrossRef]
- Graham, J.G.; Quinn, M.L.; Fabricant, D.S.; Farnsworth, N.R. Plants used against cancer—An extension of the work of Jonathan Hartwell. J. Ethnopharmacol. 2000, 73, 347–377. [Google Scholar] [CrossRef]
- Caamal-Fuentes, E.; Torres-Tapia, L.W.; Simá-Polanco, P.; Peraza-Sánchez, S.R.; Moo-Puc, R. Screening of plants used in Mayan traditional medicine to treat cancer-like symptoms. J. Ethnopharmacol. 2011, 135, 719–724. [Google Scholar] [CrossRef]
- Herrera, M.R.; Machocho, A.K.; Brun, R.; Viladomat, F.; Codina, C.; Bastida, J. Crinane and lycorane type alkaloids from Zephyranthes citrina. Planta Med. 2001, 67, 191–193. [Google Scholar] [CrossRef]
- Nair, J.J.; van Staden, J. Pharmacological and toxicological insights to the South African Amaryllidaceae. Food Chem. Toxicol. 2013, 62, 262–275. [Google Scholar] [CrossRef]
- Ingrassia, L.; Lefranc, F.; Mathieu, V.; Darro, F.; Kiss, R. Amaryllidaceae isocarbostyril alkaloids and their derivatives as promising antitumor agents. Transl. Oncol. 2008, 1, 1–13. [Google Scholar] [CrossRef]
- Kornienko, A.; Evidente, A. Chemistry, biology, and medicinal potential of narciclasine and its congeners. Chem. Rev. 2008, 108, 1982–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Kilgore, M.B.; Kutchan, T.M. The Amaryllidaceae alkaloids: Biosynthesis and methods for enzyme discovery. Phytochem. Rev. 2016, 15, 317–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, A.; Magne, K.; Massot, S.; Tallini, L.R.; Scopel, M.; Bastida, J.; Ratet, P.; Zuanazzi, J.A.S. Amaryllidaceae alkaloids: Identification and partial characterization of montanine production in Rhodophiala bifida plant. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Desgagné-Penix, I. Biosynthesis of the Amaryllidaceae alkaloids. Plant Sci. Today 2014, 1, 114–120. [Google Scholar] [CrossRef]
- Wang, R.; Xu, S.; Wang, N.; Xia, B.; Jiang, Y.; Wang, R. Transcriptome analysis of secondary metabolism pathway, transcription factors, and transporters in response to methyl jasmonate in Lycoris aurea. Front. Plant Sci. 2017, 7, 1971. [Google Scholar] [CrossRef] [Green Version]
- Ferdausi, A. A Metabolomics and Transcriptomics Comparison of Narcissus pseudonarcissus cv. Carlton Feld and In Vitro Tissues in Relation to Alkaloid Production. Ph.D. Thesis, University of Liverpool, Liverpool, UK, 2017. [Google Scholar]
- Singh, A.; Desgagné-Penix, I. Transcriptome and metabolome profiling of Narcissus pseudonarcissus ‘King Alfred’ reveal components of Amaryllidaceae alkaloid metabolism. Sci. Rep. 2017, 7, 17356. [Google Scholar] [CrossRef]
- Fuganti, C.; Ghiringhelli, D.; Grasselli, P. Stereochemistry of hydrogen removal β to nitrogen in the biological conversion of O-methylnorbelladine into the montanine-type alkaloids. J. Chem. Soc. Chem. Commun. 1973, 13, 430–431. [Google Scholar] [CrossRef]
- Wildman, W.C.; Olesen, B. Biosynthesis of montanine. J. Chem. Soc. Chem. Commun. 1976, 14, 551. [Google Scholar] [CrossRef]
- Feinstein, A.I.; Wildman, W.C. Biosynthetic oxidation and rearrangement of vittatine and its derivatives. J. Org. Chem. 1976, 41, 2447–2450. [Google Scholar] [CrossRef]
- Laurain-Mattar, D.; Ptak, A. Amaryllidaceae alkaloid accumulation by plant in vitro system. In Bioprocessing of Plant In Vitro Systems, Reference Series in Phytochemistry; Pavlov, A., Bley, T., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–22. [Google Scholar]
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2007, 24, 886–905. [Google Scholar] [CrossRef] [PubMed]
- Wildman, W.C.; Kaufman, C.J. Alkaloids of the Amaryllidaceae. III. Isolation of five new alkaloids from Haemanthus species1. J. Am. Chem. Soc. 1955, 77, 1248–1252. [Google Scholar] [CrossRef]
- Cedrón, J.C.; Oberti, J.C.; Estévez-Braun, A.; Ravelo, A.G.; del Arco-Aguilar, M.; López, M. Pancratium canariense as an important source of Amaryllidaceae alkaloids. J. Nat. Prod. 2009, 72, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Viladomat, F.; Bastida, J.; Codina, C.; Campbell, W.E.; Mathee, S. Alkaloids from Boophane flava. Phytochemistry 1995, 40, 307–311. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, H.; Pan, C.; Wang, J.; Huang, R.; Li, Q. Flexible synthesis of montanine-like alkaloids: Revisiting the structure of montabuphine. Org. Biomol. Chem. 2012, 10, 3812–3814. [Google Scholar] [CrossRef] [PubMed]
- Matveenko, M.; Banwell, M.G.; Willis, A.C. A chemoenzymatic total synthesis of the structure assigned to the alkaloid (+)-montabuphine. Org. Lett. 2008, 10, 4693–4696. [Google Scholar] [CrossRef]
- Farinon, M.; Clarimundo, V.S.; Pedrazza, G.P.; Gulko, P.S.; Zuanazzi, J.A.; Xavier, R.M.; de Oliveira, P.G. Disease modifying anti-rheumatic activity of the alkaloid montanine on experimental arthritis and fibroblast-like synoviocytes. Eur. J. Pharmacol. 2017, 799, 180–187. [Google Scholar] [CrossRef]
- Wildman, W.C.; Brown, C.L. Mass spectra of 5,11-methanomorphanthridine alkaloids. The structure of pancracine. J. Am. Chem. Soc. 1968, 90, 6439–6446. [Google Scholar] [CrossRef]
- Masi, M.; Van Slambrouck, S.; Gunawardana, S.; van Rensburg, M.J.; James, P.C.; Mochel, J.G.; Heliso, P.S.; Albalawi, A.S.; Cimmino, A.; van Otterlo, W.A.L.; et al. Alkaloids isolated from Haemanthus humilis Jacq., an indigenous South African Amaryllidaceae: Anticancer activity of coccinine and montanine. S. Afr. J. Bot. 2019, 126, 277–281. [Google Scholar] [CrossRef]
- Stafford, G.I.; Birer, C.; Brodin, B.; Christensen, S.B.; Eriksson, A.H.; Jäger, A.K.; Rønsted, N. Serotonin transporter protein (SERT) and P-glycoprotein (P-gp) binding activity of montanine and coccinine from three species of Haemanthus L.(Amaryllidaceae). S. Afr. J. Bot. 2013, 88, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.F.S.; de Andrade, J.P.; Machado, K.R.B.; Rocha, A.B.; Apel, M.A.; Sobral, M.E.G.; Henriques, A.T.; Zuanazzi, J.A. Screening for cytotoxic activity of extracts and isolated alkaloids from bulbs of Hippeastrum vittatum. Phytomedicine 2008, 15, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.E.; Pigni, N.B.; Andujar, S.A.; Roitman, G.; Suvire, F.D.; Enriz, R.D.; Tapia, A.; Bastida, J.; Feresin, G.E. Alkaloids from Hippeastrum argentinum and their cholinesterase-inhibitory activities: An in vitro and in silico study. J. Nat. Prod. 2016, 79, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Cahlíková, L.; Benešová, N.; Macáková, K.; Urbanová, K.; Opletal, L. GC/MS analysis of three Amaryllidaceae species and their cholinesterase activity. Nat. Prod. Commun. 2011, 6, 1255–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffield, A.M.; Aplin, R.T.; Budzikiewicz, H.; Djerassi, C.; Murphy, C.F.; Wildman, W.C. Mass spectrometry in structural and stereochemical problems. LXXXII. 1 A study of the fragmentation of some Amaryllidaceae alkaloids2. J. Am. Chem. Soc. 1965, 87, 4902–4912. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, M.; Hoshino, O.; Iitaka, Y. Total synthesis of montanine-type Amaryllidaceae alkaloids, which possess a 5, 11-methanomorphanthridine ring system, through cyclization with sodium bis (2-methoxyethoxy) aluminum hydride (SMEAH): The first stereoselective total syntheses of (±)-montanine, (±)-coccinine, (±)-O-acetylmontanine, (±)-pancracine, and (±)-brunsvigine. J. Org. Chem. 1992, 57, 7285–7295. [Google Scholar]
- Bao, X.; Cao, Y.X.; Chu, W.D.; Qu, H.; Du, J.Y.; Zhao, X.H.; Ma, X.Y.; Wang, C.T.; Fan, C.A. Bioinspired total synthesis of montanine-type Amaryllidaceae alkaloids. Angew. Chem. Int. Edit. 2013, 52, 14167–14172. [Google Scholar] [CrossRef] [PubMed]
- Crouch, N.R.; Pohl, T.L.; Mulholland, D.A.; Ndlovu, E.; Van staden, J. Alkaloids from three ethnomedicinal Haemanthus species: H. albiflos, H. deformis and H. pauculifolius (Amaryllidaceae). S. Afr. J. Bot. 2005, 71, 49–52. [Google Scholar] [CrossRef] [Green Version]
- da Silva, A.F.S.; de Andrade, J.P.; Bevilaqua, L.R.M.; de Souza, M.M.; Izquierdo, I.; Henriques, A.T.; Zuanazzi, J.A.S. Anxiolytic-, antidepressant- and anticonvulsant-like effects of the alkaloid montanine isolated from Hippeastrum vittatum. Pharmacol. Biochem. Behav. 2006, 85, 148–154. [Google Scholar] [CrossRef]
- Al Shammari, L.; Al Mamun, A.; Koutová, D.; Majorošová, M.; Hulcová, D.; Šafratová, M.; Breiterová, K.; Maříková, J.; Havelek, R.; Cahlíková, L. Alkaloid profiling of Hippeastrum cultivars by GC-MS, isolation of Amaryllidaceae alkaloids and evaluation of their cytotoxicity. Rec. Nat. Prod. 2020, 14, 154–159. [Google Scholar] [CrossRef]
- Berkov, S.; Evstatieva, L.; Popov, S. Alkaloids in Bulgarian Pancratium maritimum L. Zeitschrift für Naturforschung C 2004, 59, 65–69. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kaya, G.I.; Somer, N.U. Chemical composition and enzyme inhibitory activities of Turkish Pancratium maritimum bulbs. Nat. Prod. Commun. 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Labraña, J.; Machocho, A.K.; Kricsfalusy, V.; Brun, R.; Codina, C.; Viladomat, F.; Bastida, J. Alkaloids from Narcissus angustifolius subsp. transcarpathicus. Phytochemistry 2002, 60, 847–852. [Google Scholar] [CrossRef]
- Cedrón, J.C.; Ravelo, A.G.; León, L.G.; Padrón, J.M.; Estévez-Braun, A. Antiproliferative and structure activity relationships of Amaryllidaceae alkaloids. Molecules 2015, 20, 13854–13863. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.Y.; Wang, Z.Y.; Pi, H.F.; Zhang, P.; Ruan, H.L. Neuroprotective compounds from the bulbs of Lycoris radiata. Fitoterapia 2013, 88, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.W.; Cheng, T.H.; Raghukumar, V.; Sha, C.K. An expedient route to montanine-type Amaryllidaceae alkaloids: Total syntheses of (−)-brunsvigine and (−)-manthine. J. Org. Chem. 2008, 73, 7580–7585. [Google Scholar] [CrossRef] [PubMed]
- Inubushi, Y.; Fales, H.M.; Warnhoff, E.W.; Wildman, W.C. Structures of montanine, coccinine, and manthine. J. Org. Chem. 1960, 25, 2153–2164. [Google Scholar] [CrossRef]
- Clark, R.C.; Warren, F.L.; Pachler, K.G.R. Alkaloids of the Amaryllidaceae: Brunsvigine: NMR, ORD/CD and mass spectrometry, degradation and interconversion studies. Tetrahedron 1975, 31, 1855–1859. [Google Scholar] [CrossRef]
- Dry, L.J.; Poynton, M.; Thompson, M.E.; Warren, F.L. The alkaloids of the Amaryllidaceae. Part IV. The alkaloids of Brunsvigia cooperi Baker. J. Chem. Soc. 1958, 4701–4704. [Google Scholar] [CrossRef]
- Wildman, W.C.; Brown, C.L.; Michel, K.H.; Bailey, D.T.; Heimer, N.E.; Shaffer, R.; Murphy, C.F. Alkaloids from Rhodophiala bifida, Crinum erubescens and Sprekelia formisissima. Pharmazie 1967, 22, 725. [Google Scholar]
- Nair, J.J.; Bastida, J.; Viladomat, F.; van Staden, J. Cytotoxic agents of the crinane series of Amaryllidaceae alkaloids. Nat. Prod. Comm. 2012, 7, 1677–1688. [Google Scholar] [CrossRef]
- He, M.; Qu, C.; Gao, O.; Hu, X.; Hong, X. Biological and pharmacological activities of Amaryllidaceae alkaloids. RSC Adv. 2015, 5, 16562–16574. [Google Scholar] [CrossRef]
- Sener, B.; Orhan, I.; Satayavivad, J. Antimalarial activity screening of some alkaloids and the plant extracts from Amaryllidaceae. Phytother. Res. 2003, 17, 1220–1223. [Google Scholar] [CrossRef] [PubMed]
- Osorio, E.J.; Berkov, S.; Brun, R.; Codina, C.; Viladomat, F.; Cabezas, F.; Bastida, J. In vitro antiprotozoal activity of alkaloids from Phaedranassa dubia (Amaryllidaceae). Phytochem. Lett. 2010, 3, 161–163. [Google Scholar] [CrossRef]
- Kulhánková, A.; Cahlíková, L.; Novák, Z.; Macáková, K.; Kuneš, J.; Opletal, L. Alkaloids from Zephyranthes robusta Baker and their acetylcholinesterase and butyrylcholinesterase inhibition activity. Chem. Biodivers. 2013, 10, 1120–1127. [Google Scholar] [CrossRef]
- Havelek, R.; Seifrtová, M.; Královec, K.; Bručková, L.; Cahlíková, L.; Dalecká, M.; Vávrová, J.; Řezáčová, M.; Opletal, L.; Bílková, Z. The effect of Amaryllidaceae alkaloids Haemanthamine and Haemanthidine on cell cycle progression and apoptosis in p53-negative human leukemic Jurkat cells. Phytomedicine 2014, 21, 479–490. [Google Scholar] [CrossRef]
- Nair, J.J.; Van Staden, J.; Bastida, J. Cytotoxic alkaloids constituents of the Amaryllidaceae. In Studies in Natural Products Chemistry, 1st ed.; Rahman, A.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 107–156. [Google Scholar]
- Fuchs, S.; Hsieh, L.T.; Saarberg, W.; Erdelmeier, C.A.J.; Wichelhaus, T.A.; Schaefer, L.; Koch, E.; Fürst, R. Haemanthus coccineus extract and its main bioactive component narciclasine display profound anti-inflammatory activities in vitro and in vivo. J. Cell Mol. Med. 2015, 19, 1021–1032. [Google Scholar] [CrossRef]
- Breiterová, K.; Koutová, D.; Maříková, J.; Havelek, R.; Kuneš, J.; Majorošová, M.; Opletal, L.; Hošťálková, A.; Jenčo, J.; Řezáčová, M.; et al. Amaryllidaceae alkaloids of different structural types from Narcissus L. cv. Professor Einstein and their cytotoxic activity. Plants 2020, 9, 137. [Google Scholar] [CrossRef] [Green Version]
- Govindaraju, K.; Ingels, A.; Hasan, M.N.; Sun, D.; Mathieu, V.; Masi, M.; Evidente, A.; Kornienko, A. Synthetic analogues of the montanine-type alkaloids with activity against apoptosis-resistant cancer cells. Bioorg. Med. Chem. Lett. 2018, 28, 589–593. [Google Scholar] [CrossRef]
- Pagliosa, L.B.; Monteiro, S.C.; Silva, K.B.; De Andrade, J.P.; Dutilh, J.; Bastida, J.; Cammarota, M.; Zuanazzi, J.A.S. Effect of isoquinoline alkaloids from two Hippeastrum species on in vitro acetylcholinesterase activity. Phytomedicine 2010, 17, 698–701. [Google Scholar] [CrossRef]
- Evidente, A.; Andolfi, A.; Abou-Donia, A.H.; Touema, S.M.; Hammoda, H.M.; Shawky, E.; Motta, A. (−)-Amarbellisine, a lycorine-type alkaloid from Amaryllis belladonna L. growing in Egypt. Phytochemistry. 2004, 65, 2113–2118. [Google Scholar] [CrossRef]
- Mathew, S.; Faheem, M.; Al-Malki, A.L.; Kumosani, T.A.; Qadri, I. In silico inhibition of GABARAP activity using antiepileptic medicinal derived compounds. Bioinformation 2015, 11, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Castilhos, T.S.; Giordani, R.B.; Henriques, A.T.; Menezes, F.S.; Zuanazzi, J.A.S. Availacao in vitro das atividades antiinflamatoria, antioxidante e antimicrobiana do alcaloide montanina. Rev. Bras. Pharmacogn. 2007, 17, 209–214. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, P.G.; Pedrazza, G.P.R.; Farinon, M.; Machado, X.R.; Zuanazzi, J.A.S.; Spies, F. Process for Extracting the Alkaloid Fraction of Rhodophiala bifida (Herb.) Traub and Uses Threof. US Patent 2020/0000798 A1, 2 January 2020. [Google Scholar]
- Kohelová, E.; Peřinová, R.; Maafi, N.; Korábečný, J.; Hulcová, D.; Maříková, J.; Kučera, T.; Martínez González, L.; Hrabinova, M.; Vorčáková, K.; et al. Derivatives of the β-crinane Amaryllidaceae alkaloid haemanthamine as multi-target directed ligands for Alzheimer’s Disease. Molecules 2019, 24, 1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tallini, L.R.; Bastida, J.; Cortes, N.; Osorio, E.H.; Theoduloz, C.; Schmeda-Hirschmann, G. Cholinesterase inhibition activity, alkaloid profiling and molecular docking of Chilean Rhodophiala (Amaryllidaceae). Molecules 2018, 23, 1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masondo, N.A.; Stafford, G.I.; Aremu, A.O.; Makunga, N.P. Acetylcholinesterase inhibitors from southern African plants: An overview of ethnobotanical, pharmacological potential and phytochemical research including and beyond Alzheimer’s disease treatment. S. Afr. J. Bot. 2019, 120, 39–64. [Google Scholar] [CrossRef]
- Cahlíková, L.; Pérez, D.I.; Štěpánková, S.; Chlebek, J.; Šafratová, M.; Hošt’álková, A.; Opletal, L. In vitro inhibitory effects of 8-O-demethylmaritidine and undulatine on acetylcholinesterase and their predicted penetration across the blood–brain barrier. J. Nat. Prod. 2015, 78, 1189–1192. [Google Scholar] [CrossRef]
- Hulcová, D.; Maříková, J.; Korábečný, J.; Hošťálková, A.; Jun, D.; Kuneš, J.; Chlebek, J.; Opletal, L.; De Simone, A.; Nováková, L.; et al. Amaryllidaceae alkaloids from Narcissus pseudonarcissus L. cv. Dutch Master as potential drugs in treatment of Alzheimer’s disease. Phytochemistry 2019, 165, 112055. [Google Scholar] [CrossRef]
- Ishizaki, M.; Hoshino, O.; Iitaka, Y. A first total synthesis of montanine-type Amaryllidaceae alkaloids, (±)-coccinine, (±)-montanine, and (±)-pancracine. Tetrahedron Lett. 1991, 32, 7079–7082. [Google Scholar] [CrossRef]
- Overman, L.E.; Shim, J. Synthesis applications of cationic aza-Cope rearrangements. 23. First total synthesis of Amaryllidaceae alkaloids of the 5,11-methano morphanthridine type. An efficient total synthesis of (±)-pancracine. J. Org. Chem. 1991, 56, 5005–5007. [Google Scholar] [CrossRef]
- Ishizaki, M.; Kurihara, K.I.; Tanazawa, E.; Hoshino, O. Radical-mediated synthesis of the 5,11-methanomorphanthridine ring system: Formal total synthesis of montanine-type Amaryllidaceae alkaloids, (±)-montanine, (±)-coccinine and (±)-pancracine. J. Chem. Soc. Perkin Trans. 1 1993, 1, 101–110. [Google Scholar] [CrossRef]
- Overman, L.E.; Shim, J. Total synthesis of Amaryllidaceae alkaloids of the 5,11-methanomorphanthridine type. Efficient total synthesis of (-)-pancracine and (±)-pancracine. J. Org. Chem. 1993, 58, 4662–4672. [Google Scholar] [CrossRef]
- Jin, J.; Weinreb, S.M. Application of a stereospecific intramolecular allenylsilane imino ene reaction to enantioselective total synthesis of the 5,11-methanomorphanthridine class of Amaryllidaceae alkaloids. J. Am. Chem. Soc. 1997, 119, 5773–5784. [Google Scholar] [CrossRef]
- Pearson, W.H.; Lian, B.W. Application of the 2-azaallyl anion cycloaddition method to an enantioselective total synthesis of (+)-coccinine. Angew. Chem. Int. Ed. Engl. 1998, 37, 1724–1726. [Google Scholar] [CrossRef]
- Ikeda, M.; Hamada, M.; Yamashita, T.; Matsui, K.; Sato, T.; Ishibashi, H. Stereoselective synthesis of (3R*, 3aS*, 7aS*)-3-aryloctahydroindol-2-ones using radical cyclisation: A formal synthesis of (±)-pancracine. J. Chem. Soc. Perkin Trans. 1 1999, 14, 1949–1956. [Google Scholar] [CrossRef]
- Banwell, M.G.; Edwards, A.J.; Jolliffe, K.A.; Kemmler, M. An operationally simple and fully regiocontrolled formal total synthesis of the montanine-type Amaryllidaceae alkaloid (±)-pancracine. J. Chem. Soc. Perkin Trans. 1 2001, 12, 1345–1348. [Google Scholar] [CrossRef]
- Sha, C.K.; Hong, A.W.; Huang, C.M. Synthesis of aza bicyclic enones via anionic cyclization: Application to the total synthesis of (−)-Brunsvigine. Org. Lett. 2001, 3, 2177–2179. [Google Scholar] [CrossRef]
- Pandey, G.; Banerjee, P.; Kumar, R.; Puranik, V.G. Stereospecific route to 5,11-methanomorphanthridine alkaloids via intramolecular 1,3-dipolar cycloaddition of nonstabilized azomethine ylide: Formal total synthesis of (±)-pancracine. Org. Lett. 2005, 7, 3713–3716. [Google Scholar] [CrossRef]
- Banwell, M.G.; Kokas, O.J.; Willis, A.C. Chemoenzymatic approaches to the montanine alkaloids: A total synthesis of (+)-brunsvigine. Org. Lett. 2007, 9, 3503–3506. [Google Scholar] [CrossRef]
- Kokas, O.J.; Banwell, M.G.; Willis, A.C. Chemoenzymatic approaches to the montanine alkaloids: A total synthesis of (+)-nangustine. Tetrahedron 2008, 64, 6444–6451. [Google Scholar] [CrossRef]
- Anada, M.; Tanaka, M.; Shimada, N.; Nambu, H.; Yamawaki, M.; Hashimoto, S. Asymmetric formal synthesis of (−)-pancracine via catalytic enantioselective C–H amination process. Tetrahedron 2009, 65, 3069–3077. [Google Scholar] [CrossRef]
- Pansare, S.V.; Lingampally, R.; Kirby, R.L. Stereoselective synthesis of 3-aryloctahydroindoles and application in a formal synthesis of (−)-pancracine. Org. Lett. 2010, 12, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Kumar, R.; Banerjee, P.; Puranik, V.G. One-step stereospecific strategy for the construction of the core structure of the 5,11-methanomorphanthridine alkaloids in racemic as well as in optically pure form: Synthesis of (±)-pancracine and (±)-brunsvigine. Eur. J. Org. Chem. 2011, 2011, 4571–4587. [Google Scholar] [CrossRef]
- Pandey, G.; Gadre, S.R. Stereoselective construction of 5,11-methanomorphanthridine and 5,10b-phenanthridine structural frameworks: Total syntheses of (±)-pancracine, (±)-brunsvigine, (±)-maritidine, and (±)-crinine. Pure Appl. Chem. 2012, 84, 1597–1619. [Google Scholar] [CrossRef]
- Yang, H.; Hou, S.; Tao, C.; Liu, Z.; Wang, C.; Cheng, B.; Li, Y.; Zhai, H. Rhodium-catalyzed denitrogenative [3 + 2] cycloaddition: Access to functionalized hydroindolones and the framework of montanine-type Amaryllidaceae alkaloids. Chem. Eur. J. 2017, 23, 12930–12936. [Google Scholar] [CrossRef]
- Pandey, G.; Dey, D.; Tiwari, S.K. Synthesis of biologically active natural products by [3 + 2] cycloaddition of non-stabilized azomethine ylides (AMY): Concepts and realizations. Tetrahedron Lett. 2017, 58, 699–705. [Google Scholar] [CrossRef]
- Cedrón, J.C.; Estévez-Braun, A.; Ravelo, A.; Gutiérrez, D.; Flores, N.; Bucio, M.A.; Pérez-Hernández, N.; Joseph-Nathan, P. Bioactive montanine derivatives from halide-induced rearrangements of haemanthamine-type alkaloids. Absolute configuration by VCD. Org. Lett. 2009, 11, 1491–1494. [Google Scholar] [CrossRef]







| Alkaloid | Amaryllidaceae Family Plants | References | Ref. for Spectroscopic Data (NMR, MS, UV, IR) |
|---|---|---|---|
| Montanine | Rhodophiala bifida | [16,31,32] | [33,34,35,36,37,38,39,40] |
| Haemanthus humilis | [33] | ||
| Haemanthus amarylloides | [26] | ||
| Haemanthus coccineus | [26,34] | ||
| Haemanthus montanus | [26,34] | ||
| Haemanthus sanguineus | [34] | ||
| Haemanthus pauculifolius | [41] | ||
| Haemanthus deformis | [41] | ||
| Hippeastrum vittatum | [35,42] | ||
| Hippeastrum cv. Ferrari | [43] | ||
| Hippeastrum cv. Double King | [43] | ||
| Hippeastrum cv. Pretty Nymph | [43] | ||
| Hippeastrum cv. Spartacus | [43] | ||
| Hippeastrum argentinum | [36] | ||
| Scadoxus multiflorus | [37] | ||
| Pancracine | Rhodophiala bifida | [16,32] | [36,39,40,44,45,46] |
| Pancratium canariense | [27,47] | ||
| Pancratium maritimum | [44,45] | ||
| Narcissus angustifolius subsp. transcarpathicus | [46] | ||
| Lycoris radiata | [48] | ||
| Hippeastrum cv. Ferrari | [43] | ||
| Hippeastrum cv.Double King | [43] | ||
| Hippeastrum cv. Pretty Nymph | [43] | ||
| Hippeastrum argentinum | [36] | ||
| Coccinine | Haemanthus humilis | [33] | [33,34,38,39,40] |
| Haemanthus amarylloides | [26] | ||
| Haemanthus coccineus | [26] | ||
| Haemanthus montanus | [34] | ||
| Haemanthus sanguineus | [34] | ||
| Haemanthus deformis | [41] | ||
| Manthine | Haemanthus amarylloides | [26] | [32,49] |
| Haemanthus montanus | [34] | ||
| Haemanthus tigrinus | [50] | ||
| Manthidine | Haemanthus coccineus | [26] | [40] |
| Haemanthus pauculifolius | [41] | ||
| Haemanthus deformis | [41] | ||
| Brunsvigine | Brunsvigia radulosa | [50] | [40,49,51] |
| Brunsvigia cooperi | [52] | ||
| Pancratinine B | Pancratium canariense | [27] | [27] |
| Pancratinine C (reported also as Squamigine) | Pancratium canariense | [27] | [27,45] |
| Pancratium maritimum | [45] | ||
| Lycoris radiata | [48] | ||
| Nangustine | Narcissus angustifolius subsp. transcarpathicus | [46] | [46] |
| 3-O-Methylpancracine (reported also as isohaemanthamine) | Lycoris radiata | [48] | [32,49] |
| 3-O-Acetylpancracine | Rhodophiala bifida | [16] | [32] |
| 4-O-Methylnangustine | Hippeastrum argentinum | [36] | [36] |
| Montabuphine | Boophone flava | [28] | [28,29,30] |
| O-Acetylmontanine | Rhodophiala bifida | [53] | [39] |
| Montanine Type Alkaloid | Cell Line | Method of Assay/Time of Treatment | Value and Type of Half-Maximal Inhibitory Concentration | References | |
|---|---|---|---|---|---|
| Montanine | Jurkat | WST-1/48 h | 1.04 ± 0.14 | a | [43] |
| MOLT-4 | WST-1/48 h | 1.26 ± 0.11 | a | [43] | |
| A549 | WST-1/48 h | 1.09 ± 0.31 | a | [43] | |
| HT-29 | WST-1/48 h | 1.35 ± 0.47 | a | [43] | |
| PANC-1 | WST-1/48 h | 2.30 ± 0.45 | a | [43] | |
| A2780 | WST-1/48 h | 1.67 ± 0.29 | a | [43] | |
| HeLa | WST-1/48 h | 1.99 ± 0.22 | a | [43] | |
| MCF-7 | WST-1/48 h | 1.39 ± 0.21 | a | [43] | |
| SAOS-2 | WST-1/48 h | 1.36 ± 0.49 | a | [43] | |
| MRC-5 | WST-1/48 h | 1.79 ± 0.50 | a | [43] | |
| A549 | MTT/48 h | 1.9 ± 0.4 | a | [33] | |
| HCT-15 | MTT/48 h | 6.8 ± 0.5 | a | [33] | |
| SK-MEL-28 | MTT/48 h | 23.2 ± 1.9 | a | [33] | |
| MCF-7 | MTT/48 h | 4.4 ± 0.4 | a | [33] | |
| MDA-MB-231 | MTT/48 h | 3.4 ± 0.9 | a | [33] | |
| Hs578T | MTT/48 h | 3.6 ± 1.7 | a | [33] | |
| HT-29 | SRB/not specified | 0.71 ± 0.1 µg/mL | a | [35] | |
| H460 | SRB/not specified | 0.57 ± 0.57 µg/mL | a | [35] | |
| RXF393 | SRB/not specified | 0.65 ± 0.01 µg/mL | a | [35] | |
| MCF7 | SRB/not specified | 0.74 ± 0.02 µg/mL | a | [35] | |
| OVCAR3 | SRB/not specified | 0.84 ± 0.11 µg/mL | a | [35] | |
| Pancracine | Jurkat | WST-1/48 h | 5.07 ± 0.31 | a | [62] |
| MOLT-4 | WST-1/48 h | 2.71 ± 0.25 | a | [62] | |
| A549 | WST-1/48 h | 2.29 ± 0.43 | a | [62] | |
| HT-29 | WST-1/48 h | 2.60 ± 0.51 | a | [62] | |
| A2780 | WST-1/48 h | 5.08 ± 0.43 | a | [62] | |
| HeLa | WST-1/48 h | 5.03 ± 0.36 | a | [62] | |
| MCF-7 | WST-1/48 h | 2.68 ± 0.37 | a | [62] | |
| SAOS-2 | WST-1/48 h | 2.20 ± 0.25 | a | [62] | |
| MRC-5 | WST-1/48 h | 5.15 ± 0.34 | a | [62] | |
| A2780 | SRB/48 h | 8.3 ± 0.5 | b | [47] | |
| SW1573 | SRB/48 h | 4.3 ± 0.7 | b | [47] | |
| T47-D | SRB/48 h | 6.5 ± 2.5 | b | [47] | |
| WiDr | SRB/48 h | 9.1 ± 1.0 | b | [47] | |
| Coccinine | A549 | MTT/48 h | 5.9 ± 0.8 | a | [33] |
| HCT-15 | MTT/48 h | 16.8 ± 1.8 | a | [33] | |
| SK-MEL-28 | MTT/48 h | >50 | a | [33] | |
| MCF-7 | MTT/48 h | 7.9 ± 0.9 | a | [33] | |
| MDA-MB-231 | MTT/48 h | 13.8 ± 0.8 | a | [33] | |
| Hs578T | MTT/48 h | 5.3 ± 0.4 | a | [33] | |
| Manthine | A549 | MTT/72 h | 3 | b | [63] |
| SKMEL-28 | MTT/72 h | 4 | b | [63] | |
| U373 | MTT/72 h | 5 | b | [63] | |
| MCF7 | MTT/72 h | 4 | b | [63] | |
| Hs683 | MTT/72 h | 3 | b | [63] | |
| B16F10 | MTT/72 h | 3 | b | [63] | |
| Parasite | Trypanosoma brucei rhodesiense | Trypanosoma cruzi | Leishmania donovani | Plasmodium falciparum | |
|---|---|---|---|---|---|
| Stage | Trypomastigote | Trypomastigote | Amastigotes | Erythrocytic form | |
| Strain | STIB 900 IC50 (µg/mL) | Tulahuen C4 IC50 (µg/mL) | MHOM-ET-67/L82 IC50 (µg/mL) | K1 IC50 (µg/mL) | NF54 IC50 (µg/mL) |
| Nangustine | 9.6 | 54.6 | >30 | 2.14 | 2.93 |
| Pancracine | 0.7 | 7.1 | >30 | 0.75 | 0.70 |
| Compound | GI50 In Vitro Values (µM) | |||||
|---|---|---|---|---|---|---|
| Resistant to Apoptosis | Sensitive to Apoptosis | |||||
| A549 | SKMEL-28 | U373 | MCF7 | Hs683 | B16F10 | |
| 1 | 6 | 26 | 51 | 17 | 6 | 7 |
| 2 | 5 | 8 | 31 | 13 | 4 | 8 |
| Manthine | 3 | 4 | 5 | 4 | 3 | 3 |
| 3 | 59 | >100 | >100 | 82 | >100 | 40 |
| 4 | 10 | 14 | 20 | 20 | 7 | 7 |
| 5 | 23 | 28 | 42 | 28 | 24 | 10 |
| 6 | 59 | 65 | 72 | 44 | 67 | 10 |
| 7 | 18 | 9 | 9 | 23 | 24 | 4 |
| 8 | 86 | 67 | >100 | 68 | 95 | 11 |
| 9 | >100 | >100 | >100 | >100 | >100 | >100 |
| 10 | >100 | >100 | >100 | >100 | >100 | >100 |
| 11 | >100 | >100 | >100 | >100 | >100 | >100 |
| 12 | 78 | >100 | >100 | 78 | 71 | 39 |
| 13 | 9 | 18 | 25 | 19 | 5 | 7 |
| 14 | >100 | >100 | >100 | >100 | >100 | 72 |
| Compound | IC50 (µg/mL) |
|---|---|
| Pancracine | 0.9 ± 0.04 |
| 1 | 0.4 ± 0.02 |
| 15 | 0.6 ± 0.04 |
| 16 | 0.7 ± 0.04 |
| Chloroquine | 0.013 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koutová, D.; Maafi, N.; Havelek, R.; Opletal, L.; Blunden, G.; Řezáčová, M.; Cahlíková, L. Chemical and Biological Aspects of Montanine-Type Alkaloids Isolated from Plants of the Amaryllidaceae Family. Molecules 2020, 25, 2337. https://doi.org/10.3390/molecules25102337
Koutová D, Maafi N, Havelek R, Opletal L, Blunden G, Řezáčová M, Cahlíková L. Chemical and Biological Aspects of Montanine-Type Alkaloids Isolated from Plants of the Amaryllidaceae Family. Molecules. 2020; 25(10):2337. https://doi.org/10.3390/molecules25102337
Chicago/Turabian StyleKoutová, Darja, Negar Maafi, Radim Havelek, Lubomír Opletal, Gerald Blunden, Martina Řezáčová, and Lucie Cahlíková. 2020. "Chemical and Biological Aspects of Montanine-Type Alkaloids Isolated from Plants of the Amaryllidaceae Family" Molecules 25, no. 10: 2337. https://doi.org/10.3390/molecules25102337







