Evaluation of Antioxidant and Antibacterial Activities, Cytotoxicity of Acacia seyal Del Bark Extracts and Isolated Compounds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Phenolic, Flavonoid Contents and Extractable Tannins
2.2. Antioxidant Activity
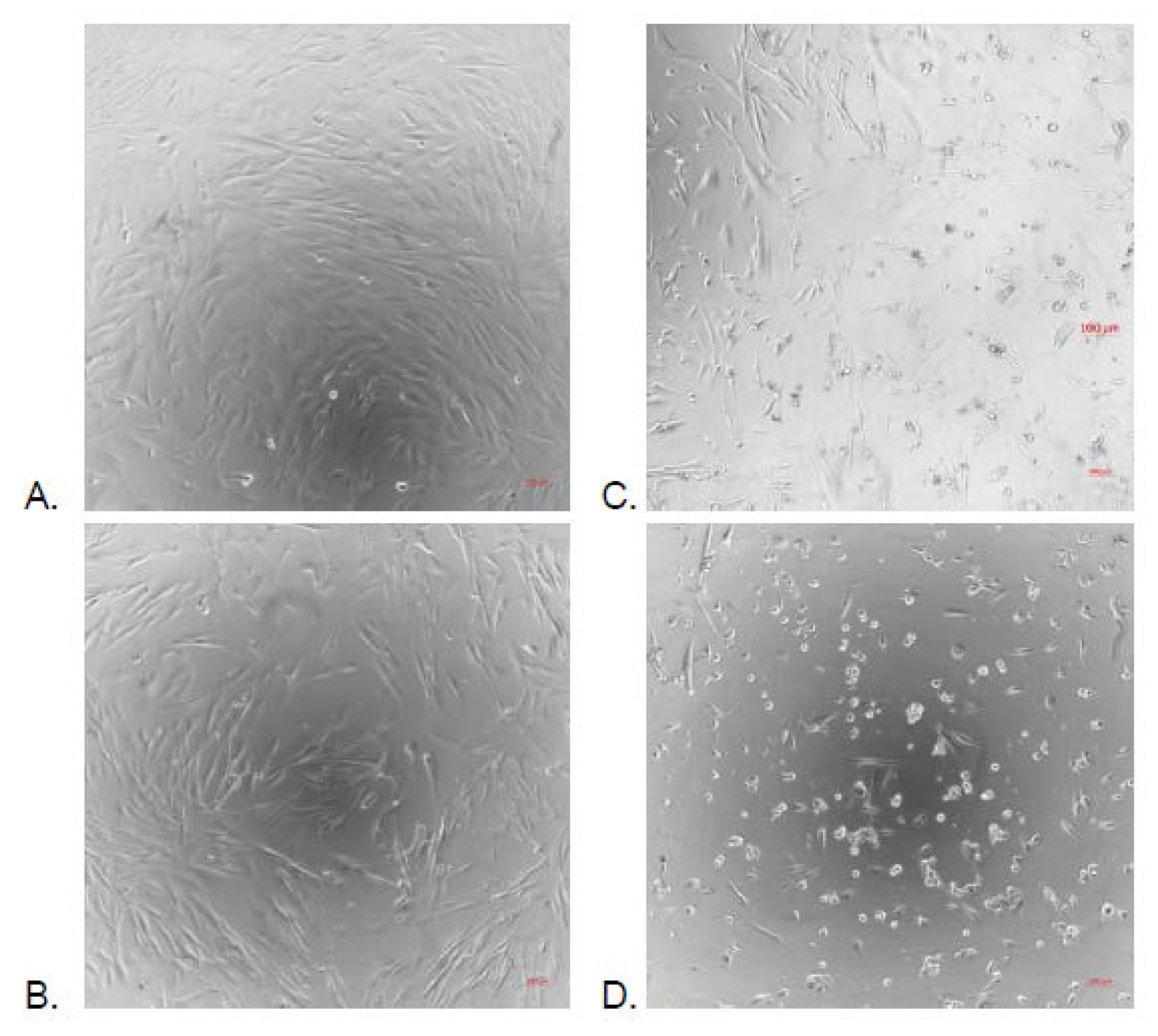
2.3. Cytotoxicity Activity
2.4. Antibacterial Activity
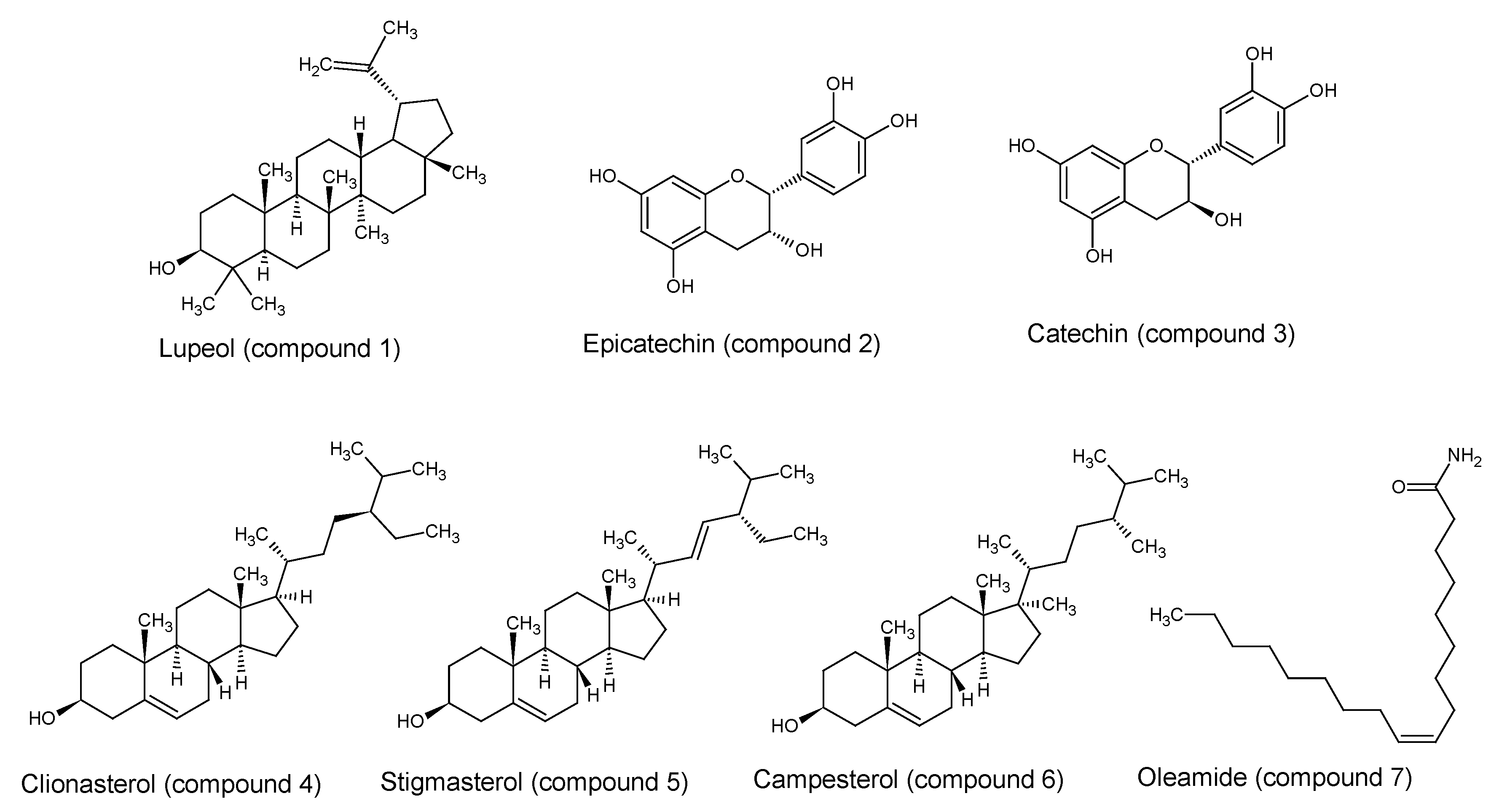
2.5. Chemical Constituents
2.6. Characterization of Condensed Tannins by MALDI-TOF MS Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Plant Materials
3.3. Preparation of Plant Extracts
3.4. Precipitation of Tannins from Crude Extracts
3.5. Determination of the Total Phenol Content (TPC) in the Extracts
3.6. Determination of the Total Flavonoid Content (TFC) in the Extracts
3.7. Preparation of Antioxidant Activities Tests
3.7.1. DPPH Method
3.7.2. ABTS Method
3.7.3. FRAP Method
3.8. Cytotoxicity Assay
3.9. Antibacterial Assay
3.10. Isolation and Characterization of Pure Compounds
3.10.1. Isolation of Pure Compounds
3.10.2. MALDI-TOF MS for Characterization of Condensed Tannins
3.10.3. GC-MS
3.10.4. NMR
3.10.5. LC-HRESIMS
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| VC | Vitamin C |
| QE | Quercetin Equivalent |
| VCE | Vitamin C Equivalent |
| GAE | Gallic acid Equivalent |
| TPC | Total polyphenols content |
| TFC | Total flavonoids content |
| FM | filtrate devoid of tannin from methanolic extract |
| PM | precipitate of tannins from methanolic extract |
| FW | filtrate devoid of tannin from water extract |
| PW | precipitate of tannins from water extract |
References
- Nacro, M.; Millogo-Rasolodimbi, J. Plantes tinctoriales et plantes à tanins du Burkina Faso; ScientifikA: Amiens, France, 1993; ISBN 978-2-909894-09-6. [Google Scholar]
- Nie, S.-P.; Wang, C.; Cui, S.W.; Wang, Q.; Xie, M.-Y.; Phillips, G.O. The core carbohydrate structure of Acacia seyal var. seyal (Gum arabic). Food Hydrocoll. 2013, 32, 221–227. [Google Scholar] [CrossRef]
- Rather, L.J.; ul-Islam, S.; Mohammad, F. Acacia nilotica (L.): A review of its traditional uses, phytochemistry, and pharmacology. Sustain. Chem. Pharm. 2015, 2, 12–30. [Google Scholar] [CrossRef]
- Revathi, S.; Govindarajan, R.K.; Rameshkumar, N.; Hakkim, F.L.; Mohammed, A.-B.; Krishnan, M.; Kayalvizhi, N. Anti-cancer, anti-microbial and anti-oxidant properties of Acacia nilotica and their chemical profiling. Biocatal. Agric. Biotechnol. 2017, 11, 322–329. [Google Scholar] [CrossRef]
- Abdel-Farid, I.B.; Sheded, M.G.; Mohamed, E.A. Metabolomic profiling and antioxidant activity of some Acacia species. Saudi J. Biol. Sci. 2014, 21, 400–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulrazak, S.A.; Fujihara, T.; Ondiek, J.K.; Ørskov, E.R. Nutritive evaluation of some Acacia tree leaves from Kenya. Anim. Feed Sci. Technol. 2000, 85, 89–98. [Google Scholar] [CrossRef]
- Abdoul-latif, F.M.; Osman, D.A.; Fourreh, A.E.; Abdallah, A.H.; Merito, A.; Hassan, S.; Asfaw, Z.; Kelbessa, E. Candidate medicinal plant species of djiboutian pharmacopeia for testing pharmacological activities on common microbial diseases. Int. J. Pharm. Pharm. Sci. 2016, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Geissler, P.W.; Harris, S.A.; Prince, R.J.; Olsen, A.; Odhiambo, R.A.; Oketch-Rabah, H.; Madiega, P.A.; Andersen, A.; Mølgaard, P. Medicinal plants used by Luo mothers and children in Bondo district, Kenya. J. Ethnopharmacol. 2002, 83, 39–54. [Google Scholar] [CrossRef]
- Wambugu, S.N.; Mathiu, P.M.; Gakuya, D.W.; Kanui, T.I.; Kabasa, J.D.; Kiama, S.G. Medicinal plants used in the management of chronic joint pains in Machakos and Makueni counties, Kenya. J. Ethnopharmacol. 2011, 137, 945–955. [Google Scholar] [CrossRef]
- Hassan-Abdallah, A.; Merito, A.; Hassan, S.; Aboubaker, D.; Djama, M.; Asfaw, Z.; Kelbessa, E. Medicinal plants and their uses by the people in the Region of Randa, Djibouti. J. Ethnopharmacol. 2013, 148, 701–713. [Google Scholar] [CrossRef]
- Hammiche, V.; Maiza, K. Traditional medicine in Central Sahara: Pharmacopoeia of Tassili N’ajjer. J. Ethnopharmacol. 2006, 105, 358–367. [Google Scholar] [CrossRef]
- Eldeen, I.M.S.; Van Staden, J. Cyclooxygenase inhibition and antimycobacterial effects of extracts from Sudanese medicinal plants. South. Afr. J. Bot. 2008, 74, 225–229. [Google Scholar] [CrossRef] [Green Version]
- Eldeen, I.M.S.; Van Staden, J. In vitro pharmacological investigation of extracts from some trees used in Sudanese traditional medicine. South Afr. J. Bot. 2007, 73, 435–440. [Google Scholar] [CrossRef] [Green Version]
- Muthaura, C.N.; Keriko, J.M.; Mutai, C.; Yenesew, A.; Gathirwa, J.W.; Irungu, B.N.; Nyangacha, R.; Mungai, G.M.; Derese, S. Antiplasmodial potential of traditional antimalarial phytotherapy remedies used by the Kwale community of the Kenyan Coast. J. Ethnopharmacol. 2015, 170, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.E.M.; Abdelgadir, H.; Sugimoto, Y.; Khalid, H.E.; Efferth, T. Cytotoxicity of 35 medicinal plants from Sudan towards sensitive and multidrug-resistant cancer cells. J. Ethnopharmacol. 2015, 174, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Tang, C.-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Mugedo, J.Z.A.; Waterman, P.G. Sources of tannin: Alternatives to wattle (Acacia mearnsii) among indigenous Kenyan species. Econ. Bot. 1992, 46, 55–63. [Google Scholar] [CrossRef]
- Wei, S.-D.; Zhou, H.-C.; Lin, Y.-M.; Liao, M.-M.; Chai, W.-M. MALDI-TOF MS analysis of condensed tannins with potent antioxidant activity from the leaf, stem bark and root bark of Acacia confusa. Mol. Basel Switz. 2010, 15, 4369–4381. [Google Scholar] [CrossRef] [Green Version]
- Tung, Y.-T.; Wu, J.-H.; Huang, C.-Y.; Kuo, Y.-H.; Chang, S.-T. Antioxidant activities and phytochemical characteristics of extracts from Acacia confusa bark. Bioresour. Technol. 2009, 100, 509–514. [Google Scholar] [CrossRef]
- Arnao, M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Halake, K.; Birajdar, M.; Lee, J. Structural implications of polyphenolic antioxidants. J. Ind. Eng. Chem. 2016, 35, 1–7. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mandal, S.M.; Jana, S.K.; Das, S.; Mandal, M. Studies on the phenolic profiling, anti-oxidant and cytotoxic activity of Indian honey: In vitro evaluation. Nat. Prod. Res. 2010, 24, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-García, V.; Trindade, C.; Lima, E.; Guecheva, T.N.; Villela, I.; Martinez-Lopez, W.; Corrêa, D.S.; de Ferraz, A.B.F.; Moura, S.; Sosa, M.Q.; et al. Chemical characterization and cytotoxic, genotoxic, and mutagenic properties of Baccharis trinervis (Lam, Persoon) from Colombia and Brazil. J. Ethnopharmacol. 2018, 213, 210–220. [Google Scholar] [CrossRef]
- Marzouk, M.M. Flavonoid constituents and cytotoxic activity of Erucaria hispanica (L.) Druce growing wild in Egypt. Arab. J. Chem. 2016, 9, 411–415. [Google Scholar] [CrossRef] [Green Version]
- Sundarraj, S.; Thangam, R.; Sreevani, V.; Kaveri, K.; Gunasekaran, P.; Achiraman, S.; Kannan, S. γ-Sitosterol from Acacia nilotica L. induces G2/M cell cycle arrest and apoptosis through c-Myc suppression in MCF-7 and A549 cells. J. Ethnopharmacol. 2012, 141, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Tittikpina, N.K.; Nana, F.; Fontanay, S.; Philippot, S.; Batawila, K.; Akpagana, K.; Kirsch, G.; Chaimbault, P.; Jacob, C.; Duval, R.E. Antibacterial activity and cytotoxicity of Pterocarpus erinaceus Poir extracts, fractions and isolated compounds. J. Ethnopharmacol. 2018, 212, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Mulaudzi, R.B.; Ndhlala, A.R.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Antimicrobial properties and phenolic contents of medicinal plants used by the Venda people for conditions related to venereal diseases. J. Ethnopharmacol. 2011, 135, 330–337. [Google Scholar] [CrossRef]
- Tafesh, A.; Najami, N.; Jadoun, J.; Halahlih, F.; Riepl, H.; Azaizeh, H. Synergistic Antibacterial Effects of Polyphenolic Compounds from Olive Mill Wastewater. Available online: https://www.hindawi.com/journals/ecam/2011/431021/ (accessed on 25 April 2020).
- Donovan, J.L.; Luthria, D.L.; Stremple, P.; Waterhouse, A.L. Analysis of (+)-catechin, (-)-epicatechin and their 3′- and 4′-O-methylated analogs. A comparison of sensitive methods. J. Chromatogr. B. Biomed. Sci. App. 1999, 726, 277–283. [Google Scholar] [CrossRef]
- NIST Standard Reference Databases: Analytical Chemistry. Available online: https://www.nist.gov/srd/nist-standard-reference-databases-analytical-chemistry (accessed on 25 April 2020).
- Zhang, L.L.; Lin, Y.M. HPLC, NMR and MALDI-TOF MS analysis of condensed tannins from Lithocarpus glaber leaves with potent free radical scavenging activity. Mol. Basel Switz. 2008, 13, 2986–2997. [Google Scholar] [CrossRef] [Green Version]
- Navarrete, P.; Pizzi, A.; Pasch, H.; Rode, K.; Delmotte, L. MALDI-TOF and 13C NMR characterization of maritime pine industrial tannin extract. Ind. Crop. Prod. 2010, 32, 105–110. [Google Scholar] [CrossRef]
- Duval, A.; Avérous, L. Characterization and physicochemical properties of condensed tannins from Acacia catechu. J. Agric. Food Chem. 2016, 64, 1751–1760. [Google Scholar] [CrossRef]
- Lhuillier, A. Contribution à l’étude Phytochimique de Quatre Plantes Malgaches: Agauria salicifolia Hook.f ex Oliver, Agauria polyphylla Baker (Ericaceae), Tambourissa trichophylla Baker (Monimiaceae) et Embelia concinna Baker (Myrsinaceae). Ph.D. Thesis, National Polytechnic Institute of Toulouse, Toulouse, France, 2007. [Google Scholar]
- Butsat, S.; Siriamornpun, S. Antioxidant capacities and phenolic compounds of the husk, bran and endosperm of Thai rice. Food Chem. 2010, 119, 606–613. [Google Scholar] [CrossRef]
- Dowd, L.E. Spectrophotometric determination of quercetin. Anal. Chem. 1959, 31, 1184–1187. [Google Scholar] [CrossRef]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of propolis extract and identification of principal constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar]
- Huang, B.; Ban, X.; He, J.; Tong, J.; Tian, J.; Wang, Y. Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves. Food Chem. 2010, 120, 873–878. [Google Scholar] [CrossRef]
- Huang, B.; Ke, H.; He, J.; Ban, X.; Zeng, H.; Wang, Y. Extracts of Halenia elliptica exhibit antioxidant properties in vitro and in vivo. Food Chem. Toxicol. 2011, 49, 185–190. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Clinical Laboratory Testing and in vitro Diagnostic Test Systems—Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Reference Method for Testing the in Yitro Activity of Antimicrobial Agents Against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases. Available online: https://www.iso.org/standard/41630.html (accessed on 25 April 2020).
- Cockerill, F. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Third Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013; ISBN 978-1-56238-865-2. [Google Scholar]
- EUCAST: MIC Determination. Available online: https://eucast.org/ast_of_bacteria/mic_determination/ (accessed on 25 April 2020).
Sample Availability: Samples of the crude extracts and compounds 1, 2 and 3 are available from the authors. |


| Extracts | TPC (mg GAE/100 g) | TFC (mg QE/100 g) | Extractable Tannins (%) |
|---|---|---|---|
| Methanolic extract | 1927.1 ± 11.1 | 30.9 ± 1.5 | 82.8 ± 2.3% |
| Water extract | 1820.5 ± 13.6 | 13.8 ± 5.3 | 80.7 ± 1.1% |
| Extracts, Fractions and Standards | DPPH IC50 (µg/mL) | ABTS IC50 (µg/mL) | FRAP (µg VE/g) |
|---|---|---|---|
| Methanolic crude extract | 150 ± 2.2 | 27 ± 1.3 | 45.7 ± 6.1 |
| Filtrate FM | 250 ± 12 | 50 ± 3 | 20.7 ± 2.3 |
| Precipitate PM | 95 ± 3 | 16 ± 0.8 | 54.4 ± 9.3 |
| Water crude extract | 183 ± 5.3 | 33 ± 2 | 5.7 ± 1.0 |
| Filtrate FW | 321 ± 13 | 41 ± 1 | 3.7 ± 1.6 |
| Precipitate PW | 136 ± 5 | 21 ± 1 | 6.7 ± 1.1 |
| Vitamin C | 70 ± 7 | 70 ± 1.5 | ND |
| Trolox | 110 ± 2.5 | 18 ± 3.3 | ND |
| Extracts, Fractions and Pure Compounds | Staphylococcus aureus | Pseudomonas aeruginosa | Corynebacterium urealyticum |
|---|---|---|---|
| Water extract | 512 | >1024 | >1024 |
| Methanolic extract | 64 | 512 | 64 |
| PM | 512 | >1024 | >1024 |
| FM | 32 | 512 | 32 |
| F1 | >256 | >256 | >256 |
| F2 | 128 | >256 | 256 |
| F3 | >256 | >256 | >256 |
| F4 | >256 | >256 | >256 |
| F5 | 128 | 128 | 128 |
| F6 | 256 | 256 | >256 |
| F7 | 128 | 256 | >256 |
| F8 | 128 | 128 | >256 |
| F9 | 256 | >256 | >256 |
| F10 | >256 | >256 | >256 |
| F11 | >256 | 128 | 256 |
| F12 | 32 | >1024 | 128 |
| Compound 1 | 512 | >1024 | 512 |
| Compound 2 | >1024 | >1024 | >1024 |
| Compound 3 | 1024 | 1024 | 1024 |
| Fraction | Series | [M+Na] Experimental | [M+Na] Calculated | N1 | N2 | N3 | N2+ DHB | DP | Polymer |
|---|---|---|---|---|---|---|---|---|---|
| Precipitate (PM) from Methanol Extract | |||||||||
| PM | S1 | 737.3 | 737.1 | 0 | 2 | 0 | 1 | 2 | Dimer |
| PM | S1 | 1025.3 | 1025.2 | 0 | 3 | 0 | 1 | 3 | Trimer |
| PM | S1 | 1329.4 | 1329.3 | 1 | 3 | 0 | 1 | 4 | Tetramer |
| PM | S1 | 1633.4 | 1633.3 | 2 | 3 | 0 | 1 | 5 | Pentamer |
| PM | S2 | 889.3 | 889.2 | 0 | 3 | 0 | 0 | 3 | Trimer |
| PM | S2 | 905.2 | 905.2 | 1 | 2 | 0 | 0 | 3 | Trimer |
| PM | S2 | 921.2 | 921.2 | 2 | 1 | 0 | 0 | 3 | Trimer |
| PM | S2 | 1193.0 | 1193.3 | 1 | 3 | 0 | 0 | 4 | Tetramer |
| PM | S2 | 1481.4 | 1481.3 | 1 | 4 | 0 | 0 | 5 | Pentamer |
| PM | S2 | 1769.5 | 1769.4 | 1 | 5 | 0 | 0 | 6 | Hexamer |
| PM | S2 | 2057.5 | 2057.4 | 1 | 6 | 0 | 0 | 7 | Heptamer |
| PM | S2 | 2345.6 | 2345.6 | 1 | 7 | 0 | 0 | 8 | Octamer |
| Precipitate (PW) from Water Extract | |||||||||
| PW | S3 | 617.2 | 617.1 | 1 | 1 | 0 | 0 | 2 | Dimer |
| PW | S3 | 905.2 | 905.2 | 1 | 2 | 0 | 0 | 3 | Trimer |
| PW | S3 | 1193.3 | 1193.3 | 1 | 3 | 0 | 0 | 4 | Tetramer |
| PW | S3 | 1481.4 | 1481.3 | 1 | 4 | 0 | 0 | 5 | Pentamer |
| PW | S3 | 1769.4 | 1769.4 | 1 | 5 | 0 | 0 | 6 | Hexamer |
| PW | S3 | 2057.5 | 2057.4 | 1 | 6 | 0 | 0 | 7 | Heptamer |
| PW | S3 | 2345.6 | 2345.5 | 1 | 7 | 0 | 0 | 8 | Octamer |
| PW | S3 | 2633.6 | 2633.6 | 1 | 8 | 0 | 0 | 9 | Nonamer |
| PW | S4 | 769.2 | 769.1 | 1 | 1 | 1 | 0 | 2 | Dimer |
| PW | S4 | 1057.2 | 1057.2 | 1 | 2 | 1 | 0 | 3 | Trimer |
| PW | S4 | 1345.3 | 1345.3 | 1 | 3 | 1 | 0 | 4 | Tetramer |
| PW | S4 | 1633.4 | 1633.3 | 1 | 4 | 1 | 0 | 5 | Pentamer |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmi, A.; Spina, R.; Risler, A.; Philippot, S.; Mérito, A.; Duval, R.E.; Abdoul-latif, F.M.; Laurain-Mattar, D. Evaluation of Antioxidant and Antibacterial Activities, Cytotoxicity of Acacia seyal Del Bark Extracts and Isolated Compounds. Molecules 2020, 25, 2392. https://doi.org/10.3390/molecules25102392
Elmi A, Spina R, Risler A, Philippot S, Mérito A, Duval RE, Abdoul-latif FM, Laurain-Mattar D. Evaluation of Antioxidant and Antibacterial Activities, Cytotoxicity of Acacia seyal Del Bark Extracts and Isolated Compounds. Molecules. 2020; 25(10):2392. https://doi.org/10.3390/molecules25102392
Chicago/Turabian StyleElmi, Abdirahman, Rosella Spina, Arnaud Risler, Stéphanie Philippot, Ali Mérito, Raphaël E. Duval, Fatouma Mohamed Abdoul-latif, and Dominique Laurain-Mattar. 2020. "Evaluation of Antioxidant and Antibacterial Activities, Cytotoxicity of Acacia seyal Del Bark Extracts and Isolated Compounds" Molecules 25, no. 10: 2392. https://doi.org/10.3390/molecules25102392
APA StyleElmi, A., Spina, R., Risler, A., Philippot, S., Mérito, A., Duval, R. E., Abdoul-latif, F. M., & Laurain-Mattar, D. (2020). Evaluation of Antioxidant and Antibacterial Activities, Cytotoxicity of Acacia seyal Del Bark Extracts and Isolated Compounds. Molecules, 25(10), 2392. https://doi.org/10.3390/molecules25102392








