Trioxolone Methyl, a Novel Cyano Enone-Bearing 18?H-Glycyrrhetinic Acid Derivative, Ameliorates Dextran Sulphate Sodium-Induced Colitis in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of TM
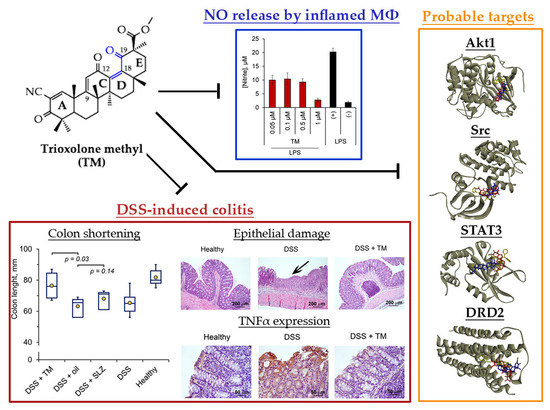
2.2. TM Inhibits NO Production by LPS-Stimulated J774 Macrophages
2.3. TM Inhibits DSS-Induced Colitis in Mice
2.4. Akt1, STAT3 and Dopamine Receptor D2 are Probable Primary Targets of TM, Associated with Its Anti-Colitic Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Synthesis of the Methyl 2-cyano-3,12,19-trioxo-olean-1(2),9(11),13(18)-trien-30-oate (Trioxolone methyl) (TM)
3.3. Cell Cultures and Semi-Synthetic Triterpenoids SM and TM
3.4. Cell Viability analysis by MTT Assay
3.5. Measurement of LPS-induced NO Production by Macrophages
3.6. Mice
3.7. Induction of Acute Colitis
3.8. Histopathological Analysis
3.9. Glutathione (GSH) Assay
3.10. GEO Datasets Processing
3.11. Prediction of Probable Primary Targets of TM
3.12. PPI Network Reconstruction
3.13. Molecular Docking
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krenske, E.H.; Petter, R.C.; Houk, K.N. Kinetics and Thermodynamics of Reversible Thiol Additions to Mono- and Diactivated Michael Acceptors: Implications for the Design of Drugs That Bind Covalently to Cysteines. J. Org. Chem. 2016, 81, 11726–11733. [Google Scholar] [CrossRef]
- Eric, A.; Gary, B.; Deborah, F.; Xin, J.; Robert, K., Jr.; Patrick, O.; Melean, V. Compounds Including an Anti-Inflammatory Pharmacore and Methods of Use Background of the Invention. Patent WO/2009/146218, 20 April 2009. [Google Scholar]
- Popadyuk, I.I.; Markov, A.V.; Salomatina, O.V.; Logashenko, E.B.; Shernyukov, A.V.; Zenkova, M.A.; Salakhutdinov, N.F. Synthesis and biological activity of novel deoxycholic acid derivatives. Bioorganic Med. Chem. 2015, 23, 5022–5034. [Google Scholar] [CrossRef] [PubMed]
- Liby, K.T.; Sporn, M.B. Synthetic Oleanane Triterpenoids: Multifunctional Drugs with a Broad Range of Applications for Prevention and Treatment of Chronic Disease. Pharmacol. Rev. 2012, 64, 972–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markov, A.V.; Zenkova, M.A.; Logashenko, E.B. Modulation of Tumour-Related Signaling Pathways by Natural Pentacyclic Triterpenoids and their Semisynthetic Derivatives. Curr. Med. Chem. 2017, 24, 1277–1320. [Google Scholar] [CrossRef] [PubMed]
- You, Y.J.; Kim, Y.; Nam, N.H.; Ahn, B.Z. Synthesis and cytotoxic activity of A-ring modified betulinic acid derivatives. Bioorganic Med. Chem. Lett. 2003, 13, 3137–3140. [Google Scholar] [CrossRef]
- Subba Rao, G.S.R.; Kondaiah, P.; Singh, S.K.; Ravanan, P.; Sporn, M.B. Chemical modifications of natural triterpenes-glycyrrhetinic and boswellic acids: Evaluation of their biological activity. Tetrahedron 2008, 64, 11541–11548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.; Kaur, R.; Shah, B.A.; Malik, F.; Kumar, A.; Bhushan, S.; Jain, S.K.; Taneja, S.C.; Singh, J. A Novel cyano derivative of 11-Keto-β-Boswellic acid causes apoptotic death by disrupting PI3K/AKT/Hsp-90 cascade, mitochondrial integrity, and other cell survival signaling events in HL-60 cells. Mol. Carcinog. 2012, 51, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Rounds, B.V.; Bore, L.; Finlay, H.J.; Favaloro, F.G.; Suh, N.; Wang, Y.; Sporn, M.B.; Gribble, G.W. Synthetic oleanane and ursane triterpenoids with modified rings A and C: A series of highly active inhibitors of nitric oxide production in mouse macrophages. J. Med. Chem. 2000, 43, 4233–4246. [Google Scholar] [CrossRef]
- Logashenko, E.B.; Salomatina, O.V.; Markov, A.V.; Korchagina, D.V.; Salakhutdinov, N.F.; Tolstikov, G.A.; Vlassov, V.V.; Zenkova, M.A. Synthesis and Pro-Apoptotic Activity of Novel Glycyrrhetinic Acid Derivatives. ChemBioChem 2011, 12, 784–794. [Google Scholar] [CrossRef] [Green Version]
- Song, D.; Gao, Y.; Wang, R.; Liu, D.; Zhao, L.; Jing, Y. Downregulation of c-FLIP, XIaP and Mcl-1 protein as well as depletion of reduced glutathione contribute to the apoptosis induction of glycyrrhetinic acid derivatives in leukemia cells. Cancer Biol. Ther. 2010, 9, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Lin, Q.X.; Liby, K.T.; Sporn, M.B.; Gribble, G.W. An efficient synthesis of methyl 2-cyano-3,12-dioxoursol-1,9-dien-28-oate (CDDU-methyl ester): Analogues, biological activities, and comparison with oleanolic acid derivatives. Org. Biomol. Chem. 2014, 12, 5192–5200. [Google Scholar] [CrossRef] [PubMed]
- An Extended Access Program for Bardoxolone Methyl in Patients with CKD. Available online: https://clinicaltrials.gov/ct2/show/NCT03749447 (accessed on 18 April 2020).
- A Phase 2/3 Trial of the Efficacy and Safety of Bardoxolone Methyl in Patients with Alport Syndrome. Available online: https://clinicaltrials.gov/ct2/show/NCT03019185 (accessed on 18 April 2020).
- Markov, A.V.; Sen’kova, A.V.; Zenkova, M.A.; Logashenko, E.B. Novel Glycyrrhetinic Acid Derivative Soloxolone Methyl Inhibits the Inflammatory Response and Tumor Growth in vivo. Mol. Biol. 2018, 52, 262–268. [Google Scholar] [CrossRef]
- Salomatina, O.V.; Markov, A.V.; Logashenko, E.B.; Korchagina, D.V.; Zenkova, M.A.; Salakhutdinov, N.F.; Vlassov, V.V.; Tolstikov, G.A. Synthesis of novel 2-cyano substituted glycyrrhetinic acid derivatives as inhibitors of cancer cells growth and NO production in LPS-activated J-774 cells. Bioorganic Med. Chem. 2014, 22, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.V.; Sen’Kova, A.V.; Warszycki, D.; Salomatina, O.V.; Salakhutdinov, N.F.; Zenkova, M.A.; Logashenko, E.B. Soloxolone methyl inhibits influenza virus replication and reduces virus-induced lung inflammation. Sci. Rep. 2017, 7, 13968. [Google Scholar] [CrossRef] [Green Version]
- Urban, M.; Klinot, J.; Tislerova, I.; Biedermann, D.; Hajduch, M.; Cisarova, I.; Sarek, J. Reactions of activated lupane oxo-compounds with diazomethane: An approach to new derivatives of cytotoxic triterpenes. Synthesis 2006, 3979–3986. [Google Scholar]
- Hussain, H.; Green, I.R.; Ali, I.; Khan, I.A.; Ali, Z.; Al-Sadi, A.M.; Ahmed, I. Ursolic acid derivatives for pharmaceutical use: A patent review (2012–2016). Expert Opin. Ther. Pat. 2017, 27, 1061–1072. [Google Scholar] [CrossRef]
- Ruzicka, L.; Jeger, O.; Winter, M. Zur Kenntnis der Triterpene. (75. Mitteilung). Zur Lage der Carboxylgruppe bei der Oleanolsäure und der Glycyrrhetinsäure. Helv. Chim. Acta 1943, 26, 265–279. [Google Scholar] [CrossRef]
- Liu, B.; Piao, X.; Guo, L.; Liu, S.; Chai, F.; Gao, L. Ursolic acid protects against ulcerative colitis via anti-inflammatory and antioxidant effects in mice. Mol. Med. Rep. 2016, 13, 4779–4785. [Google Scholar] [CrossRef] [Green Version]
- Anthoni, C.; Laukoetter, M.G.; Rijcken, E.; Vowinkel, T.; Mennigen, R.; Müller, S.; Senninger, N.; Russell, J.; Jauch, J.; Bergmann, J.; et al. Mechanisms underlying the anti-inflammatory actions of boswellic acid derivatives in experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1131–G1137. [Google Scholar] [CrossRef] [Green Version]
- Jeon, Y.D.; Kang, S.H.; Bang, K.S.; Chang, Y.N.; Lee, J.H.; Jin, J.S. Glycyrrhetic acid ameliorates dextran sulfate sodium-induced ulcerative colitis in vivo. Molecules 2016, 21, 523. [Google Scholar] [CrossRef] [Green Version]
- Kang, G.D.; Lim, S.; Kim, D.H. Oleanolic acid ameliorates dextran sodium sulfate-induced colitis in mice by restoring the balance of Th17/Treg cells and inhibiting NF-κB signaling pathway. Int. Immunopharmacol. 2015, 29, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, L.R.; Stonesifer, E.; Small, J.S.; Liby, K.T. The synthetic triterpenoid (CDDO-Im) inhibits STAT3, as well as IL-17, and improves DSS-induced colitis in mice. Inflammopharmacology 2014, 22, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Ogita, T.; Tari, H.; Arakawa, T.; Suzuki, T. Supplemental psyllium fibre regulates the intestinal barrier and inflammation in normal and colitic mice. Br. J. Nutr. 2017, 118, 661–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Lin, C.H.; Nagarajan, B.; Sankaranarayanan, N.V.; Desai, U.R.; Liu, M.Y. Mucoadhesive role of tamarind xyloglucan on inflammation attenuates ulcerative colitis. J. Funct. Foods 2018, 47, 1–10. [Google Scholar] [CrossRef]
- Mauricio, I.; Francischetti, B.; Monteiro, R.Q.; Guimarães, J.A. Identification of Glycyrrhizin as a thrombin inhibitor. Biochem. Biophys. Res. Commun. 1997, 235, 259–263. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, S.H.; Lee, S.Y.; Kim, E.K.; Kwon, J.E.; Seo, H.B.; Lee, H.H.; Lee, B.I.; Park, S.H.; Cho, M. La Grim19 attenuates DSS induced colitis in an animal model. PLoS ONE 2016, 11, e0155853. [Google Scholar]
- Vassilyadi, P.; Harding, S.V.; Nitschmann, E.; Wykes, L.J. Experimental colitis and malnutrition differentially affect the metabolism of glutathione and related sulfhydryl metabolites in different tissues. Eur. J. Nutr. 2016, 55, 1769–1776. [Google Scholar] [CrossRef]
- Yang, L.; Calingasan, N.Y.; Thomas, B.; Charturvedi, R.K.; Kiaei, M.; Wille, E.J.; Liby, K.T.; Williams, C.; Royce, D.; Risingson, R.; et al. Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2-mediated transcription. PLoS ONE 2009, 4, e5757. [Google Scholar] [CrossRef] [Green Version]
- Awale, M.; Reymond, J.L. Polypharmacology Browser PPB2: Target Prediction Combining Nearest Neighbors with Machine Learning. J. Chem. Inf. Model. 2019, 59, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Santos, A.; Von Mering, C.; Jensen, L.J.; Bork, P.; Kuhn, M. STITCH 5: Augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2016, 44, D380–D384. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, S.; Henry, K.; Pei, H.; Clayton, J.; Rempala, M.; Johns, D.; De Frutos, O.; Garcia, P.; Mateos, C.; Pleite, S.; et al. Discovery of chiral dihydropyridopyrimidinones as potent, selective and orally bioavailable inhibitors of AKT. Bioorganic Med. Chem. Lett. 2018, 28, 1887–1891. [Google Scholar] [CrossRef] [PubMed]
- Sakkiah, S.; Arullaperumal, V.; Hwang, S.; Lee, K.W. Ligand-based pharmacophore modeling and Bayesian approaches to identify c-Src inhibitors. J. Enzyme Inhib. Med. Chem. 2014, 29, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, Z.; Ding, C.; Xiong, A.; Wild, C.; Wang, L.; Ye, N.; Cai, G.; Flores, R.M.; Ding, Y.; et al. Discovery of potent anticancer agent HJC0416, an orally bioavailable small molecule inhibitor of signal transducer and activator of transcription 3 (STAT3). Eur. J. Med. Chem. 2014, 82, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Tan, L.; Chen, Z.; Qi, J.; Nie, F.; Luo, Z.; Cheng, J.; Wang, S. Haloperidol bound D2 dopamine receptor structure inspired the discovery of subtype selective ligands. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Bhasin, D.; Cisek, K.; Pandharkar, T.; Regan, N.; Li, C.; Pandit, B.; Lin, J.; Li, P.K. Design, synthesis, and studies of small molecule STAT3 inhibitors. Bioorganic Med. Chem. Lett. 2008, 18, 391–395. [Google Scholar] [CrossRef]
- Guo, S.; Luo, W.; Liu, L.; Pang, X.; Zhu, H.; Liu, A.; Lu, J.; Ma, D.L.; Leung, C.H.; Wang, Y.; et al. Isocryptotanshinone, a STAT3 inhibitor, induces apoptosis and pro-death autophagy in A549 lung cancer cells. J. Drug Target. 2016, 24, 934–942. [Google Scholar] [CrossRef]
- Yang, Y.X.; Li, S.Y.; Zhang, Q.; Chen, H.; Xia, Z.N.; Yang, F.Q. Characterization of phenolic acids binding to thrombin using frontal affinity chromatography and molecular docking. Anal. Methods 2017, 9, 5174–5180. [Google Scholar] [CrossRef]
- Pereira, R.C.C.; Lourenço, A.L.; Terra, L.; Abreu, P.A.; Teixeira, V.L.; Castro, H.C. Marine diterpenes: Molecular modeling of thrombin inhibitors with potential biotechnological application as an antithrombotic. Mar. Drugs 2017, 15, 79. [Google Scholar] [CrossRef] [Green Version]
- Vijayalakshmi, J.; Padmanabhan, K.P.; Tulinsky, A.; Mann, K.G. The isomorphous structures of prethrombin2, hirugen–, and PPACK–thrombin: Changes accompanying activation and exosite binding to thrombin. Protein Sci. 1994, 3, 2254–2271. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.L.; Xu, J.; Zhang, X.H.; Qiu, B.Y.; Peng, L.; Zhang, M.; Gan, H.T. PI3K/Akt signaling pathway is involved in the pathogenesis of ulcerative colitis. Inflamm. Res. 2011, 60, 727–734. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; He, X.; Chen, Z.; Ke, J.; He, X.; Yuan, R.; Cai, Z.; Chen, X.; Wu, X.; Lan, P. Activation of the mTORC1 and STAT3 pathways promotes the malignant transformation of colitis in mice. Oncol. Rep. 2014, 32, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Tolstanova, G.; Deng, X.; Ahluwalia, A.; Paunovic, B.; Prysiazhniuk, A.; Ostapchenko, L.; Tarnawski, A.; Sandor, Z.; Szabo, S. Role of Dopamine and D2 Dopamine Receptor in the Pathogenesis of Inflammatory Bowel Disease. Dig. Dis. Sci. 2015, 60, 2963–2975. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Raina, D.; Meyer, C.; Kufe, D. Triterpenoid CDDO-methyl ester inhibits the Janus-activated kinase-1 (JAK1)→signal transducer and activator of transcription-3 (STAT3) pathway by direct inhibition of JAK1 and STAT3. Cancer Res. 2008, 68, 2920–2926. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Wang, H.; Han, C.; Cao, X. Src promotes anti-inflammatory (M2) macrophage generation via the IL-4/STAT6 pathway. Cytokine 2018, 111, 209–215. [Google Scholar] [CrossRef]
- Lim, W.C.; Wang, Y.; Macdonald, J.K.; Hanauer, S. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database Syst. Rev. 2016, 2016, CD008870. [Google Scholar] [CrossRef]
- Zhao, L.N.; Li, J.Y.; Yu, T.; Chen, G.C.; Yuan, Y.H.; Chen, Q.K. 5-Aminosalicylates reduce the risk of colorectal neoplasia in patients with ulcerative colitis: An updated meta-analysis. PLoS ONE 2014, 9, e94208. [Google Scholar] [CrossRef] [Green Version]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [Green Version]
- Heberle, H.; Meirelles, V.G.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Assenov, Y.; Ramírez, F.; Schelhorn, S.E.S.E.; Lengauer, T.; Albrecht, M. Computing topological parameters of biological networks. Bioinformatics 2008, 24, 282–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trott, O.; Olson, A.J. Software news and update AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [PubMed] [Green Version]








| Protein | PDB ID | Center | Size | ||||
|---|---|---|---|---|---|---|---|
| x | Y | z | x | y | z | ||
| Thrombin | 2ZDA | 15.88 | −13.212 | 22.865 | 34 | 20 | 16 |
| Akt1 | 6CCY | −10.177 | 15.902 | −31.523 | 40 | 34 | 26 |
| Src | 4O2P | −8.493 | −24.568 | −6.5 | 32 | 30 | 30 |
| STAT3 | 6NJS | 12.405 | 54.061 | 0.521 | 28 | 58 | 30 |
| DRD2 | 6LUQ | 8.946 | 6.635 | −8.471 | 20 | 18 | 42 |
| OPRM1 | 5C1M | 2.022 | 15.93 | −59.7 | 18 | 28 | 18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markov, A.V.; Sen’kova, A.V.; Salomatina, O.V.; Logashenko, E.B.; Korchagina, D.V.; Salakhutdinov, N.F.; Zenkova, M.A. Trioxolone Methyl, a Novel Cyano Enone-Bearing 18?H-Glycyrrhetinic Acid Derivative, Ameliorates Dextran Sulphate Sodium-Induced Colitis in Mice. Molecules 2020, 25, 2406. https://doi.org/10.3390/molecules25102406
Markov AV, Sen’kova AV, Salomatina OV, Logashenko EB, Korchagina DV, Salakhutdinov NF, Zenkova MA. Trioxolone Methyl, a Novel Cyano Enone-Bearing 18?H-Glycyrrhetinic Acid Derivative, Ameliorates Dextran Sulphate Sodium-Induced Colitis in Mice. Molecules. 2020; 25(10):2406. https://doi.org/10.3390/molecules25102406
Chicago/Turabian StyleMarkov, Andrey V., Aleksandra V. Sen’kova, Oksana V. Salomatina, Evgeniya B. Logashenko, Dina V. Korchagina, Nariman F. Salakhutdinov, and Marina A. Zenkova. 2020. "Trioxolone Methyl, a Novel Cyano Enone-Bearing 18?H-Glycyrrhetinic Acid Derivative, Ameliorates Dextran Sulphate Sodium-Induced Colitis in Mice" Molecules 25, no. 10: 2406. https://doi.org/10.3390/molecules25102406
APA StyleMarkov, A. V., Sen’kova, A. V., Salomatina, O. V., Logashenko, E. B., Korchagina, D. V., Salakhutdinov, N. F., & Zenkova, M. A. (2020). Trioxolone Methyl, a Novel Cyano Enone-Bearing 18?H-Glycyrrhetinic Acid Derivative, Ameliorates Dextran Sulphate Sodium-Induced Colitis in Mice. Molecules, 25(10), 2406. https://doi.org/10.3390/molecules25102406