Sample Preparation Using Graphene-Oxide-Derived Nanomaterials for the Extraction of Metals
Abstract
1. Introduction
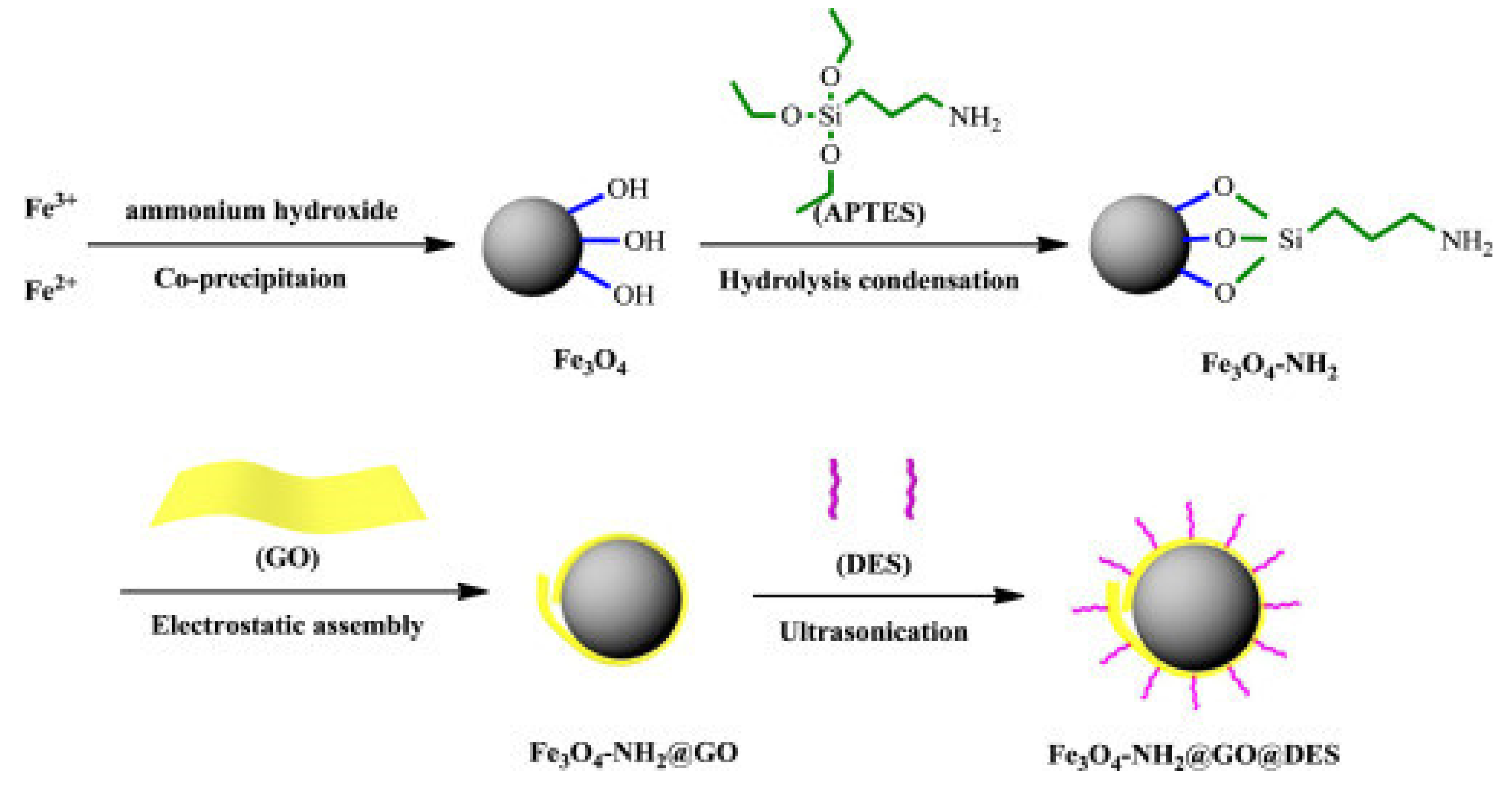
2. Synthesis of Graphene-Oxide-Derived Materials
3. Extraction of Metal Ions with Graphene-Oxide-Derived Materials
3.1. Extraction of Mercury
3.2. Extraction of Chromium
3.3. Extraction of Cadmium
3.4. Extraction of Gold
3.5. Extraction of Cobalt
3.6. Extraction of Zinc
3.7. Extraction of Copper
3.8. Extraction of Lead
3.9. Extraction of Thallium
3.10. Extraction of Cerium
3.11. Extraction of Samarium
4. Multielement Extraction with Graphene-Oxide-Derived Materials
5. Application of Ionic Liquids and Deep Eutectic Solvents for the Modification of GO
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Censi, P.; Spoto, S.E.; Saiano, F.; Sprovieri, M.; Mazzola, S.; Nardone, G.; Di Geronimo, S.I.; Punturo, R.; Ottonello, D. Heavy metals in coastal water systems. A case study from the northwestern Gulf of Thailand. Chemosphere 2016, 64, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Squadrone, S.; Prearo, M.; Brizio, P.; Gavinelli, S.; Pellegrino, M.; Scanzio, T.; Guarise, S.; Benetto, A.; Abete, M.C. Heavy metals distribution in muscle, liver, kidney and gill of European catfish (Silurus glanis) from Italian Rivers. Chemosphere 2013, 90, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Canli, M.; Atli, G. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ. Pollut. 2013, 121, 129–136. [Google Scholar] [CrossRef]
- Zachariadis, G.; Anthemidis, A.N.; Bettas, P.G.; Stratis, J.A. Determination of lead by on-line solid phase extraction using a PTFE micro-column and flame atomic absorption spectrometry. Talanta 2002, 57, 919–927. [Google Scholar] [CrossRef]
- Mousavi, H.Z.; Asghari, A.; Shirkhanloo, H. Determination of Hg in water and wastewater samples by CV-AAS following on-line preconcentration with silver trap. J. Anal. Chem. 2010, 65, 935–939. [Google Scholar] [CrossRef]
- Daftsis, E.; Zachariadis, G. Analytical performance of ETAAS method for Cd, Co, Cr and Pb determination in blood fractions samples. Talanta 2007, 71, 722–730. [Google Scholar] [CrossRef]
- Momen, A.; Zachariadis, G.; Anthemidis, A.; Stratis, J. Optimization and comparison of two digestion methods for multi-element analysis of certified reference plant materials by ICP-AES. Application of Plackett-Burman and central composite designs. Microchim. Acta 2007, 160, 397–403. [Google Scholar] [CrossRef]
- Manousi, N.; Gomez-Gomez, B.; Madrid, Y.; Deliyanni, E.; Zachariadis, G. Determination of rare earth elements by inductively coupled plasma-mass spectrometry after dispersive solid phase extraction with novel oxidized graphene oxide and optimization with response surface methodology and central composite design. Microchem. J. 2019, 152. [Google Scholar] [CrossRef]
- Feist, B.; Mikula, B. Preconcentration of heavy metals on activated carbon and their determination in fruits by inductively coupled plasma optical emission spectrometry. Food Chem. 2014, 147, 302–306. [Google Scholar] [CrossRef]
- Giakisikli, G.; Anthemidis, A. Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review. Anal. Chim. Acta 2013, 789, 1–16. [Google Scholar] [CrossRef]
- Rosillo-Lopez, M.; Salzmann, C. Highly efficient heavy-metal extraction from water with carboxylated graphene nanoflakes. RSC Adv. 2018, 8, 11043–11050. [Google Scholar] [CrossRef]
- Mejias Carpio, I.; Mangadlao, J.; Nguyen, H.; Advincula, R.; Rodrigues, D. Graphene oxide functionalized with ethylenediamine triacetic acid for heavy metal adsorption and anti-microbial applications. Carbon 2014, 77, 289–301. [Google Scholar] [CrossRef]
- Ensafi, A.; Rabiei, S.; Rezaei, B.; Allafchian, A. Magnetic solid-phase extraction to preconcentrate ultra trace amounts of lead(ii) using modified-carbon nanotubes decorated with NiFe2O4 magnetic nanoparticles. Anal. Methods 2013, 5, 3903–3908. [Google Scholar] [CrossRef]
- Tadjarodi, A.; Abbaszadeh, A. A magnetic nanocomposite prepared from chelator-modified magnetite (Fe3O4) and HKUST-1 (MOF-199) for separation and preconcentration of mercury(II). Microchim. Acta. 2016, 183, 1391–1399. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Zhao, C.; Hao, J.; Fang, G.; Wang, S. Fabrication of porous covalent organic frameworks as selective and advanced adsorbents for the on-line preconcentration of trace elements against the complex sample matrix. J. Hazard. Mater. 2018, 344, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Wang, S.; Jia, J.; Xu, F.; Long, Z.; Hou, X. Ultrasensitive determination of inorganic arsenic by hydride generation-atomic fluorescence spectrometry using Fe3O4@ZIF-8 nanoparticles for preconcentration. Microchem. J. 2016, 124, 578–583. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, G.; Wei, F.; Song, Q.; Tang, T.; Wang, X.; Hu, Q. Determination of Hg (II) in tea and mushroom samples based on metal-organic frameworks as solid-phase extraction sorbents. Microporous Mesoporous Mater. 2016, 235, 204–210. [Google Scholar] [CrossRef]
- Kalantari, H.; Manoochehri, M. A nanocomposite consisting of MIL-101(Cr) and functionalized magnetite nanoparticles for extraction and determination of selenium(IV) and selenium(VI). Microchim. Acta 2018, 185. [Google Scholar] [CrossRef]
- Rezaei Kahkha, M.; Daliran, S.; Oveisi, A.; Kaykhaii, M.; Sepehri, Z. The Mesoporous Porphyrinic Zirconium Metal-Organic Framework for Pipette-Tip Solid-Phase Extraction of Mercury from Fish Samples Followed by Cold Vapor Atomic Absorption Spectrometric Determination. Food Anal. Methods 2017, 10, 2175–2184. [Google Scholar] [CrossRef]
- Abolghasemi, M.; Yousefi, V.; Piryaei, M. Synthesis of a metal-organic framework confined in periodic mesoporous silica with enhanced hydrostability as a novel fiber coating for solid-phase microextraction. J. Sep. Sci. 2015, 38, 1187–1193. [Google Scholar] [CrossRef]
- Huang, X.; Qiu, N.; Yuan, D.; Huang, B. A novel stir bar sorptive extraction coating based on monolithic material for apolar, polar organic compounds and heavy metal ions. Talanta 2009, 78, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Gheim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, Z.; Xia, J.; Chen, S.; Zhang, X.; Ding, M. Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Talanta 2012, 101, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shi, J.; Jiang, G. Application of graphene in analytical sample preparation. TrAC Trends Anal. Chem. 2012, 37, 1–11. [Google Scholar] [CrossRef]
- Lin, L.; Liu, Y.; Zhao, X.; Li, J. Sensitive and Rapid Screening of T4 Polynucleotide Kinase Activity and Inhibition Based on Coupled Exonuclease Reaction and Graphene Oxide Platform. Anal. Chem. 2011, 83, 8396–8402. [Google Scholar] [CrossRef]
- Tang, L.; Wang, Y.; Li, Y.; Feng, H.; Lu, J.; Li, J. Preparation, Structure, and Electrochemical Properties of Reduced Graphene Sheet Films. Adv. Funct. Mater. 2009, 19, 2782–2789. [Google Scholar] [CrossRef]
- Wang, X.; Bai, H.; Yao, Z.; Liu, A.; Shi, G. Electrically conductive and mechanically strong biomimetic chitosan/reduced graphene oxide composite films. J. Mater. Chem. 2010, 20, 9032–9036. [Google Scholar] [CrossRef]
- Yu, I.; Xiong, X.; Tsang, D.; Ng, Y.; Clark, J.; Fan, J.; Zhang, S.; Hu, C.; Ok, Y. Graphite oxide- and graphene oxide-supported catalysts for microwave-assisted glucose isomerisation in water. Green Chem. 2019, 21, 4341–4353. [Google Scholar] [CrossRef]
- Gadipelli, S.; Guo, Z. Graphene-based materials: Synthesis and gas sorption, storage and separation. Prog. Mater. Sci. 2015, 69, 1–60. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano. Res. 2008, 1, 203–212. [Google Scholar] [CrossRef]
- Sitko, R.; Zawisza, B.; Malicka, E. Graphene as a new sorbent in analytical chemistry. Trac. Trends Anal. Chem. 2013, 51, 33–43. [Google Scholar] [CrossRef]
- Hummers, W.; Offeman, R. Preparation of Graphitic Oxide. J. Amer. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Dai, B.; Shao, X.P.; Ma, Y.J.; Pei, Z.H. Review of new carbon materials—Graphene. Mater. Rev. 2010, 24, 17–21. [Google Scholar]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-Based Composite Materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Maggira, M.; Deliyanni, E.; Samanidou, V. Synthesis of Graphene Oxide Based Sponges and Their Study as Sorbents for Sample Preparation of Cow Milk Prior to HPLC Determination of Sulfonamides. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Manousi, N.; Giannakoudakis, D.; Rosenberg, E.; Zachariadis, G. Extraction of Metal Ions with Metal–Organic Frameworks. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Li, N.; Chen, J.; Shi, Y. Magnetic reduced graphene oxide functionalized with β-cyclodextrin as magnetic solid-phase extraction adsorbents for the determination of phytohormones in tomatoes coupled with high performance liquid chromatography. J. Chromatogr. A 2016, 1441, 24–33. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, H.; Zhang, Z.; Wu, X.; Chen, W.; Zhu, Y.; Fang, C.; Zhao, Y. Three-dimensional ionic liquid functionalized magnetic graphene oxide nanocomposite for the magnetic dispersive solid phase extraction of 16 polycyclic aromatic hydrocarbons in vegetable oils. J. Chromatogr. A 2017, 1489, 29–38. [Google Scholar] [CrossRef]
- Amiri, A.; Baghayeri, M.; Sedighi, M. Magnetic solid-phase extraction of polycyclic aromatic hydrocarbons using a graphene oxide/Fe3O4@polystyrene nanocomposite. Microchim. Acta 2018, 185. [Google Scholar] [CrossRef]
- Kyzas, C.Z.; Deliyanni, E.A.; Bikiaris, D.N.; Mitropoulos, A.C. Graphene composites as dye adsorbents: Review. Chem. Eng. Res. Des. 2018, 129, 75–88. [Google Scholar] [CrossRef]
- Travlou, N.A.; Kyzas, C.Z.; Lazaridis, N.K.; Deliyanni, E.A. Functionalization of graphite oxide with magnetic chitosan for the preparation of a nanocomposite dye adsorbent. Langmuir 2013, 29, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Travlou, N.A.; Kalogirou, O.; Deliyanni, E. Magnetic graphene oxide: Effect of preparation route on Reactive Black 5 adsorption. Materials 2013, 6, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Deliyanni, E.; Matis, K.A. Graphene oxide and its application as adsorbent to waste water treatment. J. Chem. Technol. Biotechnol 2014, 89, 196–205. [Google Scholar] [CrossRef]
- Rekos, K.; Kampouraki, Z.C.; Sarafidis, C.; Samanidou, V.; Deliyanni, E. Graphene Oxide Based Magnetic Nanocomposites with Polymers as Effective Bisphenol–A Nanoadsorbents. Materials 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Scida, K.; Stege, P.W.; Haby, G.; Messina, G.A.; García, C.D. Recent applications of carbon-based nanomaterials in analytical chemistry: Critical review. Anal. Chim. Acta 2011, 691, 6–17. [Google Scholar] [CrossRef]
- Chabot, V.; Higgins, D.; Yu, A.; Xiao, X.; Chen, Z.; Zhang, J. A review of graphene and graphene oxide sponge: Material synthesis and applications to energy and the environment. Energy Environ. Sci. 2014, 7, 1564–1596. [Google Scholar] [CrossRef]
- Manousi, N.; Rosenberg, E.; Deliyanni, E.; Zachariadis, G.A.; Samanidou, V. Magnetic Solid-Phase Extraction of Organic Compounds Based on Graphene Oxide Nanocomposites. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Grajek, H.; Jonik, J.; Witkiewicz, Z.; Wawer, T.; Purchała, M. Applications of Graphene and Its Derivatives in Chemical Analysis. Crit. Rev. Anal. Chem. 2019, 7, 1–27. [Google Scholar] [CrossRef]
- Ibrahim, W.; Nodeh, H.; Sanagi, M. Graphene-Based Materials as Solid Phase Extraction Sorbent for Trace Metal Ions, Organic Compounds, and Biological Sample Preparation. Crit. Rev. Anal. Chem. 2015, 46, 267–283. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.; Potts, J.; Ruoff, R. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Li, W.; Yang, Y.; Qin, P.; Zhang, X.; Lu, M. Magnetic graphene oxide nanocomposites as the adsorbent for extraction and pre-concentration of azo dyes in different food samples followed by high-performance liquid chromatography analysis. Food Addit. Contam. A 2018, 35, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Costa dos Reis, L.; Vidal, L.; Canals, A. Graphene oxide/Fe3O4 as sorbent for magnetic solid-phase extraction coupled with liquid chromatography to determine 2,4,6-trinitrotoluene in water samples. Anal. Bioanal. Chem. 2017, 409, 2665–2674. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yu, L.; Ding, X.; Li, P.; Dai, X.; Chen, X.; Zhou, H.; Bai, Y.; Ding, J. Magnetic solid-phase extraction based on graphene oxide for the determination of lignans in sesame oil. Food Chem. 2017, 217, 320–325. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, R.; Singh, D. Graphene oxide: Strategies for synthesis, reduction and frontier applications. RSC. Adv. 2016, 6, 64993–65011. [Google Scholar] [CrossRef]
- Bele, S.; Samanidou, V.; Deliyanni, E. Effect of the reduction degree of graphene oxide on the adsorption of Bisphenol A. Chem. Eng. Res. Des. 2016, 109, 573–585. [Google Scholar] [CrossRef]
- Qi, T.; Huang, C.; Yan, S.; Li, X.; Pan, S. Synthesis, characterization and adsorption properties of magnetite/reduced graphene oxide nanocomposites. Talanta 2015, 144, 1116–1124. [Google Scholar] [CrossRef]
- Sherlala, A.; Raman, A.; Bello, M.; Asghar, A. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 2018, 193, 1004–1017. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Seidi, S.; Fotouhi, M. Magnetic dispersive solid phase extraction based on polythiophene modified magnetic graphene oxide for mercury determination in seafood followed by flow-injection cold vapor atomic absorption spectrometry. Anal. Methods 2017, 9, 803–813. [Google Scholar] [CrossRef]
- Keramat, A.; Zare-Dorabei, R. Ultrasound-assisted dispersive magnetic solid phase extraction for preconcentration and determination of trace amount of Hg (II) ions from food samples and aqueous solution by magnetic graphene oxide (Fe3O4@GO/2-PTSC): Central composite design optimization. Ultrason. Sonochem. 2017, 38, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Ziaei, E.; Mehdinia, A.; Jabbari, A. A novel hierarchical nanobiocomposite of graphene oxide–magnetic chitosan grafted with mercapto as a solid phase extraction sorbent for the determination of mercury ions in environmental water samples. Anal. Chim. Acta 2014, 850, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, E.; Haji Shabani, A.; Dadfarnia, S.; Izadi, F. Speciation and determination of chromium ions by dispersive micro solid phase extraction using magnetic graphene oxide followed by flame atomic absorption spectrometry. Int. J. Environ. Anal. Chem. 2017, 97, 1080–1093. [Google Scholar] [CrossRef]
- Islam, A.; Ahmad, H.; Zaidi, N.; Kumar, S. A graphene oxide decorated with triethylenetetramine-modified magnetite for separation of chromium species prior to their sequential speciation and determination via FAAS. Microchim. Acta 2015, 183, 289–296. [Google Scholar] [CrossRef]
- Sarikhani, Z.; Manoochehri, M. Determination of Ultra Trace Cr(III) and Cr(VI) Species by Electrothermal Atomic Absorption Spectrometry after Simultaneous Magnetic Solid Phase Extraction with the Aid of a Novel Imidazolium-Functionalized Magnetite Graphene Oxide Nanocomposite. Bull. Chem. Soc. Jpn. 2017, 90, 746–753. [Google Scholar] [CrossRef]
- Seidi, S.; Majd, M. Polyaniline-functionalized magnetic graphene oxide for dispersive solid-phase extraction of Cr(VI) from environmental waters followed by graphite furnace atomic absorption spectrometry. J. Iran. Chem. Soc. 2017, 14, 1195–1206. [Google Scholar] [CrossRef]
- Etezadi, H.; Baramakeh, L. Application of Graphene Oxide- Magnetic Nanoparticle for Solid Phase Extraction of Trace Amounts of Cadmium Ions in Environmental Samples. J. Phys. Chem. Electrochem. 2014, 2, 137–147. [Google Scholar]
- Kazemi, E.; Dadfarnia, S.; Haji Shabani, A. Dispersive solid phase microextraction with magnetic graphene oxide as the sorbent for separation and preconcentration of ultra-trace amounts of gold ions. Talanta 2015, 141, 273–278. [Google Scholar] [CrossRef]
- Ahmad, H.; Jalil, A.; Triwahyono, S. Dispersive solid phase extraction of gold with magnetite-graphene oxide prior to its determination via microwave plasma-atomic emission spectrometry. RSC Adv. 2016, 6, 88110–88116. [Google Scholar] [CrossRef]
- AlKinani, A.; Eftekhari, M.; Gheibi, M. Ligandless dispersive solid phase extraction of cobalt ion using magnetic graphene oxide as an adsorbent followed by its determination with electrothermal atomic absorption spectrometry. Int. J. Environ. Anal. Chem. 2019, 1–18. [Google Scholar] [CrossRef]
- Babaei, A.; Zeeb, M.; Es-haghi, A. Magnetic dispersive solid-phase extraction based on graphene oxide/Fe3O4@polythionine nanocomposite followed by atomic absorption spectrometry for zinc monitoring in water, flour, celery and egg. J. Sci. Food Agr. 2018, 98, 3571–3579. [Google Scholar] [CrossRef]
- Kazemi, E.; Dadfarnia, S.; Haji Shabani, A.; Ranjbar, M. Synthesis, characterization, and application of a Zn (II)-imprinted polymer grafted on graphene oxide/magnetic chitosan nanocomposite for selective extraction of zinc ions from different food samples. Food Chem. 2017, 237, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Bahar, S.; Karami, F. Amino-functionalized Fe3O4–graphene oxide nanocomposite as magnetic solid-phase extraction adsorbent combined with flame atomic absorption spectrometry for copper analysis in food samples. J. Iran. Chem. Soc. 2015, 12, 2213–2220. [Google Scholar] [CrossRef]
- Akbarzade, S.; Chamsaz, M.; Rounaghi, G. Highly selective preconcentration of ultra-trace amounts of lead ions in real water and food samples by dispersive solid phase extraction using modified magnetic graphene oxide as a novel sorbent. Anal. Methods 2018, 10, 2081–2087. [Google Scholar] [CrossRef]
- Islam, A.; Ahmad, H.; Zaidi, N.; Kumar, S. Graphene Oxide Sheets Immobilized Polystyrene for Column Preconcentration and Sensitive Determination of Lead by Flame Atomic Absorption Spectrometry. ACS Appl. Mater. Interfaces 2014, 6, 13257–13265. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.; Mehri, A.; Hassannia, A. Fe3O4-modified graphene oxide as a sorbent for sequential magnetic solid phase extraction and dispersive liquid phase microextraction of thallium. Microchim. Acta 2017, 184, 3239–3246. [Google Scholar] [CrossRef]
- Farzin, L.; Shamsipur, M.; Shanehsaz, M.; Sheibani, S. A new approach to extraction and preconcentration of Ce(III) from aqueous solutions using magnetic reduced graphene oxide decorated with thioglycolic-acid-capped CdTe QDs. Int. J. Environ. Anal. Chem. 2017, 97, 854–867. [Google Scholar] [CrossRef]
- Farzin, L.; Amiri, M. Determination of samarium (III) ions in environmental samples after magnetic solid phase extraction using 1, 10- phenanthroline-2, 9-dicarboxilic acid modified Fe3O4/GO nanosheets. J. Nanoanal. 2018, 5, 241–248. [Google Scholar]
- Zawisza, B.; Sitko, R.; Malicka, E.; Talik, E. Graphene oxide as a solid sorbent for the preconcentration of cobalt, nickel, copper, zinc and lead prior to determination by energy-dispersive X-ray fluorescence spectrometry. Anal. Methods 2013, 5, 6425–6430. [Google Scholar] [CrossRef]
- Pytlakowska, K. Dispersive micro solid-phase extraction of heavy metals as their complexes with 2-(5-bromo-2-pyridylazo) -5-diethylaminophenol using graphene oxide nanoparticles. Microchim. Acta 2016, 183, 91–99. [Google Scholar] [CrossRef]
- Ghazaghi, M.; Mousavi, H.; Rashidi, A.; Shirkhanloo, H.; Rahighi, R. Innovative separation and preconcentration technique of coagulating homogenous dispersive micro solid phase extraction exploiting graphene oxide nanosheets. Anal. Chim. Acta 2016, 902, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Pourjavid, M.; Arabieh, M.; Yousefi, S.; Jamali, M.; Rezaee, M.; Hosseini, M.; Sehat, A. Study on column SPE with synthesized graphene oxide and FAAS for determination of trace amount of Co(II) and Ni(II) ions in real samples. Mat. Sci. Eng. C 2015, 47, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Sitko, R.; Zawisza, B.; Talik, E.; Janik, P.; Osoba, G.; Feist, B.; Malicka, E. Spherical silica particles decorated with graphene oxide nanosheets as a new sorbent in inorganic trace analysis. Anal. Chim. Acta 2014, 834, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Chen, B.; He, M.; Hu, B. Graphene oxide–silica composite coating hollow fiber solid phase microextraction online coupled with inductively coupled plasma mass spectrometry for the determination of trace heavy metals in environmental water samples. Talanta 2014, 123, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liang, Q.; Han, Q.; Zhang, X.; Ding, M. One-step synthesis of magnetic graphene oxide nanocomposite and its application in magnetic solid phase extraction of heavy metal ions from biological samples. Talanta 2015, 132, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Suo, L.; Zhao, J.; Dong, X.; Gao, X.; Li, X.; Xu, J.; Lu, X.; Zhao, L. Functionalization of a SiO2-coated magnetic graphene oxide composite with polyaniline–polypyrrole for magnetic solid phase extraction of ultra-trace Cr(III) and Pb(II) in water and food samples using a Box–Behnken design. New J. Chem. 2019, 43, 12126–12136. [Google Scholar] [CrossRef]
- Molaei, K.; Bagheri, H.; Asgharinezhad, A.; Ebrahimzadeh, H.; Shamsipur, M. SiO2-coated magnetic graphene oxide modified with polypyrrole–polythiophene: A novel and efficient nanocomposite for solid phase extraction of trace amounts of heavy metals. Talanta 2017, 167, 607–616. [Google Scholar] [CrossRef]
- Khan, M.; Yilmaz, E.; Sevinc, B.; Sahmetlioglu, E.; Shah, J.; Jan, M.; Soylak, M. Preparation and characterization of magnetic allylamine modified graphene oxide-poly(vinyl acetate-co-divinylbenzene) nanocomposite for vortex assisted magnetic solid phase extraction of some metal ions. Talanta 2016, 146, 130–137. [Google Scholar] [CrossRef]
- Zawisza, B.; Baranik, A.; Malicka, E.; Talik, E.; Sitko, R. Preconcentration of Fe(III), Co(II), Ni(II), Cu(II), Zn(II) and Pb(II) with ethylenediamine-modified graphene oxide. Microchim. Acta 2015, 183, 231–240. [Google Scholar] [CrossRef]
- Aliyari, E.; Alvand, M.; Shemirani, F. Simultaneous separation and preconcentration of lead and cadmium from water and vegetable samples using a diethylenetriamine-modified magnetic graphene oxide nanocomposite. Anal. Methods 2015, 7, 7582–7589. [Google Scholar] [CrossRef]
- Pytlakowska, K.; Pilch, M.; Hachuła, B.; Nycz, J.; Kornaus, K.; Pisarski, W. Energy dispersive X-ray fluorescence spectrometric determination of copper, zinc, lead and chromium species after preconcentration on graphene oxide chemically modified with mercapto-groups. J. Anal. At. Spectrom. 2019, 34, 1416–1425. [Google Scholar] [CrossRef]
- Neyestani, M.; Shemirani, F.; Mozaffari, S.; Alvand, M. A magnetized graphene oxide modified with 2-mercaptobenzothiazole as a selective nanosorbent for magnetic solid phase extraction of gold(III), palladium(II) and silver(I). Microchim. Acta 2017, 184, 2871–2879. [Google Scholar] [CrossRef]
- Dahaghin, Z.; Mousavi, H.; Sajjadi, S. Trace amounts of Cd(II), Cu(II) and Pb(II) ions monitoring using Fe3O4@graphene oxide nanocomposite modified via 2-mercaptobenzothiazole as a novel and efficient nanosorbent. J. Mol. Liq. 2017, 231, 386–395. [Google Scholar] [CrossRef]
- Sitko, R.; Janik, P.; Zawisza, B.; Talik, E.; Margui, E.; Queralt, I. Green Approach for Ultratrace Determination of Divalent Metal Ions and Arsenic Species Using Total-Reflection X-ray Fluorescence Spectrometry and Mercapto-Modified Graphene Oxide Nanosheets as a Novel Adsorbent. Anal. Chem. 2015, 87, 3535–3542. [Google Scholar] [CrossRef] [PubMed]
- Mosavi, S.; Ghanemi, K.; Nickpour, Y. Graphene oxide nanosheets modified with trithiocyanuric acid for extraction and enrichment of Pb (II) and Cu (II) ions in seawater. Water Environ. J. 2018, 32, 377–383. [Google Scholar] [CrossRef]
- Pourjavid, M.; Sehat, A.; Arabieh, M.; Yousefi, S.; Hosseini, M.; Rezaee, M. Column solid phase extraction and flame atomic absorption spectrometric determination of manganese(II) and iron(III) ions in water, food and biological samples using 3-(1-methyl-1H-pyrrol-2-yl)-1H-pyrazole-5-carboxylic acid on synthesized graphene oxide. Mat. Sci. Eng. 2014, 35, 370–378. [Google Scholar] [CrossRef]
- Zhu, X.; Cui, Y.; Chang, X.; Wang, H. Selective solid-phase extraction and analysis of trace-level Cr(III), Fe(III), Pb(II), and Mn(II) Ions in waste water using diethylenetriamine-functionalized carbon nanotubes dispersed in graphene oxide colloids. Talanta 2016, 146, 358–363. [Google Scholar] [CrossRef]
- Dahaghin, R.; Mousavi, H.Z.; Sajjadi, M. Synthesis and Application of Magnetic Graphene Oxide Modified with 8--Hydroxyquinoline for Extraction and Preconcentration of Trace Heavy Metal Ions. Chem. Select. 2017, 2, 1282–1289. [Google Scholar] [CrossRef]
- Pytlakowska, K.; Kozik, V.; Matussek, M.; Pilch, M.; Hachuła, B.; Kocot, K. Glycine modified graphene oxide as a novel sorbent for preconcentration of chromium, copper, and zinc ions from water samples prior to energy dispersive X-ray fluorescence spectrometric determination. RSC Adv. 2016, 6, 42836–42844. [Google Scholar] [CrossRef]
- Garcia-Mesa, J.C.; Leal, P.M.; Guerrero, M.M.L.; Alonso, E.I.V. Simultaneous determination of noble metals, Sb and Hg by magnetic solid phase extraction on line ICP OES based on a new functionalized magnetic graphene oxide. Microchem. J. 2019, 150. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, C.; Zhang, Q.; Chen, B.; He, M.; Hu, B. Graphene oxide–TiO2 composite as a novel adsorbent for the preconcentration of heavy metals and rare earth elements in environmental samples followed by on-line inductively coupled plasma optical emission spectrometry detection. RSC Adv. 2015, 5, 5996–6005. [Google Scholar] [CrossRef]
- Su, S.; Chen, B.; He, M.; Hu, B.; Xiao, Z. Determination of trace/ultratrace rare earth elements in environmental samples by ICP-MS after magnetic solid phase extraction with Fe3O4@SiO2@polyaniline–graphene oxide composite. Talanta 2014, 119, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Chatzimitakos, T.; Stalikas, C. Carbon-Based Nanomaterials Functionalized with Ionic Liquids for Microextraction in Sample Preparation. Separations 2017, 4. [Google Scholar] [CrossRef]
- Han, D.; Row, K.H. Recent applications of ionic liquids in separation technology. Molecules 2010, 15, 2405–2426. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Chatzimitakos, T.; Gallego, M.; Stalikas, C.D. 1-butyl-3-aminopropyl imidazolium functionalized graphene oxide as a nanoadsorbent for the simultaneous extraction of steroids and β-blockers via dispersive solid-phase microextraction. J. Chromatogr. A 2016, 1436, 9–18. [Google Scholar] [CrossRef] [PubMed]
- García-Alvarez-Coque, M.; Ruiz-Angel, M.; Berthod, A.; Carda-Broch, S. On the use of ionic liquids as mobile phase additives in high-performance liquid chromatography. A review. Anal. Chim. Acta 2015, 883, 1–21. [Google Scholar] [CrossRef]
- Shi, X.; Qiao, L.; Xu, G. Recent development of ionic liquid stationary phases for liquid chromatography. J. Chromatogr. A 2015, 1420, 1–15. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis, 2nd ed.; Wiley-VCH: Berlin, Germany, 2003. [Google Scholar]
- Alvand, M.; Shemirani, F. Fabrication of Fe3O4@graphene oxide core-shell nanospheres for ferrofluid-based dispersive solid phase extraction as exemplified for Cd(II) as a model analyte. Microchim. Acta 2016, 183, 1749–1757. [Google Scholar] [CrossRef]
- Lotfi, Z.; Mousavi, H.Z.; Sajjadi, S.M. Covalently bonded double-charged ionic liquid on magnetic graphene oxide as a novel, efficient, magnetically separable and reusable sorbent for extraction of heavy metals from medicine capsules. RSC Adv. 2016, 6, 90360–90370. [Google Scholar] [CrossRef]
- Aliyari, E.; Alvand, M.; Shemirani, F. Modified surface-active ionic liquid-coated magnetic graphene oxide as a new magnetic solid phase extraction sorbent for preconcentration of trace nickel. RSC Adv. 2016, 6, 64193–64202. [Google Scholar] [CrossRef]
- Gu, W.; Zhu, X. Nanoparticles of type Fe3O4-SiO2-graphene oxide and coated with an amino acid-derived ionic liquid for extraction of Al(III), Cr(III), Cu(II), Pb(II) prior to their determination by ICP-OES. Microchim. Acta 2017, 184, 4279–4286. [Google Scholar] [CrossRef]
- Rofouei, M.; Jamshidi, S.; Seidi, S.; Saleh, A. A bucky gel consisting of Fe3O4 nanoparticles, graphene oxide and ionic liquid as an efficient sorbent for extraction of heavy metal ions from water prior to their determination by ICP-OES. Microchim. Acta 2017, 184, 3425–3432. [Google Scholar] [CrossRef]
- Smith, E.; Abbott, A.; Ryder, K. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Reviews 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, Y.; Ding, X.; Huang, Y.; Li, N.; Wen, Q. Magnetic solid-phase extraction of protein with deep eutectic solvent immobilized magnetic graphene oxide nanoparticles. Talanta 2016, 148, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Qiu, H. Application of deep eutectic solvents in chromatography: A review. TrAC Trends Anal. Chem. 2019, 120. [Google Scholar] [CrossRef]
- Shishov, A.; Bulatov, A.; Locatelli, M.; Carradori, S.; Andruch, V. Application of deep eutectic solvents in analytical chemistry. A review. Microchem. J. 2017, 135, 33–38. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Wei, X.; Xu, P.; Xu, W.; Ni, R.; Meng, J. Magnetic solid-phase extraction for the removal of mercury from water with ternary hydrosulphonyl-based deep eutectic solvent modified magnetic graphene oxide. Talanta 2018, 188, 454–462. [Google Scholar] [CrossRef]
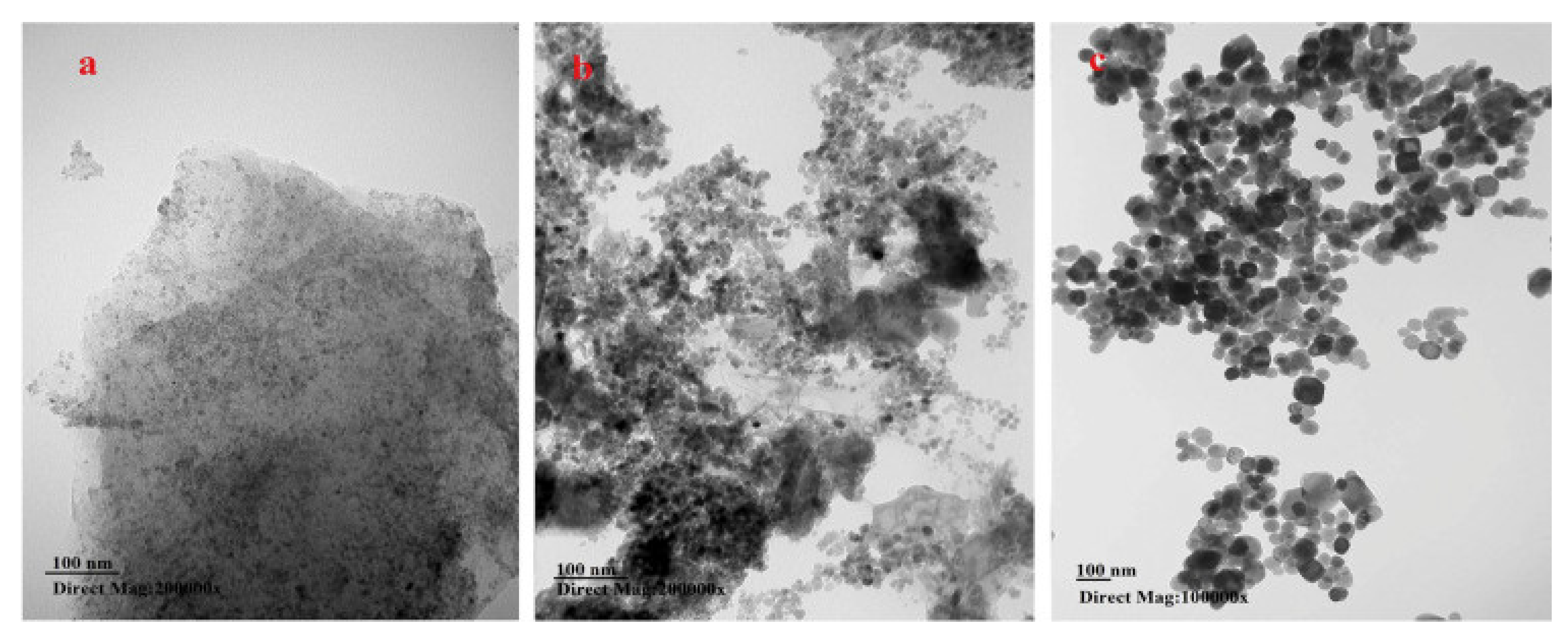

| Analyte | Sample Matrix | Sorbent | Functional Groups | Analytical Technique 1 | LODs (µg L−1) | Adsorption Time (min)/ Desorption Time (min) | Recovery (%) | Adsorption Capacity (mg g−1) | Reusability | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Hg(II) | Seafood | GO/Fe3O4 | Polythiophene | FI-CVAAS | 0.03 | 21/2 | 85 | 1 | [60] | |
| Fish, rice, tea, milk | GO/Fe3O4 | 2-Pyridinecarboxaldehyde | ICP-OES | 0.008 | 3/4 | 97 | NA | [61] | ||
| Water | GO/Fe3O4 | Chitosan, Mercaptopropyltrimethoxysilane | CVAAS | 0.06 | 10/10 | >95 | >400 | [62] | ||
| Cr(VI) & Cr(III) species | Water | GO/Fe3O4 | FAAS | 0.1 | >5 min/3 | 97–103 | 60 | At least 10 times | [63] | |
| Water | GO/Fe3O4 | Triethylenetetramine | FAAS | 1.4–1.6 | 30/- | >96 | 9.6–16.4 | [64] | ||
| Water | GO/Fe3O4 | Imidazolium, thioamine | GFAAS | 1.2 × 10−3 | 9/16.5 | >95 | 304 (total) | [65] | ||
| Cr(VI) | Water | GO/Fe3O4 | Polyaniline | GFAAS | 5 × 10−3 | 20/4 | 68 | 14.8 | [66] | |
| Cd(II) | Water, rice | GO/Fe3O4 | FAAS | 0.21 × 10−3 | 2/1 | >95 | 11.1 | [67] | ||
| Au(II) | Water | GO/Fe3O4 | FI-FAAS | 4 × 10−3 | Rapid/40 s | 98–102 | 9.8 | At least 10 times | [68] | |
| Water | GO/Fe3O4 | MP-AES | 5 × 10−3 | 10/5 | 97–101 | 192.1 | Up to 20 times | [69] | ||
| Co(II) | Water, food, biological samples | GO/Fe3O4 | ETAAS | 0.02 | ½ | 70–106 | 60 | [70] | ||
| Zn(II) | Water, food | GO/Fe3O4 | Polythionine | FAAS | 0.08 | 7/- | >87 | At least 5 times | [71] | |
| Water, food | GO/Fe3O4 | Chitosan, Zn-imprinted polymer | FAAS | 0.09 | 10/5 | >96 | 71.4 | At least 9 times | [72] | |
| Cu(II) | Eggplant, red lentil and mushroom | GO/Fe3O4 | 1,6-Hexadiamine | FAAS | 0.9 | 10/2 | >97 | Up to 5 times | [73] | |
| Pb(II) | Water, food | GO/Fe3O4 | 4-(2-pyridylazo)resorcinol | ETAAS | 0.18 × 10−3 | -/3 | >98 | 133 | [74] | |
| Water, food | GO | Polystyrene | FAAS | 2.5 | Not applicable | >99 | 227.9 | Up to 50 times | [75] | |
| Tl(III) | Water | GO/Fe3O4 | 4-methyl-2(2-pyrazinyl)-1,3-thiazole-5-carboxy acid | GFAAS | 12 × 10−3 | 8/3 | 65 | 20.0 | [76] | |
| Ce(III) | Water | RGO/Fe3O4 | Thioglycolic-acid-capped Cadmium–tellurium quantum dots | ICP-OES | 0.1 | 10/6 | >96 | 56.8 | At least 12 times | [77] |
| Sa(III) | Water | GO/Fe3O4 | 10-phenanthroline-2,9-dicarboxilic acid | ICP-OES | 1.4 | 20/12 | >97 | [78] |
| Analytes | Sample Matrix | Sorbent | Modification | Analytical Technique 1 | LODs (µg L−1) | Adsorption Time (min)/ Desorption Time (min) | Recovery (%) | Adsorption Capacity (mg g−1) | Reusability | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Co(II), Ni(II), Cu(II), Zn(II), Pb(II) | Water | GO | ICP-OES | 0.5–1.8 | 5/- | 94–106 | 294–1119 | [79] | ||
| Cr(III), Co(II), Ni(II), Cu(II), Zn(II), Pb(II) | Water | GO | EDXRF | 0.07–0.25 | 15/- | 94–104 | [80] | |||
| Cr(III), Cd(II), Pb(II) | Water, saliva, urine | GO | ETAAS | 5-12 × 10−3 | Few seconds/- | 94–103 | [81] | |||
| Co(II), Ni(II) | Water, black tea, tomato | GO | FAAS | 0.18–0.25 | Not applicable | >95 | 6.8–7 | [82] | ||
| Cu(II), Pb(II) | Water | GO | SiO2 | FAAS | 0.08–0.27 | Not applicable | >95 | 6.0–13.6 | At least 50 times | [83] |
| Mn(II), Co(II), Ni(II), Cu(II), Cd(II), Pb(II) | Water | GO | Silica | ICP-MS | 0.39–22 × 10−3 | 5/1 | 85–119 | 4.6–25 | At least 50 times | [84] |
| Co(II), Ni(II), Cu(II), Cd(II), Pb(II) | Plasma, Urine | GO/Fe3O4 | ICP-MS | 0.02–0.40 | 7/7 | 81–113 | 1.3–9.7 | At least 20 times | [85] | |
| Cr(III), Pb(II) | Rice, milk, wine, water | GO/Fe3O4 | Polyaniline–polypyrrole, SiO2 | ICP-MS | 3.4–4.8 × 10−3 | 6.3/3.7 | 96–103 | 188.9–213.3 | At least 6 times | [86] |
| Cu(II), Pb(II), Zn(II), Cr(III), Cd(II) | Water, agricultural samples | GO/Fe3O4 | Polypyrrole–polythiophene, SiO2 | FAAS | 0.15–0.65 | 6.5/12 | 90–106 | 80–230 | At least 5 times | [87] |
| Pb(II), Cd(II), Cu(II), Ni(II), Co(II) | Water, food samples | GO/Fe3O4 | Poly(vinylacetate-co-divinylbenzene) | FAAS | 0.37–2.39 | -/- | >95 | [88] | ||
| Fe(III), Co(II), Ni(II), Cu(II), Zn(II),Pb(II) | Water | GO | Ethylene diamine | EDXRF | 0.06–0.1 | 5/- | >90 | [89] | ||
| Cd(II), Pb(II) | Water, vegetables | GO/Fe3O4 | Diethylenetriamine (DETA) | FAAS | 0.38–0.40 | 10/2 | >99 | 59.9–172.4 | [90] | |
| Cr(III), Cu(II), Zn(II), Pb(II) | Water | GO/Fe3O4 | Mercapto-groups | EDXRF | 0.06–0.10 | 10/- | >95 | 191.5–487.3 | [91] | |
| Au(III), Pd(II), Ag(I) | Water, ore and automobile catalyst | Magnetic GO | 2-mercaptobenzothiazole | ICP-OES | 0.05–0.08 | 10/3 | 90–103 | 28–45 | At least 5 times | [92] |
| Cd(II), Cu(II), Pb(II) | Water, vegetables | GO/Fe3O4 | 2-mercaptobenzothiazole | FAAS | 0.19–0.35 | 4/5 | >99 | 156–179 | [93] | |
| Co(II), Ni(II), Cu(II), As(III), Cd(II), Pb(II) | Water | GO | (3-mercaptopropyl)-trimethoxysilane | TXRF | 0.05–9.11 | 10/2 | >94 | 18.1–108.3 | [94] | |
| Pb(II), Cu(II) | Water | GO/Fe3O4 | Trithiocyanuric acid | FAAS | 0.13–0.32 | Not applicable | >90 | 0.46–0.75 | [95] | |
| Mn(II), Fe(III) | Water, food and biological samples | GO | 3-(1-methyl-1H-pyrrol-2-yl)-1H-pyrazole-5-carboxylic acid | FAAS | 0.31–355 | Not applicable | >95 | 21.6–24.0 | [96] | |
| Cr(III), Fe(III), Pb(II), Mn(II) | Wastewater | GO | Multi-walled carbon nanotubes -DETA | ICP-OES | 0.16–0.50 | Not applicable | >95 | 5.4–13.8 | [97] | |
| Cd(II), Pb(II) | Vegetables, fish, lipstick | GO/Fe3O4 | 8-Hydroxyquinoline | FAAS | 0.09–0.27 | 5/5 | >96 | 133–150 | [98] | |
| Cr(III), Zn(II), Cu(II) | Water | GO/Fe3O4 | Glycine | EDXRF | 0.07–0.15 | 10/- | >97 | [99] | ||
| Noble metals, Sb(III), Hg(II) | Seawater | GO/Fe3O4 | 1,5-bis(di-2-pyridyl)methylene thiocarbohydrazide | ICP-OES | 0.05–2.60 | Not applicable | 90–106 | 4.5–9.7 | [100] | |
| Cu (II), Pb(II), La(III), Ce(III), Eu(III), Dy(III), Yb(III) | Water | GO | TiO2 | ICP-OES | 0.13–2.64 | Not applicable | >90 | 0.8–13.5 | At least 90 times | [101] |
| REEs | Water | GO/Fe3O4 | Polyaniline, SiO2 | ICP-MS | 0.04–1.49 × 10−3 | 2/5 | 80–121 | 7.7–16.3 | At least 30 times | [102] |
| REEs | Nuts, water | Oxidized GO | ICP-MS | 0.03–1.8 | 15/1 | 60–90 | 6.1–12.2 | At least 12 times | [8] |
| Analyte | Sample Matrix | Sorbent | Ionic Liquids | Analytical Technique 1 | LODs (µgL−1) | Adsorption Time (min)/ Desorption Time (min) | Recovery (%) | Adsorption Capacity (mg g−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Cd(II) | River and seawater, carrot, lettuce and tobacco | GO/Fe3O4 | 1-ethyl-3-methylimidazolium tetrafluoroborate | FAAS | 0.12 | Few seconds/1 min | 98–102 | 33.7 | [109] |
| Pb(II), Cd(II), Ni(II), Cu(II) and Cr(III) | Medicine capsules | GO/Fe3O4 modified with (3-mercaptopropyl)trimethoxysilane | 1,4-diazabicyclo [2.2.2]octane | FAAS | 0.2–1.8 | 4 min/1 min | 95–102 | 18.1–47.6 | [110] |
| Ni(II) | Sea and river water, tea, spinach, cacao powder, cigarette | GO/Fe3O4 | 1-hexadecyl-3-methylimidazolium chloride | FAAS | 0.16 | 15 min/2 min | 97–99 | 129.9 | [111] |
| Al(III), Cr(III), Cu(II), Pb(II) | Environmental water | Fe3O4-SiO2−GO | N-(3- Dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride | ICP-OES | 0.5–30 × 10−3 | 5 min/3 min | 89–118 | 5.0–11.7 | [112] |
| Cu(II), Zn(II), Cd(II), Cr(III), Pb(II) and Co(II) | Environmental water | GO/Fe3O4 | 1-butyl-3-methylimidazolium hexafluorophosphate | ICP-OES | 0.1–1 | 10 min/6 min | 34–94 | 312.5 | [113] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manousi, N.; Rosenberg, E.; Deliyanni, E.A.; Zachariadis, G.A. Sample Preparation Using Graphene-Oxide-Derived Nanomaterials for the Extraction of Metals. Molecules 2020, 25, 2411. https://doi.org/10.3390/molecules25102411
Manousi N, Rosenberg E, Deliyanni EA, Zachariadis GA. Sample Preparation Using Graphene-Oxide-Derived Nanomaterials for the Extraction of Metals. Molecules. 2020; 25(10):2411. https://doi.org/10.3390/molecules25102411
Chicago/Turabian StyleManousi, Natalia, Erwin Rosenberg, Eleni A. Deliyanni, and George A. Zachariadis. 2020. "Sample Preparation Using Graphene-Oxide-Derived Nanomaterials for the Extraction of Metals" Molecules 25, no. 10: 2411. https://doi.org/10.3390/molecules25102411
APA StyleManousi, N., Rosenberg, E., Deliyanni, E. A., & Zachariadis, G. A. (2020). Sample Preparation Using Graphene-Oxide-Derived Nanomaterials for the Extraction of Metals. Molecules, 25(10), 2411. https://doi.org/10.3390/molecules25102411









