Antiprotozoal Activity Against Entamoeba histolytica of Flavonoids Isolated from Lippia graveolens Kunth
Abstract
1. Introduction
2. Results
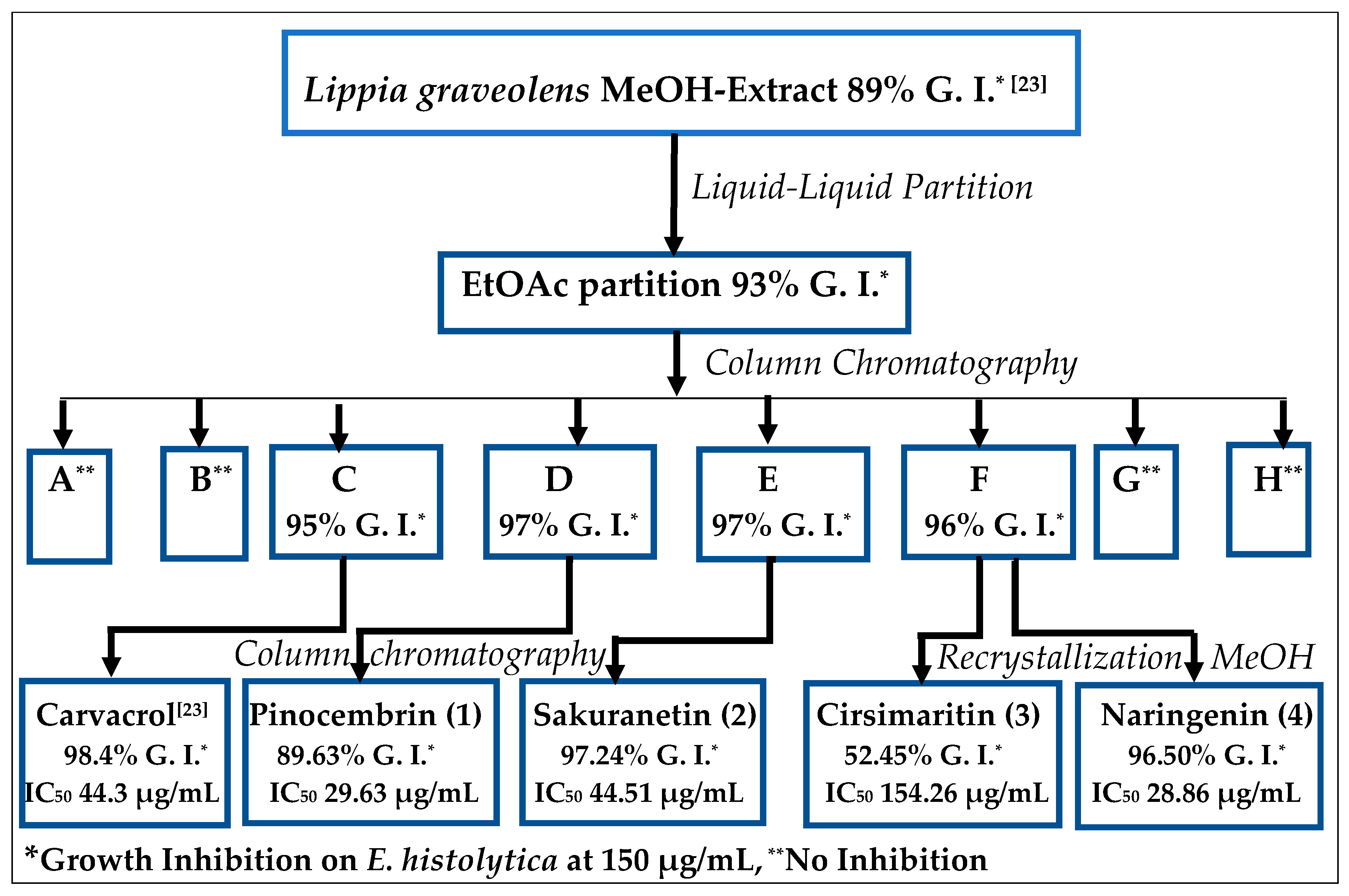
2.1. Bioguided Isolation of Flavonoids from Lippia graveolens Kunth
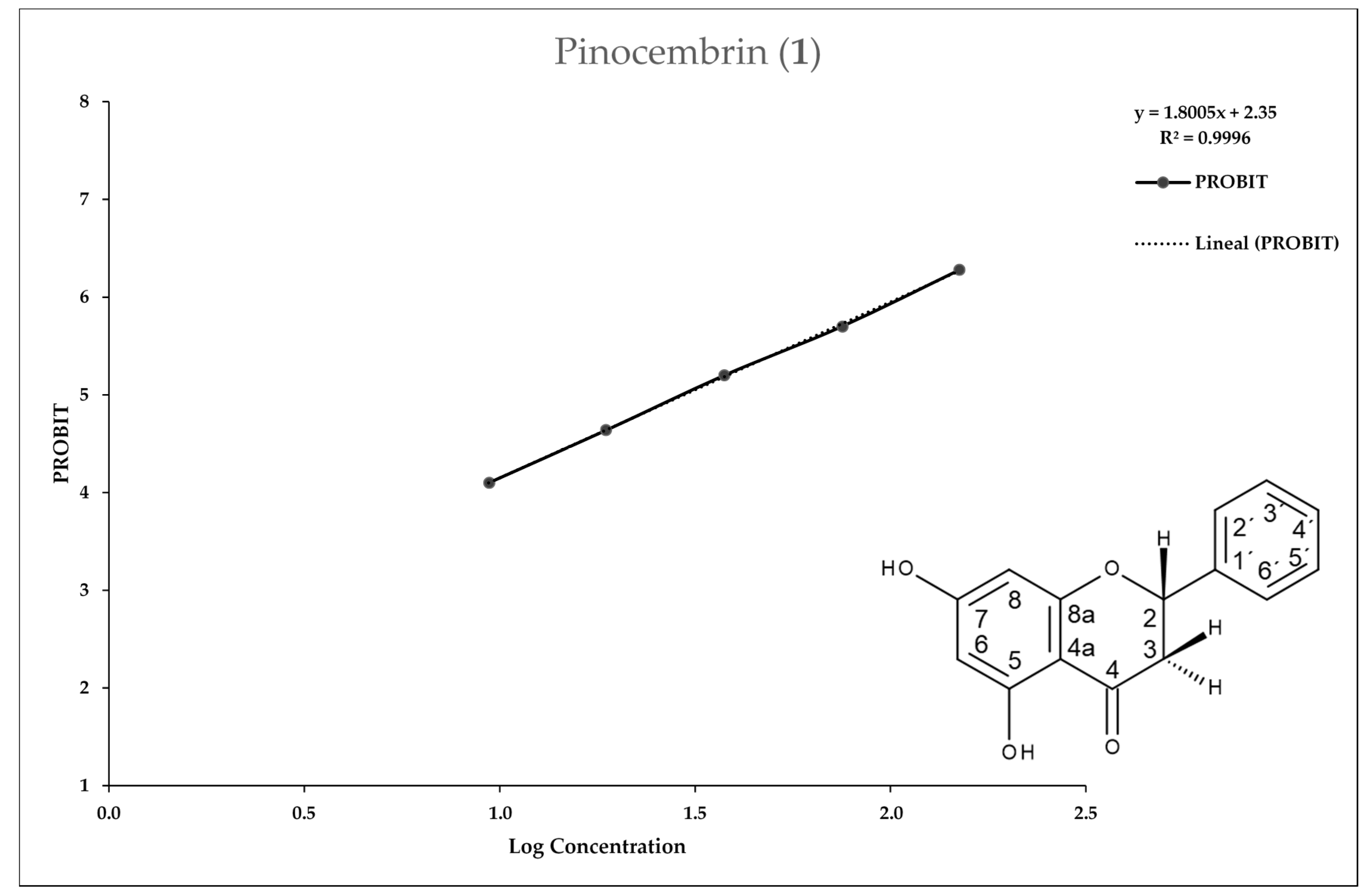
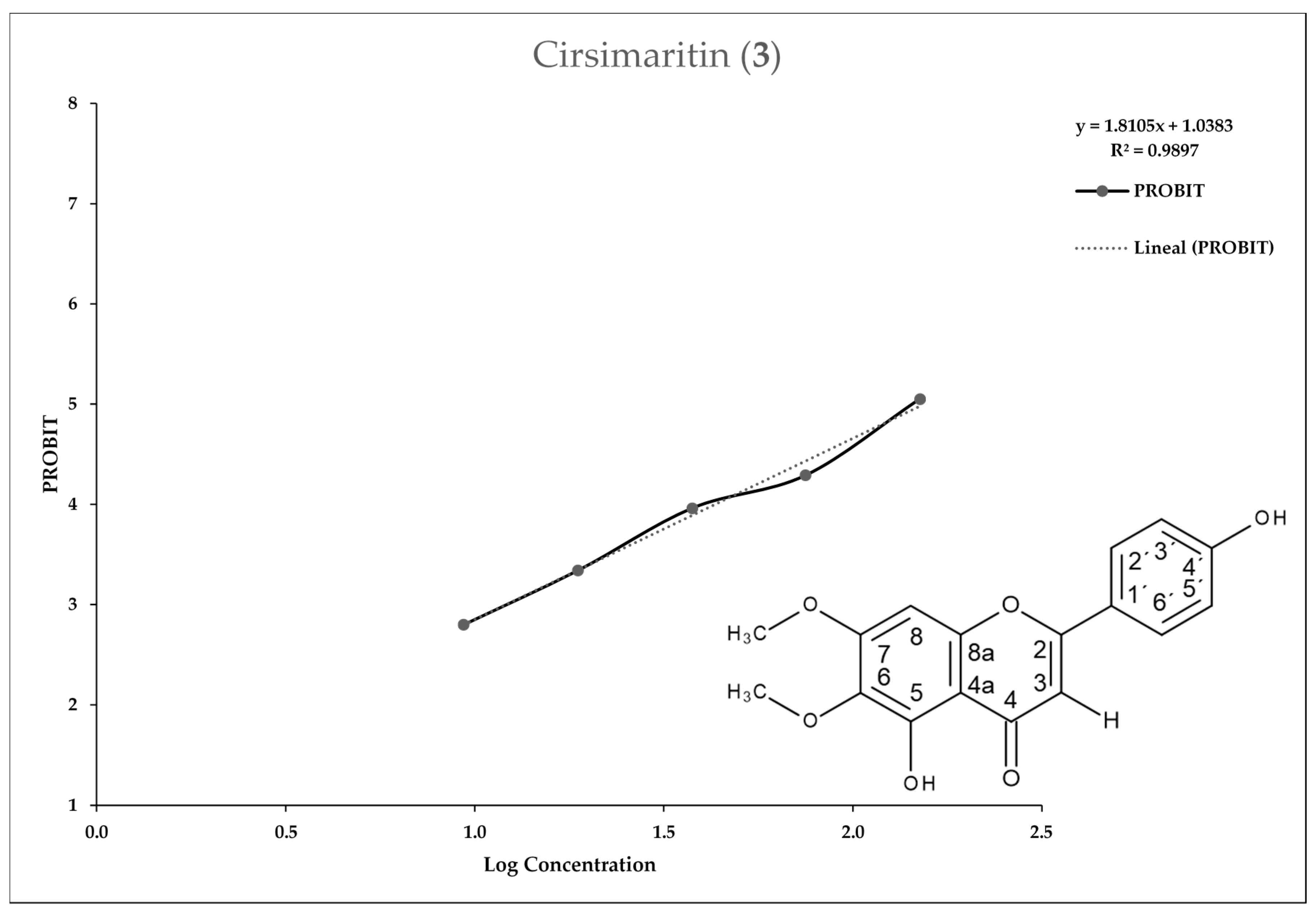
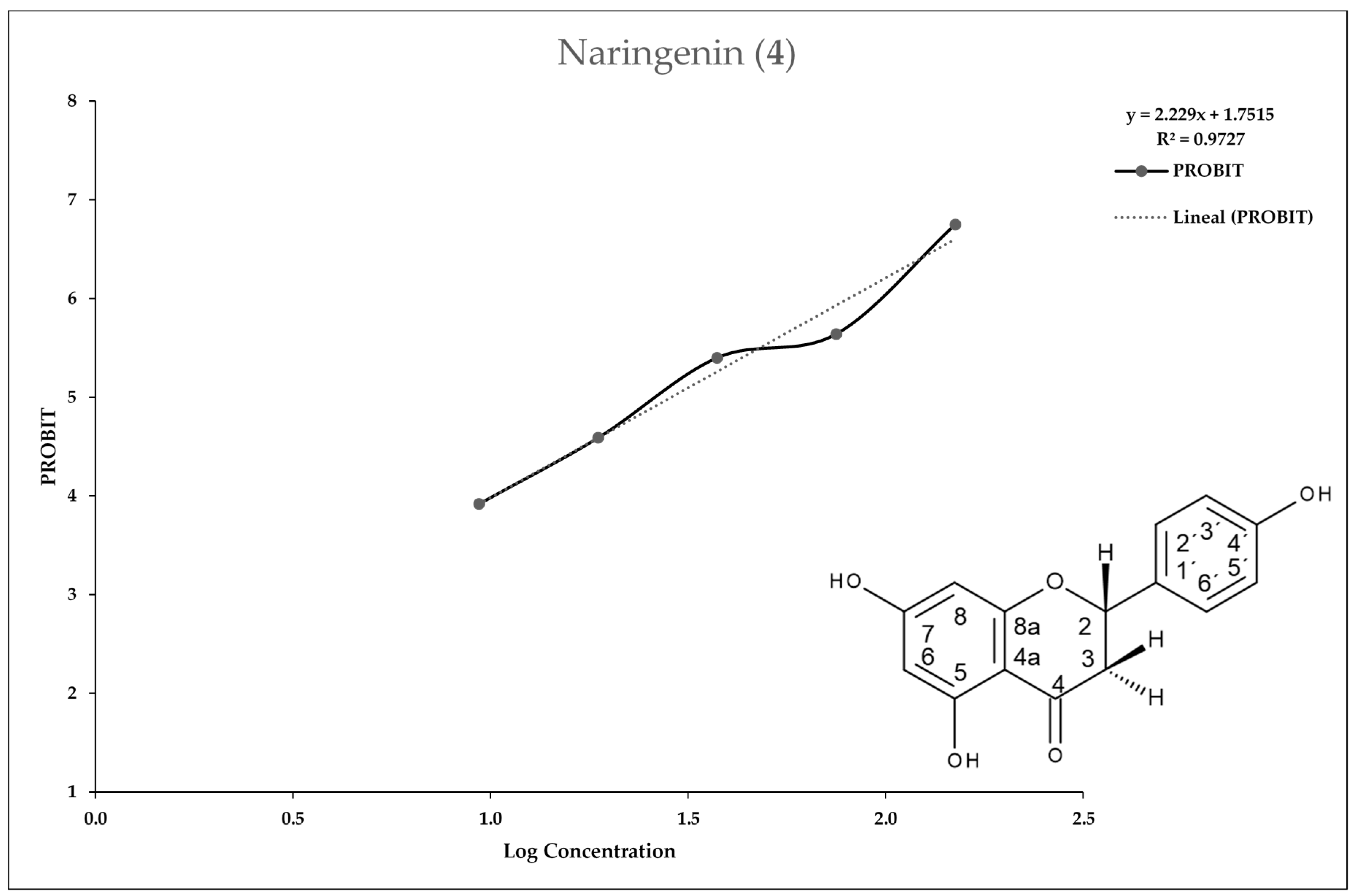
2.2. Entamoeba Histolytica Growth Parameters
2.3. In Vitro Assay for Entamoeba histolytica
3. Discussion
4. Materials and Methods
4.1. General
4.2. Plant Material
4.3. Plant Extraction and Bioguided Isolation of Antiamoebic Compounds from Lippia graveolens Kunth
4.4. Antiprotozoal Assay
4.4.1. Test Microorganisms
4.4.2. In Vitro Assay for Entamoeba histolytica
4.4.3. In Vitro IC50 Determination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ávila-García, R.; Valdés, J.; Jáuregui-Wade, J.M.; Ayala-Sumuano, J.T.; Cerbón-Solórzano, J. The metabolic pathway of sphingolipids biosynthesis and signaling in Entamoeba histolytica. Biochem. Biophys. Res. Commun. 2020, 522, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.C.; Reyes-López, M.; Serrano-Luna, J.; Shibayama, M.; Unzueta, J.; León-Sicairos, N.; De La Garza, M. Intestinal amoebiasis: 160 years of its first detection and still remains as a health problem in developing countries. Int. J. Med. Microbiol. 2020, 310, 151358. [Google Scholar] [CrossRef] [PubMed]
- Broucke, S.V.D.; Verschueren, J.; Van Esbroeck, M.; Bottieau, E.; Ende, J.V.D. Clinical and microscopic predictors of Entamoeba histolytica intestinal infection in travelers and migrants diagnosed with Entamoeba histolytica/dispar infection. PLoS Negl. Trop. Dis. 2018, 12, e0006892. [Google Scholar] [CrossRef]
- Aguilar-Rojas, A.; Olivo-Marin, J.-C.; Guillen, N. The motility of Entamoeba histolytica: Finding ways to understand intestinal amoebiasis. Curr. Opin. Microbiol. 2016, 34, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.L.A.; Minchaca, A.Z.; Mares, R.E.; Ramos-Ibarra, M.A. Activity, stability and folding analysis of the chitinase from Entamoeba histolytica. Parasitol. Int. 2016, 65, 70–77. [Google Scholar] [CrossRef]
- Guo, F.; Forde, M.; Werre, S.R.; Krecek, R.C.; Zhu, G. Seroprevalence of five parasitic pathogens in pregnant women in ten Caribbean countries. Parasitol. Res. 2016, 116, 347–358. [Google Scholar] [CrossRef]
- Singh, R.S.; Walia, A.K.; Kanwar, J.R.; Kennedy, J.F. Amoebiasis vaccine development: A snapshot on E. histolytica with emphasis on perspectives of Gal/GalNAc lectin. Int. J. Boil. Macromol. 2016, 91, 258–268. [Google Scholar] [CrossRef]
- Iyer, L.R.; Verma, A.K.; Paul, J.; Bhattacharya, A. Phagocytosis of Gut Bacteria by Entamoeba histolytica. Front. Microbiol. 2019, 9, 34. [Google Scholar] [CrossRef]
- Institut-Pasteur Amoebiasis. Available online: https://www.pasteur.fr/en/medical-center/disease-sheets/amoebiasis-0 (accessed on 19 April 2020).
- Gómez-García, C.; Marchat, L.A.; López-Cánovas, L.; Pérez-Ishiwara, D.G.; Rodriguez, M.A.; Orozco, E. Drug Resistance Mechanisms in Entamoeba histolytica, Giardia lamblia, Trichomonas vaginalis, and Opportunistic Anaerobic Protozoa. In Antimicrobial Drug Resistance. Mechanisms of Drug Resistance; Mayers, D.L., Sobel, J.D., Oullette, M., Kaye, K.S., Marchaim, D., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 613–628. ISBN 978-3-319-47264-5. [Google Scholar]
- Luevano, J.H.E.; Castro-Ríos, R.; Sánchez-García, E.; Hernández-García, M.E.; Villarreal, J.V.; Rodríguez-Luis, O.E.; Chávez-Montes, A. In Vitro Study of Antiamoebic Activity of Methanol Extracts of Argemone mexicana on Trophozoites of Entamoeba histolytica HM1-IMSS. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Bioactive compounds in banana and their associated health benefits—A review. Food Chem. 2016, 206, 1–11. [Google Scholar] [CrossRef]
- Hayat, F.; Azam, A.; Shin, D. Recent progress on the discovery of antiamoebic agents. Bioorganic Med. Chem. Lett. 2016, 26, 5149–5159. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.S.; Ghosh, S.K. Stress-responsive Entamoeba topoisomerase II: A potential antiamoebic target. FEBS Lett. 2020, 594, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Leyva-López, N.; Nair, V.; Bang, W.Y.; Cisneros-Zevallos, L.; Heredia, J.B. Protective role of terpenes and polyphenols from three species of Oregano (Lippia graveolens, Lippia palmeri and Hedeoma patens) on the suppression of lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. J. Ethnopharmacol. 2016, 187, 302–312. [Google Scholar] [CrossRef]
- Ramirez-Moreno, E.; Soto-Sanchez, J.; Rivera, G.; Marchat, L.A. Mexican Medicinal Plants as an Alternative for the Development of New Compounds Against Protozoan Parasites. In Natural Remedies in the Fight Against Parasites; Hanem, K., Govindarajan, M., Benelli, G., Eds.; INTECH: London, UK, 2017; pp. 61–91. ISBN 978-953-51-3290-5. [Google Scholar]
- Martínez-Rocha, A.; Puga, R.; Hernández-Sandoval, L.; Loarca-Piña, G.; Mendoza, S. Antioxidant and Antimutagenic Activities of Mexican Oregano (Lippia graveolens Kunth). Plant Foods Hum. Nutr. 2007, 63, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Arana-Sánchez, A.; Estarrón-Espinosa, M.; Obledo-Vázquez, E.; Padilla-Camberos, E.; Silva-Vázquez, R.; Lugo-Cervantes, E. Antimicrobial and antioxidant activities of Mexican oregano essential oils (Lippia graveolens H. B. K.) with different composition when microencapsulated inβ-cyclodextrin. Lett. Appl. Microbiol. 2010, 50, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Cruz, I.; Duarte, G.; Navarrete, A.; Bye, R.; Linares, E.; Mata, R. Chemical composition and antimicrobial and spasmolytic properties of Poliomintha longiflora and Lippia graveolens essential oils. J. Food Sci. 2011, 76, C309–C317. [Google Scholar] [CrossRef]
- Adame-Gallegos, J.; Ochoa, S.A.; Nevárez-Moorillón, G.V. Potential Use of Mexican Oregano Essential Oil against Parasite, Fungal and Bacterial Pathogens. J. Essent. Oil Bear. Plants 2016, 19, 553–567. [Google Scholar] [CrossRef]
- García-Heredia, A.; Garcia, S.; Merino-Mascorro, J.Á.; Feng, P.; Heredia, N. Natural plant products inhibits growth and alters the swarming motility, biofilm formation, and expression of virulence genes in enteroaggregative and enterohemorrhagic Escherichia coli. Food Microbiol. 2016, 59, 124–132. [Google Scholar] [CrossRef]
- Quintanilla-Licea, R.; Mata-Cárdenas, B.D.; Villarreal, J.V.; Bazaldúa-Rodríguez, A.F.; Kavimngeles-Hernández, I.; Garza-González, J.N.; Hernández-García, M.E.; Ángeles-Hernández, I.K. Antiprotozoal Activity against Entamoeba histolytica of Plants Used in Northeast Mexican Traditional Medicine. Bioactive Compounds from Lippia graveolens and Ruta chalepensis. Molecules 2014, 19, 21044–21065. [Google Scholar] [CrossRef]
- Ching, A.Y.L.; Wah, T.S.; Sukari, M.A.; Lian, G.E.C.; Rahmani, M.; Khalid, K. Characterization of Flavonoid Derivatives From Boesenbergia Rotunda (L.). Malays. J. Anal. Sci. 2007, 11, 154–159. [Google Scholar]
- Jerz, G.; Waibel, R.; Achenbach, H. Cyclohexanoid protoflavanones from the stem-bark and roots of Ongokea gore. Phytochemistry 2005, 66, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Suleimen, Y.; Raldugin, V.A.; Adekenov, S.M. Cirsimaritin from Stizolophus balsamita. Chem. Nat. Compd. 2008, 44, 398. [Google Scholar] [CrossRef]
- Jain, R.; Mittal, M. Naringenin, a flavonone from the stem of Nyctanthes arbortristis Linn. Int. J. Biol. Pharm. Allied Sci. 2012, 1, 964–972. [Google Scholar]
- Bertelli, D.; Papotti, G.; Bortolotti, L.; Marcazzan, G.L.; Plessi, M. 1H-NMR Simultaneous Identification of Health-Relevant Compounds in Propolis Extracts. Phytochem. Anal. 2011, 23, 260–266. [Google Scholar] [CrossRef]
- Katerere, D.R.; Gray, A.I.; Nash, R.J.; Waigh, R.D. Phytochemical and antimicrobial investigations of stilbenoids and flavonoids isolated from three species of Combretaceae. Fitoterapia 2012, 83, 932–940. [Google Scholar] [CrossRef]
- Ramírez, J.; Cartuche, L.; Morocho, V.; Aguilar, S.; Malagón, O. Antifungal activity of raw extract and flavanons isolated from Piper ecuadorense from Ecuador. Rev. Bras. Farm. 2013, 23, 370–373. [Google Scholar] [CrossRef]
- Guzmán-Avendaño, A.J.; Torrenegra-Guerrero, R.D.; Rodríguez-Aguirre, O.E. Flavonoids from Chromolaena subscandens (Hieron.) R.M. King & H. Rob. Rev. Prod. Nat. 2008, 2, 1–5. [Google Scholar]
- McNulty, J.; Nair, J.J.; Bollareddy, E.; Keskar, K.; Thorat, A.; Crankshaw, D.; Holloway, A.C.; Khan, G.; Wright, G.D.; Ejim, L. Isolation of flavonoids from the heartwood and resin of Prunus avium and some preliminary biological investigations. Phytochemistry 2009, 70, 2040–2046. [Google Scholar] [CrossRef]
- Grecco, S.D.S.; Dorigueto, A.C.; Landre, I.M.; Soares, M.G.; Martho, K.; Lima, R.; Pascon, R.C.; Vallim, M.A.; Capello, T.M.; Romoff, P.; et al. Structural Crystalline Characterization of Sakuranetin—An Antimicrobial Flavanone from Twigs of Baccharis retusa (Asteraceae). Molecules 2014, 19, 7528–7542. [Google Scholar] [CrossRef]
- Yamashita, Y.; Hanaya, K.; Shoji, M.; Sugai, T. Simple Synthesis of Sakuranetin and Selinone via a Common Intermediate, Utilizing Complementary Regioselectivity in the Deacetylation of Naringenin Triacetate. Chem. Pharm. Bull. 2016, 64, 961–965. [Google Scholar] [CrossRef]
- Alwahsh, M.A.A.; Khairuddean, M.; Chong, W.K. Chemical constituents and antioxidant activity of Teucrium barbeyanum Aschers. Rec. Nat. Prod. 2015, 9, 159–163. [Google Scholar]
- Exarchou, V.; Kanetis, L.; Charalambous, Z.; Apers, S.; Pieters, L.; Gekas, V.; Goulas, V. HPLC-SPE-NMR Characterization of Major Metabolites in Salvia fruticosa Mill. Extract with Antifungal Potential: Relevance of Carnosic Acid, Carnosol, and Hispidulin. J. Agric. Food Chem. 2015, 63, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Jang, H.-J.; Kim, Y.; Oh, H.-M.; Lee, S.; Jung, K.; Kim, Y.-H.; Lee, W.-S.; Lee, S.W.; Rho, M.-C. Inhibitory effects of IL-6-induced STAT3 activation of bio-active compounds derived from Salvia plebeia R.Br. Process. Biochem. 2016, 51, 2222–2229. [Google Scholar] [CrossRef]
- Gaggeri, R.; Rossi, D.; Christodoulou, M.S.; Passarella, D.; Leoni, F.; Azzolina, O.; Collina, S. Chiral Flavanones from Amygdalus lycioides Spach: Structural Elucidation and Identification of TNFalpha Inhibitors by Bioactivity-guided Fractionation. Molecules 2012, 17, 1665–1674. [Google Scholar] [CrossRef]
- Grecco, S.D.S.; Reimão, J.Q.; Tempone, A.G.; Sartorelli, P.; Cunha, R.L.O.R.; Romoff, P.; Ferreira, M.J.P.; Fávero, O.A.; Lago, J. In vitro antileishmanial and antitrypanosomal activities of flavanones from Baccharis retusa DC. (Asteraceae). Exp. Parasitol. 2012, 130, 141–145. [Google Scholar] [CrossRef]
- Kyriakou, E.; Primikyri, A.; Charisiadis, P.; Katsoura, M.; Stamatis, H.; Gerothanassis, I.P.; Tzakos, A. Unexpected enzyme-catalyzed regioselective acylation of flavonoid aglycones and rapid product screening. Org. Biomol. Chem. 2012, 10, 1739. [Google Scholar] [CrossRef]
- Ashour, M.A.-G. New diacyl flavonoid derivatives from the Egyptian plant Blepharis edulis (Forssk.) Pers. Bull. Fac. Pharm. Cairo Univ. 2015, 53, 11–17. [Google Scholar] [CrossRef][Green Version]
- Joseph-Nathan, P.; Gordillo-Román, B. Vibrational Circular Dichroism Absolute Configuration Determination of Natural Products. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A.D., Falk, H., Kobayashi, J., Eds.; Springer International Publishing Switzerland: Cham, Switzerland, 2015; Volume 100, pp. 311–452. [Google Scholar]
- Silverstein, R.M.; Bassler, G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Grotewold, E. The Science of Flavonoids; Springer Science+Business Media, Inc.: New York, NY, USA, 2006; ISBN 9780387288222. [Google Scholar]
- Pinheiro, P.F.; Justino, G.C. Structural analysis of flavonoids and related compounds—A review of spectroscopic applications. In Phytochemicals–A Global Perspective of Their Role in Nutrition and Health; Rao, V., Ed.; InTech Europe: Rijeka, Croatia, 2012; pp. 33–56. ISBN 978-953-51-0296-0. [Google Scholar]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Ruedi, P. Antitrypanosomal and Antileishmanial Activities of Flavonoids and Their Analogues: In Vitro, In Vivo, Structure-Activity Relationship, and Quantitative Structure-Activity Relationship Studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Plochmann, K.; Korte, G.; Koutsilieri, E.; Richling, E.; Riederer, P.; Rethwilm, A.; Schreier, P.; Scheller, C. Structure–activity relationships of flavonoid-induced cytotoxicity on human leukemia cells. Arch. Biochem. Biophys. 2007, 460, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Delgado, L.; Fernandes, I.; González-Manzano, S.; De Freitas, V.; Mateus, N.; Santos-Buelga, C. Anti-proliferative effects of quercetin and catechin metabolites. Food Funct. 2014, 5, 797. [Google Scholar] [CrossRef] [PubMed]
- Resende, F.A.; Almeida, C.P.D.S.; Vilegas, W.; Varanda, E.A. Differences in the hydroxylation pattern of flavonoids alter their chemoprotective effect against direct- and indirect-acting mutagens. Food Chem. 2014, 155, 251–255. [Google Scholar] [CrossRef]
- Martínez-Castillo, M.; Pacheco-Yépez, J.; Flores, N.; Guzman, P.G.; Jarillo-Luna, R.A.; Cárdenas-Jaramillo, L.M.; Campos-Rodríguez, R.; Shibayama, M. Flavonoids as a Natural Treatment Against Entamoeba histolytica. Front. Microbiol. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Vaya, J.; Tamir, S. The relation between the chemical structure of flavonoids and their estrogen-like activities. Curr. Med. Chem. 2004, 11, 1333–1343. [Google Scholar] [CrossRef]
- Agrawal, A.D. Pharmacological activities of flavonoids: A review. Int. J. Pharm. Sci. Nanotechnol. 2011, 4, 1394–1398. [Google Scholar]
- Beekmann, K.; Actis-Goretta, L.; Van Bladeren, P.J.; Dionisi, F.; Destaillats, F.; Rietjens, I.M.C.M. A state-of-the-art overview of the effect of metabolic conjugation on the biological activity of flavonoids. Food Funct. 2012, 3, 1008. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Z.; Mukhopadhyay, S.; Robbins, R.J.; Harnly, J.M. Identification and quantification of flavonoids of Mexican oregano (Lippia graveolens) by LC-DAD-ESI/MS analysis. J. Food Compos. Anal. 2007, 20, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, S.; Sánchez, H.; Suárez, M.; Baldas, J.; González, M.D.R. Chemical Constituents of Lippia graveolens. Planta Med. 1989, 55, 208–209. [Google Scholar] [CrossRef]
- González-Güereca, M.C.; Soto-Hernández, M.; Kite, G.; Martínez-VÁzquez, M. Antioxidant activity of flavonoids from the stem of the Mexican oregano (Lippia graveolens HBK var. berlandieri Schauer). Rev. Fitotec. Mex. 2007, 30, 43–49. [Google Scholar]
- Rufino-González, Y.; Ponce-Macotela, M.; González-Maciel, A.; Reynoso-Robles, R.; Jiménez-Estrada, M.; Sanchez-Contreras, A.; Martínez-Gordillo, M.N. In vitro activity of the F-6 fraction of oregano against Giardia intestinalis. Parasitology 2012, 139, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rodríguez, A.; Vázquez-Medrano, J.; Hernández-Portilla, L.B.; Peñalosa-Castro, I.; Canales-Martínez, M.; Orozco-Segovia, A.; Jiménez-Estrada, M.; Colville, L.; Pritchard, H.W.; Flores-Ortiz, C.M. The effect of light and soil moisture on the accumulation of three flavonoids in the leaves of Mexican oregano (Lippia graveolens Kunth). J. Food, Agric. Environ. 2014, 12, 1272–1279. [Google Scholar]
- Grael, C.; De Albuquerque, S.; Lopes, J.L. Chemical constituents of Lychnophora pohlii and trypanocidal activity of crude plant extracts and of isolated compounds. Fitoterapia 2005, 76, 73–82. [Google Scholar] [CrossRef]
- Alday-Provencio, S.; Diaz, G.; Rascon, L.; Quintero, J.; Alday, E.; Robles-Zepeda, R.; Garibay-Escobar, A.; Astiazaran, H.; Hernández, J.; Velázquez, C. Sonoran Propolis and Some of its Chemical Constituents Inhibit in vitro Growth of Giardia lamblia Trophozoites. Planta Med. 2015, 81, 742–747. [Google Scholar] [CrossRef]
- Calzada, F.; Meckes, M.; Cedillo-Rivera, R. Antiamoebic and Antigiardial Activity of Plant Flavonoids. Planta Med. 1999, 65, 78–80. [Google Scholar] [CrossRef]
- Tasdemir, D.; Lack, G.; Brun, R.; Rüedi, P.; Scapozza, L.; Perozzo, R. Inhibition of Plasmodium falciparum fatty acid biosynthesis: Evaluation of FabG, FabZ, and FabI as drug targets for flavonoids. J. Med. Chem. 2006, 49, 3345–3353. [Google Scholar] [CrossRef]
- Calzada, F.; Velazquez, C.; Cedillo-Rivera, R.; Esquivel, B. Antiprotozoal activity of the constituents ofTeloxys graveolens. Phytother. Res. 2003, 17, 731–732. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, V.; Díaz-Martínez, A.; Soto, J.; Marchat, L.A.; Sánchez-Monroy, V.; Ramírez-Moreno, E. Kaempferol inhibits Entamoeba histolytica growth by altering cytoskeletal functions. Mol. Biochem. Parasitol. 2015, 204, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Gupta, P.; Agarwal, S.M. Insight into structural requirements of antiamoebic flavonoids: 3D-QSAR and G-QSAR studies. Chem. Boil. Drug Des. 2018, 92, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.; Gómez, C.; Calzada, F.; Ramírez, M.E. Ultrastructural Changes on Entamoeba histolytica HM1-IMSS Caused by the Flavan-3-Ol, (-)-Epicatechin. Planta Med. 2009, 76, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, V.; Díaz-Martínez, A.; Soto, J.; Rodríguez, M.A.; López-Camarillo, C.; Marchat, L.A.; Ramírez-Moreno, E. The flavonoid (-)-epicatechin affects cytoskeleton proteins and functions in Entamoeba histolytica. J. Proteom. 2014, 111, 74–85. [Google Scholar] [CrossRef]
- Yi, W.; Wei, Q.; Di, G.; Jing-Yu, L.; Luo, Y.-L. Phenols and flavonoids from the aerial part of Euphorbia hirta. Chin. J. Nat. Med. 2012, 10, 40–42. [Google Scholar]
- Vasconcelos, J.M.; Silva, A.M.S.; Cavaleiro, J.A.S. Chromones and flavanones from artemisia campestris subsp. maritima. Phytochemistry 1998, 49, 1421–1424. [Google Scholar] [CrossRef]
- Oyama, K.-I.; Kondo, T. Total Synthesis of Flavocommelin, a Component of the Blue Supramolecular Pigment fromCommelinacommunis, on the Basis of Direct 6-C-Glycosylation of Flavan. J. Org. Chem. 2004, 69, 5240–5246. [Google Scholar] [CrossRef]
- Isobe, T.; Doe, M.; Morimoto, Y.; Nagata, K.; Ohsaki, A. The anti-Helicobacter pylori flavones in a Brazilian plant, Hyptis fasciculata, and the activity of methoxyflavones. Boil. Pharm. Bull. 2006, 29, 1039–1041. [Google Scholar] [CrossRef]
- Mata-Cárdenas, B.D.; Vargas-Villarreal, J.; González-Salazar, F.; Palacios-Corona, R.; Salvador, S.-F. A new vial microassay to screen antiprotozoal drugs. Pharmacologyonline 2008, 1, 529–537. [Google Scholar]
- González-Salazar, F.; Mata-Cárdenas, B.D.; Vargas-Villarreal, J. Sensibilidad de trofozoitos de Entamoeba histolytica a Ivermectina. Medicina (Buenos Aires) 2009, 69, 318–320. [Google Scholar]
- Hirudkar, J.R.; Parmar, K.M.; Prasad, R.S.; Sinha, S.K.; Jogi, M.S.; Itankar, P.R.; Prasad, S.K. Quercetin a major biomarker of Psidium guajava L. inhibits SepA protease activity of Shigella flexneri in treatment of infectious diarrhoea. Microb. Pathog. 2019, 138, 103807. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintanilla-Licea, R.; Vargas-Villarreal, J.; Verde-Star, M.J.; Rivas-Galindo, V.M.; Torres-Hernández, Á.D. Antiprotozoal Activity Against Entamoeba histolytica of Flavonoids Isolated from Lippia graveolens Kunth. Molecules 2020, 25, 2464. https://doi.org/10.3390/molecules25112464
Quintanilla-Licea R, Vargas-Villarreal J, Verde-Star MJ, Rivas-Galindo VM, Torres-Hernández ÁD. Antiprotozoal Activity Against Entamoeba histolytica of Flavonoids Isolated from Lippia graveolens Kunth. Molecules. 2020; 25(11):2464. https://doi.org/10.3390/molecules25112464
Chicago/Turabian StyleQuintanilla-Licea, Ramiro, Javier Vargas-Villarreal, María Julia Verde-Star, Verónica Mayela Rivas-Galindo, and Ángel David Torres-Hernández. 2020. "Antiprotozoal Activity Against Entamoeba histolytica of Flavonoids Isolated from Lippia graveolens Kunth" Molecules 25, no. 11: 2464. https://doi.org/10.3390/molecules25112464
APA StyleQuintanilla-Licea, R., Vargas-Villarreal, J., Verde-Star, M. J., Rivas-Galindo, V. M., & Torres-Hernández, Á. D. (2020). Antiprotozoal Activity Against Entamoeba histolytica of Flavonoids Isolated from Lippia graveolens Kunth. Molecules, 25(11), 2464. https://doi.org/10.3390/molecules25112464






