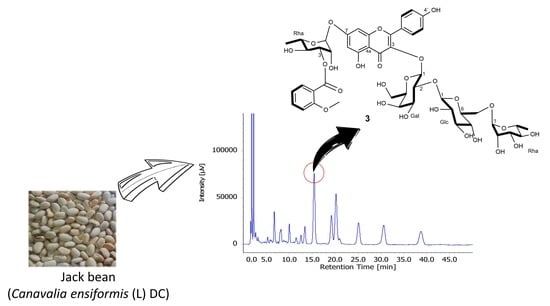
Identification and Characterization of α-Glucosidase Inhibition Flavonol Glycosides from Jack Bean (Canavalia ensiformis (L.) DC
Abstract
1. Introduction
2. Results and Discussion
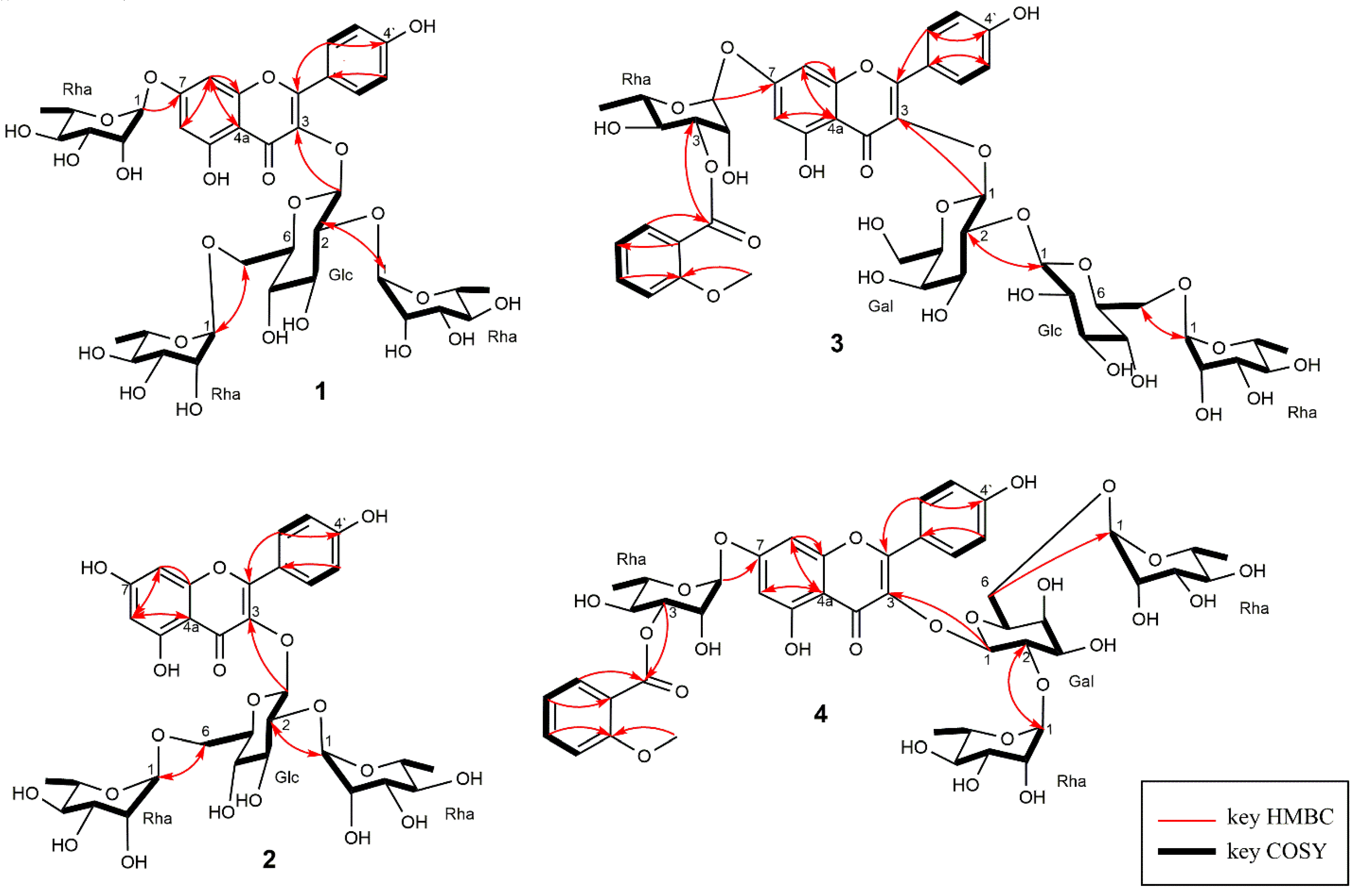
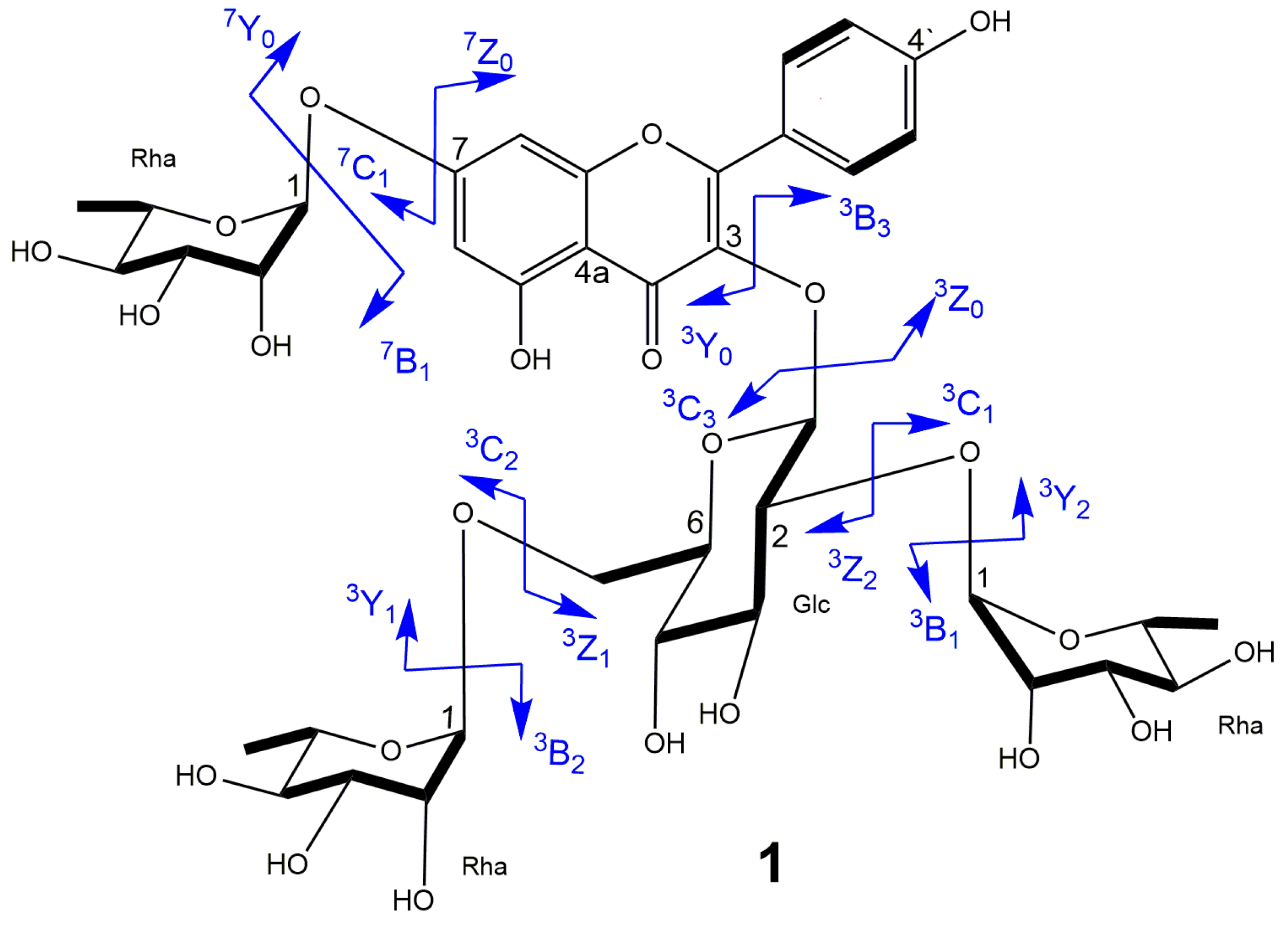
2.1. Structure Elucidation of 1–4
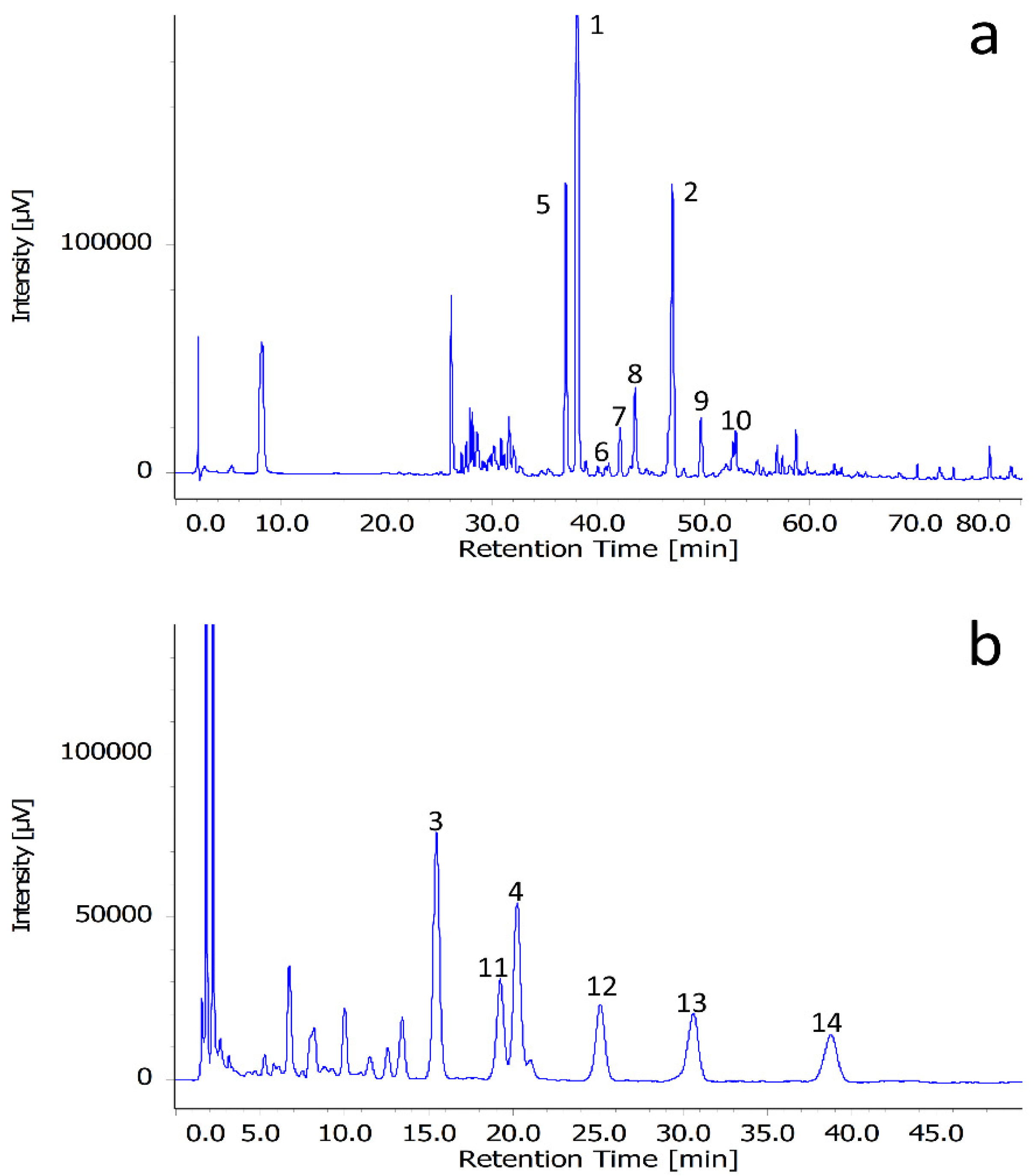
2.2. UPLC-ESI-MS/MS Analysis
2.3. Quantitation of Kaempferol Content in Methanolic Jack Bean Extract
2.4. In Vitro α-Glucosidase Inhibition Activity
3. Materials and Methods
3.1. Plant Materials
3.2. Reagents and Standards
3.3. Extraction and Isolation
3.4. HPLC Condition
3.5. Acid Hydrolysis of 1–4
3.6. GC-MS Condition
3.7. Ultrahigh-Performance Liquid Chromatography–Time-of-Flight Mass Spectrometry (UPLC-TOF-MS) Conditions
3.8. Spectroscopic Data of Compounds 1–4
3.8.1. Kaempferol 3-O-α-l-rhamnopyranosyl (1→2) [α-l-rhamnopyranosyl (1→6)]-β-d-glucopyranoside-7-O- α-l-rhamnopyranoside (1)
3.8.2. Kaempferol 3-O-(2,6-di-α-l-rhamnopyranosyl)-β-d-glucopyranoside (2)
3.8.3. Kaempferol 3-O-α-l-rhamnopyranosyl (1→6)-β-d-glucopyranosyl (1→2)-β-d-galactopyranosyl-7-O-[3-O-o-anisoyl]-α-l-rhamnopyranoside (3)
3.8.4. Kaempferol 3-O-(2,6-di-α-l-rhamnopyranosyl)-β-d-galactopyranoside-7-O-[3-O-o-anisoyl]- α-l-rhamnopyranoside (4)
3.9. Quantitative Analysis
3.10. In Vitro α-Glucosidase Inhibition Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; ISBN 978-2-930229-87-4. [Google Scholar]
- BischoffAg, H.B.B. Pharmacology of α-glucosidase inhibition. Eur. J. Clin. Investig. 2010, 24, 3–10. [Google Scholar] [CrossRef]
- Kumar, V.; Prakash, O.; Kumar, S.; Narwal, S. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef]
- Murad, M.H.; Coto-Yglesias, F.; Wang, A.T.; Sheidaee, N.; Mullan, R.J.; Elamin, M.B.; Erwin, P.J.; Montori, V.M. Drug-Induced Hypoglycemia: A Systematic Review. J. Clin. Endocrinol. Metab. 2009, 94, 741–745. [Google Scholar] [CrossRef]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; Del Cañizo-Gómez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354–395. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef]
- Ade-Omowaye, B.I.O.; Tucker, G.A.; Smetanska, I. Nutritional Potential of Nine Underexploited Legumes in Southwest Nigeria. Int. Food Res. J. 2015, 22, 798–806. [Google Scholar]
- Doss, A.; Pugalenthi, M.; Vadivel, V. Nutritional Evaluation of Wild Jack Bean (Canavalia ensiformis DC) Seeds in Different Locations of South India. World Appl. Sci. J. 2011, 13, 1606–1612. [Google Scholar]
- Chel-Guerrero, L.A.; Delgado-Herrera, A.; Betancur-Ancona, D.A.; Pérez-Aviña, P.; Castellanos-Ruelas, A.F. Protein and Energy Evaluation of Detoxified Canavalia Seeds as a Feedstuff for Poultry in the Tropics. Nov. Sci. 2016, 8, 219–232. [Google Scholar]
- Vadivel, V.; Biesalski, H.K. Effect of certain indigenous processing methods on the bioactive compounds of ten different wild type legume grains. J. Food Sci. Technol. 2011, 49, 673–684. [Google Scholar] [CrossRef]
- Doss, A.; Pugalenthi, M.; Vadivel, V.G.; Subhashini, G.; Anitha Subash, R. Effects of Processing Technique on the Nutritional Composition and Antinutrients Content of Under-utilized Food Legume Canavalia ensiformis L. DC. Int. Food Res. J. 2011, 18, 965–970. [Google Scholar]
- Akpapunam, M.; Sefa-Dedeh, S. Some physicochemical properties and anti-nutritional factors of raw, cooked and germinated Jack bean (Canavalia ensiformis). Food Chem. 1997, 59, 121–125. [Google Scholar] [CrossRef]
- Akande, K. Effects of Anti-nutritive Constituents of Unprocessed Jack Bean (Canavalia ensiformis) (L) (DC) Seed Meal on Poultry Performance. Am. J. Exp. Agric. 2016, 10, 1–10. [Google Scholar] [CrossRef]
- Akande, K.; Fabiyi, E. Effect of Processing Methods on Some Antinutritional Factors in Legume Seeds for Poultry Feeding. Int. J. Poult. Sci. 2010, 9, 996–1001. [Google Scholar] [CrossRef]
- Belmarl, R.; Nava-Montero, R.; Sandoval-Castro, C.; McNab, J.M. Jack Bean (Canavalia ensiformis L. DC) in Poultry Diets: Antinutritional Factors and Detoxification Studies—A Review. World’s Poult. Sci. J. 1999, 55, 37–59. [Google Scholar] [CrossRef]
- Olugboyega, S.K.; Edem, A.R. Comparative Phytochemicals and In Vitro Antioxidative Effects of Jack Beans (Canavalia ensiformis) and Sword Beans (Canavalia gladiata). Ann. Food Sci. Technol. 2018, 19, 499–505. [Google Scholar]
- Sowndhararajan, K.; Siddhuraju, P.; Manian, S.; Sowndhararajan, K. Antioxidant activity of the differentially processed seeds of Jack bean (Canavalia ensiformis L. DC). Food Sci. Biotechnol. 2011, 20, 585–591. [Google Scholar] [CrossRef]
- Vadivel, V.; Cheong, J.; Biesalski, H. Antioxidant and type II diabetes related enzyme inhibition properties of methanolic extract of an underutilized food legume, Canavalia ensiformis (L.) DC: Effect of traditional processing methods. LWT 2012, 47, 255–260. [Google Scholar] [CrossRef]
- Kite, G.C.; Stoneham, C.; Veitch, N.C. Flavonol tetraglycosides and other constituents from leaves of Styphnolobium japonicum (Leguminosae) and related taxa. Phytochemistry 2007, 68, 1407–1416. [Google Scholar] [CrossRef]
- Kazuma, K. Malonylated flavonol glycosides from the petals of Clitoria ternatea. Phytochem. 2003, 62, 229–237. [Google Scholar] [CrossRef]
- Murakami, T.; Kohno, K.; Kishi, A.; Matsuda, H.; Yoshikawa, M. Absolute Stereostructures of Canavalioside, a New Ent-kaurane-type Diterpene Glycoside and Gladiatosides A1, A2, A3, B1, B2, B3, C1 and C2, New Acylated Flavonol Glycosides, From Sword Bean, the Seeds of Canavalia gladiata. Chem. Pharm. Bull. 2000, 48, 1673–1680. [Google Scholar] [CrossRef]
- Kachlicki, P.; Piasecka, A.; Stobiecki, M.; Marczak, Ł. Structural Characterization of Flavonoid Glycoconjugates and Their Derivatives with Mass Spectrometric Techniques. Molecules 2016, 21, 1494. [Google Scholar] [CrossRef]
- Stobiecki, M.; Skirycz, A.; Kerhoas, L.; Kachlicki, P.; Muth, D.; Einhorn, J.; Mueller-Roeber, B. Profiling of phenolic glycosidic conjugates in leaves of Arabidopsis thaliana using LC/MS. Metabolomics 2006, 2, 197–219. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Cuyckens, F.; Heuvel, H.V.D.; Claeys, M. Mass spectrometric methods for the characterisation and differentiation of isomeric O-diglycosyl flavonoids. Phytochem. Anal. 2001, 12, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Cuyckens, F.; Rozenberg, R.; De Hoffmann, E.; Claeys, M. Structure characterization of flavonoid O-diglycosides by positive and negative nano-electrospray ionization ion trap mass spectrometry. J. Mass Spectrom. 2001, 36, 1203–1210. [Google Scholar] [CrossRef]
- Ferreres, F.; Andrade, P.B.; Valentão, P.; Gil-Izquierdo, A. Further knowledge on barley (Hordeum vulgare L.) leaves O-glycosyl-C-glycosyl flavones by liquid chromatography-UV diode-array detection-electrospray ionisation mass spectrometry. J. Chromatogr. A 2008, 1182, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in Dietary Polyphenols as α-Glucosidases Inhibitors: A Review on Structure-Activity Relationship Aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kwon, C.-S.; Son, K.H. Inhibition of Alpha-glucosidase and Amylase by Luteolin, a Flavonoid. Biosci. Biotechnol. Biochem. 2000, 64, 2458–2461. [Google Scholar] [CrossRef]
- Sutedja, A.M.; Yanase, E.; Batubara, I.; Fardiaz, D.; Lioe, H.N. Antidiabetic components from the hexane extract of red kidney beans (Phaseolus vulgaris L.): Isolation and structure determination. Biosci. Biotechnol. Biochem. 2019, 84, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| C | 1 a | 2 a | 3 a | 4 b | ||||
|---|---|---|---|---|---|---|---|---|
| δH (mult., J = Hz) | δC | δH (mult., J = Hz) | dC | δH (mult., J = Hz) | δC | δH (mult., J = Hz) | δC | |
| Aglycone | ||||||||
| 2 | - | 159.22 | - | 158.64 | - | 161.79 | - | 159.22 |
| 3 | - | 134.72 | - | 134.40 | - | 135.15 | - | 134.73 |
| 4 | - | 179.52 | - | 179.40 | - | 179.94 | - | 179.50 |
| 5 | - | 157.92 | - | 163.13 | - | 162.87 | - | 163.17 |
| 6 | 6.36 (d, 2.4) | 100.42 | 6.19 (d, 2.0) | 99.75 | 6.55 (s) | 100.70 | 6.52 (s) | 100.30 |
| 7 | - | 163.40 | - | 165.56 | - | 163.40 | - | 163.17 |
| 8 | 6.63 (d,2.4) | 95.62 | 6.38 (d, 2.0) | 94.64 | 6.82 (s) | 95.91 | 6.78 (s) | 95.66 |
| 9 | - | 162.90 | - | 158.40 | - | 158.00 | - | 157.95 |
| 10 | - | 107.50 | - | 105.90 | - | 107.30 | - | 107.68 |
| 1′ | 122.80 | 123.00 | 122.40 | 122.80 | ||||
| 2′, 6′ | 8.00 (d, 8.8) | 132.33 | 8.06 (d, 8.8) | 132.30 | 8.13 (d, 8.8) | 132.65 | 8.11 (d, 8.6) | 132.34 |
| 3′, 5′ | 6.81 (d, 8.8) | 116.25 | 6.90 (d, 8.8) | 116.20 | 6.93 (d, 8.8) | 116.33 | 6.92 (d, 8.2) | 116.23 |
| 4′ | - | 161.50 | - | 161.30 | - | 159.64 | - | 161.40 |
| 3-O-Glc/3-O-Galc | ||||||||
| 1 | 5.53 (d, 8.0) | 100.82 | 5.60 (d, 8.0) | 100.83 | 5.28 (d, 7.0) | 101.64 | 5.63 (br s) | 100.79 |
| 2 | 3.84 (br s) | 77.57 | 3.92 (dd, 9.6, 8.0) | 77.54 | 4.07 (dd, 7.8, 9.4) | 80.14 | 3.95 (t, 8.4) | 77.58 |
| 3 | 3.61 (dd, 3.2, 9.6) | 75.69 | 3.69 (dd, 3.2, 9.6) | 75.70 | 3.73 (m) | 74.74 | 3.70 (br s) | 75.58 |
| 4 | 3.68 (d, 3.2) | 70.67 | 3.76 (d, 3.2) | 70.68 | 3.81 (m) | 70.92 | 3.78 (d, 3.0) | 70.63 |
| 5 | 3.54 (t, 6.4) | 75.34 | 3.63 (t, 6.4) | 75.29 | 3.35 (br s) | 78.20 | 3.48 (br s) | 72.34 |
| 6 | 3.36 (m); 3.68 (m) | 67.15 | 3.49 (m); 3.72 (m) | 67.11 | 3.70 (m) | 62.56 | 3.65 (m), 3.70 (m) | 67.14 |
| 2′′-O-Rha/2′′-O-Glcd | ||||||||
| 1 | 5.12 (s) | 102.59 | 5.21 (br s) | 102.60 | 4.77 (d, 7.2) | 104.80 | 5.25 (s) | 102.54 |
| 2 | 3.91 (br s) | 71.68 | 4.00 (m) | 72.40 | 3.41 (m) | 71.23 | 4.01 (br s) | 72.34 |
| 3 | 3.69 (dd, 3.2, 9.6) | 72.37 | 3.80 (dd, 3.2, 9.6) | 72.44 | 3.42 (m) | 77.85 | 3.82 (br s) | 72.01 |
| 4 | 3,25 (t, 9.6) | 74.02 | 3.34 (t, 5.6) | 74.06 | 3.40 (d, 2.4) | 75.56 | 3.40 (m) | 74.03 |
| 5 | 3.95 (dd, 6.4, 9.6) | 69.83 | 4.06 (m) | 69.81 | 3.61 (t, 6.0) | 75.26 | 3.50 (m) | 69.79 |
| 6 | 0.88 (d, 6.4) | 17.54 | 0.97 (d, 5.6) | 17.53 | 3.65 (br s); 3.40 (m) | 67.21 | 1.17 (d, 6.0) | 17.94 |
| 6′′-O-Rha/1′′′-O-Rha | ||||||||
| 1 | 4.42 (s) | 101.80 | 4.52 (s) | 101.86 | 4.51 (br s) | 101.83 | 4.51 (br s) | 101.78 |
| 2 | 3.45 (br s) | 71.68 | 3.56 (br s) | 72.07 | 3.55 (br s) | 72.03 | 3.55 (br s) | 72.25 |
| 3 | 3.40 (m) | 72.23 | 3.51 (m) | 72.27 | 3.48 (dd, 9.6, 4.0) | 72.26 | 3.46 (d, 5.4) | 67.14 |
| 4 | 3.21 (m) | 73.86 | 3.27 (m) | 73.85 | 3.27 (t, 9.6) | 73.86 | 3.29 (br s) | 73.86 |
| 5 | 3.41 (m) | 69.66 | 3.53 (m) | 69.68 | 3.51 (m) | 69.67 | 4.06 (m) | 69.63 |
| 6 | 1.07 (d, 6.4) | 17.95 | 1.17 (d, 5.6) | 17.94 | 1.17 (d, 5.6) | 17.94 | 0.99 (d, 6.0) | 17.54 |
| 7-O-Rha | ||||||||
| 1 | 5.47 (s) | 99.87 | - | - | 5.63 (s) | 99.82 | 5.62 (s) | 99.81 |
| 2 | 3.93 (br s) | 71.68 | - | - | 4.35 (br s) | 69.50 | 4.35 (br s) | 69.52 |
| 3 | 3.73 (dd, 3.2, 9.6) | 72.04 | - | - | 5.34 (dd, 9.6, 3.2) | 75.42 | 5.35 (dd, 9.0, 3.6) | 75.62 |
| 4 | 3.39 (br s) | 73.59 | - | - | 3.84 (t, 9.6) | 70.92 | 3.81 (br s) | 70.91 |
| 5 | 3.51 (dd, 5.6, 9.6) | 71.24 | - | - | 3.79 (m) | 71.51 | 3.80 (m) | 71.45 |
| 6 | 1.16 (d, 5.6) | 18.10 | - | - | 1.33 (d. 6.4) | 18.15 | 1.33 (d, 6.0) | 18.14 |
| Anis | ||||||||
| -1’ | - | - | - | - | - | 120.96 | - | 121.14 |
| -2’ | - | - | - | - | - | 160.81 | - | 160.76 |
| -3’ | - | - | - | - | 7.13 (d, 8.8) | 113.37 | 7.13 (d, 8.4) | 113.37 |
| -4’ | - | - | - | - | 7.55 (t, 7.2) | 136.15 | 7.55 (t, 7.8) | 135.19 |
| -5’ | - | - | - | - | 7.03 (t, 8.0) | 121.15 | 7.03 (t, 7.8) | 121.01 |
| -6’ | - | - | - | - | 8.00 (d, 6.4) | 132.91 | 7.96 (d, 7.8) | 131.48 |
| -OCH3 | - | - | - | - | 3.90 (s) | 56.43 | 3.90 (s) | 56.45 |
| -CO | - | - | - | - | - | 167.38 | - | 167.37 |
| Compound | Fragment Ions a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3Y2 | 3Y1 | 3Y0 | 3Z2 | 3Z1 | 3Y07Y2 | 3Y07Y1 | 3Y07Y0 | [A+R]+ b | |
| 1 | 741.2214 | 595.1542 | 433.0958 | n.d c | 579.1572 | n.d | n.d | 287.0376 | − |
| 2 | 595.1542 | 449.1033 | 287.0376 | n.d | 433.0958 | − | − | − | − |
| 3 | 891.2531 | 729.1957 | 567.1334 | 875.2322 | n.d | n.d | 433.1136 | 287.0576 | 281.1014 |
| 4 | 875.2546 | 729.2129 | 567.1486 | n.d | 713.1918 | 433.1136 | n.d | 287.0576 | 281.1014 |
| Compound | Content (mg/100 g dry wt.) a | Formula | [M + H]+ | Aglycone b | Derivatives c | Detection | |||
|---|---|---|---|---|---|---|---|---|---|
| Calculated Mass | Exact Mass | Mass Error(ppm) | Glycans | Other | |||||
| 1 | 2.2862 ± 0.0745 | C39H50O23 | 887.2821 | 887.2843 | 2.4795 | K | Rha, Rha, Rha, Hex | MS, NMR | |
| 2 | 0.6651 ± 0.0205 | C33H40O19 | 741.2242 | 741.2214 | −3.7775 | K | Rha, Rha, Hex | MS, NMR | |
| 3 | 0.4906 ± 0.0176 | C47H56O26 | 1037.3138 | 1037.3076 | −5.9770 | K | Rha, Rha, Hex, Hex | Anis | MS, NMR |
| 4 | 0.3916 ± 0.0134 | C47H56O25 | 1021.3189 | 1021.3170 | −1.8603 | K | Rha, Rha, Rha, Hex | Anis | MS, NMR |
| 5 | 0.5743 ± 0.0185 | C39H50O24 | 903.2770 | 903.2747 | −2.5463 | K | Rha, Rha, Hex, Hex | MS | |
| 6 | 0.0778 ± 0.0033 | C38H48O23 | 873.2665 | 873.2609 | −6.4127 | K | Rha, Rha, Hex, Xyl | MS | |
| 7 | 0.1987 ± 0.0072 | C33H40O20 | 757.2191 | 757.2161 | −3.9617 | K | Rha, Hex, Hex | MS | |
| 8 | 0.0139 ± 0.0031 | C33H40O19 | 741.2242 | 741.2214 | −3.7775 | K | Rha, Rha, Hex | MS | |
| 9 | 0.1089 ± 0.0041 | C32H38O19 | 727.2086 | 727.2133 | 6.4631 | K | Rha, Hex, Xyl | MS | |
| 10 | 0.0520 ± 0.0015 | C27H30O15 | 595.1663 | 595.1622 | −6.8888 | K | Rha, Hex | MS | |
| 11 | 0.3045 ± 0.0162 | C47H56O26 | 1037.3138 | 1037.3076 | −5.9770 | K | Rha, Rha, Hex, Hex | Anis | MS |
| 12 | 0.2475 ± 0.0105 | C47H56O25 | 1021.3189 | 1021.3170 | −1.8603 | K | Rha, Rha, Rha, Hex | Anis | MS |
| 13 | 0.0866 ± 0.0037 | C47H56O26 | 1037.3138 | 1037.3076 | −5.9770 | K | Rha, Rha, Hex, Hex, | Anis | MS |
| 14 | 0.0722 ± 0.0030 | C47H56O25 | 1021.3189 | 1021.3170 | −1.8603 | K | Rha, Rha, Rha, Hex | Anis | MS |
| Sample * | Inhibition Activity (%) ** |
|---|---|
| F20 | trace *** |
| F50 | 24.91 ± 0.84a |
| F80 | 51.34 ± 1.23d |
| F100 | trace |
| Compound 1 | 30.96 ± 1.62b |
| Compound 2 | 39.54 ± 0.69c |
| Compound 3 | 90.07 ± 1.60g |
| Compound 4 | 77.06 ± 0.79f |
| Acarbose | 71.71 ± 0.08e |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutedja, A.M.; Yanase, E.; Batubara, I.; Fardiaz, D.; Lioe, H.N. Identification and Characterization of α-Glucosidase Inhibition Flavonol Glycosides from Jack Bean (Canavalia ensiformis (L.) DC. Molecules 2020, 25, 2481. https://doi.org/10.3390/molecules25112481
Sutedja AM, Yanase E, Batubara I, Fardiaz D, Lioe HN. Identification and Characterization of α-Glucosidase Inhibition Flavonol Glycosides from Jack Bean (Canavalia ensiformis (L.) DC. Molecules. 2020; 25(11):2481. https://doi.org/10.3390/molecules25112481
Chicago/Turabian StyleSutedja, Anita M., Emiko Yanase, Irmanida Batubara, Dedi Fardiaz, and Hanifah N. Lioe. 2020. "Identification and Characterization of α-Glucosidase Inhibition Flavonol Glycosides from Jack Bean (Canavalia ensiformis (L.) DC" Molecules 25, no. 11: 2481. https://doi.org/10.3390/molecules25112481
APA StyleSutedja, A. M., Yanase, E., Batubara, I., Fardiaz, D., & Lioe, H. N. (2020). Identification and Characterization of α-Glucosidase Inhibition Flavonol Glycosides from Jack Bean (Canavalia ensiformis (L.) DC. Molecules, 25(11), 2481. https://doi.org/10.3390/molecules25112481