Pretreatment with Warfarin Attenuates the Development of Ischemia/Reperfusion-Induced Acute Pancreatitis in Rats
Abstract
1. Introduction
2. Results
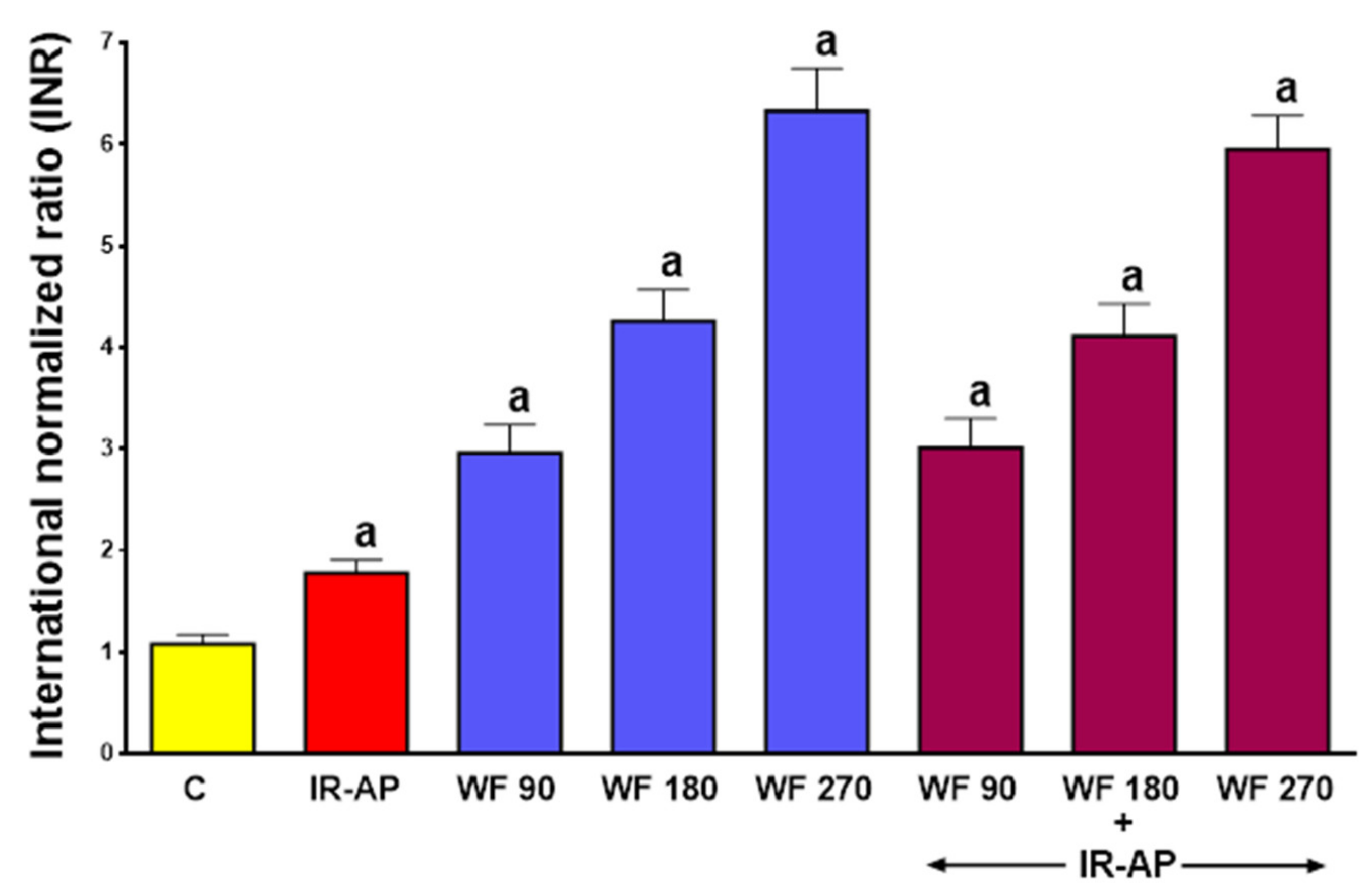
2.1. The International Normalized Ratio
2.2. Histological Examination
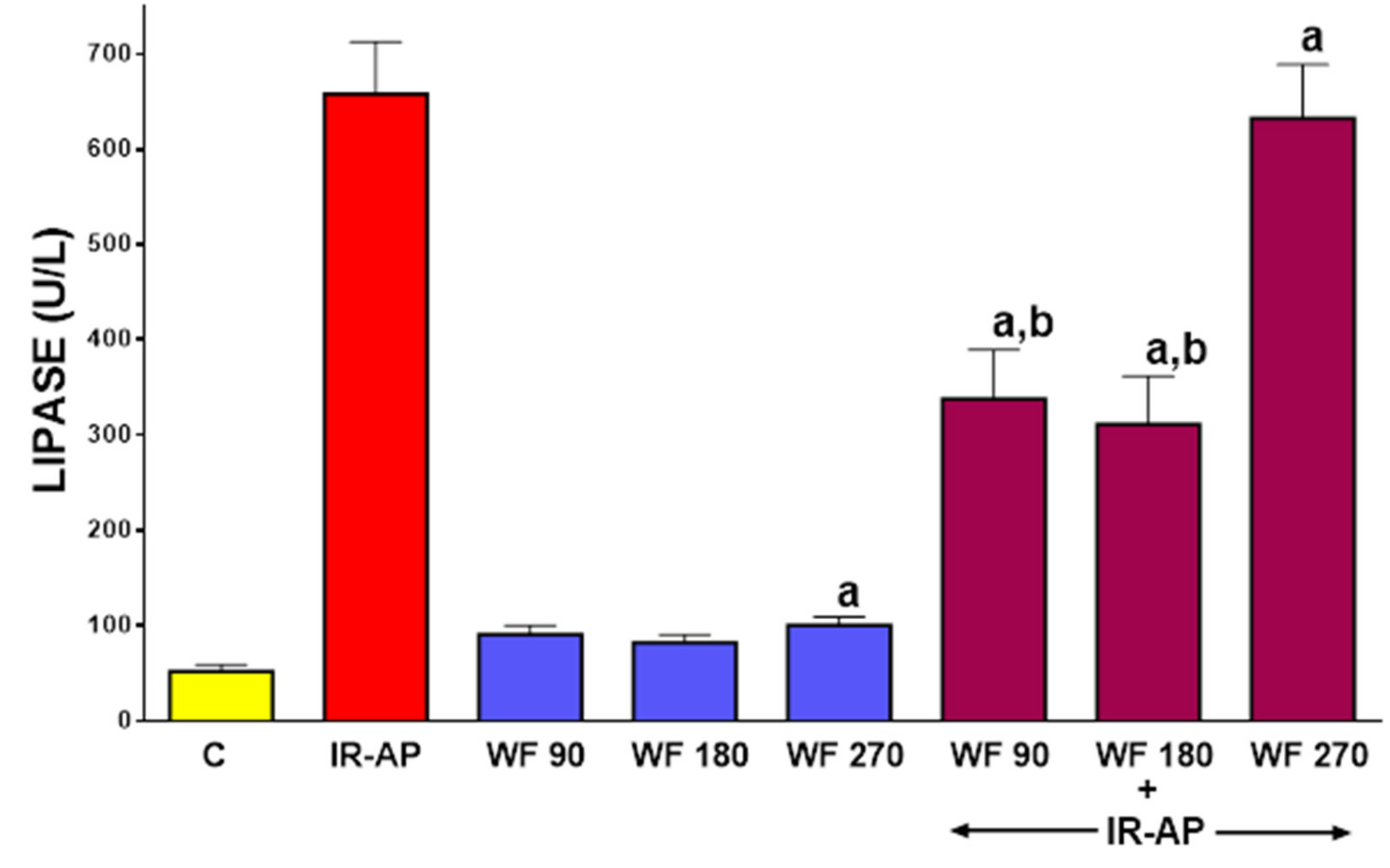
2.3. Serum Activity of Pancreatic Digestive Enzymes
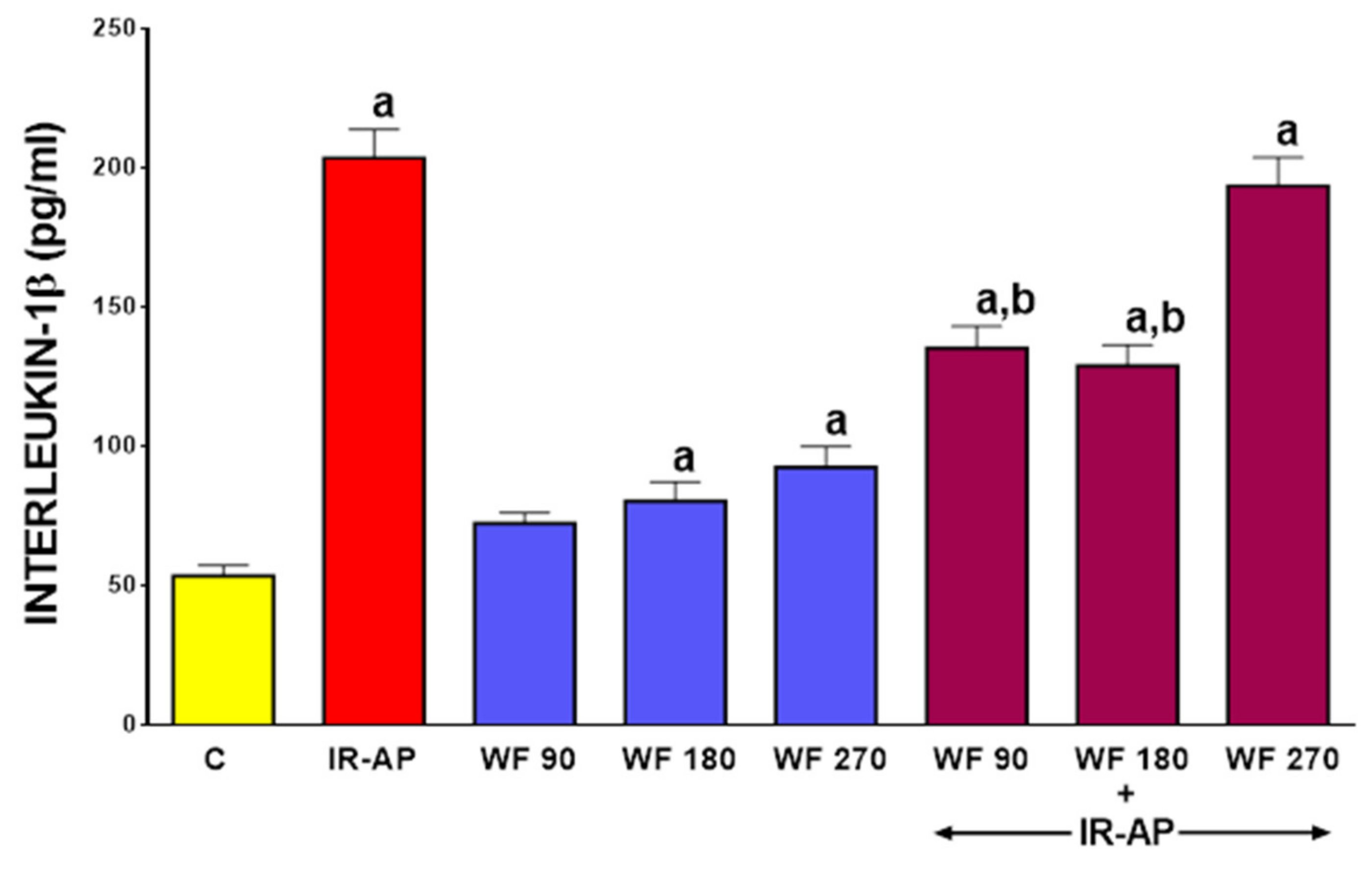
2.4. Serum Level of Interleukin-1β (IL-β)
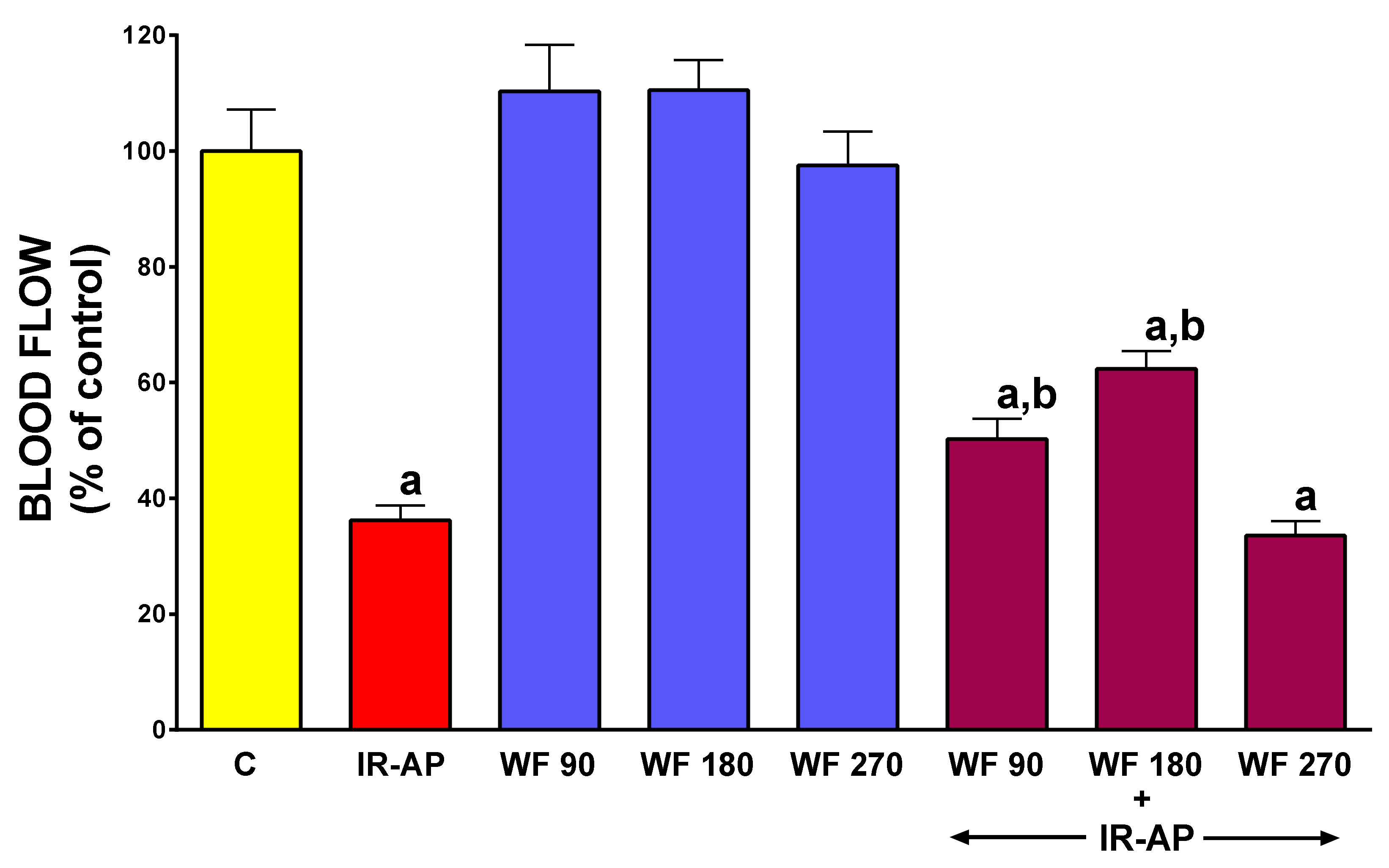
2.5. Pancreatic Blood Flow
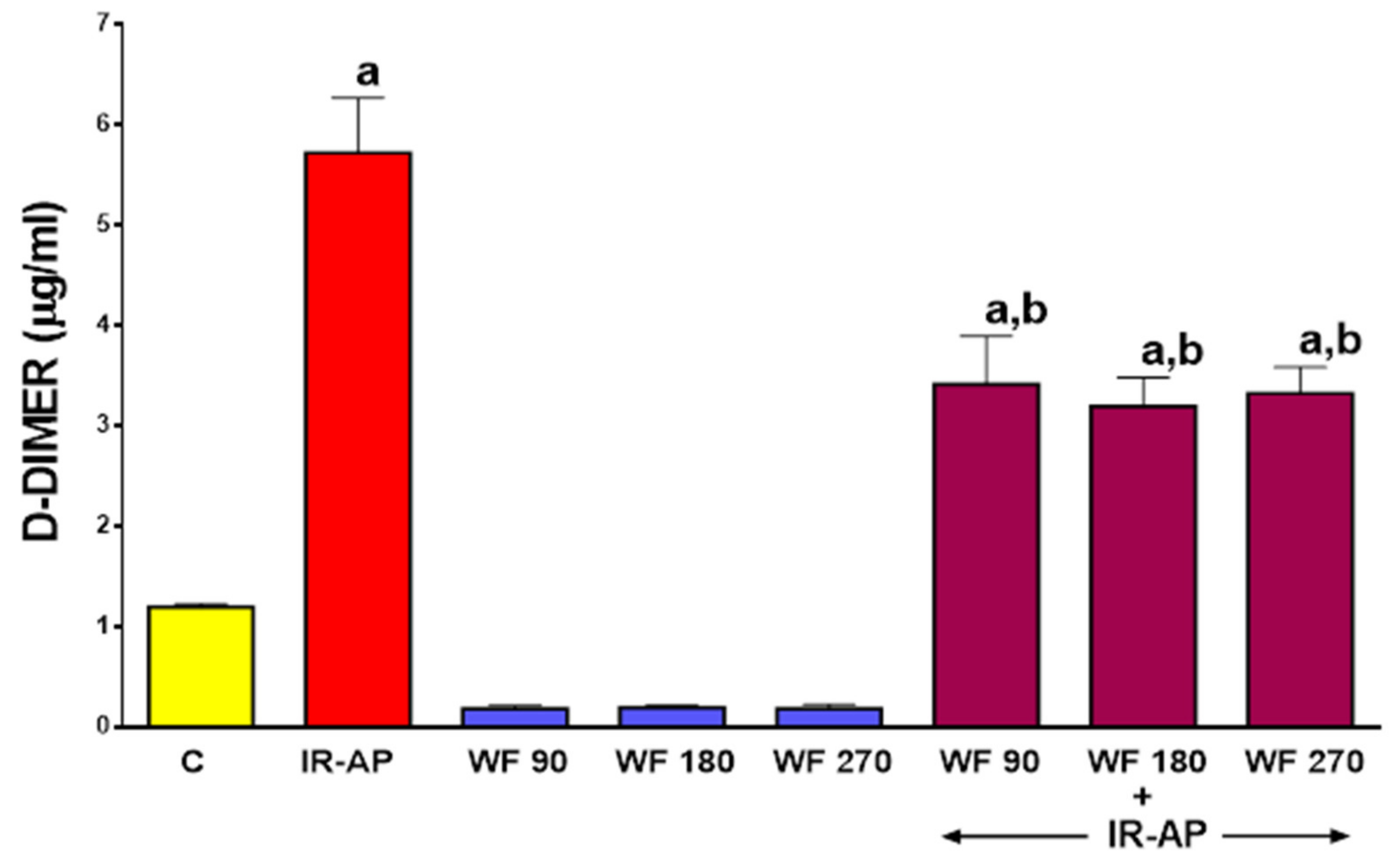
2.6. Plasma Concentration of D-dimer
2.7. Animal Mortality During Experiment
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. Determination of Pancreatic Blood Flow
4.3. Biochemical Analysis
4.4. Histological Examination of Pancreatic Damage
- (1)
- pancreatic edema (0—no edema, 1—interlobar edema, 2—interlobar and moderate intralobular edema, 3—severe interlobular and intralobular edema);
- (2)
- leukocyte inflammatory infiltration (0—absent, 1—scarce perivascular infiltration, 2—moderate perivascular and scarce diffuse infiltration, 3—abundant diffuse infiltration);
- (3)
- vacuolization of acinar cells (0—absent, 1—involving less than 25% of acinar cells, 2—involving from 25% to 50% acinar cells, 3—involving more than 50% of acinar cells);
- (4)
- necrosis of acinar cells (0—absent, 1—involving less than 15% of acinar cells, 2—involving from 15% to 35% acinar cells, 3—involving more than 35% of acinar cells);
- (5)
- hemorrhage (0—absent, 1—from 1 to 2 foci per slide, 2—from 3 to 5 foci per slide, 3—more than 5 foci per slide).
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Esmon, C.T. Reprint of Crosstalk between inflammation and thrombosis. Maturitas 2008, 61, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Danckwardt, S.; Hentze, M.W.; Kulozik, A.E. Pathologies at the nexus of blood coagulation and inflammation: Thrombin in hemostasis, cancer, and beyond. J. Mol. Med. 2013, 91, 1257–1271. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. Possible involvement of cytokines in diffuse intravascular coagulation and thrombosis. Best Pr. Res. Clin. Haematol. 1999, 12, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Van Der Poll, T.; Schultz, M. New insights into pathways that determine the link between infection and thrombosis. Neth. J. Med. 2012, 70, 114–120. [Google Scholar] [PubMed]
- Levi, M.; Keller, T.T.; Van Gorp, E.; Cate, H.T. Infection and inflammation and the coagulation system. Cardiovasc. Res. 2003, 60, 26–39. [Google Scholar] [CrossRef]
- Coughlin, S.R. Thrombin signalling and protease-activated receptors. Nature 2000, 407, 258–264. [Google Scholar] [CrossRef]
- Rezaie, A.R. Protease-activated receptor signalling by coagulation proteases in endothelial cells. Thromb. Haemost. 2014, 112, 876–882. [Google Scholar] [CrossRef]
- Camerer, E.; Røttingen, J.-A.; Gjernes, E.; Larsen, K.; Skartlien, A.H.; Iversen, J.-G.; Prydz, H. Coagulation Factors VIIa and Xa Induce Cell Signaling Leading to Up-regulation of theegr-1Gene. J. Boil. Chem. 1999, 274, 32225–32233. [Google Scholar] [CrossRef]
- Jin, Y.; Nonoyama, S.; Morio, T.; Imai, K.; Ochs, H.D.; Mizutani, S. Characterization of soluble CD40 ligand released from human activated platelets. J. Med. Dent. Sci. 2001, 48, 23–27. [Google Scholar]
- Croce, K.; Libby, P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr. Opin. Hematol. 2007, 14, 55–61. [Google Scholar] [CrossRef]
- Henn, V.; Slupsky, J.R.; Gräfe, M.; Anagnostopoulos, I.; Förster, R.; Müller-Berghaus, G.; Kroczek, R.A. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998, 391, 591–594. [Google Scholar] [CrossRef]
- Lasson, Å.; Ohlsson, K. Consumptive coagulopathy, fibrinolysis and protease antiprotease interactions during acute human pancreatitis. Thromb. Res. 1986, 41, 167–183. [Google Scholar] [CrossRef]
- Salomone, T.; Tosi, P.; Palareti, G.; Tomassetti, P.; Migliori, M.; Guariento, A.; Saieva, C.; Raiti, C.; Romboli, M.; Gullo, L. Coagulative Disorders in Human Acute Pancreatitis: Role for the D-Dimer. Pancreas 2003, 26, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Hirota, M.; Ichihara, A.; Ohmuraya, M.; Hashimoto, D.; Sugita, H.; Takamori, H.; Kanemitsu, K.; Baba, H. Applicability of Disseminated Intravascular Coagulation Parameters in the Assessment of the Severity of Acute Pancreatitis. Pancreas 2006, 32, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Dumnicka, P.; Maduzia, D.; Ceranowicz, P.; Olszanecki, R.; Drozdz, R.; Kuśnierz-Cabala, B. The Interplay between Inflammation, Coagulation and Endothelial Injury in the Early Phase of Acute Pancreatitis: Clinical Implications. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Bowman, W.C.; Rand, M.J. (Eds.) The blood: Drugs affecting coagulation, fibrinolysis, haematopoiesis and functioning of blood cells. In Textbook of Pharmacology; Blackwell Publishers: Oxford, London, UK, 1980; p. 21. [Google Scholar]
- Linhardt, R.J. Fbsbioscience Org Heparin and anticoagulation. Front. Biosci. 2016, 21, 1372–1392. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembinski, M.; Ceranowicz, P.; Dembinski, A. Heparin and its anti-inflammatory action in the gut. Gastroenterol. Pol. 2010, 17, 227–233. [Google Scholar]
- Finotti, P.; Manente, S. Heparin-induced structural and functional alterations of bovine trypsin. Biochim. Biophys. Acta 1994, 1207, 80–87. [Google Scholar] [CrossRef]
- Struβ, D.; Storck, J.; Zimmermann, R. The inhibition of thrombin and chymotrypsin by heparin-cofactor II. Thromb. Res. 1992, 68, 45–56. [Google Scholar] [CrossRef]
- Wolosowicz, N.; Prokopowicz, J.; Gabryelewicz, A. The inhibitory effect of heparin on trypsinogen activation with enterokinase. Acta Hepatogastroenterol. 1977, 24, 367–371. [Google Scholar]
- Gabryelewicz, A.; Kosidlo, S.; Prokopowicz, J.; Podkowicz, K. Does heparin modify protease-antiprotease balance in acute experimental pancreatitis in rats. Hepatogastroenterology 1986, 33, 79–82. [Google Scholar] [PubMed]
- Dobosz, M.; Wajda, Z.; Hac, S.; Mysliwska, J.; Mionskowska, L.; Bryl, E.; Roszkiewicz, A.; Mysliwski, A. Heparin and nitric oxide treatment in experimental acute pancreatitis in rats. Forum 1998, 8, 303–310. [Google Scholar] [PubMed]
- Gabryelewicz, A.; Niewiarowski, S.; Prokopowicz, J.; Chlebowski, J. Heparin and Protease Inhibitors in the Prevention of Experimental Acute Pancreatic Necrosis in Dogs. Digestion 1969, 2, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Lu, X.-S.; Huang, Y.-K. Effect of low molecular weight heparin on pancreatic micro-circulation in severe acute pancreatitis in a rodent model. Chin. Med J. 2007, 120, 2260–2263. [Google Scholar] [CrossRef] [PubMed]
- Ceranowicz, P.; Dembinski, A.; Warzecha, Z.; Dembinski, M.; Cieszkowski, J.; Rembisz, K.; Konturek, S.J.; Kusnierz-Cabala, B.; Tomaszewska, R.; Pawlik, W.W. Protective and therapeutic effect of heparin in acute pancreatitis. J. Physiol. Pharmacol. 2008, 59, 103–125. [Google Scholar] [PubMed]
- Ceranowicz, P.; Dembinski, M.; Warzecha, Z.; Cieszkowski, J.; Kusnierz-Cabala, B.; Tomaszewska, R.; Dembinski, A. Healing effect of heparin in the course of edematous, cerulein-induced acute pancreatitis. Przeglad. Gastroenterology 2009, 4, 199–205. [Google Scholar]
- Rabenstein, T.; Roggenbuck, S.; Framke, B.; Martus, P.; Fischer, B.; Nusko, G.; Muehldorfer, S.; Hochberger, J.; Ell, C.; Hahn, E.G.; et al. Complications of endoscopic sphincterotomy: Can heparin prevent acute pancreatitis after ERCP? Gastrointest. Endosc. 2002, 55, 476–483. [Google Scholar] [CrossRef]
- Xin-Sheng, L.; Fu, Q.; Jie-Qin, L.; Qin-Qiao, F.; Ri-Guang, Z.; Yu-Hang, A.; Kai-Cheng, Z.; Yi-Xiong, L. Low Molecular Weight Heparin in the Treatment of Severe Acute Pancreatitis: A Multiple Centre Prospective Clinical Study. Asian J. Surg. 2009, 32, 89–94. [Google Scholar] [CrossRef]
- Du, J.-D.; Zheng, X.; Huang, Z.-Q.; Cai, S.-W.; Tan, J.-W.; Li, Z.-L.; Yao, Y.-M.; Jiao, H.-B.; Yin, H.-N.; Zhu, Z.-M. Effects of intensive insulin therapy combined with low molecular weight heparin anticoagulant therapy on severe pancreatitis. Exp. Ther. Med. 2014, 8, 141–146. [Google Scholar] [CrossRef][Green Version]
- Tozlu, M.; Kayar, Y.; Ince, A.T.; Baysal, B.; Senturk, H. Low molecular weight heparin treatment of acute moderate and severe pancreatitis: A randomized, controlled, open-label study. Turk. J. Gastroenterol. 2020, 30, 81–87. [Google Scholar] [CrossRef]
- Alagozlu, H.; Cindoruk, M.; Karakan, T.; Unal, S. Heparin and Insulin in the Treatment of Hypertriglyceridemia-Induced Severe Acute Pancreatitis. Dig. Dis. Sci. 2006, 51, 931–933. [Google Scholar] [CrossRef]
- Kyriakidis, A.; Raitsiou, B.; Sakagianni, A.; Harisopoulou, V.; Pyrgioti, M.; Panagopoulou, A.; Vasilakis, N.; Lambropoulos, S. Management of Acute Severe Hyperlipidemic Pancreatitis. Digestion 2006, 73, 259–264. [Google Scholar] [CrossRef]
- Valdivielso, P.; Ramírez-Bueno, A.; Ewald, N. Current knowledge of hypertriglyceridemic pancreatitis. Eur. J. Intern. Med. 2014, 25, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-S.; Qiu, F.; Li, Y.-X.; Li, J.-Q.; Fan, Q.-Q.; Zhou, R.-G. Effect of Lower-Molecular Weight Heparin in the Prevention of Pancreatic Encephalopathy in the Patient With Severe Acute Pancreatitis. Pancreas 2010, 39, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, Z.; Sendur, P.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Kusnierz-Cabala, B.; Tomaszewska, R.; Dembinski, A. Pretreatment with low doses of acenocoumarol inhibits the development of acute ischemia/reperfusion-induced pancreatitis. J. Physiol. Pharmacol. 2015, 66, 731–740. [Google Scholar] [PubMed]
- Warzecha, Z.; Sendur, P.; Ceranowicz, P.; Dembiński, M.; Cieszkowski, J.; Kuśnierz-Cabala, B.; Olszanecki, R.; Tomaszewska, R.; Ambroży, T.; Dembiński, A. Protective Effect of Pretreatment with Acenocoumarol in Cerulein-Induced Acute Pancreatitis. Int. J. Mol. Sci. 2016, 17, 1709. [Google Scholar] [CrossRef]
- Warzecha, Z.; Sendur, P.; Ceranowicz, P.; Cieszkowski, J.; Dembiński, M.; Sendur, R.; Bonior, J.; Jaworek, J.; Ambroży, T.; Olszanecki, R.; et al. Therapeutic Effect of Low Doses of Acenocoumarol in the Course of Ischemia/Reperfusion-Induced Acute Pancreatitis in Rats. Int. J. Mol. Sci. 2017. [Google Scholar] [CrossRef]
- Wu, S.; Liu, S.; Davis, C.H.; Stafford, D.W.; Kulman, J.D.; Pedersen, L.G. A hetero-dimer model for concerted action of vitamin K carboxylase and vitamin K reductase in vitamin K cycle. J. Theor. Boil. 2011, 279, 143–149. [Google Scholar] [CrossRef]
- Verhoef, T.I.; Redekop, W.K.; Daly, A.K.; Van Schie, R.M.F.; De Boer, A.; Der Zee, A.H.M.-V. Pharmacogenetic-guided dosing of coumarin anticoagulants: Algorithms for warfarin, acenocoumarol and phenprocoumon. Br. J. Clin. Pharmacol. 2014, 77, 626–641. [Google Scholar] [CrossRef]
- Pattacini, C.; Manotti, C.; Pini, M.; Quintavalla, R.; Dettori, A.G. A comparative study on the quality of oral anticoagulant therapy (warfarin versus acenocoumarol). Thromb. Haemost. 1994, 71, 188–191. [Google Scholar]
- Palareti, G.; Legnani, C.; Guazzaloca, G.; Lelia, V.; Cosmi, B.; Lunghi, B.; Marchetti, G.; Poli, D.; Pengo, V. Ad hoc Study Group of the Italian Federation of Anticoagulation Clinics Risks factors for highly unstable response to oral anticoagulation: A case-control study. Br. J. Haematol. 2005, 129, 72–78. [Google Scholar] [CrossRef]
- Undas, A.; Cieśla-Dul, M.; Zółciński, M.; Tracz, W. Switching from acenocoumarol to warfarin in patients with unstable anticoagulation and its effect on anticoagulation control. Pol. Arch. Intern. Med. 2009, 119, 360–365. [Google Scholar] [CrossRef]
- Wang, S.-B.; Liu, H.; Li, G.-Y.; Li, J.; Li, X.-J.; Lei, K.; Wei, L.-C.; Quan, Z.-S.; Wang, X.-K.; Liu, R.-M. Coumarin and 3,4-dihydroquinolinone derivatives: Synthesis, antidepressant activity, and molecular docking studies. Pharmacol. Rep. 2019, 71, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wang, R.; Li, S.; Wang, Y.; Song, F.; Gu, Y.; Yuan, Y. Antifibrotic effects of Fraxetin on carbon tetrachloride-induced liver fibrosis by targeting NF-κB/IκBα, MAPKs and Bcl-2/Bax pathways. Pharmacol. Rep. 2019, 71, 409–416. [Google Scholar] [CrossRef] [PubMed]
- El-Kashef, D.H.; Shaaban, A.A.; El-Agamy, D.S. Protective role of pirfenidone against experimentally-induced pancreatitis. Pharmacol. Rep. 2019, 71, 774–781. [Google Scholar] [CrossRef]
- Bleeker, W.K.; Agterberg, J.; Rigter, G.; Hack, C.E.; Gool, J.V.; Bleeker, W.K. Protective effect of antithrombin III in acute experimental pancreatitis in rats. Dig. Dis. Sci. 1992, 37, 280–285. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Weidenbach, H.; Lührs, H.; Lerch, M.M.; Dickneite, G.; Adler, G. Combined treatment with C1 esterase inhibitor and antithrombin III improves survival in severe acute experimental pancreatitis. Gut 1997, 40, 531–535. [Google Scholar] [CrossRef]
- Hagiwara, S.; Iwasaka, H.; Shingu, C.; Matsumoto, S.; Uchida, T.; Noguchi, T. Antithrombin III Prevents Cerulein-Induced Acute Pancreatitis in Rats. Pancreas 2009, 38, 746–751. [Google Scholar] [CrossRef]
- Fertmann, J.M.; Arbogast, H.P.; Illner, W.-D.; Tarabichi, A.; Dieterle, C.; Land, W.G.; Jauch, K.-W.; Johannes, N.H. Antithrombin therapy in pancreas retransplantation and pancreas-after-kidney/pancreas-transplantation-alone patients. Clin. Transplant. 2011, 25, E499–E508. [Google Scholar] [CrossRef]
- Ageno, W.; Gallus, A.S.; Wittkowsky, A.; Crowther, M.; Hylek, E.M.; Palareti, G. Oral Anticoagulant Therapy. Chest 2012. [Google Scholar] [CrossRef]
- Harauchi, T.; Takano, K.; Matsuura, M.; Yoshizaki, T. Liver and plasma levels of descarboxyprothrombin (PIVKA II) in vitamin K deficiency in rats. Jpn. J. Pharmacol. 1986, 40, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Zabczyk, M. Antithrombotic medications and their impact on fibrin clot structure and function. J. Physiol. Pharmacol. 2018. [Google Scholar] [CrossRef]
- Drabik, L.; Wołkow, P.; Undas, A. Fibrin Clot Permeability as a Predictor of Stroke and Bleeding in Anticoagulated Patients With Atrial Fibrillation. Stroke 2017, 48, 2716–2722. [Google Scholar] [CrossRef] [PubMed]
- Beger, H.G.; Isenmann, R.; Imrie, C. Diagnosis, Objective Assessment of Severity, and Management of Acute Pancreatitis: Santorini Consensus Conference. Int. J. Pancreatol. 1999, 26, 1–4. [Google Scholar] [CrossRef]
- Warzecha, Z.; Ceranowicz, P.; Dembinski, A.; Cieszkowski, J.; Kusnierz-Cabala, B.; Tomaszewska, R.; Kuwahara, A.; Kato, I. Therapeutic effect of ghrelin in the course of cerulein-induced acute pancreatitis in rats. J. Physiol. Pharmacol. 2010, 61, 419–427. [Google Scholar]
- Bukowczan, J.; Warzecha, Z.; Ceranowicz, P.; Kuśnierz-Cabala, B.; Tomaszewska, R.; Dembiński, A. Pretreatment with obestatin reduces the severity of ischemia/reperfusion-induced acute pancreatitis in rats. Eur. J. Pharmacol. 2015, 760, 113–121. [Google Scholar] [CrossRef]
- Bukowczan, J.; Cieszkowski, J.; Warzecha, Z.; Ceranowicz, P.; Kuśnierz-Cabala, B.; Tomaszewska, R.; Dembinski, A. Therapeutic Effect of Obestatin in the Course of Cerulein-Induced Acute Pancreatitis. Pancreas 2016. [Google Scholar] [CrossRef]
- Bonior, J.; Warzecha, Z.; Ceranowicz, P.; Gajdosz, R.; Pierzchalski, P.; Kot, M.; Leja-Szpak, A.; Nawrot-Porąbka, K.; Link-Lenczowski, P.; Pędziwiatr, M.; et al. Capsaicin-Sensitive Sensory Nerves Are Necessary for the Protective Effect of Ghrelin in Cerulein-Induced Acute Pancreatitis in Rats. Int. J. Mol. Sci. 2017. [Google Scholar] [CrossRef]
- Fabre, A.; Boulogne, O.; Gaudart, J.; Mas, E.; Olives, J.-P.; Sarles, J. Evaluation of serum lipase as predictor of severity of acute pancreatitis in children. J. Pediatr. Gastroenterol. Nutr. 2014. [Google Scholar] [CrossRef]
- Keck, T.; Friebe, V.; Warshaw, A.L.; Antoniu, B.A.; Waneck, G.; Benz, S.; Hopt, U.T.; Fernandez-Del-Castillo, C. Pancreatic proteases in serum induce leukocyte-endothelial adhesion and pancreatic microcirculatory failure. Pancreatolgy 2005, 5, 241–250. [Google Scholar] [CrossRef]
- Fink, G.W.; Norman, J.G. Intrapancreatic Interleukin-1? Gene Expression by Specific Leukocyte Populations during Acute Pancreatitis. J. Surg. Res. 1996, 63, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.G.; Fink, G.W.; Denham, W.; Yang, J.; Carter, G.; Sexton, C.; Falkner, J.; Gower, W.R.; Franz, M.G. Tissue-specific cytokine production during experimental acute pancreatitis. A probable mechanism for distant organ dysfunction. Dig. Dis. Sci. 1997, 42, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Frossard, J.-L. Experimental acute pancreatitis: New insights into the pathophysiology. Front. Biosci. 2002. [Google Scholar] [CrossRef]
- A Dinarello, C. Interleukin-1 and interleukin-1 antagonism. Blood 1991, 77, 1625–1652. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Dembiński, M.; Cieszkowski, J.; Kuśnierz-Cabala, B.; Naskalski, J.W.; Jaworek, J.; Konturek, S.J.; Pawlik, W.W.; et al. Influence of ischemic preconditioning on blood coagulation, fibrinolytic activity and pancreatic repair in the course of caerulein-induced acute pancreatitis in rats. J. Physiol. Pharmacol. 2007, 58, 303–319. [Google Scholar] [PubMed]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Warzecha, A.M.; Pawlik, W.W.; Dembiński, M.; Rembiasz, K.; Sendur, P.; Kuśnierz-Cabala, B.; Tomaszewska, R.; et al. Dual, time-dependent deleterious and protective effect of anandamide on the course of cerulein-induced acute pancreatitis. Role of sensory nerves. Eur. J. Pharmacol. 2008, 591, 284–292. [Google Scholar] [CrossRef]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A.; Cieszkowski, J.; Dembinski, M.; Sendur, R.; Kusnierz-Cabala, B.; Tomaszewska, R.; Kuwahara, A.; Kato, I. Pretreatment with obestatin inhibits the development of cerulein-induced pancreatitis. J. Physiol. Pharmacol. 2009, 60, 95–101. [Google Scholar]
- Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Dembinski, M.; Sendur, R.; Cieszkowski, J.; Sendur, P.; Tomaszewska, R. Heparin inhibits protective effect of ischemic preconditioning in ischemia/reperfusion-induced acute pancreatitis. J. Physiol. Pharmacol. 2012, 63, 355–365. [Google Scholar]
- Norman, J.; Franz, M.; Messina, J.; Riker, A.; Fabri, P.J.; Rosemurgy, A.S.; Gowerjr, W. Interleukin-1 receptor antagonist decreases severity of experimental acute pancreatitis. Surgery 1995, 117, 648–655. [Google Scholar] [CrossRef]
- Marrache, F.; Tu, S.P.; Bhagat, G.; Pendyala, S.; Österreicher, C.H.; Gordon, S.; Ramanathan, V.; Penz-Österreicher, M.; Betz, K.S.; Song, Z.; et al. Overexpression of interleukin-1beta in the murine pancreas results in chronic pancreatitis. Gastroenterology 2008, 135, 1277–1287. [Google Scholar] [CrossRef]
- Park, J.W.; Choi, J.S.; Han, K.J.; Lee, S.H.; Kim, E.J.; Cho, J.H. Association of a genetic polymorphism of IL1RN with risk of acute pancreatitis in a Korean ethnic group. Korean J. Intern. Med. 2018, 33, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Lasek-Bal, A.; Jedrzejowska-Szypulka, H.; Student, S.; Warsz-Wianecka, A.; Zareba, K.; Puz, P.; Bal, W.; Pawletko, K.; Lewin-Kowalik, J. The importance of selected markers of inflammation and blood-brain barrier damage for short-term ischemic stroke prognosis. J. Physiol. Pharmacol. 2019, 70, 209–217. [Google Scholar]
- Gullo, L.; Cavicchi, L.; Tomassetti, P.; Spagnolo, C.; Freyrie, A.; D’Addato, M. Effects of ischemia on the human pancreas. Gastroenterology 1996, 111, 1033–1038. [Google Scholar] [CrossRef]
- Lonardo, A.; Grisendi, A.; Bonilauri, S.; Rambaldi, M.; Selmi, I.; Tondelli, E. Ischemic necrotizing pancreatitis after cardiac surgery. A case report and review of the literature. Ital. J. Gastroenterol. Hepatol. 1999, 31, 872–875. [Google Scholar]
- Vollmar, B.; Menger, M.D. Microcirculatory dysfunction in acute pancreatitis: A new concept of pathogenesis involving vasomotion-associated arteriolar constriction and dilation. Pancreatology 2003, 3, 181–190. [Google Scholar] [CrossRef]
- Kusterer, K.; Enghofer, M.; Zendler, S.; Blöchle, C.; Usadel, K.H. Microcirculatory changes in sodium taurocholate-induced pancreatitis in rats. Am. J. Physiol. Liver Physiol. 1991. [Google Scholar] [CrossRef]
- Gress, T.M.; Arnold, R.; Adler, G. Structural alterations of pancreatic microvasculature in cerulein-induced pancreatitis in the rat. Res. Exp. Med. (Berl) 1990, 190, 401–412. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Konturek, P.C.; Stachura, J.; Konturek, S.J.; Niemiec, J. Protective effect of calcitonin gene-related peptide against caerulein-induced pancreatitis in rats. J. Physiol. Pharmacol. 1997, 48, 775–787. [Google Scholar]
- Hernandez-Barbachano, E.; Roman, J.I.; Lopez, M.A.; Covenas, R.; Lopez-Novoa, J.M.; Calvo, J.J. Beneficial Effects of Vasodilators in Preventing Severe Acute Pancreatitis Shock. Pancreas 2006, 32, 335–342. [Google Scholar] [CrossRef]
- Cieszkowski, J.; Warzecha, Z.; Ceranowicz, P.; Ceranowicz, D.; Kusnierz-Cabala, B.; Pedziwiatr, M.; Dembinski, M.; Ambrozy, T.; Kaczmarzyk, T.; Pihut, M.; et al. Therapeutic effect of exogenous ghrelin in the healing of gingival ulcers is mediated by the release of endogenous growth hormone and insulin-like growth factor-1. J. Physiol. Pharmacol. 2017, 68, 609–617. [Google Scholar]
- Guttu, K.; Sørbye, H.; Gislason, H.; Svanes, K.; Grønbech, J.E. Role of bicarbonate in blood flow-mediated protection and repair of damaged gastric mucosa in the cat. Gastroenterology 1994, 107, 149–159. [Google Scholar] [CrossRef]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Brzozowski, T.; Dembiński, M.; Konturek, S.J.; Pawlik, W.W. Role of capsaicin-sensitive nerves and histamine H1, H2, and H3 receptors in the gastroprotective effect of histamine against stress ulcers in rats. Eur. J. Pharmacol. 2005, 508, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A.; Sendur, R.; Cieszkowski, J.; Ceranowicz, D.; Pawlik, W.W.; Kuwahara, A.; Kato, I.; Konturek, P.C. Treatment with ghrelin accelerates the healing of acetic acid-induced gastric and duodenal ulcers in rats. J. Physiol. Pharmacol. 2009, 60, 87–98. [Google Scholar] [PubMed]
- Warzecha, Z.; Ceranowicz, D.; Dembiński, A.; Ceranowicz, P.; Cieszkowski, J.; Kuwahara, A.; Kato, I.; Dembiński, M.; Konturek, P.C. Ghrelin accelerates the healing of cysteamine-induced duodenal ulcers in rats. Med. Sci. Monit. 2012. [Google Scholar] [CrossRef]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Ceranowicz, D.; Gałązka, K.; Bonior, J.; Jaworek, J.; Bartuś, K.; Gil, K.; et al. Exogenous Ghrelin Accelerates the Healing of Acetic Acid-Induced Colitis in Rats. Int. J. Mol. Sci. 2016. [Google Scholar] [CrossRef]
- Konarska, K.; Cieszkowski, J.; Warzecha, Z.; Ceranowicz, P.; Chmura, A.; Kuśnierz-Cabala, B.; Gałązka, K.; Kowalczyk, P.; Miskiewicz, A.; Konturek, T.; et al. Treatment with Obestatin—A Ghrelin Gene-Encoded Peptide—Reduces the Severity of Experimental Colitis Evoked by Trinitrobenzene Sulfonic Acid. Int. J. Mol. Sci. 2018. [Google Scholar] [CrossRef]
- Lerch, M.M.; Gorelick, F.S. Models of Acute and Chronic Pancreatitis. Gastroenterology 2013, 144, 1180–1193. [Google Scholar] [CrossRef]
- Brunk, D. Systemic anticoagulation found to benefit acute pancreatitis patients. MDedge/Internal Medicine presented by Internal Medicine News 2019. Available online: https://www.mdedge.com/internalmedicine/article/201168/gastroenterology/systemic-anticoagulation-found-benefit-acute (accessed on 25 May 2020).
- Kroner, P.T.; Raimondo, M.; Wallace, M.B.; Ji, B.; Bi, Y. Sa1381 – Systemic Anticoagulation is Associated with Decreased Mortality and Morbidity in Acute Pancreatitis. Gastroenterology 2019. [Google Scholar] [CrossRef]
- Miklosz, J.; Kalaska, B.; Mogielnicki, A. Pharmacogenetic considerations of anticoagulant medication. J. Physiol. Pharmacol. 2018. [Google Scholar] [CrossRef]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Stachura, J.; Tomaszewska, R.; Konturek, S.J.; Sendur, R.; Dembiński, M.; Pawlik, W.W. Pancreatic damage and regeneration in the course of ischemia-reperfusion induced pancreatitis in rats. J. Physiol. Pharmacol. 2001, 52, 221–235. [Google Scholar]
- Konturek, S.J.; Szlachcic, A.; Dembinski, A.; Warzecha, Z.; Jaworek, J.; Stachura, J. Nitric oxide in pancreatic secretion and hormone-induced pancreatitis in rats. Int. J. Pancreatol. 1994, 15, 19–28. [Google Scholar] [PubMed]
- Dembinski, A.; Warzecha, Z.; Konturek, P.J.; Ceranowicz, P.; Konturek, S.J. Influence of capsaicin-sensitive afferent neurons and nitric oxide (NO) on cerulein-induced pancreatitis in rats. Int. J. Pancreatol. 1996, 19, 179–189. [Google Scholar] [PubMed]
- Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Cieszkowski, J.; Konturek, S.J.; Dembiński, M.; Kuśnierz-Cabala, B.; Tomaszewska, R.; Pawlik, W.W. Ischemic preconditioning of the hindlimb or kidney does not attenuate the severity of acute ischemia/reperfusion-induced pancreatitis in rats. J. Physiol. Pharmacol. 2008, 59, 337–352. [Google Scholar] [PubMed]
Sample Availability: Samples not available from the authors. |
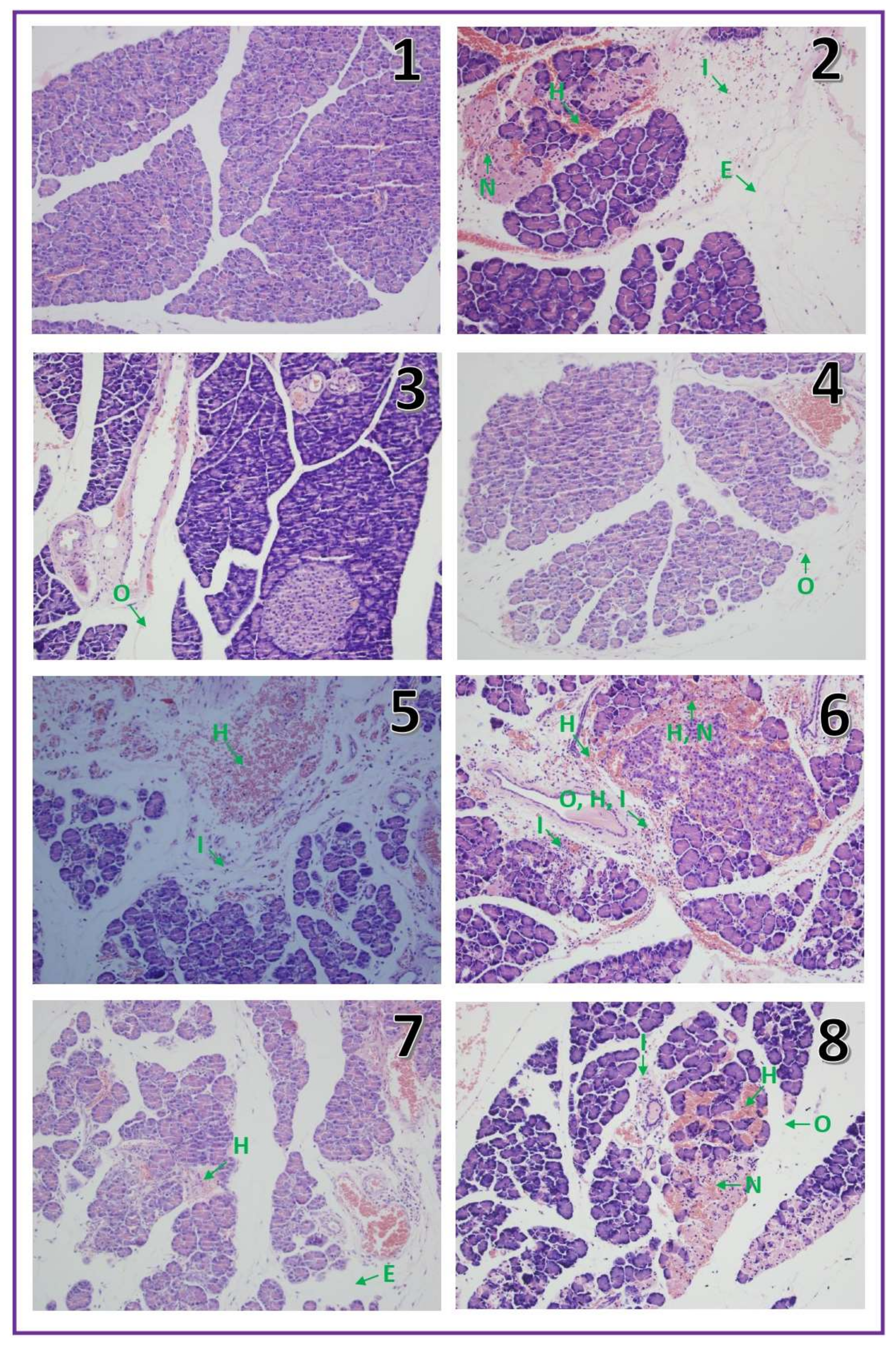

| Edema (0–3) | Inflammatory infiltration (0–3) | Vacuolization (0–3) | Necrosis (0–3) | Hemorrhages (0–3) | |
|---|---|---|---|---|---|
| Control | 0 | 0 | 0 | 0 | 0 |
| IR-AP | 2 | 2 | 0–1 | 1 | 1–2 |
| Warfarin 90 | 0–1 | 0 | 0 | 0 | 0 |
| Warfarin 180 | 0–1 | 0 | 0 | 0 | 0 |
| Warfarin 270 | 0–1 | 0 | 0 | 0 | 0–1 |
| Warfarin 90 + IR-AP | 1–2 | 1 | 0 | 1 | 1 |
| Warfarin 180 + IR-AP | 1–2 | 1 | 0 | 0–1 | 1 |
| Warfarin 270 + IR-AP | 2–3 | 2 | 1 | 1 | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maduzia, D.; Ceranowicz, P.; Cieszkowski, J.; Gałązka, K.; Kuśnierz-Cabala, B.; Warzecha, Z. Pretreatment with Warfarin Attenuates the Development of Ischemia/Reperfusion-Induced Acute Pancreatitis in Rats. Molecules 2020, 25, 2493. https://doi.org/10.3390/molecules25112493
Maduzia D, Ceranowicz P, Cieszkowski J, Gałązka K, Kuśnierz-Cabala B, Warzecha Z. Pretreatment with Warfarin Attenuates the Development of Ischemia/Reperfusion-Induced Acute Pancreatitis in Rats. Molecules. 2020; 25(11):2493. https://doi.org/10.3390/molecules25112493
Chicago/Turabian StyleMaduzia, Dawid, Piotr Ceranowicz, Jakub Cieszkowski, Krystyna Gałązka, Beata Kuśnierz-Cabala, and Zygmunt Warzecha. 2020. "Pretreatment with Warfarin Attenuates the Development of Ischemia/Reperfusion-Induced Acute Pancreatitis in Rats" Molecules 25, no. 11: 2493. https://doi.org/10.3390/molecules25112493
APA StyleMaduzia, D., Ceranowicz, P., Cieszkowski, J., Gałązka, K., Kuśnierz-Cabala, B., & Warzecha, Z. (2020). Pretreatment with Warfarin Attenuates the Development of Ischemia/Reperfusion-Induced Acute Pancreatitis in Rats. Molecules, 25(11), 2493. https://doi.org/10.3390/molecules25112493








