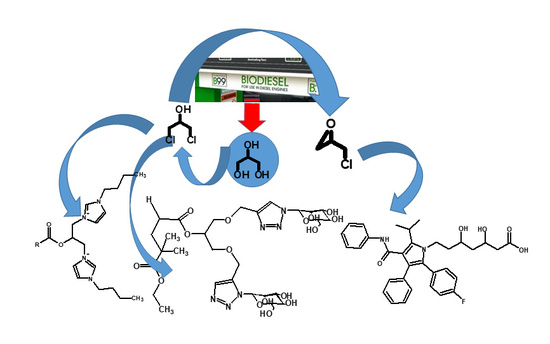
Preparation and Uses of Chlorinated Glycerol Derivatives
Abstract
:1. Introduction
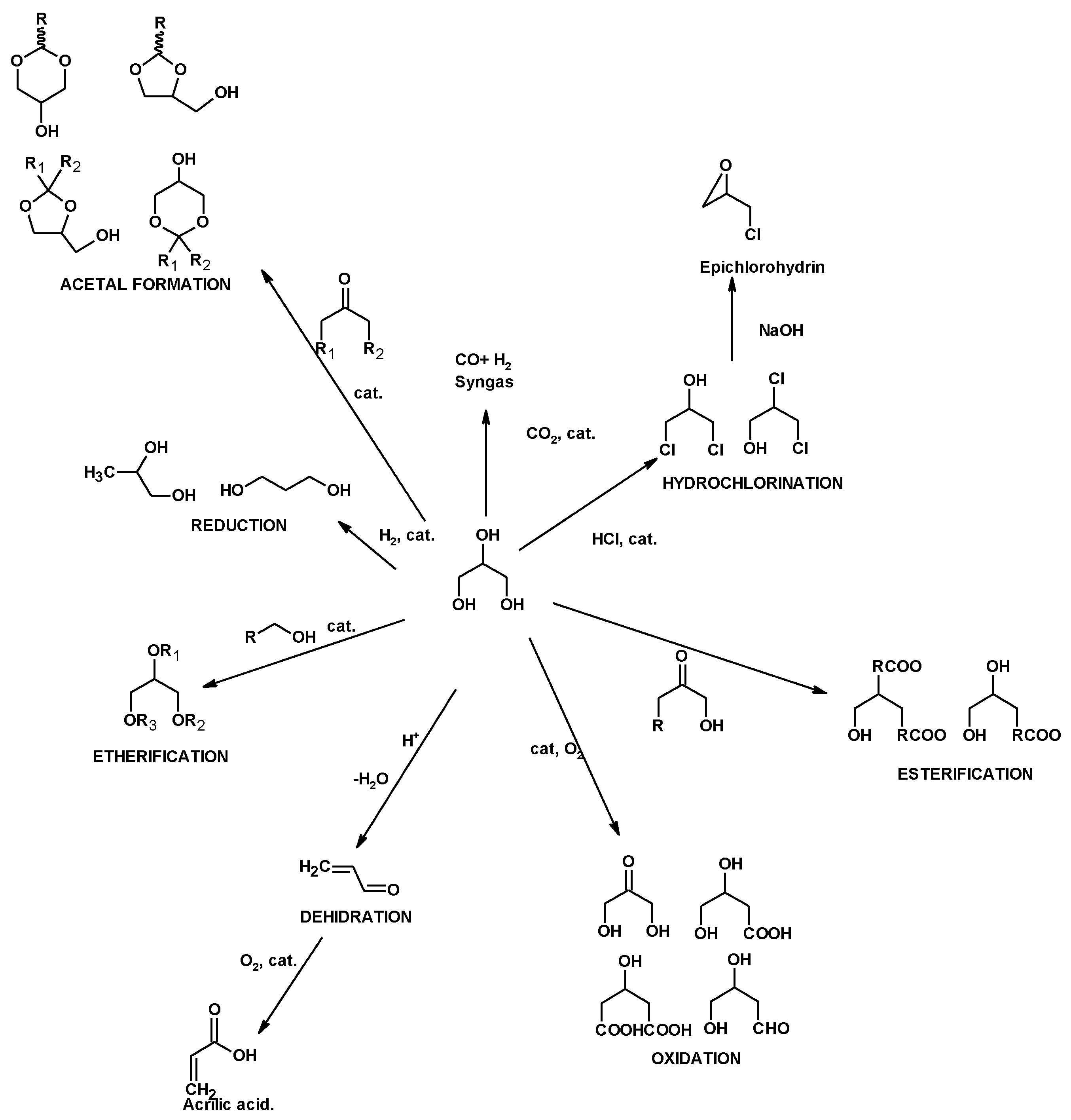
2. From Glycerol to Synthetic Intermediates
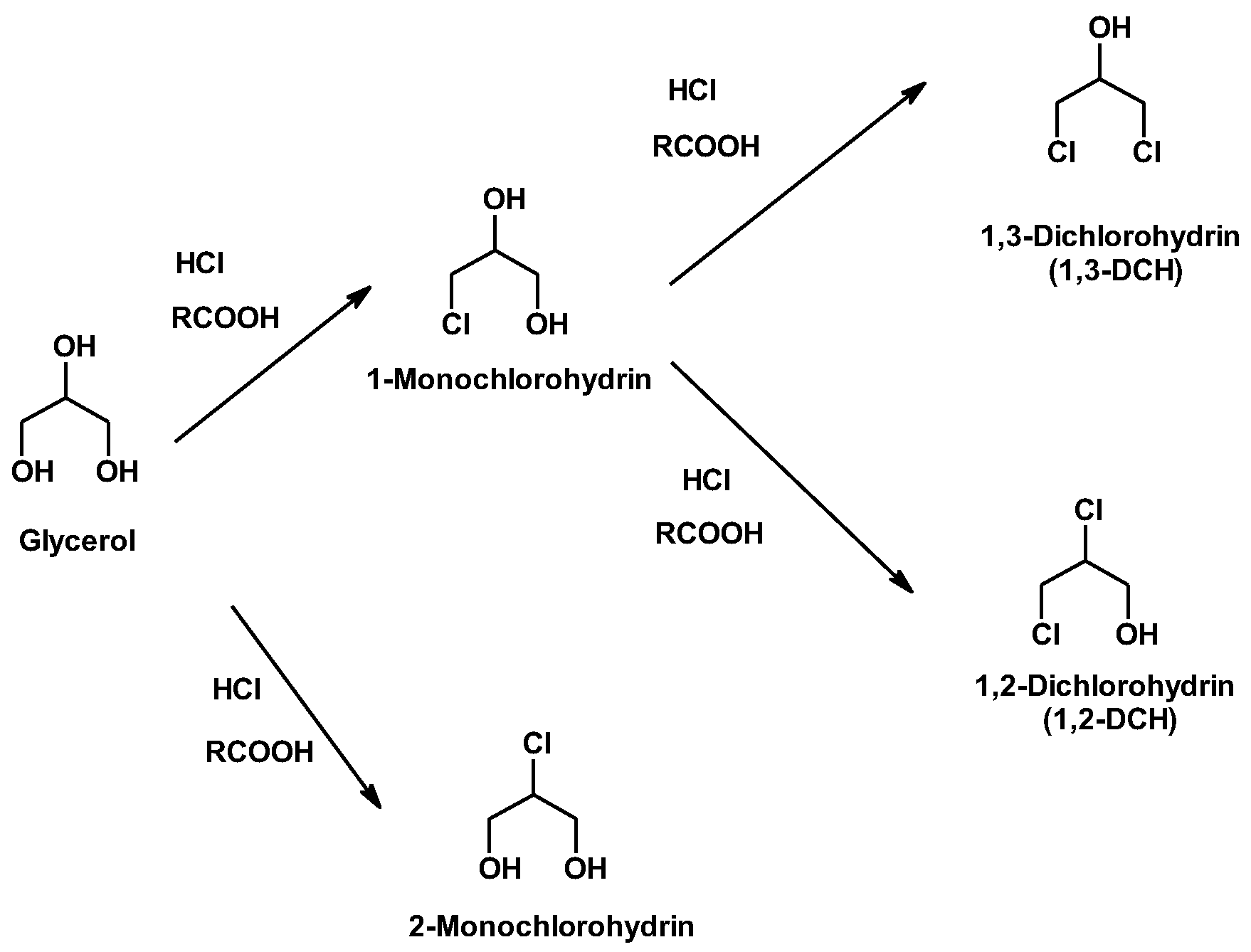
2.1. Synthesis of Chlorohydrins by Glycerol Hydrochlorination
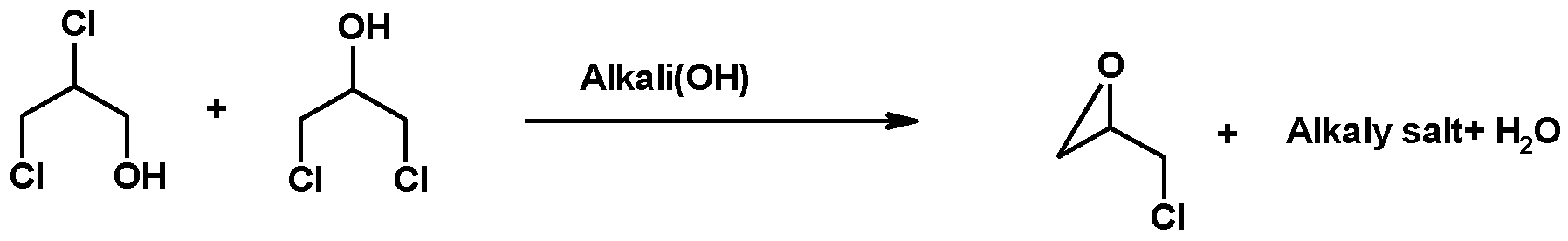
2.2. Synthesis of Epichlorohydrin
2.2.1. Enzymatically Catalyzed Synthesis of ECH
2.2.2. Chemical Synthesis of ECH
2.3. Sinthesis of Dichloropropyl Esters from Glycerol
3. From Building Blocks to End Products
3.1. Synthesis of Non-Cyclic Compounds
3.1.1. Synthesis of Allyl Esters
3.1.2. Synthesis of Nitrile Derivatives
3.1.3. Synthesis of Azide Derivatives
Synthesis of Diazides
Synthesis of Mononoamide Derivatives
3.1.4. Sulfonamides
3.1.5. Synthesis of Polynuclear Metals
3.1.6. Glycoconjugate Synthesis
3.1.7. Funcionalization of Aza-Heterocyclic Compounds
Pyridine Derivatives
Synthesis of Aziridine Derivatives
Synthesis of 1,2,4-Triazinones Derivatives
Synthesis of Pthalazine Derivatives
3.1.8. Synthesis of Polymers
3.2. Cyclic Compounds
3.2.1. Synthesis of Oxo-Heterocycles
3.2.2. Synthesis of Aza-Heterocycles
Synthesis of Oxazolidinones
Synthesis of Triazole Derivatives
3.2.3. Synthesis of Ionic Compounds Based on Quaternary Bis-Ammonium Salts
4. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, P.; Sims, E.; Bertham, O.; Symington, H.; Bell, N.; Pfaltzgraff, L.; Sjögren, P.; Wilts, C.H.; O’Brien, M. Towards a Circular Economy: Waste Management in the EU; European Parliament (STOA): Brussels, Belgium, 2017; pp. 1–140. [Google Scholar]
- Wilson, D.C.; Rodic, L.; Modak, P.; Soos, R.; Carpintero-Rogero, A.; Iyer, M.; Simonett, O. Global Waste Management Outlook; UNEP: Nairobi, Kenya, 2015; pp. 1–346. [Google Scholar]
- Gallezot, P. Process options for converting renewable feedstocks to bioproducts. Green Chem. 2007, 9, 295. [Google Scholar] [CrossRef]
- Christensen, C.H.; Marsden, C.C.; Taarning, E.; Egeblad, K.; Rass-Hansen, J. The Renewable Chemicals Industry. ChemSusChem 2008, 1, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.J.; Corma, A.; Iborra, S. Converting carbohydrates to bulk chemicals and fine chemicals over heterogeneous catalysts. Green Chem. 2011, 13, 520. [Google Scholar] [CrossRef]
- Petrus, L.; Noordermeer, M.A. Biomass to biofuels, a chemical perspective. Green Chem. 2006, 8, 861. [Google Scholar] [CrossRef]
- Görling, M.; Larsson, M.; Alvfors, P. Bio-methane via fast pyrolysis of biomass. Appl. Energy 2013, 112, 440–447. [Google Scholar] [CrossRef]
- Wilkie, A.C. Biomethane from Biomass, Biowaste, and Biofuels. In Bioenergy; American Society for Microbiology: Washington, DC, USA, 2008; pp. 195–205. [Google Scholar]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Brehmer, B.; Boom, R.M.; Sanders, J. Maximum fossil fuel feedstock replacement potential of petrochemicals via biorefineries. Chem. Eng. Res. Des. 2009, 87, 1103–1119. [Google Scholar] [CrossRef]
- Demirbas, M.F.; Balat, M.; Balat, H. Potential contribution of biomass to the sustainable energy development. Energy Convers. Manag. 2009, 50, 1746–1760. [Google Scholar] [CrossRef]
- Tuck, C.O.; Perez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Della Pina, C. From glycerol to value-added products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef]
- Zakaria, Z.; Linnekoski, J.; Amin, N. Catalyst screening for conversion of glycerol to light olefins. Chem. Eng. J. 2012, 207, 803–813. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Capron, M.; Dumeignil, F. Towards the Sustainable Production of Acrolein by Glycerol Dehydration. ChemSusChem 2009, 2, 719–730. [Google Scholar] [CrossRef]
- Gu, Y.; Azzouzi, A.; Pouilloux, Y.; Jérôme, F.; Barrault, J. Heterogeneously catalyzed etherification of glycerol: New pathways for transformation of glycerol to more valuable chemicals. Green Chem. 2008, 10, 164–167. [Google Scholar] [CrossRef]
- Teng, W.K.; Ngoh, G.C.; Yusoff, R.; Aroua, M.K. A review on the performance of glycerol carbonate production via catalytic transesterification: Effects of influencing parameters. Energy Convers. Manag. 2014, 88, 484–497. [Google Scholar] [CrossRef]
- Markočič, E.; Kramberger, B.; Van Bennekom, J.G.; Heeres, H.J.; Vos, J.; Knez, Ž. Glycerol reforming in supercritical water; a short review. Renew. Sustain. Energy Rev. 2013, 23, 40–48. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; César, A.D.S. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Christoph, R.; Schmidt, B.; Steinberner, U.; Dilla, W.; Karinen, R. Glycerol. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Rostovtsev, V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Mydock, L.K.; Demchenko, A.V. Mechanism of chemical O-glycosylation: From early studies to recent discoveries. Org. Biomol. Chem. 2010, 8, 497–510. [Google Scholar] [CrossRef]
- Roy, B.; Mukhopadhyay, B. Sulfuric Acid Immobilized on Silica: An Excellent Catalyst for Fischer Type Glycosylation. TetraChedromin Lett. 2007, 38, 483783. [Google Scholar] [CrossRef]
- Zhu, X.; Schmidt, R.R. New Principles for Glycoside-Bond Formation. Angew. Chem. Int. Ed. 2009, 48, 1900–1934. [Google Scholar] [CrossRef]
- Tanaka, T.; Nagai, H.; Noguchi, M.; Kobayashi, A.; Shoda, S.-I. One-step conversion of unprotected sugars to [small beta]-glycosyl azides using 2-chloroimidazolinium salt in aqueous solution. Chem. Commun. 2009, 23, 3378–3379. [Google Scholar] [CrossRef]
- Tomabechi, Y.; Squire, M.A.; Fairbanks, A.J. Endo-[small beta]-N-Acetylglucosaminidase catalysed glycosylation: Tolerance of enzymes to structural variation of the glycosyl amino acid acceptor. Org. Biomol. Chem. 2014, 12, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Santacesaria, E.; Vicente, G.M.; Di Di Serio, M.; Tesser, R. Main technologies in biodiesel production: State of the art and future challenges. Catal. Today 2012, 195, 2–13. [Google Scholar] [CrossRef]
- Dasari, M.A.; Kiatsimkul, P.-P.; Sutterlin, W.R.; Suppes, G. Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl. Catal. A Gen. 2005, 281, 225–231. [Google Scholar] [CrossRef]
- Alhanash, A.; Kozhevnikova, E.F.; Kozhevnikov, I. Hydrogenolysis of Glycerol to Propanediol Over Ru: Polyoxometalate Bifunctional Catalyst. Catal. Lett. 2007, 120, 307–311. [Google Scholar] [CrossRef]
- Chiu, C.-W.; Dasari, M.A.; Sutterlin, W.R.; Suppes, G.J. Removal of Residual Catalyst from Simulated Biodiesel’s Crude Glycerol for Glycerol Hydrogenolysis to Propylene Glycol. Ind. Eng. Chem. Res. 2006, 45, 791–795. [Google Scholar] [CrossRef]
- Tesser, R.; Santacesaria, E.; Di Di Serio, M.; Di Nuzzi, G.; Fiandra, V. Kinetics of Glycerol Chlorination with Hydrochloric Acid: A New Route to α,γ-Dichlorohydrin. Ind. Eng. Chem. Res. 2007, 46, 6456–6465. [Google Scholar] [CrossRef]
- Tesser, R.; Di Di Serio, M.; Vitiello, R.; Russo, V.; Ranieri, E.; Speranza, E.; Santacesaria, E. Glycerol Chlorination in Gas–Liquid Semibatch Reactor: An Alternative Route for Chlorohydrins Production. Ind. Eng. Chem. Res. 2011, 51, 8768–8776. [Google Scholar] [CrossRef]
- Santacesaria, E.; Tesser, R.; Di Di Serio, M.; Casale, L.; Verde, D. New Process for Producing Epichlorohydrin via Glycerol Chlorination. Ind. Eng. Chem. Res. 2010, 49, 964–970. [Google Scholar] [CrossRef]
- Jaecker-Voirol, A.; Durand, I.; Hillion, G.; Delfort, B.; Montagne, X. Glycerin for New Biodiesel Formulation. Oil Gas. Sci. Technol. 2008, 63, 395–404. [Google Scholar] [CrossRef]
- Melero, J.A.; Van Grieken, R.; Morales, G.; Paniagua, M. Acidic Mesoporous Silica for the Acetylation of Glycerol: Synthesis of Bioadditives to Petrol Fuel. Energy Fuels 2007, 21, 1782–1791. [Google Scholar] [CrossRef]
- Silva, P.H.; Gonçalves, V.L.; Mota, C. Glycerol acetals as anti-freezing additives for biodiesel. Bioresour. Technol. 2010, 101, 6225–6229. [Google Scholar] [CrossRef] [PubMed]
- Crotti, C.; Farnetti, E.; Guidolin, N. Alternative intermediates for glycerol valorization: Iridium-catalyzed formation of acetals and ketals. Green Chem. 2010, 12, 2225. [Google Scholar] [CrossRef]
- Johnson, D.T.; Taconi, K.A. The glycerin glut: Options for the value-added conversion of crude glycerol resulting from biodiesel production. Environ. Prog. 2007, 26, 338–348. [Google Scholar] [CrossRef]
- Atia, H.; Armbruster, U.; Martin, A. Dehydration of glycerol in gas phase using heteropolyacid catalysts as active compounds. J. Catal. 2008, 258, 71–82. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Bellière-Baca, V.; Rey, P.; Dumeignil, F.Y. Glycerol dehydration to acrolein in the context of new uses of glycerol. Green Chem. 2010, 12, 2079. [Google Scholar] [CrossRef]
- Esan, A.O.; Adeyemi, A.D.; Ganesan, S. A review on the recent application of dimethyl carbonate in sustainable biodiesel production. J. Clean. Prod. 2020, 14, 120561. [Google Scholar] [CrossRef]
- Filho, C.A.D.A.; Eranen, K.; Mikkola, J.-P.; Salmi, T. A comprehensive study on the kinetics, mass transfer and reaction engineering aspects of solvent-free glycerol hydrochlorination. Chem. Eng. Sci. 2014, 120, 88–104. [Google Scholar] [CrossRef]
- Bell, B.M.; Briggs, J.R.; Campbell, R.M.; Chambers, S.M.; Gaarenstroom, P.D.; Hippler, J.G.; Hook, B.D.; Kearns, K.; Kenney, J.M.; Kruper, W.; et al. Glycerin as a Renewable Feedstock for Epichlorohydrin Production. The GTE Process. CLEAN Soil Air Water 2008, 36, 657–661. [Google Scholar] [CrossRef]
- Song, S.H.; Lee, S.H.; Park, D.R.; Kim, H.; Woo, S.Y.; Song, W.S.; Kwon, M.S.; Song, I.K. Direct preparation of dichloropropanol from glycerol and hydrochloric acid gas in a solvent-free batch reactor: Effect of experimental conditions. Korean J. Chem. Eng. 2009, 26, 382–386. [Google Scholar] [CrossRef]
- Wolkowicz, I.R.H.; Aronzon, C.M.; Coll, C.S.P. Lethal and sublethal toxicity of the industrial chemical epichlorohydrin on Rhinella arenarum (Anura, Bufonidae) embryos and larvae. J. Hazard. Mater. 2013, 263, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.-S.; Park, N.-H.; Lee, J.-C.; Kim, K.-H.; Moon, C.; Kim, S.-H.; Shin, N.-H.; Park, S.-C.; Kim, H.-Y.; Kim, J.-C. One-generation reproductive toxicity study of epichlorohydrin in Sprague-Dawley rats. Drug Chem. Toxicol. 2010, 33, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, W.H.; Malik, M.; Turner, J.E.; Autian, J. Toxicity Profile of Epichlorohydrin. J. Pharm. Sci. 1972, 61, 1712–1717. [Google Scholar] [CrossRef] [PubMed]
- Stott, I.; Murthy, A.; Robinson, A.; Thomas, N.W.; Fry, J.R. Low-dose diethyldithiocarbamate attenuates the hepatotoxicity of 1,3-dichloro-2-propanol and selectively inhibits CYP2E1 activity in the rat. Hum. Exp. Toxicol. 1997, 16, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Escribà, M.; Eras, J.; Villorbina, G.; Balcells, M.; Blanch, C.; Barniol, N.; Canela, R.; Canela-Garayoa, R. Use of Crude Glycerol from Biodiesel Producers and Fatty Materials to Prepare Allyl Esters. Waste Biomass Valorization 2011, 2, 285–290. [Google Scholar] [CrossRef]
- Santacesaria, E.; Vitiello, R.; Tesser, R.; Russo, V.; Turco, R.; Di Di Serio, M. Chemical and Technical Aspects of the Synthesis of Chlorohydrins from Glycerol. Ind. Eng. Chem. Res. 2013, 53, 8939–8962. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.C.; Lee, W.G.; Lee, S.Y.; Chang, H.N. Succinic acid production with reduced by?product formation in the fermentation of Anaerobiospirillum succiniciproducens using glycerol as a carbon source. Biotechnol. Bioeng. 2001, 72, 41–48. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipid production by Yarrowia lipolytica growing on industrial glycerol in a single-stage continuous culture. Bioresour. Technol. 2002, 82, 43–49. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, D.; Ren, H. Economical production of vitamin K2 using crude glycerol from the by-product of biodiesel. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Kasumi, T.; Ogihara, J.; Tamura, M.; Arai, T.; Tomishige, K. Erythritol: Another C4 Platform Chemical in Biomass Refinery. ACS Omega 2020, 5, 2520–2530. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-H.; You, X.-Z.; Zhong, J. Design of a Reactive Distillation Column for Direct Preparation of Dichloropropanol from Glycerol. Ind. Eng. Chem. Res. 2009, 48, 10779–10787. [Google Scholar] [CrossRef]
- Luo, Z.-H.; You, X.-Z.; Li, H.-R. A kinetic model for glycerol chlorination in the presence of acetic acid catalyst. Korean J. Chem. Eng. 2010, 27, 66–72. [Google Scholar] [CrossRef]
- Luo, Z.-H.; You, X.-Z.; Li, H.-R. Direct Preparation Kinetics of 1,3-Dichloro-2-propanol from Glycerol Using Acetic Acid Catalyst. Ind. Eng. Chem. Res. 2009, 48, 446–452. [Google Scholar] [CrossRef]
- Filho, C.A.D.A.; Heredia, S.; Eranen, K.; Salmi, T. Advanced millireactor technology for the kinetic investigation of very rapid reactions: Dehydrochlorination of 1,3-dichloro-2-propanol to epichlorohydrin. Chem. Eng. Sci. 2016, 149, 35–41. [Google Scholar] [CrossRef]
- Lari, G.M.; Pastore, G.; Mondelli, C.; Pérez-Ramírez, J. Towards sustainable manufacture of epichlorohydrin from glycerol using hydrotalcite-derived basic oxides. Green Chem. 2018, 20, 148–159. [Google Scholar] [CrossRef]
- Almena, A.; Martín, M. Technoeconomic Analysis of the Production of Epichlorohydrin from Glycerol. Ind. Eng. Chem. Res. 2015, 55, 3226–3238. [Google Scholar] [CrossRef]
- Kruper, W.J., Jr.; Arrowood, T.; Bell, B.M.; Briggs, J.; Campbell, R.M.; Hook, B.D.; Nguyen, A.; Theriault, C.; Fitschen, R. Batch, Semi-Continuous or Continuous Hydrochlorination of Glycerin with Reduced Volatile Chlorinated Hydrocarbon by-Products and Chloracetone Levels. U.S. Patent US8404905B2, 31 January 2011. [Google Scholar]
- Hook, B.D.; Briggs, J.; Campbell, R.M.; Kruper, W.J., Jr.; Schreck, D.J.; Varjian, R.D.; Hippler, J.G. Process for the Conversion of A Crude Glycerol, Crude Mixtures of Naturally Derived Multihydroxylated Aliphatic Hydrocarbons or Esters thereof to A Chlorohydrin. U.S. Patent US7910781B2, 22 March 2011. [Google Scholar]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Ramírez-López, C.; Belsué, M. A Brief Review on Industrial Alternatives for the Manufacturing of Glycerol Carbonate, a Green Chemical. Org. Process. Res. Dev. 2012, 16, 389–399. [Google Scholar] [CrossRef]
- Gervais, M.; Brocas, A.-L.; Cendejas, G.; Deffieux, A.; Carlotti, S. Synthesis of Linear High Molar Mass Glycidol-Based Polymers by Monomer-Activated Anionic Polymerization. Macromol. 2010, 43, 1778–1784. [Google Scholar] [CrossRef]
- Britton, E.C.; Heindel, R.L. Preparation of Glycerol Dichlorohydrin. U.S. Patent US 2,144,612, 24 January 1939. [Google Scholar]
- Britton, E.C.; Slagh, H.R. Glycerol Chlorohydrin. U.S. Patent US 2,198,600, 30 April 1940. [Google Scholar]
- Morodo, R.; Gérardy, R.; Petit, G.; Monbaliu, J.-C.M. Continuous flow upgrading of glycerol toward oxiranes and active pharmaceutical ingredients thereof. Green Chem. 2019, 21, 4422–4433. [Google Scholar] [CrossRef]
- Kapkowski, M.; Popiel, J.; Siudyga, T.; Dzida, M.; Zorębski, E.; Musiał, M.; Sitko, R.; Szade, J.; Balin, K.; Klimontko, J.; et al. Mono- and bimetallic nano-Re systems doped Os, Mo, Ru, Ir as nanocatalytic platforms for the acetalization of polyalcohols into cyclic acetals and their applications as fuel additives. Appl. Catal. B Environ. 2018, 239, 154–167. [Google Scholar] [CrossRef]
- Almena, A.; Bueno, L.; Díez, M.; Martín, M. Integrated biodiesel facilities: Review of glycerol-based production of fuels and chemicals. Clean Technol. Environ. Policy 2017, 20, 1639–1661. [Google Scholar] [CrossRef]
- Shen, L.; Zhou, X.; Yin, H.; Hou, X.X.; Wang, A. Selective Chlorination of Glycerol to 3-Chloro-1,2-Propanediol Catalyzed by Brønsted Acidic Ionic Liquids. Braz. J. Chem. Eng. 2018, 35, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Lyadov, A.S.; Khadzhiev, S.N. Bioglycerol as an Alternative Raw Material for Basic Organic Synthesis. Russ. J. Appl. Chem. 2017, 90, 1727–1737. [Google Scholar] [CrossRef]
- Peng, X.; Xia, C.; Lin, M.; Shu, X.; Zhu, B.; Wang, B.; Zhang, Y.; Luo, Y.; Xuhong, M. A safer and greener chlorohydrination of allyl chloride with H2O2 and HCl over hollow titanium silicate zeolite. Appl. Catal. A Gen. 2017, 543, 17–25. [Google Scholar] [CrossRef]
- Kruper, W.J., Jr.; Schreck, D.J.; Kearns, K.L.; Varjian, R.D.; Jones, M.E.; Campbell, R.M.; Hook, B.D.; Briggs, J.R.; Hippler, J.G. Conversion of A Multihydroxylated-Aliphatic Hydrocarbon or Ester Thereof to A Chlorohydrin. U.S. Patent No. 8,088,957, 23 January 2012. [Google Scholar]
- Kubicek, P.; Sladek, P.; Buricova, I. Method of Preparing Dichloropropanols from Glycerine. Patent WO2005,021,476, 10 March 2005. [Google Scholar]
- Schuhmacher, R.; Nurmi-Legat, J.; Oberhauser, A.; Kainz, M.; Krska, R. A rapid and sensitive GC–MS method for determination of 1,3-dichloro-2-propanol in water. Anal. Bioanal. Chem. 2005, 382, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-C.; Hui, K.-Y.; Cheng, S.-C.; Chung, S.W. Sensitive method for the determination of 1,3-dichloropropan-2-ol and 3-chloropropane-1,2-diol in soy sauce by capillary gas chromatography with mass spectrometric detection. J. Chromatogr. A 2002, 952, 185–192. [Google Scholar] [CrossRef]
- Ling, X.; Lu, D.-Q.; Wang, J.; Liang, M.; Zhang, S.; Ren, W.; Chen, J.; Ouyang, P. Investigation of the kinetics and mechanism of the glycerol chlorination reaction using gas chromatography-mass spectrometry. J. Serbian Chem. Soc. 2010, 75, 101–112. [Google Scholar] [CrossRef]
- Escriba, M.; Eras, J.; Duran, M.; Simon, S.; Butchosa, C.; Villorbina, G.; Balcells, M.; Canela, R.; Noguera, G.V.; Canela-Garayoa, R. From glycerol to chlorohydrin esters using a solvent-free system. Microwave irradiation versus conventional heating. Tetrahedron 2009, 65, 10370–10376. [Google Scholar] [CrossRef]
- Huber, J.E. Epichlorohydrin. Encycl. Reag. Org. Synth. 2001, 1–11. [Google Scholar] [CrossRef]
- Kawthekar, R.B.; Bi, W.; Kim, G.-J. Synthesis and application of bimetallic chiral [Co(salen)]-type complexes: A new catalytic approach to synthesis of optically pure β-blockers via kinetic resolution of epichlorohydrin. Appl. Organomet. Chem. 2008, 22, 583–591. [Google Scholar] [CrossRef]
- Montornes, J.; Reina, J.; Ronda, J. Synthesis of New Reactive Polyethers: Poly (ω-bromoalkyl-1-glycidylether)s. Macromol. Chem. Phys. 2001, 202, 917–926. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Zhang, L.-P.; Cheng, F.; Ruan, L.-T.; Hu, Z.-C.; Zheng, Y.-G.; Shen, Y.-C. Characterization of a newly synthesized epoxide hydrolase and its application in racemic resolution of (R,S)-epichlorohydrin. Catal. Commun. 2011, 16, 133–139. [Google Scholar] [CrossRef]
- Jin, H.X.; Liu, Z.Q.; Hu, Z.C.; Zheng, Y.G. Biosynthesis of ®-epichlorohydrin at high substrate concentration by kinetic resolution of racemic epichlorohydrin with a recombinant epoxide hydrolase. Eng. Life Sci. 2013, 13, 385–392. [Google Scholar] [CrossRef]
- Jin, H.-X.; Hu, Z.-C.; Liu, Z.-Q.; Zheng, Y.-G. Nitrite-mediated synthesis of chiral epichlorohydrin using halohydrin dehalogenase from Agrobacterium radiobacter AD1. Biotechnol. Appl. Biochem. 2012, 59, 170–177. [Google Scholar] [CrossRef]
- Kim, G.; Lee, H.; Kim, S.-J. Catalytic activity and recyclability of new enantioselective chiral Co–salen complexes in the hydrolytic kinetic resolution of epichlorohydrine. Tetrahedron Lett. 2003, 44, 5005–5008. [Google Scholar] [CrossRef]
- You, Z.-Y.; Liu, Z.-Q.; Zheng, Y.-G. Properties and biotechnological applications of halohydrin dehalogenases: Current state and future perspectives. Appl. Microbiol. Biotechnol. 2012, 97, 9–21. [Google Scholar] [CrossRef]
- Janssen, D.B. Biocatalysis by Dehalogenating Enzymes. Adv. Appl. Microbiol. 2007, 61, 233–252. [Google Scholar] [CrossRef]
- Spelberg, J.H.L.; Tang, L.; Van Gelder, M.; Kellogg, R.M.; Janssen, D.B. Exploration of the biocatalytic potential of a halohydrin dehalogenase using chromogenic substrates. Tetrahedron Asymmetry 2002, 13, 1083–1089. [Google Scholar] [CrossRef] [Green Version]
- Zou, S.-P.; Du, E.-H.; Hu, Z.-C.; Zheng, Y.-G. Enhanced biotransformation of 1,3-dichloro-2-propanol to epichlorohydrin via resin-based in situ product removal process. Biotechnol. Lett. 2013, 35, 937–942. [Google Scholar] [CrossRef]
- Xue, F.; Liu, Z.-Q.; Wang, Y.-J.; Zhu, H.-Q.; Wan, N.-W.; Zheng, Y.-G. Efficient synthesis of (S)-epichlorohydrin in high yield by cascade biocatalysis with halohydrin dehalogenase and epoxide hydrolase mutants. Catal. Commun. 2015, 72, 147–149. [Google Scholar] [CrossRef]
- Xue, F.; Liu, Z.-Q.; Wang, Y.-J.; Wan, N.-W.; Zheng, Y.-G. Biochemical characterization and biosynthetic application of a halohydrin dehalogenase from Tistrella mobilis ZJB1405. J. Mol. Catal. B Enzym. 2015, 115, 105–112. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Li, Y.; Xu, Y.; Ping, L.; Zheng, Y.-G. Cloning, sequencing, and expression of a novel epoxide hydrolase gene from Rhodococcus opacus in Escherichia coli and characterization of enzyme. Appl. Microbiol. Biotechnol. 2007, 74, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Archelas, A.; Furstoss, R. Synthetic applications of epoxide hydrolases. Curr. Opin. Chem. Boil. 2001, 5, 112–119. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, J.-H.; Park, S.; Lee, E.Y. Biocatalytic preparation of chiral epichlorohydrins using recombinantPichia pastoris expressing epoxide hydrolase ofRhodotorula glutinis. Biotechnol. Bioprocess. Eng. 2004, 9, 62–64. [Google Scholar] [CrossRef]
- Lee, E.Y. Enantioselective hydrolysis of epichlorohydrin in organic solvents using recombinant epoxide hydrolase. J. Ind. Eng. Chem. 2007, 13, 159–162. [Google Scholar]
- Zhang, J.; Lu, Y.; Jin, Q.; Wang, K.; Luo, G. Determination of kinetic parameters of dehydrochlorination of dichloropropanol in a microreactor. Chem. Eng. J. 2012, 203, 142–147. [Google Scholar] [CrossRef]
- Krzyżanowska, A.M.; Milchert, E. Continuous dehydrochlorination of 1,3-dichloropropan-2-ol to epichlorohydrin: Process parameters and by-products formation. Chem. Pap. 2013, 67, 1218–1224. [Google Scholar] [CrossRef]
- Milchert, E.; Krzyżanowska, A.; Wołosiak-Hnat, A.; Paździoch, W. The Influence of Technological Parameters on Dehydrochlorination of Dichloropropanols. Ind. Eng. Chem. Res. 2012, 51, 3575–3579. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, J.W.; Yuan, X.Q.; Yue, Q. Synthesis of Epichlorohydrin from Dichloropropanols: Kinetic Aspects of the Process. Chem. Eng. Res. Des. 2007, 85, 1580–1585. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, H.; Li, F.; Ma, Y. Synthesis of epichlorohydrin from 1,3-dichlorohydrin with solid catalysts using γ-Al 2 O 3 as carrier material. Asia-Pacific J. Chem. Eng. 2020, 15. [Google Scholar] [CrossRef]
- Krzyżanowska, A.M.; Milchert, E.; Paździoch, W.M. Technological Parameters of Dehydrochlorination of 1,3-Dichloropropan-2-ol to Epichlorohydrin. Ind. Eng. Chem. Res. 2013, 52, 10890–10895. [Google Scholar] [CrossRef]
- Eras, J.; Mendez, J.J.; Balcells, M.; Canela, R.; Canela-Garayoa, R. Chlorotrimethylsilane: A Suitable Reagent for the Synthesis of Chlorohydrin Esters. J. Org. Chem. 2002, 67, 8631–8634. [Google Scholar] [CrossRef]
- Mendez, J.; Eras, J.; Balcells, M.; Canela, R. Influence of Carboxylic Acids on the Synthesis of Chlorohydrin Esters from 1,3-Butanediol. Synth. Commun. 2006, 36, 1167–1175. [Google Scholar] [CrossRef]
- Solarte, C. Obtención Mediante Procesos Quimioenzimáticos de Derivados del Glicerol. Ph.D. Thesis, UdL, Lleida, Spain, 2012. [Google Scholar]
- Villorbina, G.; Tomàs, A.; Escribà, M.; Oromí-Farrús, M.; Eras, J.; Balcells, M.; Canela, R. Combining AlCl3·6H2O and an ionic liquid to prepare chlorohydrin esters from glycerol. Tetrahedron Lett. 2009, 50, 2828–2830. [Google Scholar] [CrossRef]
- Eras, J.; Escriba, M.; Villorbina, G.; Oromí-Farrús, M.; Balcells, M.; Canela, R.; Noguera, G.V.; Canela-Garayoa, R. A tandem Finkelstein-rearrangement–elimination reaction: A straightforward synthetic route to allyl esters. Tetrahedron 2009, 65, 4866–4870. [Google Scholar] [CrossRef]
- Ojimelukwe, P.; Adler, C. Potential of zimtaldehyde, 4-allyl-anisol, linalool, terpineol and other phytochemicals for the control of the confused flour beetle (Tribolium confusum J. d. V.)(Col., Tenebrionidae). J. Pest. Sci. 1999, 72, 81–86. [Google Scholar]
- Takahashi, T.; Kanzaki, T. Flying Pest Insect Repellents Containing (Cyclo) Alkoxyacetic Acid Allyl Esters of Allyl Phenoxyacetate. Japan Patent JP 2007119375, 17 May 2007. [Google Scholar]
- Iwasaki, T.; Kanno, M. Cyclopropanecarboxylic Acid 2-Furfuryl Esters and Pesticides Containing Them. Japan Patent JP 2000063374, 29 February 2000. [Google Scholar]
- Yoshida, S.; Igarashi, R. Wood Preservatives Protecting from Insects. Japan Patent JP08133909, 28 May 1996. [Google Scholar]
- Escribà, M.; Barbut, M.; Eras, J.; Canela, R.; Avilla, J.; Balcells, M.; Canela-Garayoa, R. Synthesis of Allyl Esters of Fatty Acids and Their Ovicidal Effect on Cydia pomonella (L.). J. Agric. Food Chem. 2009, 57, 4849–4853. [Google Scholar] [CrossRef]
- Gan, L.H.; Ooi, K.S.; Goh, S.H.; Chee, K.K. Polymerization of allyl esters derived from long-chain fatty acids and palm olein. J. Appl. Polym. Sci. 1992, 46, 329–338. [Google Scholar] [CrossRef]
- Wan, N.-W.; Liu, Z.-Q.; Xue, F.; Shen, Z.-Y.; Zheng, Y.-G. A One-Step Biocatalytic Process for (S)-4-Chloro-3-hydroxybutyronitrile using Halohydrin Dehalogenase: A Chiral Building Block for Atorvastatin. ChemCatChem 2015, 7, 2446–2450. [Google Scholar] [CrossRef]
- Nakamura, T.; Nagasawa, T.; Yu, F.; Watanabe, I.; Yamada, H. A new enzymatic synthesis of ®-γ-chloro-β-hydroxybutyronitrile. Tetrahedron 1994, 50, 11821–11826. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, X.; Jia, L.; Liu, Y. Synthesis and properties of quaternary ammonium surfactants containing a methoxy benzyl substitute. RSC Adv. 2014, 4, 56918–56925. [Google Scholar] [CrossRef]
- Solarte, C.; Escriba, M.; Eras, J.; Villorbina, G.; Canela, R.; Balcells, M. From symmetric glycerol derivatives to dissymmetric chlorohydrins. Molecules 2011, 16, 2065–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canela-Xandri, A. Chemical and Enzymatic Valorization of Polyols from Biomass. Ph.D. Thesis, UdL, Lleida, Spain, 2016. [Google Scholar]
- Brase, S.; Gil, C.; Knepper, K.; Zimmermann, V. Organic azides. An exploding diversity of a unique class of compounds. Angew. Chem. Int. Ed. 2005, 44, 5188–5240. [Google Scholar] [CrossRef]
- Xandri, A.C.; Villorbina, G.; Balcells, M.; Ramis, X.; Cabeza, L.F.; Canela-Garayoa, R. Synthesis and Thermophysical Characterization of Fatty Amides for Thermal Energy Storage. Molecules 2019, 24, 3777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floros, M.; Kaller, K.L.; Palam, K.D.P.; Narine, S.S. Lipid derived diamide phase change materials for high temperature thermal energy storage. Sol. Energy 2016, 139, 23–28. [Google Scholar] [CrossRef]
- Lupașcu, D.; Tuchiluş, C.; Lupuşoru, C.; Ghiciuc, C.; Şutu, M.; Neagu, A.; Profire, L. Synthesis and biological evaluation of some new rutin semisynthetic derivatives as antibacterial agents. Farmacia 2012, 60, 556–564. [Google Scholar]
- Kanamori, K.; Yamamoto, K.; Okayasu, T.; Matsui, N.; Okamoto, K.-I.; Mori, W. Structures and Magnetic Properties of Dinuclear Vanadium(III) Complexes with Alkoxo Bridge. Bull. Chem. Soc. Jpn. 1997, 70, 3031–3040. [Google Scholar] [CrossRef]
- Haldar, S.; Patra, A.; Bera, M. Exploring the catalytic activity of new water soluble dinuclear copper (ii) complexes towards the glycoside hydrolysis. RSC Adv. 2014, 4, 62851–62861. [Google Scholar] [CrossRef]
- Patra, A.; Saha, S.; Sen, T.K.; Carrella, L.; Musie, G.T.; Bera, M.; Khuda-Bukhsh, A.R. Water-Soluble Heteronuclear [NaCuII6] Metallomacrocyclic Sandwich Complexes: Synthesis, Structure, Properties and In Vitro Biological Studies. Eur. J. Inorg. Chem. 2014, 2014, 5217–5232. [Google Scholar] [CrossRef]
- Harit, T.; Abouloifa, H.; Tillard, M.; Eddike, D.; Asehraou, A.; Malek, F. New copper complexes with bipyrazolic ligands: Synthesis, characterization and evaluation of the antibacterial and catalytic properties. J. Mol. Struct. 2018, 1163, 300–307. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Lo, K.K.-W. Luminescent polynuclear d10 metal complexes. Chem. Soc. Rev. 1999, 28, 323–334. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Li, C.-K.; Chan, C.-L. Proof of Potassium Ions by Luminescence Signaling Based on Weak Gold-Gold Interactions in Dinuclear Gold(I) Complexes. Angew. Chem. Int. Ed. 1998, 37, 2857–2859. [Google Scholar] [CrossRef]
- Sabiah, S.; Varghese, B.; Murthy, N.N. First hexanuclear copper(ii) pyrophosphate through hydrolysis of phosphodiester with a dicopper complex. Chem. Commun. 2009, 37, 5636. [Google Scholar] [CrossRef]
- Jiang, X.-Y.; Wu, X.-Y.; Yu, R.; Yuan, D.; Chen, W.-Z. A glycine ligand coordinated hybrid complex constructed from hexanuclear copper clusters and octamolybdates. Inorg. Chem. Commun. 2011, 14, 1546–1549. [Google Scholar] [CrossRef]
- Meng, B.; Zhu, Z.; Baker, D.C. 1,2-cis Alkyl glycosides: Straightforward glycosylation from unprotected 1-thioglycosyl donors. Org. Biomol. Chem. 2014, 12, 5182–5191. [Google Scholar] [CrossRef]
- Salman, S.M.; Heidelberg, T.; Tajuddin, H.A. N-linked glycolipids by Staudinger coupling of glycosylated alkyl diazides with fatty acids. Carbohydr. Res. 2013, 375, 55–62. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Freeze, H.H.; Stanley, P.; Bertozzi, C.R.; Hart, G.W.; Etzler, M.E. Nematoda—Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009. [Google Scholar]
- Zhao, J.; Liu, Y.; Park, H.-J.; Boggs, J.M.; Basu, A. Carbohydrate-Coated Fluorescent Silica Nanoparticles as Probes for the Galactose/3-Sulfogalactose Carbohydrate–Carbohydrate Interaction Using Model Systems and Cellular Binding Studies. Bioconjugate Chem. 2012, 23, 1166–1173. [Google Scholar] [CrossRef]
- Perez-Sanchez, M.; Munoz, P.; Muñoz, E.; Fernández, M.; Sinisterra, J.V.; Hernaiz, M.J. Synthesis of novel glycoconjugates and evaluation as inhibitors against β-glucosidase from almond. J. Mol. Catal. B Enzym. 2008, 52, 153–157. [Google Scholar] [CrossRef]
- Horlacher, T.; Seeberger, P.H. Carbohydrate arrays as tools for research and diagnostics. Chem. Soc. Rev. 2008, 37, 1414. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, M.-R.; Shin, I. Carbohydrate microarrays as powerful tools in studies of carbohydrate-mediated biological processes. Chem. Commun. 2008, 37, 4389. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sanders, B.; Baker, D.C. Synthesis of a glycodendrimer incorporating multiple mannosides on a glucoside core. Can. J. Chem. 2011, 89, 959–963. [Google Scholar] [CrossRef]
- Moustafa, A.H.; El-Sayed, H.A.; Haikal, A.E.-F.Z.; El Ashry, E.S.H.; El-Sayed, H.A. Synthesis of Acyclovir and HBG Analogues Having Nicotinonitrile and Its 2-methyloxy 1,2,3-triazole. Nucleotides Nucleic Acids 2011, 30, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Saad, H.A.; Abdel-Hafez, S.H. Synthesis and Biological Activity of Some Nucleoside Analogs of 3-Cyanopyridin-2- one. Curr. Org. Synth. 2012, 9, 413–426. [Google Scholar] [CrossRef]
- Shamroukh, A.; Shahat, M.E.-; Drabowicz, J.; Ali, M.M.; Rashad, A.; Ali, H.S.; Ali, M.M. Anticancer evaluation of some newly synthesized N-nicotinonitrile derivative. Eur. J. Med. Chem. 2013, 69, 521–526. [Google Scholar] [CrossRef]
- Moustafa, A.H.; El-Sayed, H.A.; El-Hady, R.A.A.; Haikal, A.Z.; El-Hashash, M.; El-Sayed, H.A. Synthesis, Antiviral, and Antimicrobial Activity ofN- andS-Alkylated Phthalazine Derivatives. J. Heterocycl. Chem. 2015, 53, 789–799. [Google Scholar] [CrossRef]
- El-Shamy, I.E.; Abdel-Mohsen, A.-M.; Alsheikh, A.A.; Fouda, M.M.; Al-Deyab, S.S.; El-Hashash, M.A. Synthesis and antimicrobial activities of S-nucleosides of 4-mesitylphthalazine-1-thiol and some new selenium-containing nucleoside analogues. Tetrahedron Lett. 2015, 56, 1183–1188. [Google Scholar] [CrossRef]
- Rizk, S.A.; Abdelwahab, S.S.; El-Badawy, A.A. Design, Regiospecific Green Synthesis, Chemical Computational Analysis, and Antimicrobial Evaluation of Novel Phthalazine Heterocycles. J. Heterocycl. Chem. 2019, 56, 2347–2357. [Google Scholar] [CrossRef]
- Bouchareb, F.; Berredjem, M.; Kaki, S.A.; Bouaricha, A.; Bouzina, A.; Belhani, B.; Aouf, N.-E. Synthesis and antibacterial activity of new chiral N-sulfamoyloxazolidin-2-ones. J. Chem. Sci. 2015, 128, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Barbey, C.; Bouasla, R.; Berredjem, M.; Dupont, N.; Retailleau, P.; Aouf, N.-E.; Lecouvey, M. Synthesis and structural study of new substituted chiral sulfamoyl oxazolidin-2-ones. Tetrahedron 2012, 68, 9125–9130. [Google Scholar] [CrossRef]
- Saad, H.A.; Moustafa, A.H. Synthesis and Anticancer Activity of Some New S-Glycosyl and S-Alkyl 1,2,4-Triazinone Derivatives. Molecules 2011, 16, 5682–5700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, H.A.; Moustafa, A.H.; Haikal, A.E.-F.Z. Synthesis, Antiviral, and Antimicrobial Activity of 1,2,4-Triazole Thioglycoside Derivatives. Phosphorus Sulfur Silicon Relat. Elements 2013, 188, 649–662. [Google Scholar] [CrossRef]
- Lebel, H.; Parmentier, M.; Leogane, O.; Ross, K.; Spitz, C. Copper bis(oxazolines) as catalysts for stereoselective aziridination of styrenes with N-tosyloxycarbamates. Tetrahedron 2012, 68, 3396–3409. [Google Scholar] [CrossRef]
- Uredi, D.; Motati, D.R.; Watkins, E.B. A Unified Strategy for the Synthesis of β-Carbolines, γ-Carbolines, and Other Fused Azaheteroaromatics under Mild, Metal-Free Conditions. Org. Lett. 2018, 20, 6336–6339. [Google Scholar] [CrossRef]
- Zumbrägel, N.; Sako, M.; Takizawa, S.; Sasai, H.; Gröger, H. Vanadium-Catalyzed Dehydrogenation of N-Heterocycles in Water. Org. Lett. 2018, 20, 4723–4727. [Google Scholar] [CrossRef]
- Motati, D.; Dilipkumar, U.; Watkins, B. A general method for the metal-free, regioselective, remote C–H halogenation of 8-substituted quinolines. Chem. Sci. 2018, 9, 1782–1788. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, J.B. Aziridines: Epoxides’ ugly cousins? Chem. Soc. Rev. 2002, 31, 247–258. [Google Scholar] [CrossRef]
- Yudin, A.K. Aziridines and Epoxides in Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Botuha, C.; Chemla, F.; Ferreira, F.; Pérez-Luna, A. Aziridines in Natural Product Synthesis. Heterocycles Nat. Prod. Synth. 2011, 1–39. [Google Scholar] [CrossRef]
- Fürmeier, S.; Metzger, J.O. Fat-Derived Aziridines and Their N-Substituted Derivatives: Biologically Active Compounds Based on Renewable Raw Materials. Eur. J. Org. Chem. 2003, 2003, 649–659. [Google Scholar] [CrossRef]
- Ballereau, S.; Andrieu-Abadie, N.; Saffon, N.; Génisson, Y. Synthesis and biological evaluation of aziridine-containing analogs of phytosphingosine. Tetrahedron 2011, 67, 2570–2578. [Google Scholar] [CrossRef]
- McCoull, W.; Davis, F.A. Recent Synthetic Applications of Chiral Aziridines. Synthesis 2000, 2000, 1347–1365. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.E. Nucleophilic ring opening of aziridines. Tetrahedron 2004, 60, 2701–2743. [Google Scholar] [CrossRef]
- Hodgson, D.M.; Humphreys, P.G.; Hughes, S.P. Widening the usefulness of epoxides and aziridines in synthesis. Pure Appl. Chem. 2007, 79, 269–279. [Google Scholar] [CrossRef]
- Schneider, C. Catalytic, enantioselective ring opening of aziridines. Angew. Chem. Int. Ed. 2009, 48, 2082–2084. [Google Scholar] [CrossRef]
- Taylor, A.M.; Schreiber, S.L. Aziridines as intermediates in diversity-oriented syntheses of alkaloids. Tetrahedron Lett. 2009, 50, 3230–3233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.-C.; Zhu, J. Asymmetric total syntheses of (−)-renieramycin M and G and (−)-jorumycin using aziridine as a lynchpin. Org. Lett. 2009, 11, 5558–5561. [Google Scholar] [CrossRef]
- Dauban, P.; Malik, G. A Masked 1,3-Dipole Revealed from Aziridines. Angew. Chem. Int. Ed. 2009, 48, 9026–9029. [Google Scholar] [CrossRef]
- De Espinosa, L.M.; Meier, M.A.R.; Ronda, J.C.; Galia, M.; Cádiz, V. Phosphorus-containing renewable polyester-polyols via ADMET polymerization: Synthesis, functionalization, and radical crosslinking. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1649–1660. [Google Scholar] [CrossRef]
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef]
- Irvine, D.; McCluskey, J.; Robinson, I. Fire hazards and some common polymers. Polym. Degrad. Stab. 2000, 67, 383–396. [Google Scholar] [CrossRef]
- Davis, O.A.; Bull, J.A. Synthesis of Di-, Tri-, and Tetrasubstituted Oxetanes by Rhodium-Catalyzed O=H Insertion and C=C Bond-Forming Cyclization. Angew. Chem. Int. Ed. 2014, 53, 14230–14234. [Google Scholar] [CrossRef] [Green Version]
- Davis, O.A.; Croft, R.A.; Bull, J.A. Synthesis of Substituted 1,4-Dioxenes through O–H Insertion and Cyclization Using Keto-Diazo Compounds. J. Org. Chem. 2016, 81, 11477–11488. [Google Scholar] [CrossRef]
- Ruider, S.A.; Müller, S.; Carreira, E. Ring Expansion of 3-Oxetanone-Derived Spirocycles: Facile Synthesis of Saturated Nitrogen Heterocycles. Angew. Chem. Int. Ed. 2013, 52, 11908–11911. [Google Scholar] [CrossRef]
- Guo, B.; Schwarzwalder, G.; Njardarson, J.T. Catalytic ring expansion of vinyl oxetanes: Asymmetric synthesis of dihydropyrans using chiral counterion catalysis. Angew. Chem. Int. Ed. 2012, 51, 5675–5678. [Google Scholar] [CrossRef]
- Gronnier, C.; Kramer, S.; Odabachian, Y.; Gagosz, F. Cu(I)-Catalyzed Oxidative Cyclization of Alkynyl Oxiranes and Oxetanes. J. Am. Chem. Soc. 2011, 134, 828–831. [Google Scholar] [CrossRef]
- Loy, R.N.; Jacobsen, E.N. Enantioselective intramolecular openings of oxetanes catalyzed by (salen) Co (III) complexes: Access to enantioenriched tetrahydrofurans. J. Am. Chem. Soc. 2009, 131, 2786–2787. [Google Scholar] [CrossRef] [Green Version]
- Lo, M.M.-C.; Fu, G.C. Applications of planar-chiral heterocycles in enantioselective catalysis: Cu (I)/bisazaferrocene-catalyzed asymmetric ring expansion of oxetanes to tetrahydrofurans. Tetrahedron 2001, 57, 2621–2634. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Sun, J. Catalytic Enantioselective Intermolecular Desymmetrization of 3-Substituted Oxetanes. Angew. Chem. Int. Ed. 2013, 52, 6685–6688. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Sun, J. Catalytic enantioselective synthesis of tetrahydroisoquinolines and their analogues bearing a C4 stereocenter: Formal synthesis of (+)-(8S,13R)-cyclocelabenzine. Chem. A Eur. J. 2013, 19, 8426–8430. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, B.; Wang, Z.; Zhu, G.; Sun, J. Complex Bioactive Alkaloid-Type Polycycles through Efficient Catalytic Asymmetric Multicomponent Aza-Diels–Alder Reaction of Indoles with Oxetane as Directing Group. Angew. Chem. Int. Ed. 2013, 52, 2027–2031. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Facer, M.E.; Louie, J. Nickel-Catalyzed Cycloaddition of 1,3-Dienes with 3-Azetidinones and 3-Oxetanones. Angew. Chem. Int. Ed. 2013, 52, 12161–12165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkhard, J.A.; Tchitchanov, B.H.; Carreira, E. Cascade Formation of Isoxazoles: Facile Base-Mediated Rearrangement of Substituted Oxetanes. Angew. Chem. Int. Ed. 2011, 50, 5379–5382. [Google Scholar] [CrossRef]
- Crivello, J.V. Hybrid free radical/cationic frontal photopolymerizations. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 4331–4340. [Google Scholar] [CrossRef]
- Bouchékif, H.; Philbin, M.I.; Colclough, E.; Amass, A.J. Pseudoperiodic “Living” and/or Controlled Cationic Ring-Opening Copolymerization of Oxetane with Tetrahydropyran: Microstructure of Polymers vs Kinetics of Chain Growth. Macromolecules 2010, 43, 845–855. [Google Scholar] [CrossRef]
- Schulte, B.; Dannenberg, C.A.; Keul, H.; Möller, M. Formation of linear and cyclic polyoxetanes in the cationic ring-opening polymerization of 3-allyloxymethyl-3-ethyloxetane and subsequent postpolymerization modification of poly(3-allyloxymethyl-3-ethyloxetane). J. Polym. Sci. Part A Polym. Chem. 2012, 51, 1243–1254. [Google Scholar] [CrossRef]
- Shibutani, R.; Tsutsumi, H. Fire-retardant solid polymer electrolyte films prepared from oxetane derivative with dimethyl phosphate ester group. J. Power Sources 2012, 202, 369–373. [Google Scholar] [CrossRef] [Green Version]
- Kudo, H.; Nishikubo, T. Catalytic reactions of oxetanes with protonic reagents and aprotic reagents leading to novel polymers. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 709–726. [Google Scholar] [CrossRef]
- Ghosh, B.; Urban, M.W. Self-Repairing Oxetane-Substituted Chitosan Polyurethane Networks. Science 2009, 323, 1458–1460. [Google Scholar] [CrossRef]
- Ghosh, B.; Chellappan, K.V.; Urban, M.W. Self-healing inside a scratch of oxetane-substituted chitosan-polyurethane (OXE-CHI-PUR) networks. J. Mater. Chem. 2011, 21, 14473. [Google Scholar] [CrossRef]
- Müller, S.S.; Frey, H. Synthesis of Oxetane-Functional Aliphatic Polyesters via Enzymatic Polycondensation. Macromol. Chem. Phys. 2012, 213, 1783–1790. [Google Scholar] [CrossRef]
- Charas, A.; Morgado, J. Oxetane-functionalized conjugated polymers in organic (opto) electronic devices. Curr. Phys. Chem. 2012, 2, 241–264. [Google Scholar] [CrossRef]
- Burkhard, J.A.; Wuitschik, G.; Müller, K.; Carreira, E.; Rogers-Evans, M. Oxetanes as Versatile Elements in Drug Discovery and Synthesis. Angew. Chem. Int. Ed. 2010, 49, 9052–9067. [Google Scholar] [CrossRef]
- Wuitschik, G.; Rogers-Evans, M.; Müller, K.; Fischer, H.; Wagner, B.; Schuler, F.; Polonchuk, L.; Carreira, E. Oxetanes as Promising Modules in Drug Discovery. Angew. Chem. Int. Ed. 2006, 45, 7736–7739. [Google Scholar] [CrossRef]
- Du, J.; Chun, B.-K.; Mosley, R.T.; Bansal, S.; Bao, H.; Espiritu, C.; Lam, A.M.; Murakami, E.; Niu, C.; Micolochick Steuer, H.M. Use of 2′-spirocyclic ethers in HCV nucleoside design. J. Med. Chem. 2014, 57, 1826–1835. [Google Scholar] [CrossRef]
- Skoda, E.M.; Sacher, J.R.; Kazancioglu, M.Z.; Saha, J.; Wipf, P. An Uncharged Oxetanyl Sulfoxide as a Covalent Modifier for Improving Aqueous Solubility. ACS Med. Chem. Lett. 2014, 5, 900–904. [Google Scholar] [CrossRef]
- Jonckers, T.H.; Vandyck, K.; Vandekerckhove, L.; Hu, L.; Tahri, A.; Van Hoof, S.; Lin, T.-I.; Vijgen, L.; Berke, J.M.; Lachau-Durand, S. Nucleotide prodrugs of 2′-deoxy-2′-spirooxetane ribonucleosides as novel inhibitors of the HCV NS5B polymerase. J. Med. Chem. 2014, 57, 1836–1844. [Google Scholar] [CrossRef]
- Estrada, A.A.; Chan, B.K.; Baker-Glenn, C.; Beresford, A.; Burdick, D.J.; Chambers, M.; Chen, H.; Dominguez, S.L.; Dotson, J.; Drummond, J.; et al. Discovery of Highly Potent, Selective, and Brain-Penetrant Aminopyrazole Leucine-Rich Repeat Kinase 2 (LRRK2) Small Molecule Inhibitors. J. Med. Chem. 2014, 57, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.E.; Alimzhanov, M.; Bao, L.; Block, M.H.; Chuaqui, C.; Cooke, E.L.; Denz, C.R.; Hird, A.; Huang, S.; Larsen, N.A.; et al. Structure and Property Based Design of Pyrazolo[1,5-a]pyrimidine Inhibitors of CK2 Kinase with Activity in Vivo. ACS Med. Chem. Lett. 2013, 4, 800–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wuitschik, G.; Carreira, E.; Wagner, B.; Fischer, H.; Parrilla, I.; Schuler, F.; Rogers-Evans, M.; MüllerK. Oxetanes in Drug Discovery: Structural and Synthetic Insights. J. Med. Chem. 2010, 53, 3227–3246. [Google Scholar] [CrossRef]
- Burkhard, J.A.; Wuitschik, G.; Plancher, J.-M.; Rogers-Evans, M.; Carreira, E. Synthesis and Stability of Oxetane Analogs of Thalidomide and Lenalidomide. Org. Lett. 2013, 15, 4312–4315. [Google Scholar] [CrossRef]
- Stepan, A.F.; Karki, K.; McDonald, W.S.; Dorff, P.H.; Dutra, J.K.; Dirico, K.J.; Won, A.; Subramanyam, C.; Efremov, I.V.; O’Donnell, C.J.; et al. Metabolism-Directed Design of Oxetane-Containing Arylsulfonamide Derivatives as γ-Secretase Inhibitors. J. Med. Chem. 2011, 54, 7772–7783. [Google Scholar] [CrossRef]
- Stepan, A.F.; Kauffman, G.W.; Keefer, C.E.; Verhoest, P.R.; Edwards, M. Evaluating the Differences in Cycloalkyl Ether Metabolism Using the Design Parameter “Lipophilic Metabolism Efficiency” (LipMetE) and a Matched Molecular Pairs Analysis. J. Med. Chem. 2013, 56, 6985–6990. [Google Scholar] [CrossRef]
- Bobbink, F.D.; Van Muyden, A.P.; Gopakumar, A.; Fei, Z.; Dyson, P.J. Synthesis of Cross-linked Ionic Poly(styrenes) and their Application as Catalysts for the Synthesis of Carbonates from CO2 and Epoxides. ChemPlusChem 2016, 82, 144–151. [Google Scholar] [CrossRef]
- Fazio, F.; Bryan, M.C.; Blixt, O.; Paulson, J.C.; Wong, C.-H. Synthesis of sugar arrays in microtiter plate. J. Am. Chem. Soc. 2002, 124, 14397–14402. [Google Scholar] [CrossRef]
- Kuang, G.-C.; Guha, P.; Brotherton, W.S.; Simmons, J.T.; Stankee, L.A.; Nguyen, B.T.; Clark, R.J.; Zhu, L. Experimental Investigation on the Mechanism of Chelation-Assisted, Copper(II) Acetate-Accelerated Azide–Alkyne Cycloaddition. J. Am. Chem. Soc. 2011, 133, 13984–14001. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Collins, J.; Anastasaki, A.; Wallis, R.; Mitchell, D.; Becer, C.R.; Haddleton, D. Sequence-Controlled Multi-Block Glycopolymers to Inhibit DC-SIGN-gp120 Binding. Angew. Chem. Int. Ed. 2013, 52, 4435–4439. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ke, C.; Zhang, H.-Y.; Cui, J.; Ding, F. Complexation-Induced Transition of Nanorod to Network Aggregates: Alternate Porphyrin and Cyclodextrin Arrays. J. Am. Chem. Soc. 2008, 130, 600–605. [Google Scholar] [CrossRef]
- Yamauchi, K.; Miyawaki, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. Switching fromaltro-α-Cyclodextrin Dimer topseudo[1]Rotaxane Dimer through Tumbling. Org. Lett. 2010, 12, 1284–1286. [Google Scholar] [CrossRef]
- Yamauchi, K.; Miyawaki, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. A Molecular Reel: Shuttling of a Rotor by Tumbling of a Macrocycle. J. Org. Chem. 2010, 75, 1040–1046. [Google Scholar] [CrossRef]
- Menuel, S.; Azaroual, N.; Landy, D.; Six, N.; Hapiot, F.; Monflier, E. Unusual Inversion Phenomenon of β-Cyclodextrin Dimers in Water. Chem. A Eur. J. 2011, 17, 3949–3955. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Legros, V.; Vanhaverbeke, C.; Souard, F.; Len, C.; Désiré, J. β-Cyclodextrin–Glycerol Dimers: Synthesis and NMR Conformational Analysis. Eur. J. Org. Chem. 2013, 2013, 2583–2590. [Google Scholar] [CrossRef]
- Defaye, J.; Fernández, J.M.M.G.; Mellet, C.O. Les cyclodextrines en pharmacie: Perspectives pour le ciblage d’actifs thérapeutiques et le contrôle d’interactions membranaires. Ann. Pharm. Françaises 2007, 65, 33–49. [Google Scholar] [CrossRef]
- Li, J.; Xiao, H.; Li, J.; Zhong, Y. Drug carrier systems based on water-soluble cationic β-cyclodextrin polymers. Int. J. Pharm. 2004, 278, 329–342. [Google Scholar] [CrossRef]
- Bhushan, R.; Kumar, R. Analytical and preparative enantioseparation of dl-penicillamine and dl-cysteine by high-performance liquid chromatography on α-acid glycoprotein and β-cyclodextrin columns using ninhydrin as a reversible tagging reagent. J. Chromatogr. A 2009, 1216, 3413–3417. [Google Scholar] [CrossRef]
- Breslow, R.; Dong, S.D. Biomimetic Reactions Catalyzed by Cyclodextrins and Their Derivatives. Chem. Rev. 1998, 98, 1997–2012. [Google Scholar] [CrossRef]
- Surpateanu, G.G.; Becuwe, M.; Lungu, N.C.; Dron, P.I.; Fourmentin, S.; Landy, D.; Surpateanu, G.; Becuwe, M. Photochemical behaviour upon the inclusion for some volatile organic compounds in new fluorescent indolizine β-cyclodextrin sensors. J. Photochem. Photobiol. A Chem. 2007, 185, 312–320. [Google Scholar] [CrossRef]
- Astray, G.; González-Barreiro, C.; Mejuto, J.; Rial-Otero, R.; Simal-Gandara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Bjerre, J.; Rousseau, C.; Marinescu, L.; Bols, M. Artificial enzymes,“Chemzymes”: Current state and perspectives. Appl. Microbiol. Biotechnol. 2008, 81, 1–11. [Google Scholar] [CrossRef]
- Harada, A. Cyclodextrin-Based Molecular Machines†. Accounts Chem. Res. 2001, 34, 456–464. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y. Cooperative Binding and Multiple Recognition by Bridged Bis(β-cyclodextrin)s with Functional Linkers. Accounts Chem. Res. 2006, 39, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Aime, S.; Gianolio, E.; Arena, F.; Barge, A.; Martina, K.; Heropoulos, G.; Cravotto, G. New cyclodextrin dimers and trimers capable of forming supramolecular adducts with shape-specific ligands. Org. Biomol. Chem. 2009, 7, 370–379. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Liu, J.; Huo, S.; Deng, Q.; Yan, T.; Ding, L.; Zhang, C.; Meng, L.; Lu, Q. Synthesis and Surface Properties of Novel Gemini Imidazolium Surfactants. J. Surfactants Deterg. 2014, 17, 1107–1116. [Google Scholar] [CrossRef]
- Song, Z.; Wang, H.; Wu, Y.; Gu, J.; Li, S.; Han, H. Fabrication of Bis-Quaternary Ammonium Salt as an Efficient Bactericidal Weapon Against Escherichia coli and Staphylococcus aureus. ACS Omega 2018, 3, 14517–14525. [Google Scholar] [CrossRef] [Green Version]
- Tehrani, A.; Karnbratt, J.; Löfroth, J.-E.; Holmberg, K. Cationic ester-containing gemini surfactants: Determination of aggregation numbers by time-resolved fluorescence quenching. J. Colloid Interface Sci. 2012, 376, 126–132. [Google Scholar] [CrossRef] [PubMed]
- McBain, A.J.; Ledder, R.G.; Moore, L.E.; Catrenich, C.E.; Gilbert, P. Effects of Quaternary-Ammonium-Based Formulations on Bacterial Community Dynamics and Antimicrobial Susceptibility. Appl. Environ. Microbiol. 2004, 70, 3449–3456. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jiang, Y.; Geng, T.; Ju, H.; Duan, S. Synthesis, surface/interfacial properties, and biological activity of amide-based Gemini cationic surfactants with hydroxyl in the spacer group. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 1–10. [Google Scholar] [CrossRef]
- Cao, G.; Guo, X.; Tian, X.; Jia, L. Aggregation behaviours and bactericidal activities of novel cationic surfactants functionalized with amides and ether groups. RSC Adv. 2015, 5, 27197–27204. [Google Scholar] [CrossRef]
- Hegazy, M.; Rashwan, S.; Kamel, M.; El Kotb, M. Synthesis, surface properties and inhibition behavior of novel cationic gemini surfactant for corrosion of carbon steel tubes in acidic solution. J. Mol. Liq. 2015, 211, 126–134. [Google Scholar] [CrossRef]
- Banno, T.; Toshima, K.; Kawada, K.; Matsumura, S. Synthesis and Properties of Gemini-type Cationic Surfactants Containing Carbonate Linkages in the Linker Moiety Directed Toward Green and Sustainable Chemistry. J. Surfactants Deterg. 2009, 12, 249–259. [Google Scholar] [CrossRef]
- Yan, H.-C.; Li, Q.; Geng, T.; Jiang, Y. Properties of the Quaternary Ammonium Salts with Novel Counterions. J. Surfactants Deterg. 2012, 15, 593–599. [Google Scholar] [CrossRef]
- Menger, F.M.; Keiper, J.S. Gemini Surfactants. Angew. Chem. Int. Ed. 2000, 39, 1906–1920. [Google Scholar] [CrossRef]
- Murguía, M.C.; Vaillard, V.A.; Sánchez, V.G.; Di Conza, J.; Grau, R.J. Synthesis, surface-active properties, and antimicrobial activities of new double-chain gemini surfactants. J. Oleo Sci. 2008, 57, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, A. Cationic gemini surfactant with cleavable spacer: Emulsion stability. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 79–84. [Google Scholar] [CrossRef]
- Garcia, M.T.; Kaczerewska, O.; Ribosa, I.; Brycki, B.; Materna, P.; Drgas, M. Biodegradability and aquatic toxicity of quaternary ammonium-based gemini surfactants: Effect of the spacer on their ecological properties. Chemosphere 2016, 154, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Bharmoria, P.; Mehta, M.; Pancha, I.; Kumar, A. Structural and Functional Stability of Cellulase in Aqueous-Biamphiphilic Ionic Liquid Surfactant Solution. J. Phys. Chem. B 2014, 118, 9890–9899. [Google Scholar] [CrossRef]
- Abo-Riya, M.; Tantawy, A.H.; El-Dougdoug, W. Synthesis and evaluation of novel cationic gemini surfactants based on Guava crude fat as petroleum-collecting and dispersing agents. J. Mol. Liq. 2016, 221, 642–650. [Google Scholar] [CrossRef]
- Murguía, M.C.; Machuca, L.M.; Fernandez, M.E. Cationic gemini compounds with antifungal activity and wood preservation potentiality. J. Ind. Eng. Chem. 2019, 72, 170–177. [Google Scholar] [CrossRef]
- Yang, J.; Yun, L.; Zhao, G.; Zhang, F.; Chen, Y.; Wang, R. Fabrication of pH-responsive system based on cationic gemini surfactant/sodium octanedioate and its application on controlled release of paclitaxel. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 101–108. [Google Scholar] [CrossRef]
- Macfarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Simon, P.; Angell, C.A. Energy applications of ionic liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef] [Green Version]
- Valkenburg, M.E.; Vaughn, R.L.; Williams, M.; Wilkes, J.S. Thermochemistry of ionic liquid heat-transfer fluids. Thermochim. Acta 2005, 425, 181–188. [Google Scholar] [CrossRef]
- Tatsumi, T.; Imai, Y.; Kawaguchi, K.; Miyano, N.; Ikeda, I. Antimicrobial Activity of Cationic Gemini Surfactant Containing an Oxycarbonyl Group in the Lipophilic Portion against Gram-positive and Gram-negative Microorganisms. J. Oleo Sci. 2014, 63, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Massi, L.; Guittard, F.; Géribaldi, S.; Levy, R.; Duccini, Y. Antimicrobial properties of highly fluorinated bis-ammonium salts. Int. J. Antimicrob. Agents 2003, 21, 20–26. [Google Scholar] [CrossRef]
- Obłąk, E.; Piecuch, A.; Krasowska, A.; Łuczyński, J. Antifungal activity of gemini quaternary ammonium salts. Microbiol. Res. 2013, 168, 630–638. [Google Scholar] [CrossRef]
- Caillier, L.; De Givenchy, E.T.; Lévy, R.; Vandenberghe, Y.; Geribaldi, S.; Guittard, F. Polymerizable semi-fluorinated gemini surfactants designed for antimicrobial materials. J. Colloid Interface Sci. 2009, 332, 201–207. [Google Scholar] [CrossRef]
- Ranu, B.C.; Jana, R. Ionic Liquid as Catalyst and Reaction Medium–A Simple, Efficient and Green Procedure for Knoevenagel Condensation of Aliphatic and Aromatic Carbonyl Compounds Using a Task-Specific Basic Ionic Liquid. Eur. J. Org. Chem. 2006, 2006, 3767–3770. [Google Scholar] [CrossRef]
- Socha, A.M.; Parthasarathi, R.; Shi, J.; Pattathil, S.; Whyte, D.; Bergeron, M.; George, A.; Tran, K.; Stavila, V.; Venkatachalam, S.; et al. Efficient biomass pretreatment using ionic liquids derived from lignin and hemicellulose. Proc. Natl. Acad. Sci. USA 2014, 111, E3587–E3595. [Google Scholar] [CrossRef] [Green Version]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Jadhav, S.N.; Kumbhar, A.S.; Mali, S.S.; Hong, C.K.; Salunkhe, R.S. A Merrifield resin supported Pd–NHC complex with a spacer(Pd–NHC@SP–PS) for the Sonogashira coupling reaction under copper- and solvent-free conditions. New J. Chem. 2015, 39, 2333–2341. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Deng, Y. Recent advances in ionic liquid catalysis. Green Chem. 2011, 13, 2619. [Google Scholar] [CrossRef]
- Niknam, K.; Khataminejad, M.; Zeyaei, F. Diethylene glycol-bis (3-methylimidazolium) dihydroxide as a dicationic ionic liquid catalyst for the synthesis of 4H-pyrane derivatives in aqueous medium. Tetrahedron Lett. 2016, 57, 361–365. [Google Scholar] [CrossRef]
- Wong, W.-L.; Ho, K.-P.; Lee, L.Y.S.; So, M.-H.; Chan, T.-H.; Wong, K.-Y. Controlling the selectivity of the manganese/bicarbonate/hydrogen peroxide catalytic system by a biphasic pyrrolidinium ionic liquid/n-heptane medium. Appl. Catal. A Gen. 2013, 453, 244–249. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, T.H.; Kim, N.Y.; Park, N.-G.; Ahn, K.-D. Novel thixotropic gel electrolytes based on dicationic bis-imidazolium salts for quasi-solid-state dye-sensitized solar cells. J. Power Sources 2008, 175, 692–697. [Google Scholar] [CrossRef]
- Han, X.; Armstrong, D.W. Using Geminal Dicationic Ionic Liquids as Solvents for High-Temperature Organic Reactions. Org. Lett. 2005, 7, 4205–4208. [Google Scholar] [CrossRef]
- Tang, S.; Baker, G.A.; Zhao, H. Ether-and alcohol-functionalized task-specific ionic liquids: Attractive properties and applications. Chem. Soc. Rev. 2012, 43, 4030–4066. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.-M.; Cai, C. A green procedure for the protection of carbonyl compounds catalyzed by iodine in ionic liquid. Tetrahedron Lett. 2008, 49, 7110–7112. [Google Scholar] [CrossRef]
- Ren, Y.-M.; Zhang, Z.; Jin, S. Convenient and efficient method for synthesis of 2,4,6-triarylpyridines using catalytic amount of PEG1000-based dicationic acidic ionic liquid under solvent-free conditions. Synth. Commun. 2016, 46, 528–535. [Google Scholar] [CrossRef]
- Ren, Y.-M.; Shao, J.-J.; Wu, Z.-C.; Zhang, S.; Tao, T.-X. Facile protection of carbonyl compounds as oxathiolanes and thioacetals promoted by PEG1000-based dicationic acidic ionic liquid as chemoselective and recyclable catalyst. J. Mol. Liq. 2014, 196, 392–394. [Google Scholar] [CrossRef]
- Escribà, M.; Barreneche, C.; Varón, E.Y.; Eras, J.; Solé, A.; Tomàs, A.; Cabeza, L.F.; Canela-Garayoa, R. Ionic compounds derived from crude glycerol: Thermal energy storage capability evaluation. Renew. Energy 2017, 114, 629–637. [Google Scholar] [CrossRef] [Green Version]
- Varón, E.Y.; Eras, J.; Torres, M.; Villorbina, G.; Espart, A.; Canela-Garayoa, R. Entrapment in polymeric material of resting cells of Aspergillus flavus with lipase activity. Application to the synthesis of ethyl laurate. RSC Adv. 2014, 4, 38418–38424. [Google Scholar] [CrossRef]
- Torres, P.; Balcells, M.; Cequier, E.; Canela-Garayoa, R. Effect of Four Novel Bio-Based DES (Deep Eutectic Solvents) on Hardwood Fractionation. Molecules 2020, 25, 2157. [Google Scholar] [CrossRef] [PubMed]
- Ardi, M.; Aroua, M.K.; Hashim, N.A. Progress, prospect and challenges in glycerol purification process: A review. Renew. Sustain. Energy Rev. 2015, 42, 1164–1173. [Google Scholar] [CrossRef] [Green Version]
- Directorate-General for Research and Innovation (European Commission); Fraunhofer ISI; University of Bologna. Task 3 of “Study on Support. to R&I Policy in the Area of Bio-Based Products and Services”—Study; Top. 20 Innovative Bio-Based Products; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
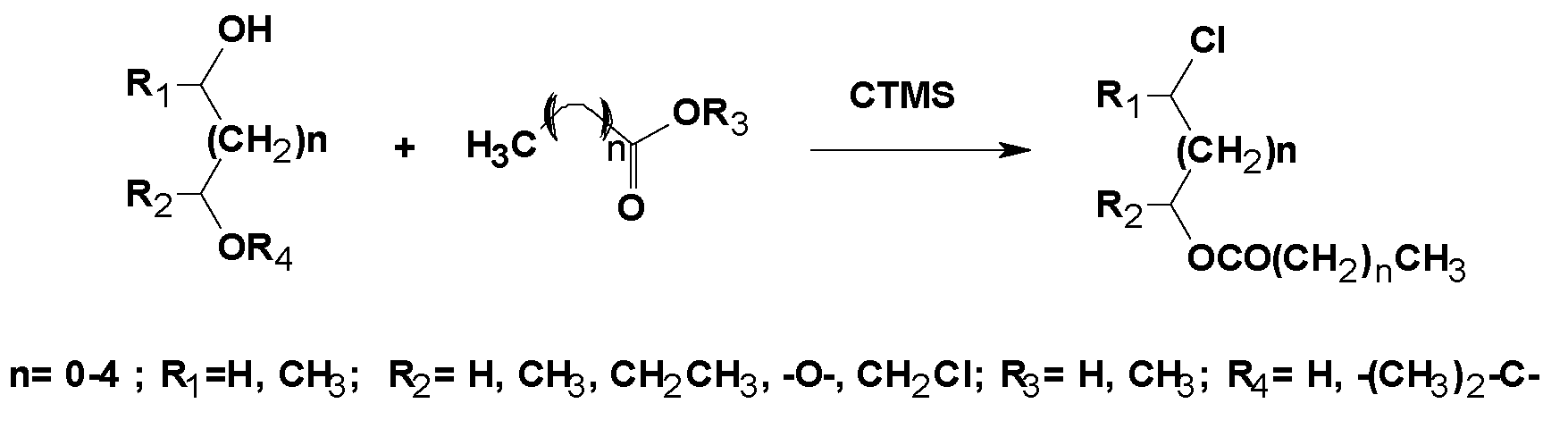




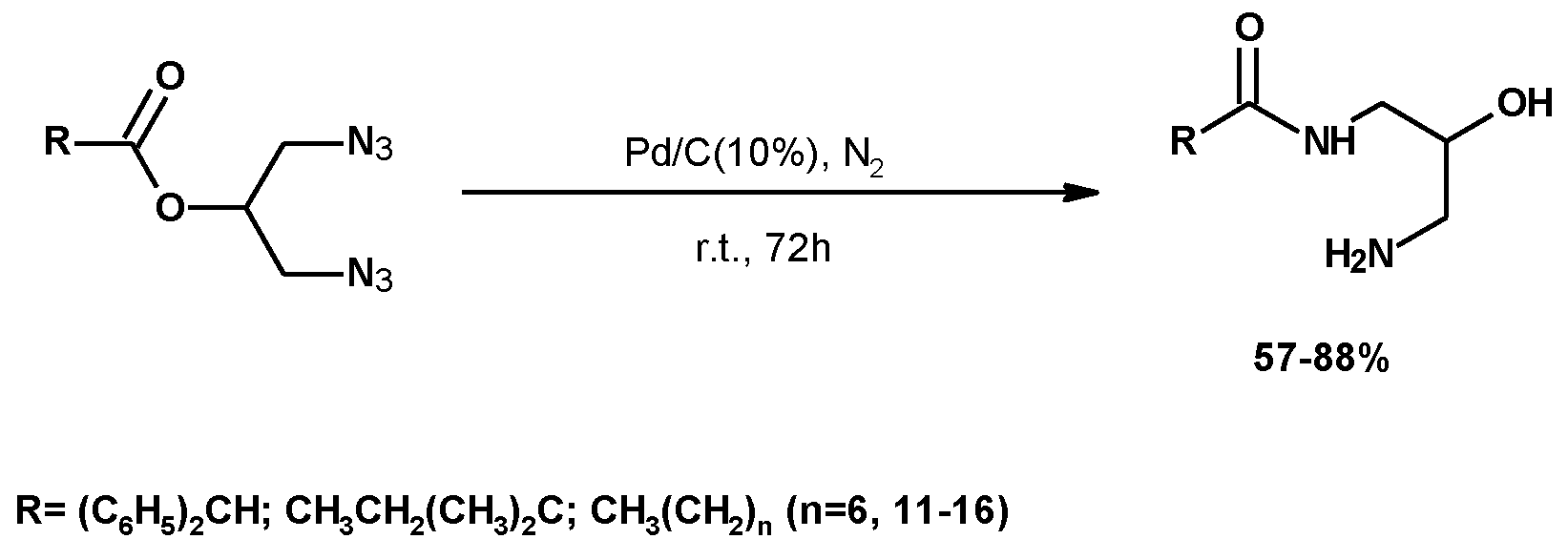







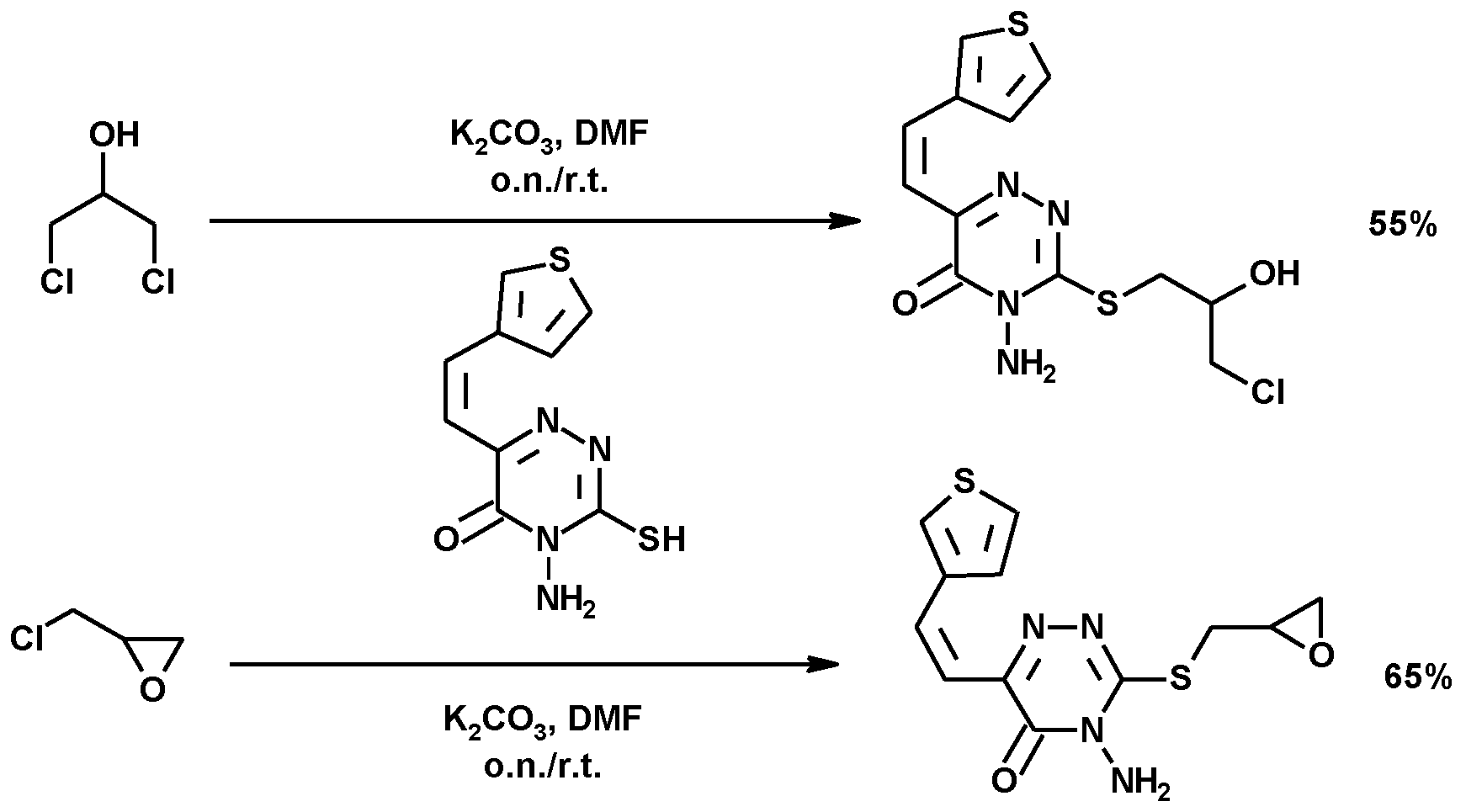











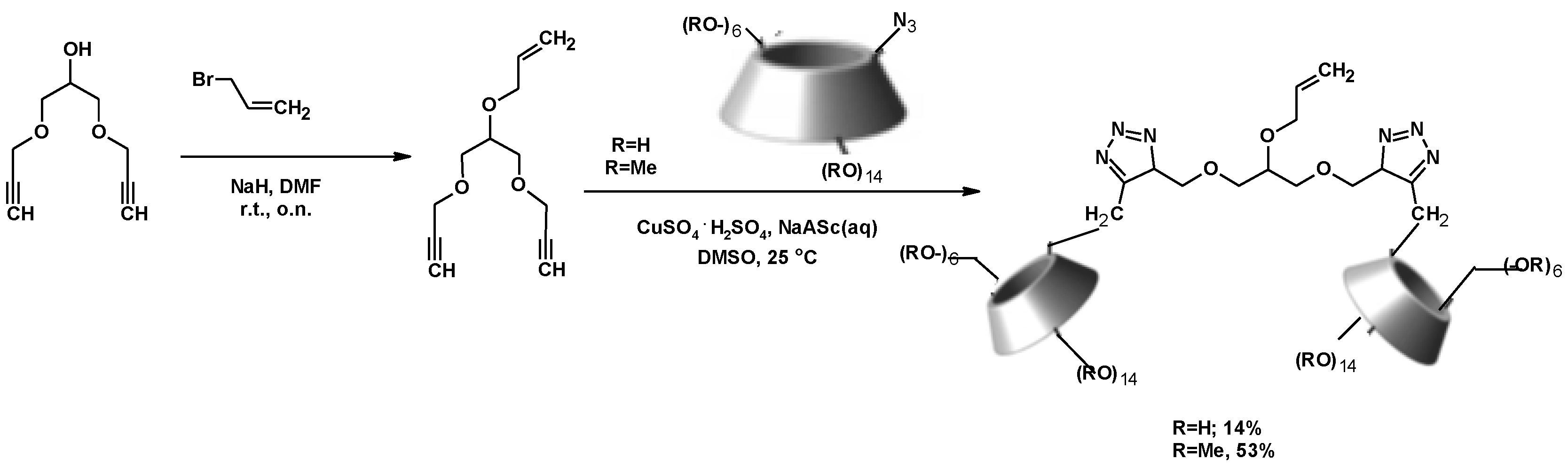


| Reagents | Catalyst | P (atm)/T (°C) | Procedure | Reaction Period | 1,3-DCH (Yield %) | Comments | Ref. |
|---|---|---|---|---|---|---|---|
| HCl(g) +wet glycerol (9%) | Acetic acid (5%) | 7.5/110 | Batch (glycerol) Continuous (HCl) | 4 | 93% DCH (46:1) (1,3-DCH:2,3-DCH) | HCl pressure has a great effect on glycerol consumption rate and product distribution. | [44] |
| HCl(g) +glycerol | Acetic acid (0–50%) | 0.25–1/105 | Semibatch | 3 | N.P. | Non-catalytic hydrochlorination is a major inconvenient at high temperatures... | [43] |
| HCl(g) +glycerol | Propionic acid 8% | 1/100 | Batch(glycerol) Continuous (HCl) | 3 | 41% | No correlation between the acidity strength of the catalyst and the reaction activity was demonstrated. | [32] |
| HCl (g) +glycerol | Hexanoic acid (5%) | 7.5/110 | Semibacth | 3 | N.P. | [61] | |
| HCl(g) +glycerol | Carboxylic acid studied | N.P. | Batch(glycerol) Continuous (HCl) | N.P. | N.P. | Correlation between catalyst pKa value and its selectivity toward mono- (pKa < 3) or dichlorinated (pKa > 4) compounds was found. | [34] |
| Entry | Enzyme Type | Enzyme from/Mutant | Isomer | ee (%) | Yield (%) | Comments | Ref. |
|---|---|---|---|---|---|---|---|
| 2.1 | HHDH | Tistrella mobilis ZJB1405 (E. coli) | S-ECH | N.P. | 75 | Alkaline pH, 45 °C | [92] |
| 2.2 | HHDH | E.coli BL21(DE3) | ECH | N.P. | 88.3 | HZD-9 resin at 10% (w/v) | [90] |
| 2.3 | HHDH | Agrobacterium radiobacter | R-ECH | 99 | 41 | NO2, pH5, 37 °C, 18 min | [85] |
| 2.4 | HHDH | P175S/W249P | S-ECH | 92.3 | 93.2. | pH = 10 | [91] |
| 2.5 | HHDH + EH | N.P. | S-ECH | 99 | 91.2 | Enzyme combination | [91] |
| 2.6 | EH | Pichia pastoris harboring the Rhodotorula glutinis EH | R-ECH | 100 | 26.4 | [95] | |
| 2.7 | EH | N.P. | R-ECH | 99 | 28.5 | [96] | |
| 2.8 | EH | A. radiobacter | R-ECH | ≥99 | 42.7 | Subtract and product inhibition | [84] |
| Reagent | Catalyst | Reactor System | Temperature (°C) | Yield % | Ref. |
|---|---|---|---|---|---|
| 1,3-DCH | NaOH | Continuous millireactor | 30–70 | 50–99 | [59] |
| 1,3-DCH:1,2-DCH(98:2) | Ca(OH)2:CaCO3:H2O (96:4:1, w/w%) | Pre-reactor/reactor Stripping column | 51/64 | 85–90 | [98,102] |
| 1,3-DCH: 1,2-DCH | NaOH | Microreactor | 50–80 | 92 | [97] |
| 1,3-DCH | Ba, Ca and Ba/γ-Al2O32 | Fixed-bed reactor | 150–300 | 10–90 | [101] |
| 1,3-DCH:1,2-DCH Aqueous (5–10 wt%) | Heterogeneous hydrotalcite | Continuous-flow fixed-bed | 200 | 60 | [60] |
| Field of Application | Property | Current Status | Chemical Compounds | Starting Materials | Section |
|---|---|---|---|---|---|
| Agricul-ture | Pesticide | Research | Allyl esters | Chorohydrin esters | 3.1.1 |
| Antimicrobial | Commercial product | 1,2,4-Triazinones | DCH/ECH | 3.1.7 | |
| Chemis-try | Reagent | Commercial product | DCH | Glycerol | 2.1 |
| Reagent | Commercial product | ECH/(S)-CHBN | DCH | 2.2/3.1.2 | |
| Reagent | Research | Chlorohydrin esters | Glycerol | 2.3 | |
| Reagent | Research | Diazides/Monoamides | Chorohydrin esters | 3.1.3 | |
| Reagent | Research | Alkyl glycosides/Azidirines/Oxetanes | DCH | 3.1.6/3.1.7/3.1.8 | |
| Reagent | Commercial product | Cyclic carbonates | ECH | 3.1.8 | |
| Reagent | Research | Oxazolidinones | DCH/Chorohydrin esters | 3.2.2 | |
| Analytic sensors | Research | Polynuclear metals /Alkyl glycosides | DCH | 3.1.5/3.1.6 | |
| Analytic sensors | Research | Triazoles | DCH/ECH | 3.2.2 | |
| Catalyst | Research | Polynuclear metals | DCH | 3.1.5 | |
| Health | Anti-microbial | Commercial product | Sulfonamides | DCH | 3.1.4 |
| Anti-microbial | Research | Pyridine derivatives | DCH/ECH | 3.1.7 | |
| Anti-microbial | Research | Azidirines/Phthalazines/Oxazolidinones/gemini imidazolium salts | DCH | 3.1.7/3.2.2/3.2.3 | |
| Anticancer | Research | Azidirines | DCH | 3.1.7 | |
| Anticancer | Research | Pyridine derivatives | DCH/ECH | 3.1.7 | |
| Antiviral | Sulfonamides/Polynuclear metals | DCH | 3.1.4/3.1.5 | ||
| Anti-hyper-tensive | Sulfonamides | DCH | 3.1.4 | ||
| Diuretic | Sulfonamides | DCH | 3.1.4 | ||
| Hypo-glycemic | Sulfonamides | DCH | 3.1.4 | ||
| Materials | Polymers | Research | Allyl esters | Chorohydrin esters | 3.1.1 |
| Polymers | Research | Polyesters | DCH | 3.1.8 | |
| Flame retar-dants | Research | Polyesters | DCH | 3.1.8 | |
| Surfactants | Research | Gemini imidazolium and ammonium salts | DCH | 3.2.3 | |
| Ionic Solvents | Research | Gemini imidazolium and ammonium salts | DCH | 3.2.3 | |
| PCM | Research | Monoamides/gemini imidazolium and ammonium salts | Chorohydrin esters/DCH | 3.1.3/3.2.3 | |
| Magnetic materials | Research | Polynuclear metals | DCH | 3.1.5 | |
| Photo-voltaic component | Research | Polynuclear metals | DCH | 3.1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canela-Xandri, A.; Balcells, M.; Villorbina, G.; Christou, P.; Canela-Garayoa, R. Preparation and Uses of Chlorinated Glycerol Derivatives. Molecules 2020, 25, 2511. https://doi.org/10.3390/molecules25112511
Canela-Xandri A, Balcells M, Villorbina G, Christou P, Canela-Garayoa R. Preparation and Uses of Chlorinated Glycerol Derivatives. Molecules. 2020; 25(11):2511. https://doi.org/10.3390/molecules25112511
Chicago/Turabian StyleCanela-Xandri, Anna, Mercè Balcells, Gemma Villorbina, Paul Christou, and Ramon Canela-Garayoa. 2020. "Preparation and Uses of Chlorinated Glycerol Derivatives" Molecules 25, no. 11: 2511. https://doi.org/10.3390/molecules25112511
APA StyleCanela-Xandri, A., Balcells, M., Villorbina, G., Christou, P., & Canela-Garayoa, R. (2020). Preparation and Uses of Chlorinated Glycerol Derivatives. Molecules, 25(11), 2511. https://doi.org/10.3390/molecules25112511