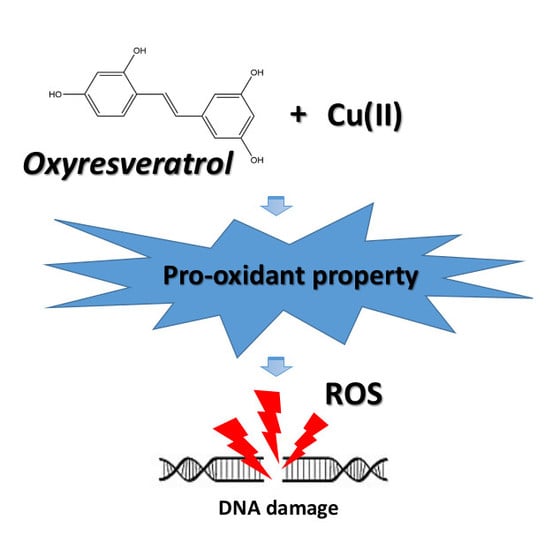
Oxyresveratrol Possesses DNA Damaging Activity
Abstract
:1. Introduction
2. Results and Discussion
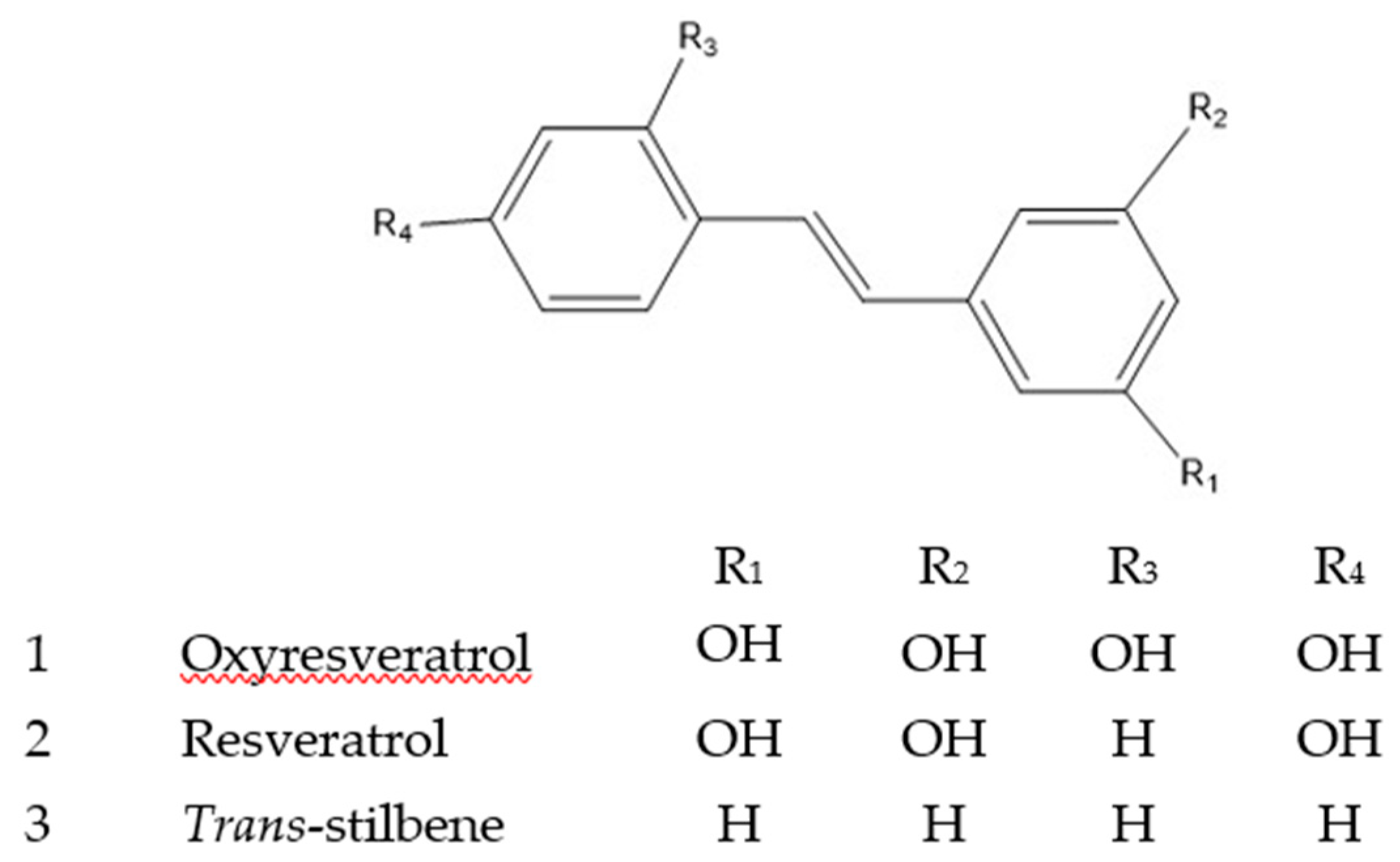
2.1. HPLC Analysis
2.2. DNA Nicking Assay
2.3. Pro-oxidant Activity of ROS Generation and Copper Reduction
3. Materials and Methods
3.1. Standard
3.2. Plant Material
3.3. Extraction
3.4. Chemical Analysis
3.5. DNA Nicking Assay
3.6. Reactive Oxygen Species Assay
3.7. Copper Reducing Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riviere, C.; Pawlus, A.D.; Merillon, J.-M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant Foods and Herbal Sources of Resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Maneechai, S.; Likhitwitayawuid, K.; Sritularak, B.; Palanuvej, C.; Ruangrungsi, N.; Sirisa-Ard, P. Quantitative analysis of oxyresveratrol content in Artocarpus lakoocha and ‘Puag-Haad’. Med Princ Pr. 2009, 18, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Borah, H.J.; Singhal, R.; Hazarika, S. Artocarpus lakoocha roxb.: An untapped bioresource of resveratrol from North East India, its extractive separation and antioxidant activity. Ind. Crop. Prod. 2017, 95, 75–82. [Google Scholar] [CrossRef]
- Akinwumi, B.C.; Bordun, K.-A.M.; Anderson, H.D. Biological activities of stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galindo, I.; Hernaez, B.; Berna, J.; Fenoll, J.; Cenis, J.L.; Escribano, J.M.; Alonso, C. Comparative inhibitory activity of the stilbenes resveratrol and oxyresveratrol on African swine fever virus replication. Antivir. Res. 2011, 91, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Preyavichyapugdee, N.; Sangfuang, M.; Chaiyapum, S.; Sriburin, S.; Pootaeng-on, Y.; Chusongsang, P.; Jiraungkoorskul, W.; Preyavichyapugdee, M.; Sobhon, P. Schistosomicidal Activity of the Crude Extract of Artocarpus Lakoocha. Southeast Asian J. Trop. Med. Public Health 2016, 47, 1–15. [Google Scholar] [PubMed]
- Singhatong, S.; Leelarungrayub, D.; Chaiyasut, C. Antioxidant and toxicity activities of Artocarpus lakoocha Roxb. heartwood extract. J. Med. Plants Res. 2010, 4, 947–953. [Google Scholar]
- Azmi, A.S.; Bhat, S.H.; Hadi, S.M. Resveratrol-Cu(II) induced DNA breakage in human peripheral lymphocytes: Implications for anticancer properties. Febs Lett. 2005, 579, 3131–3135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, M.; Shadakshari, U.; Chattopadhyay, S. A mechanistic study on the nuclease activities of some hydroxystilbenes. Bioorg. Med. Chem. 2004, 12, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Shirahata, S.; Murakami, H.; Nishiyama, K.; Shinohara, K.; Omura, H. DNA breakage by phenyl compounds. Agric. Biol. Chem. 1985, 49, 1423–1428. [Google Scholar]
- Kalinowski, D.S.; Stefani, C.; Toyokuni, S.; Ganz, T.; Anderson, G.J.; Subramaniam, N.V.; Trinder, D.; Olynyk, J.K.; Chua, A.; Jansson, P.J.; et al. Redox cycling metals: Pedaling their roles in metabolism and their use in the development of novel therapeutics. Biochim. Biophys. ActaMol. Cell Res. 2016, 1863, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.F.; Klucas, R.V.; Grayer, R.J.; Abian, J.; Becana, M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: Prooxidant and antioxidant properties. Free Radic. Biol. Med. 1997, 22, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Alarcon de la lastra, C.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.W.; Chen, S.F.; Wu, C.C.; Chen, D.R.; Lee, J.H. Serum and tissue trace elements in patients with breast cancer in Taiwan. Biol. Trace Elem. Res. 2002, 89, 1–11. [Google Scholar] [CrossRef]
- Gupte, A.; Mumper, R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



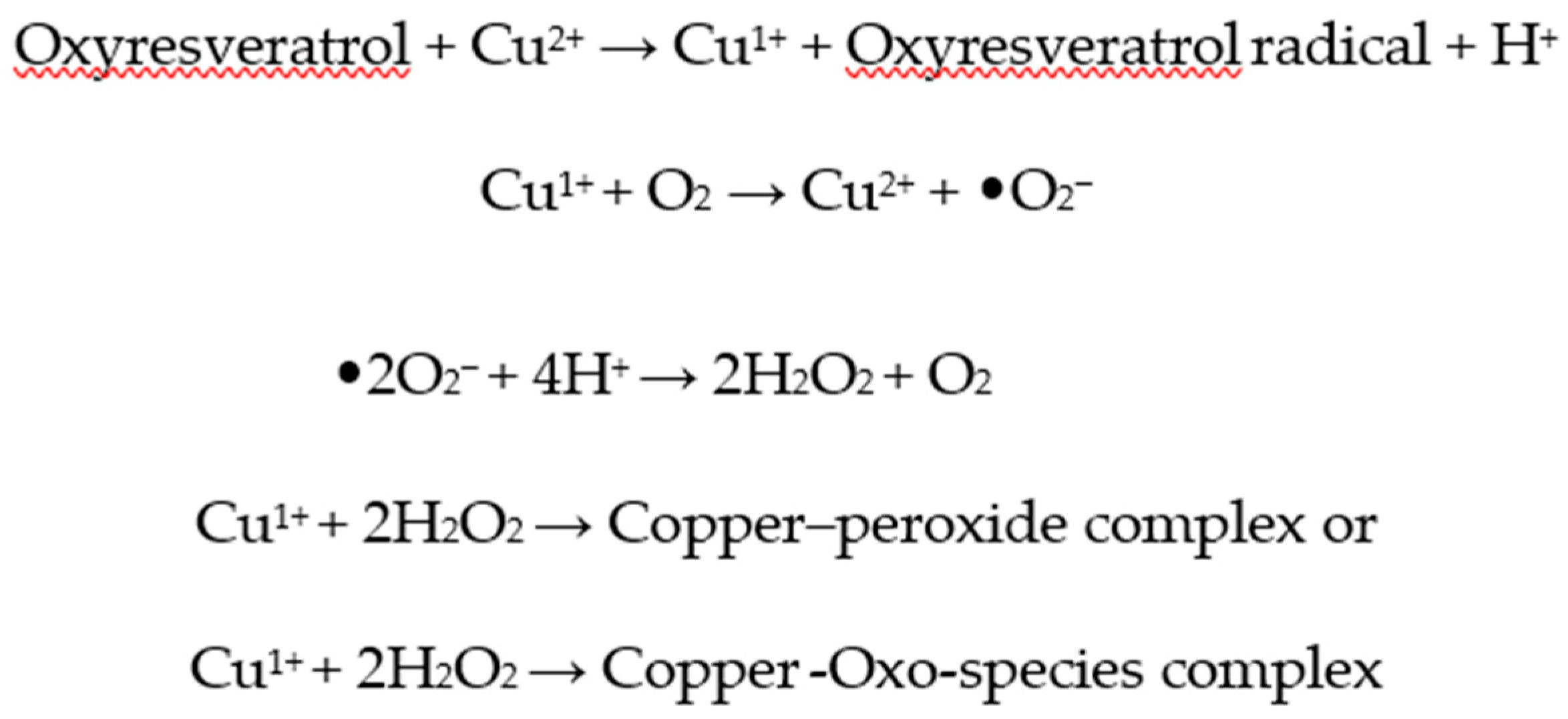

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radapong, S.; Sarker, S.D.; Ritchie, K.J. Oxyresveratrol Possesses DNA Damaging Activity. Molecules 2020, 25, 2577. https://doi.org/10.3390/molecules25112577
Radapong S, Sarker SD, Ritchie KJ. Oxyresveratrol Possesses DNA Damaging Activity. Molecules. 2020; 25(11):2577. https://doi.org/10.3390/molecules25112577
Chicago/Turabian StyleRadapong, Sarayut, Satyajit D. Sarker, and Kenneth J. Ritchie. 2020. "Oxyresveratrol Possesses DNA Damaging Activity" Molecules 25, no. 11: 2577. https://doi.org/10.3390/molecules25112577
APA StyleRadapong, S., Sarker, S. D., & Ritchie, K. J. (2020). Oxyresveratrol Possesses DNA Damaging Activity. Molecules, 25(11), 2577. https://doi.org/10.3390/molecules25112577