Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste
Abstract
:1. Tannins Overview
2. Tannins Organoleptic Taste Properties
2.1. Bitter Taste
2.2. Astringency
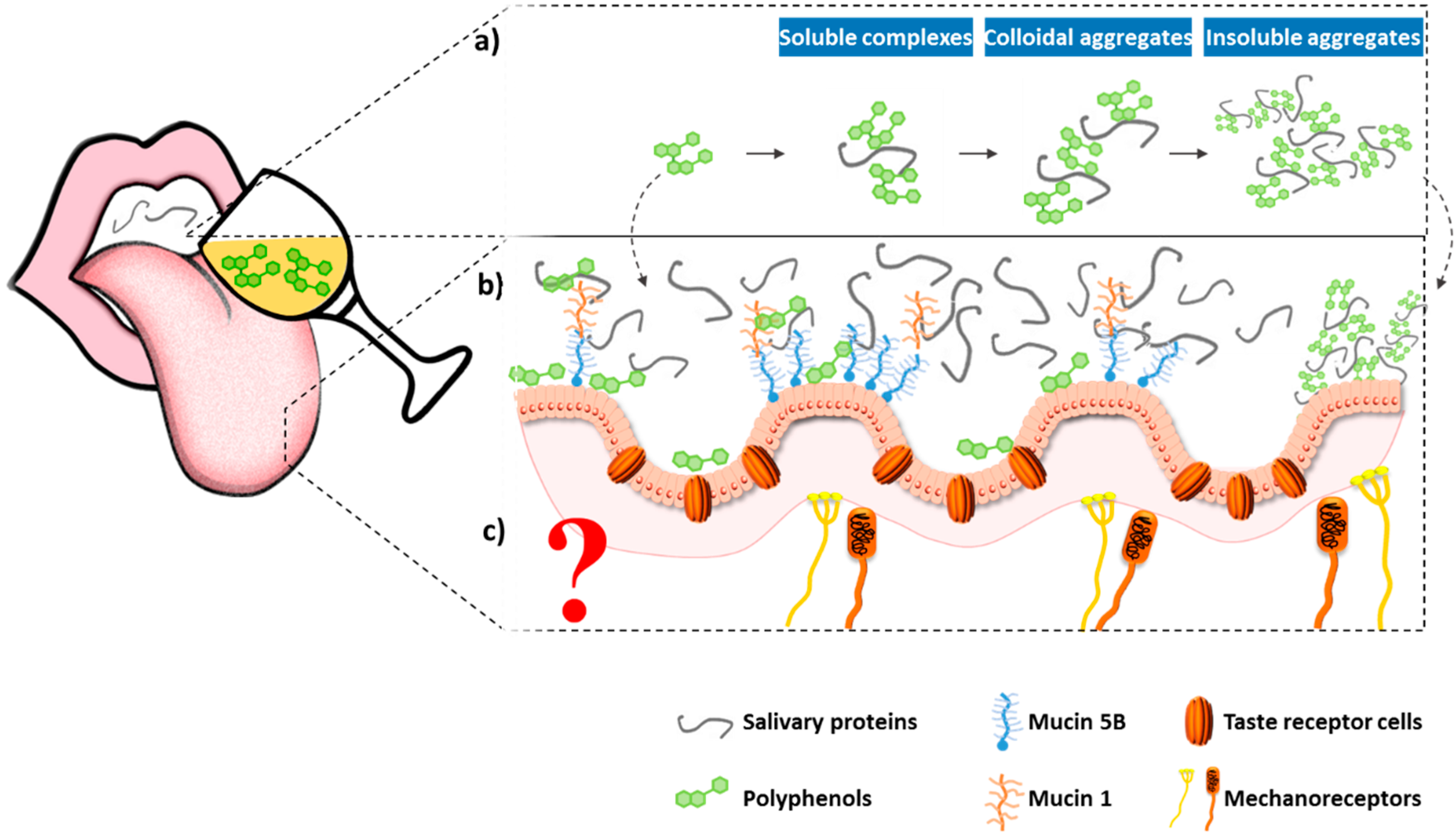
2.2.1. Interaction between Tannins and Proteins
2.2.2. Other Mechanisms for Astringency
3. Tannin–Macromolecule Interactions: Effects on Organoleptic Properties
3.1. Tannin-Protein Interactions: Beyond Astringency
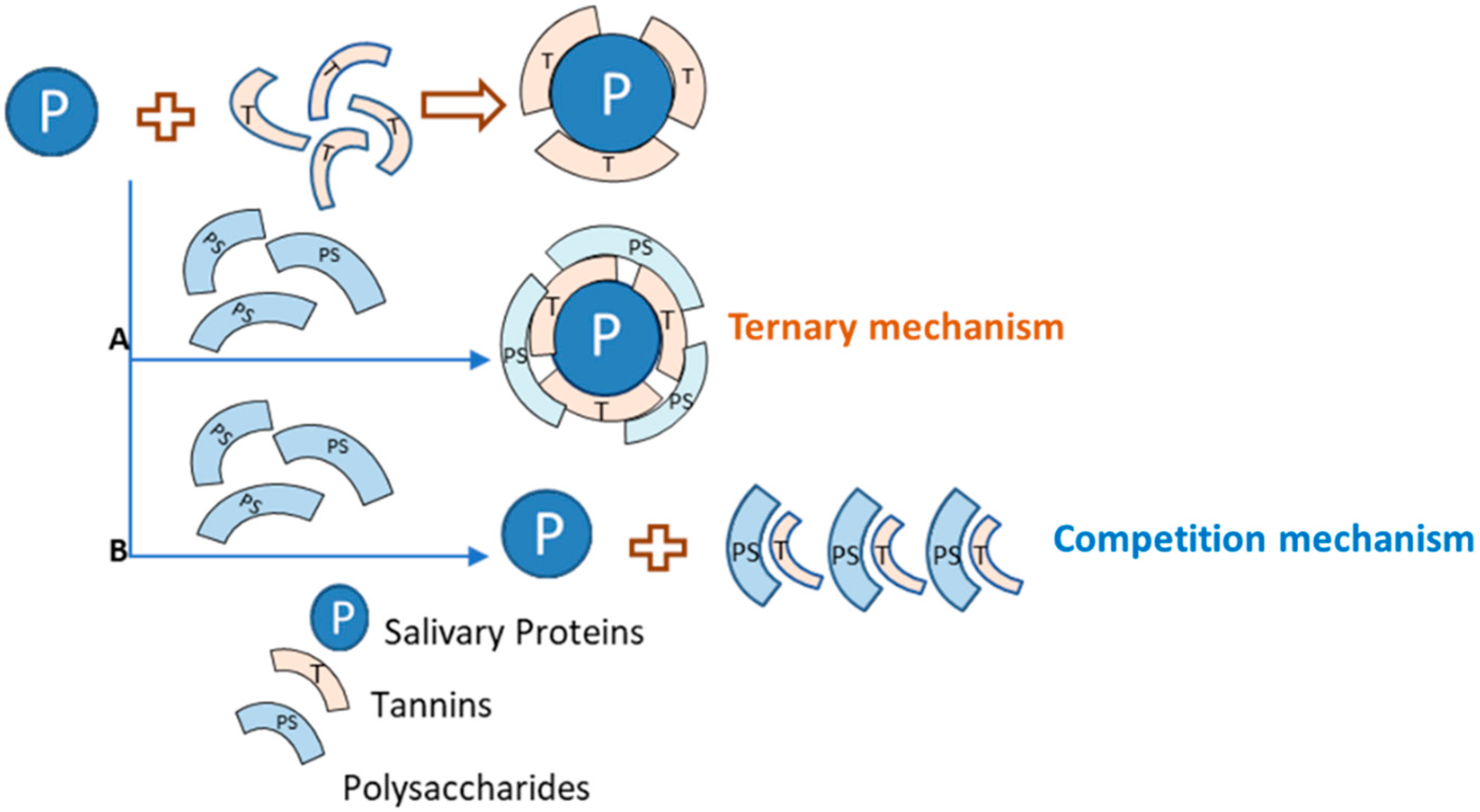
3.2. Tannin–Polysaccharide Interaction: Modulation of Taste Properties
3.2.1. Polysaccharide Effects on Astringency
3.2.2. Effect of Polysaccharides on Bitterness
4. Final Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Quideau, S.; Deffieux, D.; Douat, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural Phenol Polymers: Recent Advances in Food and Health Applications. Antioxidants 2017, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Arranz, S.; Saura-Calixto, F.; Shaha, S.; Kroon, P.A. High Contents of Nonextractable Polyphenols in Fruits Suggest That Polyphenol Contents of Plant Foods Have Been Underestimated. J. Agric. Food Chem. 2009, 57, 7298–7303. [Google Scholar] [CrossRef]
- Hellstrom, J.K.; Mattila, P.H. HPLC Determination of Extractable and Unextractable Proanthocyanidins in Plant Materials. J. Agric. Food Chem. 2008, 56, 7617–7624. [Google Scholar] [CrossRef]
- Bate-Smith, E.C.; Swain, T. Flavonoid compounds. In Comparative Biochemistry; Mason, H.S., Florkin, A.M., Eds.; Academic Press: New York, NY, USA, 1962; Volume 3. [Google Scholar]
- Janet, A.M.K.; Garry, G.D. Flavonoids in Foods, in Flavonoids. Chemistry, Biochemistry and Applications; Andersen, O.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Haslam, E. Vegetable tannins—Lessons of a phytochemical lifetime. Phytochemistry 2007, 68, 2713–2721. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Haslam, E.C. Polyphenols-structure and biosynthesis. In Pratical Polyphenolics: From Structure to Molecular Recognition and Physiological Action; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Bowsher, C.; Steer, M.; Tobin, A. Phenolics. In Plant. Biochemistry; CRC Press: Boca Raton, FL, USA, 2008; Volume 1. [Google Scholar]
- Spranger, I.; Sun, B.; Mateus, A.M.; De Freitas, V.; Ricardo-Da-Silva, J. Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem. 2008, 108, 519–532. [Google Scholar] [CrossRef] [Green Version]
- Arnold, R.A.; Noble, A.C.; Singleton, V.L. Bitterness and astringency of phenolic fractions in wine. J. Agric. Food Chem. 1980, 28, 675–678. [Google Scholar] [CrossRef]
- Green, B.G. Oral astringency: A tactile component of flavor. Acta Psychol. 1993, 84, 119–125. [Google Scholar] [CrossRef]
- Carpenter, G.; Cleaver, L.; Blakeley, M.; Hasbullah, N.; Houghton, J.; Gardner, A. Wine astringency reduces flavor intensity of Brussels sprouts. J. Text. Stud. 2018, 50, 71–74. [Google Scholar] [CrossRef]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J. Sci. Food Agric. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Robichaud, J.L.; Noble, A.C. Astringency and bitterness of selected phenolics in wine. J. Sci. Food Agric. 1990, 53, 343–353. [Google Scholar] [CrossRef]
- Hufnagel, J.C.; Hofmann, T. Quantitative Reconstruction of the Nonvolatile Sensometabolome of a Red Wine. J. Agric. Food Chem. 2008, 56, 9190–9199. [Google Scholar] [CrossRef]
- Behrens, M.; Meyerhof, W. Signaling in the Chemosensory Systems. Cell. Mol. Life Sci. 2006, 63, 1501–1509. [Google Scholar] [CrossRef]
- Montmayeur, J.-P. Receptors for bitter and sweet taste. Curr. Opin. Neurobiol. 2002, 12, 366–371. [Google Scholar] [CrossRef]
- Chandrashekar, J.; Mueller, K.L.A.; Hoon, M.; Adler, E.; Feng, L.; Guo, W.; Zuker, C.S.; Ryba, N.J. T2Rs Function as Bitter Taste Receptors. Cell 2000, 100, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Kinnamon, S.C. Taste transduction: Linkage between molecular mechanisms and psychophysics. Food Qual. Prefer. 1996, 7, 153–159. [Google Scholar] [CrossRef]
- Pérez, C.A.; Huang, L.; Rong, M.; Kozak, J.A.; Preuss, A.K.; Zhang, H.; Max, M.; Margolskee, R.F. A transient receptor potential channel expressed in taste receptor cells. Nat. Neurosci. 2002, 5, 1169–1176. [Google Scholar] [CrossRef]
- Fontoin, H.; Saucier, C.; Teissedre, P.-L.; Glories, Y. The role of pannexin 1 hemichannels in ATP release and cell–cell communication in mouse taste buds. Proc. Natl. Acad. Sci. USA 2007, 104, 6436. [Google Scholar]
- Fontoin, H.; Saucier, C.; Teissedre, P.-L.; Glories, Y. Effect of pH, ethanol and acidity on astringency and bitterness of grape seed tannin oligomers in model wine solution. Food Qual. Prefer. 2008, 19, 286–291. [Google Scholar] [CrossRef]
- Vérette, E.; Noble, A.C.; Somers, T.C. Hydroxycinnamates ofVitis vinifera: Sensory assessment in relation to bitterness in white wines. J. Sci. Food Agric. 1988, 45, 267–272. [Google Scholar] [CrossRef]
- Limayem, F.; Yannou, B. Selective assessment of judgmental inconsistencies in pairwise comparisons for group decision rating. Comput. Oper. Res. 2007, 34, 1824–1841. [Google Scholar] [CrossRef] [Green Version]
- Laubstein, A. Inconsistency and Ambiguity in Lichtheim’s Model. Brain Lang. 1993, 45, 588–603. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.; Francis, L.; Guyot, S.; Marnet, N.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E.J. The mouth-feel properties of grape and apple proanthocyanidins in a wine-like medium. J. Sci. Food Agric. 2003, 83, 564–573. [Google Scholar] [CrossRef]
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different Phenolic Compounds Activate Distinct Human Bitter Taste Receptors. J. Agric. Food Chem. 2013, 61, 1525–1533. [Google Scholar] [CrossRef]
- Soares, S.; Silva, M.S.; García-Estévez, I.; Groβmann, P.; Brás, N.F.; Brandão, E.; Mateus, N.; De Freitas, V.; Behrens, M.; Meyerhof, W.; et al. Human Bitter Taste Receptors Are Activated by Different Classes of Polyphenols. J. Agric. Food Chem. 2018, 66, 8814–8823. [Google Scholar] [CrossRef]
- Narukawa, M.; Noga, C.; Ueno, Y.; Sato, T.; Misaka, T.; Watanabe, T. Evaluation of the bitterness of green tea catechins by a cell-based assay with the human bitter taste receptor hTAS2R39. Biochem. Biophys. Res. Commun. 2011, 405, 620–625. [Google Scholar] [CrossRef]
- Yamazaki, T.; Narukawa, M.; Mochizuki, M.; Misaka, T.; Watanabe, T. Activation of the hTAS2R14 Human Bitter-Taste Receptor by (−)-Epigallocatechin Gallate and (−)-Epicatechin Gallate. Biosci. Biotechnol. Biochem. 2013, 77, 1981–1983. [Google Scholar] [CrossRef]
- Roland, W.S.U.; Van Buren, L.; Gruppen, H.; Driesse, M.; Gouka, R.J.; Smit, G.; Vincken, J.-P. Bitter Taste Receptor Activation by Flavonoids and Isoflavonoids: Modeled Structural Requirements for Activation of hTAS2R14 and hTAS2R39. J. Agric. Food Chem. 2013, 61, 10454–10466. [Google Scholar] [CrossRef]
- Roland, W.S.; Vincken, J.-P.; Gouka, R.J.; Van Buren, L.; Gruppen, H.; Smit, G. Soy Isoflavones and Other Isoflavonoids Activate the Human Bitter Taste Receptors hTAS2R14 and hTAS2R39. J. Agric. Food Chem. 2011, 59, 11764–11771. [Google Scholar] [CrossRef]
- Kuroda, Y.; Ikeda, R.; Yamazaki, T.; Ito, K.; Uda, K.; Wakabayashi, K.; Watanabe, T. Activation of human bitter taste receptors by polymethoxylated flavonoids. Biosci. Biotechnol. Biochem. 2016, 80, 2014–2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narukawa, M.; Kimata, H.; Noga, C.; Watanabe, T. Taste characterisation of green tea catechins. Int. J. Food Sci. Technol. 2010, 45, 1579–1585. [Google Scholar] [CrossRef]
- Intelmann, D.; Batram, C.; Kühn, C.; Haseleu, G.; Meyerhof, W.; Hofmann, T. Three TAS2R Bitter Taste Receptors Mediate the Psychophysical Responses to Bitter Compounds of Hops (Humulus lupulus L.) and Beer. Chemosens. Percept. 2009, 2, 118–132. [Google Scholar] [CrossRef]
- Roland, W.S.U.; Gouka, R.J.; Gruppen, H.; Driesse, M.; Van Buren, L.; Smit, G.; Vincken, J.-P. 6-Methoxyflavanones as Bitter Taste Receptor Blockers for hTAS2R39. PLoS ONE 2014, 9, e94451. [Google Scholar] [CrossRef]
- Bohin, M.C.; Vincken, J.-P.; Van Der Hijden, H.T.W.M.; Gruppen, H. Efficacy of Food Proteins as Carriers for Flavonoids. J. Agric. Food Chem. 2012, 60, 4136–4143. [Google Scholar] [CrossRef]
- Bohin, M.C.; Roland, W.S.U.; Gruppen, H.; Gouka, R.J.; Van Der Hijden, H.T.W.M.; Dekker, P.; Smit, G.; Vincken, J.-P. Evaluation of the Bitter-Masking Potential of Food Proteins for EGCG by a Cell-Based Human Bitter Taste Receptor Assay and Binding Studies. J. Agric. Food Chem. 2013, 61, 10010–10017. [Google Scholar] [CrossRef]
- Bate-Smith, E. Haemanalysis of tannins: The concept of relative astringency. Phytochem. 1973, 12, 907–912. [Google Scholar] [CrossRef]
- Haslam, E.; Lilley, T.H.; Butler, L.G. Natural astringency in foodstuffs—A molecular interpretation. Crit. Rev. Food Sci. Nutr. 1988, 27, 1–40. [Google Scholar] [CrossRef]
- Bate-Smith, E.C. Astringency in foods. Food 1954, 23, 124. [Google Scholar]
- Bate-Smith, E.C. Flavonoid Compounds in Foods. In Advances in Food Research; Mrak, E.M., Stewart, G.F., Eds.; Academic Press: Cambridge, MA, USA, 1954; pp. 261–300. [Google Scholar]
- Hagerman, A.E.; Butler, L.G. Determination of protein in tannin-protein precipitates. J. Agric. Food Chem. 1980, 28, 944–947. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. The specificity of proanthocyanidin-protein interactions. J. Biol. Chem. 1981, 256, 4494–4497. [Google Scholar]
- Mehansho, H.; Hagerman, A.; Clements, S.; Butler, L.; Rogler, J.; Carlson, D.M. Modulation of proline-rich protein biosynthesis in rat parotid glands by sorghums with high tannin levels. Proc. Natl. Acad. Sci. USA 1983, 80, 3948–3952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehansho, H.; Clements, S.; Sheares, B.T.; Smith, S.; Carlson, D.M. Induction of proline-rich glycoprotein synthesis in mouse salivary glands by isoproterenol and by tannins. J. Biol. Chem. 1985, 260, 4418–4423. [Google Scholar]
- Mehansho, H.; Butler, L.G.; Carlson, D.M. Dietary Tannins and Salivary Proline-Rich Proteins: Interactions, Induction, and Defense Mechanisms. Annu. Rev. Nutr. 1987, 7, 423–440. [Google Scholar] [CrossRef]
- Jansman, A.J.; Frohlich, A.A.; Marquardt, R.R. Production of Proline-Rich Proteins by the Parotid Glands of Rats Is Enhanced by Feeding Diets Containing Tannins from Faba Beans (Vicia faba L.). J. Nutr. 1994, 124, 249–258. [Google Scholar] [CrossRef]
- McArthur, C.; Sanson, G.D.; Beal, A.M. Salivary proline-rich proteins in mammals: Roles in oral homeostasis and counteracting dietary tannin. J. Chem. Ecol. 1995, 21, 663–691. [Google Scholar] [CrossRef]
- Bennick, A. Salivary proline-rich proteins. Mol. Cell. Biochem. 1982, 45, 83–99. [Google Scholar] [CrossRef]
- De Freitas, V.; Mateus, N. Protein/Polyphenol Interactions: Past and Present Contributions. Mechanisms of Astringency Perception. Curr. Org. Chem. 2012, 16, 724–746. [Google Scholar] [CrossRef] [Green Version]
- Bacon, J.R.; Rhodes, M.J.C. Development of a Competition Assay for the Evaluation of the Binding of Human Parotid Salivary Proteins to Dietary Complex Phenols and Tannins Using a Peroxidase-Labeled Tannin. J. Agric. Food Chem. 1998, 46, 5083–5088. [Google Scholar] [CrossRef]
- Bacon, J.R.; Rhodes, M.J.C. Binding affinity of hydrolyzable tannins to parotid saliva and to proline-rich proteins derived from it. J. Agric. Food Chem. 2000, 48, 838–843. [Google Scholar] [CrossRef]
- De Freitas, V.; Mateus, N. Structural features of procyanidin interactions with salivary proteins. J. Agric. Food Chem. 2001, 49, 940–945. [Google Scholar] [CrossRef]
- De Freitas, V.; Mateus, N. Nephelometric study of salivary protein-tannin aggregates. J. Sci. Food Agric. 2001, 82, 113–119. [Google Scholar] [CrossRef]
- Charlton, A.J.; Baxter, N.J.; Khan, M.L.; Moir, A.J.G.; Haslam, E.; Davies, A.P.; Williamson, M.P. Polyphenol/Peptide Binding and Precipitation. J. Agric. Food Chem. 2002, 50, 1593–1601. [Google Scholar] [CrossRef]
- Pascal, C.; Poncet-Legrand, C.; Cabane, B.; Vernhet, A. Aggregation of a Proline-Rich Protein Induced by Epigallocatechin Gallate and Condensed Tannins: Effect of Protein Glycosylation. J. Agric. Food Chem. 2008, 56, 6724–6732. [Google Scholar] [CrossRef]
- Sarni-Manchado, P.; Canals, J.M.; Mazerolles, G.; Cheynier, V. Influence of the Glycosylation of Human Salivary Proline-Rich Proteins on Their Interactions with Condensed Tannins. J. Agric. Food Chem. 2008, 56, 9563–9569. [Google Scholar] [CrossRef]
- Canon, F.; Giuliani, A.; Paté, F.; Sarni-Manchado, P. Ability of a salivary intrinsically unstructured protein to bind different tannin targets revealed by mass spectrometry. Anal. Bioanal. Chem. 2010, 398, 815–822. [Google Scholar] [CrossRef]
- Cala, O.; Dufourc, E.J.; Fouquet, E.; Manigand, C.; Laguerre, M.; Pianet, I. The Colloidal State of Tannins Impacts the Nature of Their Interaction with Proteins: The Case of Salivary Proline-Rich Protein/Procyanidins Binding. Langmuir 2012, 28, 17410–17418. [Google Scholar] [CrossRef]
- Lei, X.; Zhu, Y.; Wang, X.; Zhao, P.; Liu, P.; Zhang, Q.; Chen, T.; Yuan, H.; Guo, Y. Wine polysaccharides modulating astringency through the interference on interaction of flavan-3-ols and BSA in model wine. Int. J. Biol. Macromol. 2019, 139, 896–903. [Google Scholar] [CrossRef]
- Kawamoto, H.; Nakatsubo, F. Solubility of protein complexed with galloylglucoses. Phytochemistry 1997, 46, 485–488. [Google Scholar] [CrossRef]
- Kraus, T.; Yu, Z.; Preston, C.M.; Dahlgren, R.A.; Zasoski, R.J. Linking chemical reactivity and protein precipitation to structural characteristics of foliar tannins. J. Chem. Ecol. 2003, 29, 703–730. [Google Scholar] [CrossRef]
- Watrelot, A.; Renard, C.M.; Le Bourvellec, C. Comparison of microcalorimetry and haze formation to quantify the association of B-type procyanidins to poly-l-proline and bovine serum albumin. LWT 2015, 63, 376–382. [Google Scholar] [CrossRef]
- Soares, S.; Mateus, N.; De Freitas, V. Interaction of Different Polyphenols with Bovine Serum Albumin (BSA) and Human Salivary α-Amylase (HSA) by Fluorescence Quenching. J. Agric. Food Chem. 2007, 55, 6726–6735. [Google Scholar] [CrossRef]
- Poncetlegrand, C.; Edelmann, A.; Putaux, J.-L.; Cartalade, D.; Sarnimanchado, P.; Vernhet, A. Poly(l-proline) interactions with flavan-3-ols units: Influence of the molecular structure and the polyphenol/protein ratio. Food Hydrocoll. 2006, 20, 687–697. [Google Scholar] [CrossRef]
- Zhuang, J.; Dai, X.; Zhu, M.; Zhang, S.; Dai, Q.; Jiang, X.; Liu, Y.; Gao, L.; Xia, T. Evaluation of astringent taste of green tea through mass spectrometry-based targeted metabolic profiling of polyphenols. Food Chem. 2020, 305, 125507. [Google Scholar] [CrossRef]
- Morgan, A.A.; Rubenstein, E. Proline: The Distribution, Frequency, Positioning, and Common Functional Roles of Proline and Polyproline Sequences in the Human Proteome. PLoS ONE 2013, 8, e53785. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, T.; Glabasnia, A.; Schwarz, B.; Wisman, K.N.; Gangwer, K.A.; Hagerman, A.E. Protein Binding and Astringent Taste of a Polymeric Procyanidin, 1,2,3,4,6-Penta-O-galloyl-β-d-glucopyranose, Castalagin, and Grandinin. J. Agric. Food Chem. 2006, 54, 9503–9509. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Bennick, A. Interaction of tannin with human salivary proline-rich proteins. Arch. Oral Biol. 1998, 43, 717–728. [Google Scholar] [CrossRef]
- Soares, S.; Vitorino, R.; Osorio, H.; Fernandes, A.; Venâncio, A.; Mateus, N.; Amado, F.; De Freitas, V. Reactivity of Human Salivary Proteins Families Toward Food Polyphenols. J. Agric. Food Chem. 2011, 59, 5535–5547. [Google Scholar] [CrossRef]
- Soares, S.; Mateus, N.; De Freitas, V. Interaction of different classes of salivary proteins with food tannins. Food Res. Int. 2012, 49, 807–813. [Google Scholar] [CrossRef]
- Brandão, E.; Soares, S.; Mateus, N.; De Freitas, V. In Vivo Interactions between Procyanidins and Human Saliva Proteins: Effect of Repeated Exposures to Procyanidins Solution. J. Agric. Food Chem. 2014, 62, 9562–9568. [Google Scholar] [CrossRef]
- Pascal, C.; Poncet-Legrand, C.; Imberty, A.; Gautier, C.; Sarni-Manchado, P.; Cheynier, V.; Vernhet, A. Interactions between a Non Glycosylated Human Proline-Rich Protein and Flavan-3-ols Are Affected by Protein Concentration and Polyphenol/Protein Ratio. J. Agric. Food Chem. 2007, 55, 4895–4901. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.J.; Williamson, M.P.; Lilley, T.H.; Haslam, E. Study of the interaction between salivary proline-rich proteins and a polyphenol by 1H-NMR spectroscopy. JBIC J. Biol. Inorg. Chem. 1994, 219, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.I.; Hoff, J.E.; Armstrong, G.S.; Haff, L.A. Hydrophobic interaction in tannin-protein complexes. J. Agric. Food Chem. 1980, 28, 394–398. [Google Scholar] [CrossRef]
- Jöbstl, E.; Howse, J.R.; Fairclough, J.P.A.; Williamson, M.P. Noncovalent Cross-Linking of Casein by Epigallocatechin Gallate Characterized by Single Molecule Force Microscopy. J. Agric. Food Chem. 2006, 54, 4077–4081. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; García-Estévez, I.; Ferrer-Gallego, R.; Brás, N.F.; Brandão, E.; Silva, M.S.; Teixeira, N.; Fonseca, F.; Sousa, S.F.; Silva, F.; et al. Study of human salivary proline-rich proteins interaction with food tannins. Food Chem. 2017, 243, 175–185. [Google Scholar] [CrossRef]
- McRae, J.; Falconer, R.J.; Kennedy, J.A. Thermodynamics of Grape and Wine Tannin Interaction with Polyproline: Implications for Red Wine Astringency. J. Agric. Food Chem. 2010, 58, 12510–12518. [Google Scholar] [CrossRef]
- Cala, O.; Pinaud, N.; Simon, C.; Fouquet, E.; Laguerre, M.; Dufourc, E.J.; Pianet, I. NMR and molecular modeling of wine tannins binding to saliva proteins: Revisiting astringency from molecular and colloidal prospects. FASEB J. 2010, 24, 4281–4290. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. Protein precipitation method for the quantitative determination of tannins. J. Agric. Food Chem. 1978, 26, 809–812. [Google Scholar] [CrossRef]
- Naczk, M.; Oickle, D.; Pink, D.; Shahidi, F. Protein Precipitating Capacity of Crude Canola Tannins: Effect of pH, Tannin, and Protein Concentrations. J. Agric. Food Chem. 1996, 44, 2144–2148. [Google Scholar] [CrossRef]
- Naczk, M.; Grant, S.; Zadernowski, R.; Barré, E. Protein precipitating capacity of phenolics of wild blueberry leaves and fruits. Food Chem. 2006, 96, 640–647. [Google Scholar] [CrossRef]
- Brandão, E.; Silva, M.S.; García-Estévez, I.; Mateus, N.; De Freitas, V.; Soares, S. Molecular study of mucin-procyanidin interaction by fluorescence quenching and Saturation Transfer Difference (STD)-NMR. Food Chem. 2017, 228, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Maiani, G.; Ferro-Luzzi, A. Effect of Ethanol on Red Wine Tannin−Protein (BSA) Interactions. J. Agric. Food Chem. 1997, 45, 3148–3151. [Google Scholar] [CrossRef]
- Obreque-Sliíer, E.; Peña-Neira, Á.; Loópez-Soliís, R. Enhancement of Both Salivary Protein−Enological Tannin Interactions and Astringency Perception by Ethanol. J. Agric. Food Chem. 2010, 58, 3729–3735. [Google Scholar]
- Gombau, J.; Nadal, P.; Canela, N.; Gómez-Alonso, S.; García-Romero, E.; Smith, P.; Hermosín-Gutiérrez, I.; Canals, J.; Zamora, F. Measurement of the interaction between mucin and oenological tannins by Surface Plasmon Resonance (SPR); relationship with astringency. Food Chem. 2019, 275, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Yokotsuka, K.; Singleton, V.L. Interactive Precipitation between Graded Peptides from Gelatin and Specific Grape Tannin Fractions in Wine-like Model Solutions. Am. J. Enol. Vitic. 1987, 38, 199. [Google Scholar]
- Scharbert, S.; Holzmann, N.; Hofmann, T. Identification of the Astringent Taste Compounds in Black Tea Infusions by Combining Instrumental Analysis and Human Bioresponse. J. Agric. Food Chem. 2004, 52, 3498–3508. [Google Scholar] [CrossRef]
- Schwarz, B.; Hofmann, T. Is there a direct relationship between oral astringency and human salivary protein binding? Eur. Food Res. Technol. 2008, 227, 1693–1698. [Google Scholar] [CrossRef]
- Nayak, A.; Carpenter, G. A physiological model of tea-induced astringency. Physiol. Behav. 2008, 95, 290–294. [Google Scholar] [CrossRef]
- Breslin, P.A.S.; Gilmore, M.; Beauchamp, G.; Green, B. Psychophysical evidence that oral astringency is a tactile sensation. Chem. Senses 1993, 18, 405–417. [Google Scholar] [CrossRef]
- Mo, L.; Chen, J.; Wang, X. A novel experimental set up for in situ oral lubrication measurements. Food Hydrocoll. 2019, 95, 396–405. [Google Scholar] [CrossRef]
- Prinz, J.F.; Lucas, P.W. Saliva tannin interactions. J. Oral Rehabil. 2000, 27, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Laguna, L.; Álvarez, M.D.; Simone, E.; Moreno-Arribas, M.V.; Bartolomé, B. Oral Wine Texture Perception and Its Correlation with Instrumental Texture Features of Wine-Saliva Mixtures. Foods 2019, 8, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, U.; Boulton, R.; Noble, A. Physiological factors contributing to the variability of sensory assessments: Relationship between salivary flow rate and temporal perception of gustatory stimuli. Food Qual. Prefer. 1994, 5, 55–64. [Google Scholar] [CrossRef]
- Condelli, N.; Dinnella, C.; Cerone, A.; Monteleone, E.; Bertuccioli, M. Prediction of perceived astringency induced by phenolic compounds II: Criteria for panel selection and preliminary application on wine samples. Food Qual. Prefer. 2006, 17, 96–107. [Google Scholar] [CrossRef]
- Payne, C.; Bowyer, P.K.; Herderich, M.J.; Bastian, S.E. Interaction of astringent grape seed procyanidins with oral epithelial cells. Food Chem. 2009, 115, 551–557. [Google Scholar] [CrossRef]
- Soares, S.; Ferrer-Gallego, R.; Brandão, E.; Silva, M.; Mateus, N.; De Freitas, V. Contribution of Human Oral Cells to Astringency by Binding Salivary Protein/Tannin Complexes. J. Agric. Food Chem. 2016, 64, 7823–7828. [Google Scholar] [CrossRef]
- Ramos-Pineda, A.M.; Carpenter, G.; García-Estévez, I.; Escribano-Bailon, M.T. Influence of Chemical Species on Polyphenol–Protein Interactions Related to Wine Astringency. J. Agric. Food Chem. 2019, 68, 2948–2954. [Google Scholar] [CrossRef]
- Ployon, S.; Morzel, M.; Belloir, C.; Bonnotte, A.; Bourillot, E.; Briand, L.; Lesniewska, E.; Lherminier, J.; Aybeke, E.N.; Canon, F. Mechanisms of astringency: Structural alteration of the oral mucosal pellicle by dietary tannins and protective effect of bPRPs. Food Chem. 2018, 253, 79–87. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Mateus, N.; De Freitas, V.; Soares, S. Development of a New Cell-Based Oral Model to Study the Interaction of Oral Constituents with Food Polyphenols. J. Agric. Food Chem. 2019, 67, 12833–12843. [Google Scholar] [CrossRef]
- Weiffenbach, J.M. Touch and taste in the mouth: Presence and character of sapid solutions. Acta Psychol. 1993, 84, 127–130. [Google Scholar] [CrossRef]
- Jacobs, R.; Wu, C.-H.; Goossens, K.; Van Loven, K.; Van Hees, J.; Van Steenberghe, D. Oral mucosal versus cutaneous sensory testing: A review of the literature. J. Oral Rehabil. 2002, 29, 923–950. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, I.-S. Ultrastructures of mechanoreceptors in the oral mucosa. Anat. Sci. Int. 2004, 79, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Biedenbach, M.; Chan, K. Tongue mechanoreceptors: Comparison of afferent fibers in the lingual nerve and chorda tympani. Brain Res. 1971, 35, 584–588. [Google Scholar] [CrossRef]
- Schöbel, N.; Radtke, D.; Kyereme, J.; Wollmann, N.; Cichy, A.; Obst, K.; Kallweit, K.; Kletke, O.; Minovi, A.; Dazert, S.; et al. Astringency Is a Trigeminal Sensation That Involves the Activation of G Protein-Coupled Signaling by Phenolic Compounds. Chem. Senses 2014, 39, 471–487. [Google Scholar] [CrossRef]
- Fernandes, I. Wine. In Fermented Foods in Health and Disease Prevention; Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Boston, MA, USA, 2017; Chapter 26; pp. 593–621. [Google Scholar]
- Siebert, K.J. Haze in Beverages. In Advances in Food and Nutrition Research; Academic Press: Boston, MA, USA, 2009; Chapter 2; pp. 53–86. [Google Scholar]
- Millet, M.; Poupard, P.; Le Quéré, J.-M.; Bauduin, R.; Guyot, S. Haze in Apple-Based Beverages: Detailed Polyphenol, Polysaccharide, Protein, and Mineral Compositions. J. Agric. Food Chem. 2017, 65, 6404–6414. [Google Scholar] [CrossRef]
- Aron, P.M.; Shellhammer, T.H. A Discussion of Polyphenols in Beer Physical and Flavour Stability. J. Inst. Brew. 2010, 116, 369–380. [Google Scholar] [CrossRef]
- Wu, L.-C.; Siebert, K.J. Characterization of Haze-Active Proteins in Apple Juice. J. Agric. Food Chem. 2002, 50, 3828–3834. [Google Scholar] [CrossRef]
- Wu, L.-C.; Lu, Y.-W. Electrophoretic Method for the Identification of a Haze-Active Protein in Grape Seeds. J. Agric. Food Chem. 2004, 52, 3130–3135. [Google Scholar] [CrossRef]
- Ricardo-Da-Silva, J.; Cheynier, V.; Souquet, J.-M.; Moutounet, M.; Cabanis, J.-C.; Bourzeix, M. Interaction of grape seed procyanidins with various proteins in relation to wine fining. J. Sci. Food Agric. 1991, 57, 111–125. [Google Scholar] [CrossRef]
- Siebert, K.J.; Troukhanova, N.V.; Lynn, P.Y. Nature of Polyphenol−Protein Interactions. J. Agric. Food Chem. 1996, 44, 80–85. [Google Scholar] [CrossRef]
- Siebert, K.J. Effects of protein-polyphenol interactions on beverage haze, stabilization, and analysis. J. Agric. Food Chem. 1999, 47, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Sanborn, M.; Edwards, C.G.; Ross, C.F. Impact of Fining on Chemical and Sensory Properties of Washington State Chardonnay and Gewürztraminer Wines. Am. J. Enol. Vitic. 2010, 61, 31. [Google Scholar]
- Stankovic, S.; Jovic, S.; Zivkovic, J.; Pavlovic, R. Influence of Age on Red Wine Colour During Fining with Bentonite and Gelatin. Int. J. Food Prop. 2012, 15, 326–335. [Google Scholar] [CrossRef]
- Sarni-Manchado, P.; Deleris, A.; Avallone, S.; Cheynier, V.; Moutounet, M. Analysis and Characterization of Wine Condensed Tannins Precipitated by Proteins Used as Fining Agent in Enology. Am. J. Enol. Vitic. 1999, 50, 81. [Google Scholar]
- Marangon, M.; Vincenzi, S.; Curioni, A. Wine Fining with Plant Proteins. Molecules 2019, 24, 2186. [Google Scholar] [CrossRef] [Green Version]
- Bindon, K.A.; Smith, P. Comparison of the affinity and selectivity of insoluble fibres and commercial proteins for wine proanthocyanidins. Food Chem. 2013, 136, 917–928. [Google Scholar] [CrossRef]
- Simonato, B.; Mainente, F.; Selvatico, E.; Violoni, M.; Pasini, G. Assessment of the fining efficiency of zeins extracted from commercial corn gluten and sensory analysis of the treated wine. LWT 2013, 54, 549–556. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae-Leaw, T.; Balange, A.K.; Maqsood, S. Protein–polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Dinnella, C.; Recchia, A.; Tuorila, H.; Monteleone, E. Individual astringency responsiveness affects the acceptance of phenol-rich foods. Appetite 2011, 56, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, 330S–335S. [Google Scholar] [CrossRef] [Green Version]
- Poli, A.; Anzelmo, G.; Fiorentino, G.; Nicolaus, B.; Tommonaro, G.; Di Donato, P. Polysaccharides from wastes of vegetable industrial processing: New opportunities for their eco-friendly re-use. In Biotechnology of Biopolymers; InTech: Rijeka, Croatia, 2011; pp. 33–56. [Google Scholar]
- Vidal, S.; Courcoux, P.; Francis, L.; Kwiatkowski, M.; Gawel, R.; Williams, P.; Waters, E.; Cheynier, V. Use of an experimental design approach for evaluation of key wine components on mouth-feel perception. Food Qual. Prefer. 2004, 15, 209–217. [Google Scholar] [CrossRef]
- Ozawa, T.; Lilley, T.H.; Haslam, E. Polyphenol interactions: Astringency and the loss of astringency in ripening fruit. Phytochemistry 1987, 26, 2937–2942. [Google Scholar] [CrossRef]
- Mateus, N.; Carvalho, E.; Luís, C.; De Freitas, V. Influence of the tannin structure on the disruption effect of carbohydrates on protein–tannin aggregates. Anal. Chim. Acta 2004, 513, 135–140. [Google Scholar] [CrossRef]
- Luck, G.; Liao, H.; Murray, N.J.; Grimmer, H.R.; Warminski, E.E.; Williamson, M.P.; Lilley, T.H.; Haslam, E. Polyphenols, astringency and proline-rich proteins. Phytochemistry 1994, 37, 357–371. [Google Scholar] [CrossRef]
- Haslam, E.C. Maturation—Changes in astringency. In Pratical Polyphenolics: From Structure to Molecular Recognition and Physiological Action; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Gaffney, S.H.; Martin, R.; Lilley, T.H.; Haslam, E.; Magnolato, D. The association of polyphenols with caffeine and alpha-cyclodextrin and beta-cyclodextrin in aqueous-media. J. Chem. Soc. Chem. Commun. 1986, 2, 107–109. [Google Scholar] [CrossRef]
- Ishizu, T.; Kintsu, K.; Yamamoto, H. NMR Study of the Solution Structures of the Inclusion Complexes of β-Cyclodextrin with (+)-Catechin and (−)-Epicatechin. J. Phys. Chem. B 1999, 103, 8992–8997. [Google Scholar] [CrossRef]
- Irwin, P.L.; Pfeffer, P.E.; Doner, L.W.; Sapers, G.M.; Brewster, J.D.; Nagahashi, G.; Hicks, K.B. Binding geometry, stoichiometry, and thermodynamics of cyclomalto-oligosaccharide (cyclodextrin) inclusion complex formation with chlorogenic acid, the major substrate of apple polyphenol oxidase. Carbohydr. Res. 1994, 256, 13–27. [Google Scholar] [CrossRef]
- Hostettmann, K.; Lederer, M.; Marston, A.; Leipzig-Pagani, E. A study of the cyclodextrin complexes of flavonoids by thin layer chromatography. Phytochem. Anal. 1997, 8, 173–175. [Google Scholar] [CrossRef]
- Fernandes, A.; Brás, N.F.; Mateus, N.; De Freitas, V. Understanding the Molecular Mechanism of Anthocyanin Binding to Pectin. Langmuir 2014, 30, 8516–8527. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Guyot, S.; Renard, C. Non-covalent interaction between procyanidins and apple cell wall material Part I. Effect of some environmental parameters. Biochim. Biophys. Acta-Gen. Subj. 2004, 1672, 192–202. [Google Scholar] [CrossRef]
- De Freitas, V.; Carvalho, E.; Mateus, N. Study of carbohydrate influence on protein–tannin aggregation by nephelometry. Food Chem. 2003, 81, 503–509. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Watrelot, A.; Ginies, C.; Imberty, A.; Renard, C.M. Impact of Processing on the Noncovalent Interactions between Procyanidin and Apple Cell Wall. J. Agric. Food Chem. 2012, 60, 9484–9494. [Google Scholar] [CrossRef] [PubMed]
- Watrelot, A.; Le Bourvellec, C.; Imberty, A.; Renard, C.M. Interactions between Pectic Compounds and Procyanidins are Influenced by Methylation Degree and Chain Length. Biomacromolecules 2013, 14, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Watrelot, A.; Le Bourvellec, C.; Imberty, A.; Renard, C.M. Neutral sugar side chains of pectins limit interactions with procyanidins. Carbohydr. Polym. 2014, 99, 527–536. [Google Scholar] [CrossRef]
- Carvalho, E.; Póvoas, M.J.; Mateus, N.; De Freitas, V. Application of flow nephelometry to the analysis of the influence of carbohydrates on protein–tannin interactions. J. Sci. Food Agric. 2006, 86, 891–896. [Google Scholar] [CrossRef]
- Soares, S.; Gonçalves, R.; Fernandes, I.; Mateus, N.; De Freitas, V. Mechanistic Approach by Which Polysaccharides Inhibit α-Amylase/Procyanidin Aggregation. J. Agric. Food Chem. 2009, 57, 4352–4358. [Google Scholar] [CrossRef]
- Soares, S.; Mateus, N.; De Freitas, V. Carbohydrates Inhibit Salivary Proteins Precipitation by Condensed Tannins. J. Agric. Food Chem. 2012, 60, 3966–3972. [Google Scholar] [CrossRef]
- Pellerin, P.; Doco, T.; Vida, S.; Williams, P.; Brillouet, J.-M.; O’Neill, M.A. Structural characterization of red wine rhamnogalacturonan II. Carbohydr. Res. 1996, 290, 183–197. [Google Scholar] [CrossRef]
- Vidal, S.; Williams, P.; Doco, T.; Moutounet, M.; Pellerin, P. The polysaccharides of red wine: Total fractionation and characterization. Carbohydr. Polym. 2003, 54, 439–447. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Williams, P.; Romero-Cascales, I.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M.; Doco, T. Polysaccharide Composition of Monastrell Red Wines from Four Different Spanish Terroirs: Effect of Wine-Making Techniques. J. Agric. Food Chem. 2013, 61, 2538–2547. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Williams, P.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E. The mouth-feel properties of polysaccharides and anthocyanins in a wine like medium. Food Chem. 2004, 85, 519–525. [Google Scholar] [CrossRef]
- Carvalho, E.; Mateus, N.; Plet, B.; Pianet, I.; Dufourc, E.; De Freitas, V. Influence of Wine Pectic Polysaccharides on the Interactions between Condensed Tannins and Salivary Proteins. J. Agric. Food Chem. 2006, 54, 8936–8944. [Google Scholar] [CrossRef] [PubMed]
- Quijada-Morín, N.; Williams, P.; Rivas-Gonzalo, J.-C.; Doco, T.; Escribano-Bailon, M.T. Polyphenolic, polysaccharide and oligosaccharide composition of Tempranillo red wines and their relationship with the perceived astringency. Food Chem. 2014, 154, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Brandão, E.; Silva, M.S.; García-Estévez, I.; Williams, P.; Mateus, N.; Doco, T.; De Freitas, V.; Soares, S. The role of wine polysaccharides on salivary protein-tannin interaction: A molecular approach. Carbohydr. Polym. 2017, 177, 77–85. [Google Scholar] [CrossRef]
- Brandão, E.; Silva, M.S.; García-Estévez, I.; Williams, P.; Mateus, N.; Doco, T.; De Freitas, V.; Soares, S. Inhibition Mechanisms of Wine Polysaccharides on Salivary Protein Precipitation. J. Agric. Food Chem. 2019, 68, 2955–2963. [Google Scholar] [CrossRef]
- Brandão, E.; Fernandes, A.; Guerreiro, C.; Coimbra, M.A.; Mateus, N.; de Freitas, V.; Soares, S. The effect of pectic polysaccharides from grape skins on salivary protein—Procyanidin interactions. Carbohydr. Polym. 2020, 236, 116044. [Google Scholar] [CrossRef]
- Manjón, E.; Brás, N.F.; García-Estévez, I.; Escribano-Bailon, M.T. Cell Wall Mannoproteins from Yeast Affect Salivary Protein–Flavanol Interactions through Different Molecular Mechanisms. J. Agric. Food Chem. 2020. [Google Scholar] [CrossRef]
- Watrelot, A.; Schulz, D.L.; Kennedy, J.A. Wine polysaccharides influence tannin-protein interactions. Food Hydrocoll. 2017, 63, 571–579. [Google Scholar] [CrossRef]
- Riou, V.; Vernhet, A.; Doco, T.; Moutounet, M. Aggregation of grape seed tannins in model wine—Effect of wine polysaccharides. Food Hydrocoll. 2002, 16, 17–23. [Google Scholar] [CrossRef]
- Vernhet, A.; Pellerin, P.; Prieur, C.; Osmianski, J.; Moutounet, M. Charge properties of some grape and wine polysaccharide and polyphenolic fractions. Am. J. Enol. Vitic. 1996, 47, 25–30. [Google Scholar]
- Poncet-Legrand, C.; Doco, T.; Williams, P.; Vernhet, A. Inhibition of Grape Seed Tannin Aggregation by Wine Mannoproteins: Effect of Polysaccharide Molecular Weight. Am. J. Enol. Vitic. 2007, 58, 87. [Google Scholar]
- Smith, A.; June, H.; Noble, A.C. Effects of viscosity on the bitterness and astringency of grape seed tannin. Food Qual. Prefer. 1996, 7, 161–166. [Google Scholar] [CrossRef]
- Troszyńska, A.; Narolewska, O.; Wołejszo, A.; Ostaszyk, A. Effect of carboxymethyl cellulose (cmc) on perception of astringency of phenolic compounds. Pol. J. Food Nutr. Sci. 2008, 58, 241–245. [Google Scholar]
- Calvino, A.; García-Medina, M.R.; Cometto-Muniz, J.E. Interactions in caffeine–sucrose and coffee–sucrose mixtures: Evidence of taste and flavor suppression. Chem. Senses 1990, 15, 505–519. [Google Scholar] [CrossRef]
- Rashima, R.S.; Maizura, M.; Kang, W.M.; Fazilah, A.; Tan, L.X. Influence of sodium chloride treatment and polysaccharides as debittering agent on the physicochemical properties, antioxidant capacity and sensory characteristics of bitter gourd (Momordica charantia) juice. J. Food Sci. Technol. 2016, 54, 228–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jullian, C.; Miranda, S.; Zapata-Torres, G.; Mendizabal, F.; Olea-Azar, C. Studies of inclusion complexes of natural and modified cyclodextrin with (+)catechin by NMR and molecular modeling. Bioorg. Med. Chem. 2007, 15, 3217–3224. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Cano-Lechuga, M.; Ruiz-García, Y.; Gómez-Plaza, E. Interactions between grape skin cell wall material and commercial enological tannins. Practical implications. Food Chem. 2014, 152, 558–565. [Google Scholar] [CrossRef]
- Fernandes, P.A.; Le Bourvellec, C.; Renard, C.M.; Wessel, D.F.; Cardoso, S.M.; Coimbra, M.A. Interactions of arabinan-rich pectic polysaccharides with polyphenols. Carbohydr. Polym. 2019, 230, 115644. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Hydrolyzable Tannins | Grandinin | Castalagin | Punicalagin | Vescalagin | PGG | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structure |  |  |  |  |  | |||||||
| EC50 (μM) | 2.43 | 4.44 | 40.23; 3.95 | 7.26 | 8.5; 6.6 | |||||||
| Activated TAS2Rs | 7 | 7 | 5 and 7 | 7 | 5 and 7 | |||||||
| Transfected cells | HEK293T | |||||||||||
| Reference | [30] | [29] | ||||||||||
| Condensed Tannins | (−)-Epicatechin | (+)-Catechin | ECG | EGCG | EGC | Procyanidins | ||||||
| B1 | B4 | B2g | B7 | C2 | ||||||||
| Structure |  (−)-Epicatechin R1 = H, R2 = OH (−)-Catechin R1 = OH, R2 = H |  (−)-ECG R = H (−)-EGCG R = OH |  |  B1: R1 = OH; R2 = H; R3 = H; R4 = OH B4: R1 = H; R2 = OH; R3 = OH; R4 = H B2g: R1 = O-Galloyl; R2, R3 = H; R4 = OH |  |  | ||||||
| EC50 (μM) | 30151.0; 3210.0; nd; 3800 | nd; nd | 70; nd; nd; 151 | nd; 12.30; 34; nd; 8.50-161; 16.72 | nd; nd | 119.34; 123.95 | nd | 6.29; 9.11 | nd | 35.6 | ||
| Activated TAS2Rs | 4, 5, 14, 39 | 14 and 39 | 14, 30, 38, 39 | 4, 5, 14, 30, 39, 43 | 14 and 39 | 5 and 7 | 5 | 5 and 39 | 5 | 5 | ||
| Transfected cells | HEK293 and HEK293T | HEK293 | HEK293 and HEK293T | HEK293T | ||||||||
| Reference | [29,31,33] | [33] | [31,32,33] | [30,31,33] | [31,32,33] | [30] | [29] | |||||
| Binary System | ||||
| Tannin | Polysaccharide | Techniques | Ref. | |
| (+)-catechin | β-cyclodextrin | NMR; Molecular modelling | [165] | |
| (+)-catechin; (−)-epicatechin | β-cyclodextrin | NMR | [135] | |
| Apple procyanidins | Apple/citrus pectins | ITC | [141,142,143] | |
| Grape seed procyanidins | Wine polysaccharides | DLS | [158] | |
| Procyanidins | Apple/citrus pectins | UV-vis spectrophotometry | [142,143] | |
| Proanthocyanidins | Insoluble cell-wall material (CWM) | Phloroglucinolysis and SEC | [166] | |
| Apple procyanidins | Arabinan-rich pectic polysaccharides | ITC; UV-vis spectrophotometry | [167] | |
| Ternary System | ||||
| Protein | Tannin | Polysaccharide | Techniques | Ref. |
| Caffeine (similar features with proteins) | Methylgallate and Trigalloylglucose | α- and β-cyclodextrin | NMR; ITC | [134] |
| Proline-rich gelatin; Sodium caseinate | PGG | Pectin; galactomannans; carrageenans | NMR | [132] |
| BSA | Grape seed procyanidins | Pectin; xanthan; polygalacturonic acid; Arabic gum; β-cyclodextrin; arabinogalactan; dextran; glucose | Nephelometry | [131,140] |
| BSA | Grape seed procyanidins | Xanthan; Arabic gum; dextran | Flow Nephelometry | [144] |
| α-amylase; IB8c | Grape seed procyanidins | AGPs; RG II | Light scattering | [151] |
| α-amylase | Grape seed procyanidins | Pectin; Arabic gum; cyclodextrin | DLS; nephelometry and fluorescence quenching | [145] |
| Salivary proteins | Grape seed procyanidins | Pectin; Arabic gum; polygalacturonic acid | HPLC; SDS-PAGE | [146] |
| Saliva | Proanthocyanidins | Wine oligosaccharides and polysaccharides (PRAGs; RG II; MPs) | Sensory analysis | [152] |
| aPRPs and P-B peptide | Procyanidin B2 and Punicalagin | RG II and AGPs | HPLC; Nephelometry; Fluorescence quenching | [153] |
| BSA | Wine tannins | Wine polysaccharides (AGPs; MPs; RG II) | HPLC-DAD; UV-vis spectrophotometry | [157] |
| BSA | Wine flavan-3-ols | Wine polysaccharide mixture | CD; SDS-PAGE; Fluorescence spectroscopy | [63] |
| Salivary proteins | Procyanidin B2 and Punicalagin | RG II and AGPs | HPLC; Nephelometry; Fluorescence quenching; SDS-PAGE | [154] |
| Salivary proteins | Grape seed procyanidins | Grape pectic polysaccharides | HPLC; SDS-PAGE; | [155] |
| Salivary proteins | Grape seed procyanidins | Commercial yeast mannoproteins | ITC; Molecular Dynamics Simulation | [156] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. https://doi.org/10.3390/molecules25112590
Soares S, Brandão E, Guerreiro C, Soares S, Mateus N, de Freitas V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules. 2020; 25(11):2590. https://doi.org/10.3390/molecules25112590
Chicago/Turabian StyleSoares, Susana, Elsa Brandão, Carlos Guerreiro, Sónia Soares, Nuno Mateus, and Victor de Freitas. 2020. "Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste" Molecules 25, no. 11: 2590. https://doi.org/10.3390/molecules25112590
APA StyleSoares, S., Brandão, E., Guerreiro, C., Soares, S., Mateus, N., & de Freitas, V. (2020). Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules, 25(11), 2590. https://doi.org/10.3390/molecules25112590








