Impact of the Preparation Procedure on the Performance of the Microporous HKUST-1 Metal-Organic Framework in the Liquid-Phase Separation of Aromatic Compounds
Abstract
:1. Introduction
2. Results
2.1. HKUST-1 Material Synthesis
2.2. Characterization of HKUST-1 Materials
2.3. Structural Examinations
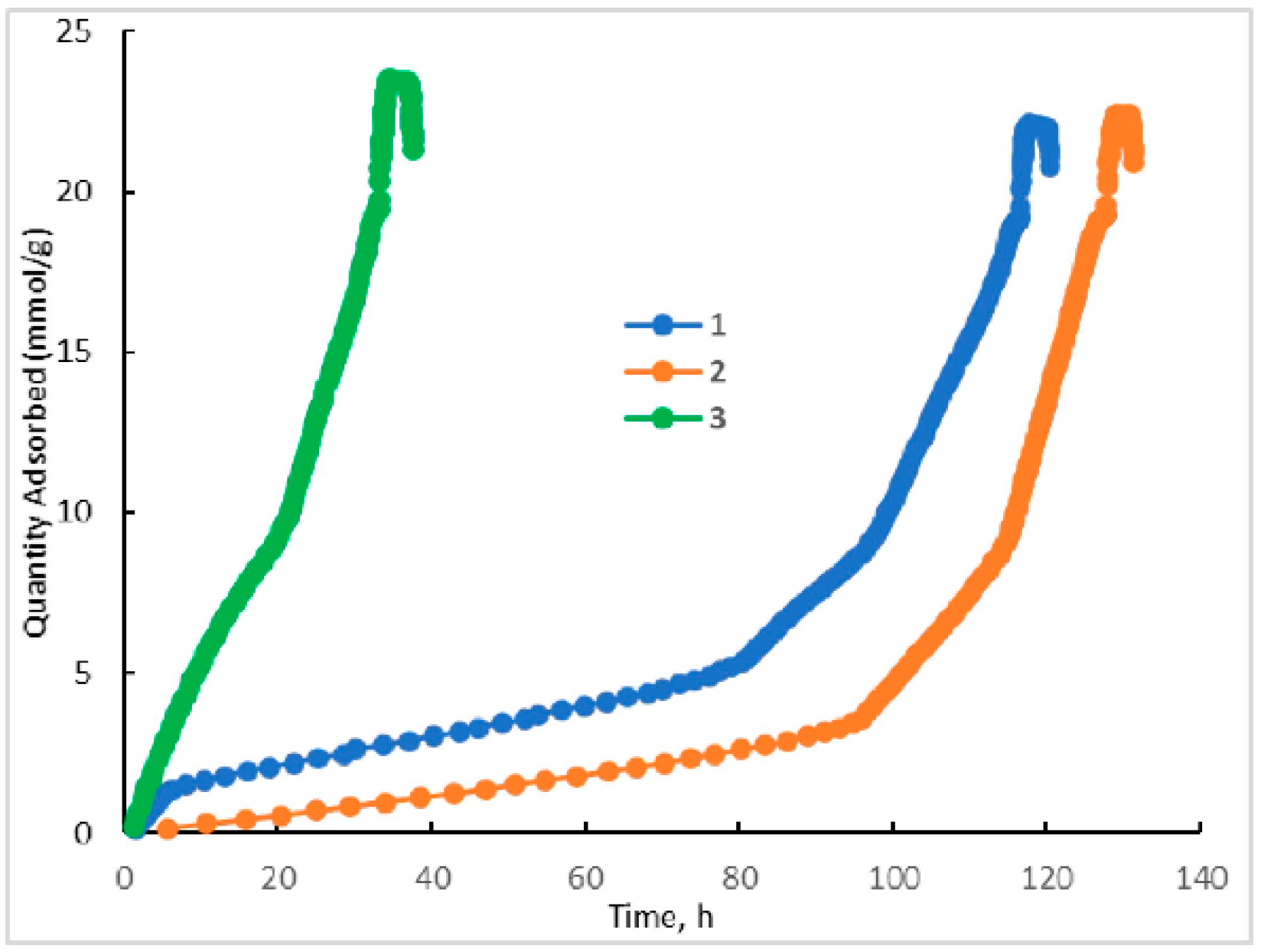
2.4. Textural Properties of HKUST-1 Materials
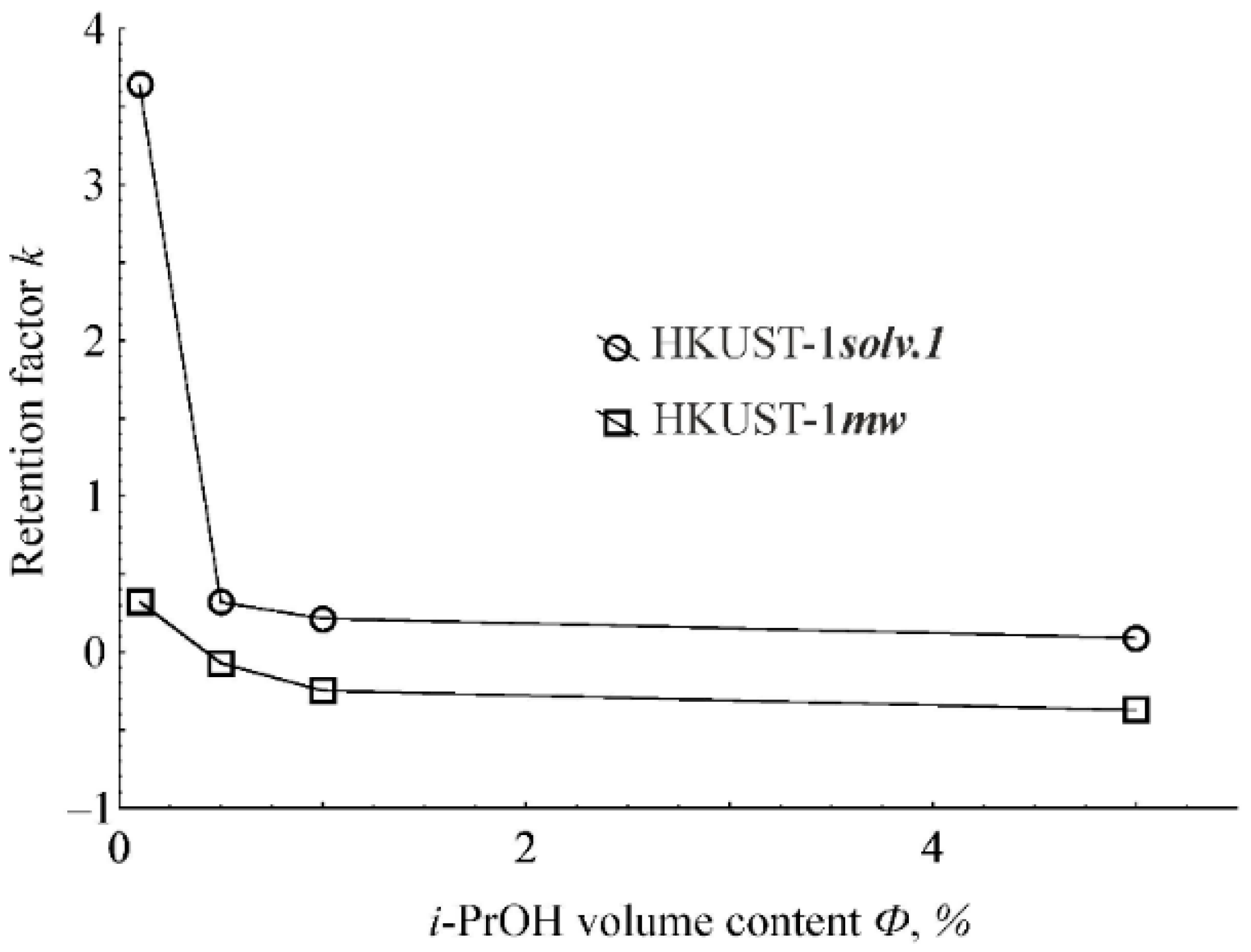
2.5. Selectivity of Aromatic Molecules Liquid-Phase Adsorption onto the HKUST-1mw Material upon HPLC
2.6. Study of the Mechanism of Liquid-Phase Adsorption onto HKUST-1 Materials
3. Discussion
4. Materials and Methods
4.1. Preparation of HKUST-1 Materials
4.1.1. HKUST-1mw Sample
4.1.2. HKUST-1solv.1
4.1.3. HKUST-1solv.2
4.2. Characterization of HKUST-1 Materials
4.2.1. N2 Adsorption Data
4.2.2. SEM Analysis
4.2.3. Powder X-ray Diffraction
4.3. Measurements of the Adsorption Selectivity in the HPLC Tests
4.4. Study of the Mechanism of Liquid-Phase Adsorption onto HKUST-1 Samples
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hao, L.; Liu, X.; Wang, J.; Wang, C.; Wu, Q.; Wang, Z. Use of ZIF-8-derived nanoporous carbon as the adsorbent for the solid phase extraction of carbamate pesticides prior to high-performance liquid chromatographic analysis. Talanta 2015, 142, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Wang, C.; Wu, Q.; Li, Z.; Zang, X.; Wang, Z. Metal–organic framework derived magnetic nanoporous carbon: Novel adsorbent for magnetic solid-phase extraction. Anal. Chem. 2014, 86, 12199–12205. [Google Scholar] [CrossRef] [PubMed]
- Kazakevich, Y.; lo Brutto, R. HPLC for Pharmaceutical Scientists, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2007; pp. 75–76. [Google Scholar]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ren, Y.; Shen, W.; Deng, H.; Gao, Z. Applications of metal-organic frameworks as stationary phases in chromatography. TrAC Trends Anal. Chem. 2013, 50, 33–41. [Google Scholar] [CrossRef]
- Yaghi, O.M. Reticular chemistry in all dimensions. ACS Central Sci. 2019, 5, 1295–1300. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Yan, X.-P. Evaluation of isostructural metal–organic frameworks coated capillary columns for the gas chromatographic separation of alkane isomers. Talanta 2012, 99, 944–950. [Google Scholar] [CrossRef]
- Münch, A.S.; Mertens, F.O.R.L. HKUST-1 as an open metal site gas chromatographic stationary phase—Capillary preparation, separation of small hydrocarbons and electron donating compounds, determination of thermodynamic data. J. Mater. Chem. 2012, 22, 10228. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Zhang, J.; Chen, Z. Metal-organic frameworks as stationary phase for application in chromatographic separation. J. Chromatogr. A 2017, 1530, 1–18. [Google Scholar] [CrossRef]
- Bell, D.C. The promise of metal–organic frameworks for use in liquid chromatography. LCGC North Am. 2018, 36, 352–354. [Google Scholar]
- McKinstry, C.; Cussen, E.; Fletcher, A.; Patwardhan, S.V.; Sefcik, J. Scalable continuous production of high quality HKUST-1 via conventional and microwave heating. Chem. Eng. J. 2017, 326, 570–577. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.-M.; Zhang, X.-H.; Wang, B.-J.; Zhang, M.; Zhang, J.-H.; Yuan, L.-M. 3D Chiral nanoporous metal–organic framework for chromatographic separation in GC. Chromatographia 2014, 77, 1359–1365. [Google Scholar] [CrossRef]
- Yusuf, K.; Aqel, A.; Alothman, Z. Metal-organic frameworks in chromatography. J. Chromatogr. A 2014, 1348, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Roy, P.K.; Ramanan, A. Hydrolytically stable ZIF-8@PDMS core–shell microspheres for gas–solid chromatographic separation. RSC Adv. 2016, 6, 13426–13432. [Google Scholar] [CrossRef]
- Férey, G.; Serre, C. Large breathing effects in three-dimensional porous hybrid matter: Facts, analyses, rules, and consequences. Chem. Soc. Rev. 2009, 38, 1380. [Google Scholar] [CrossRef]
- Grape, E.S.; Xu, H.; Cheung, O.; Calmels, M.; Zhao, J.; Dejoie, C.; Proserpio, D.M.; Zou, X.; Inge, A.K. Breathing metal–organic framework based on flexible inorganic building units. Cryst. Growth Des. 2019, 20, 320–329. [Google Scholar] [CrossRef]
- Chang, Z.; Yang, D.-H.; Xu, J.; Hu, T.-L.; Bu, X.-H. Flexible metal-organic frameworks: Recent advances and potential applications. Adv. Mater. 2015, 27, 5432–5441. [Google Scholar] [CrossRef]
- Saifutdinov, B.R.; Isaeva, V.I.; Alexandrov, E.V.; Kustov, L.M. Study of selective adsorption of aromatic compounds from solutions by the flexible MIL-53(Al) metal-organic framework. Russ. Chem. Bull. 2015, 64, 1039–1048. [Google Scholar] [CrossRef]
- Yang, C.-X.; Liu, S.-S.; Wang, H.-F.; Wang, S.-W.; Yan, X.-P. High-performance liquid chromatographic separation of position isomers using metal–organic framework MIL-53(Al) as the stationary phase. Analyst 2012, 137, 133–139. [Google Scholar] [CrossRef]
- Liu, S.-S.; Yang, C.-X.; Wang, S.-W.; Yan, X.-P. Metal–organic frameworks for reverse-phase high-performance liquid chromatography. Analyst 2012, 137, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Chui, S.S. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.J.; Dincă, M.; Long, J.R. Hydrogen storage in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Wong-Foy, A.; Matzger, A.J. Microporous coordination polymers as selective sorbents for liquid chromatography. Langmuir 2009, 25, 11977–11979. [Google Scholar] [CrossRef]
- Ameloot, R.; Liekens, A.; Alaerts, L.; Maes, M.; Galarneau, A.; Coq, B.; Desmet, G.; Sels, B.F.; Denayer, J.F.M.; de Vos, D.E. Silica-MOF composites as a stationary phase in liquid chromatography. Eur. J. Inorg. Chem. 2010, 2010, 3735–3738. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Pino, V.; Ayala, J.H.; Pasan, J.; Ruiz-Perez, C.; Afonso, A.M. A magnetic-based dispersive micro-solid-phase extraction method using the metal-organic framework HKUST-1 and ultra-high-performance liquid chromatography with fluorescence detection for determining polycyclic aromatic hydrocarbons in waters and fruit tea infusions. J. Chromatogr. A 2016, 1436, 42–50. [Google Scholar] [CrossRef]
- Granato, T.; Testa, F.; Olivo, R. Catalytic activity of HKUST-1 coated on ceramic foam. Microporous Mesoporous Mater. 2012, 153, 236–246. [Google Scholar] [CrossRef]
- Jiang, J.; Yaghi, O.M. Brønsted acidity in metal–organic frameworks. Chem. Rev. 2015, 115, 6966–6997. [Google Scholar] [CrossRef]
- Ahmed, A.; Hodgson, N.; Barrow, M.; Clowes, R.; Robertson, C.M.; Steiner, A.; McKeown, P.; Bradshaw, D.; Myers, P.; Zhang, H. Macroporous metal–organic framework microparticles with improved liquid phase separation. J. Mater. Chem. A 2014, 2, 9085–9090. [Google Scholar] [CrossRef]
- Ahmed, A.; Forster, M.; Clowes, R.; Myers, P.; Zhang, H. Hierarchical porous metal–organic framework monoliths. Chem. Commun. 2014, 50, 14314–14316. [Google Scholar] [CrossRef] [Green Version]
- Loiseau, T.; Serre, C.; Huguenard, C.; Fink, G.; Taulelle, F.; Henry, M.; Bataille, T.; Férey, G. A Rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration. Chem. A Eur. J. 2004, 10, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Ghoufi, A.; Benhamed, K.; Boukli-Hacene, L.; Maurin, G. Electrically induced breathing of the MIL-53(Cr) metal-organic framework electrically induced breathing of the MIL-53(Cr) metal–organic framework. ACS Central Sci. 2017, 3, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Knebel, A.; Zhou, C.; Huang, H.; Zhang, J.; Kustov, L.M.; Caro, J. Smart metal-organic frameworks (MOFs): Switching gas permeation through MOF membranes by external stimuli. Chem. Eng. Technol. 2018, 41, 224–234. [Google Scholar] [CrossRef]
- Getzschmann, J.; Senkovska, I.; Wallacher, D.; Tovar, M.; Fairen-Jimenez, D.; Düren, T.; van Baten, J.; Krishna, R.; Kaskel, S. Methane storage mechanism in the metal-organic framework Cu3(btc)2: An in situ neutron diffraction study. Microporous Mesoporous Mater. 2010, 136, 50–58. [Google Scholar] [CrossRef]
- Worrall, S.D.; Bissett, M.A.; Hill, P.I.; Rooney, A.; Haigh, S.J.; Attfield, M.P.; Dryfe, R.A. Metal-organic framework templated electrodeposition of functional gold nanostructures. Electrochim. Acta 2016, 222, 361–369. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, X.; Lester, E.; Wu, T. High efficiency synthesis of HKUST-1 under mild conditions with high BET surface area and CO2 uptake capacity. Prog. Nat. Sci. 2018, 28, 584–589. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Tarasov, A.L.; Chernyshev, V.V.; Kustov, L.M. Control of morphology and size of microporous framework MIL-53(Al) crystals by synthesis procedure. Mendeleev Commun. 2015, 25, 466–467. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y.; Lester, E.; Wu, T. Optimized synthesis of nano-scale high quality HKUST-1 under mild conditions and its application in CO2 capture. Microporous Mesoporous Mater. 2018, 270, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Schlichte, K.; Kratzke, T.; Kaskel, S. Improved synthesis, thermal stability, and catalytic properties of the metal-organic framework compound Cu3(BTC)2. Microporous Mesoporous Mater. 2004, 73, 81–88. [Google Scholar] [CrossRef]
- Alaerts, L.; Séguin, E.; Poelman, H.; Thibault-Starzyk, F.; Jacobs, P.A.; De Vos, D.E. Probing the Lewis acidity and catalytic activity of the metal–organic framework [Cu3(btc)2] (BTC=Benzene-1,3,5-tricarboxylate). Chem. A Eur. J. 2006, 12, 7353–7363. [Google Scholar] [CrossRef]
- Dathe, H.; Peringer, E.; Roberts, V.; Jentys, A.; Lercher, J.A. Metal organic frameworks based on Cu2+ and benzene-1,3,5-tricarboxylate as host for SO2 trapping agents. Comptes Rendus Chim. 2005, 8, 753–763. [Google Scholar] [CrossRef]
- Majano, G.; Pérez-Ramírez, J. Room temperature synthesis and size control of HKUST-1. Helvetica Chim. Acta 2012, 95, 2278–2286. [Google Scholar] [CrossRef]
- Krawiec, P.; Kramer, M.; Sabo, M.; Kunschke, R.; Fröde, H.; Kaskel, S. Improved hydrogen storage in the metal-organic framework Cu3(BTC)2. Adv. Eng. Mater. 2006, 8, 293–296. [Google Scholar] [CrossRef]
- Hartmann, M.; Kunz, S.; Himsl, D.; Tangermann, O.; Ernst, S.; Wagener, A. Adsorptive separation of isobutene and isobutane on Cu3(BTC)2. Langmuir 2008, 24, 8634–8642. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.; Bikkina, C.; Meister, D.; Dreisbach, F.; Gumma, S. Comparison of adsorption isotherms on Cu-BTC metal organic frameworks synthesized from different routes. Microporous Mesoporous Mater. 2009, 117, 406–413. [Google Scholar] [CrossRef]
- Seo, Y.-K.; Hundal, G.; Jang, I.T.; Hwang, Y.K.; Jun, C.-H.; Chang, R. Microwave synthesis of hybrid inorganic–organic materials including porous Cu3(BTC)2 from Cu (II)-trimesate mixture. Microporous Mesoporous Mater. 2009, 119, 331–337. [Google Scholar] [CrossRef]
- Ranft, A.; Betzler, S.B.; Haase, F.; Lotsch, B.V. Additive-mediated size control of MOF nanoparticles. CrystEngComm 2013, 15, 9296. [Google Scholar] [CrossRef] [Green Version]
- Isaeva, V.I.; Chernyshev, V.V.; Sokolova, N.A.; Kapustin, G.I. Modifying the hydrophobic properties of metal–organic framework HKUST-1. Russ. J. Phys. Chem. A 2018, 92, 2391–2395. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Chernyshev, V.V.; Tarasov, A.L.; Lobova, A.A.; Kapustin, G.I.; Davshan, N.A. Conditions for the formation of microporous metal–organic framework Mil-53(Al). Russ. J. Phys. Chem. A 2018, 92, 2386–2390. [Google Scholar] [CrossRef]
- Klinowski, J.; Paz, F.A.A.; Silva, P.; Rocha, J. Microwave-assisted synthesis of metal–organic frameworks. Dalton Trans. 2011, 40, 321–330. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Kustov, L.M. Microwave activation as an alternative production of metal-organic frameworks. Russ. Chem. Bull. 2016, 65, 2103–2114. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, C.; Wang, Y.; Sun, J.; Gao, Y.-A. Encapsulation of an ionic liquid into the nanopores of a 3D covalent organic framework. RSC Adv. 2017, 7, 1697–1700. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.-L.; Deng, W.; Chen, H.; Han, H.-L.; Taylor, J.M.; Wan, C.-Q.; Xu, G. A Metal-organic framework impregnated with a binary ionic liquid for safe proton conduction above 100 °C. Chem. A Eur. J. 2016, 23, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-J.; Chen, F. Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef]
- Kitchen, H.J.; Vallance, S.R.; Kennedy, J.L.; Tapia-Ruiz, N.; Carassiti, L.; Harrison, A.; Whittaker, A.G.; Drysdale, T.D.; Kingman, S.; Gregory, D.H. ChemInform abstract: Modern microwave methods in solid-state inorganic materials chemistry: From fundamentals to manufacturing. Chem. Rev. 2014, 45. [Google Scholar] [CrossRef]
- Jhung, S.H.; Jin, T.; Hwang, Y.K.; Chang, R. Microwave effect in the fast synthesis of microporous materials: Which stage between nucleation and crystal growth is accelerated by microwave irradiation? Chem. A Eur. J. 2007, 13, 4410–4417. [Google Scholar] [CrossRef]
- Seetharaj, R.; Vandana, P.; Arya, P.; Mathew, S. Dependence of solvents, pH, molar ratio, and temperature in tuning metal organic framework architecture. Arab. J. Chem. 2019, 12, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Saifutdinov, B.R.; Konnova, M.E.; Isaeva, V.I.; Il’in, M.M.; Kustov, L.M. Thermodynamics of adsorption of aromatic compounds from non-aqueous solutions by MIL-53(Al) metal-organic framework Thermodynamics of adsorption of aromatic compounds from non-aqueous solutions by MIL-53(Al) metal-organic framework. Russ. Chem. Bull. 2017, 66, 16–22. [Google Scholar] [CrossRef]
- Gritti, F.; Kazakevich, Y.; Guiochon, G. Measurement of hold-up volumes in reverse-phase liquid chromatography. J. Chromatogr. A 2007, 1161, 157–169. [Google Scholar] [CrossRef]
- Davankov, V.A.; Tsyurupa, M.P. Hypercross-Linked Polymeric Networks and Adsorbing Materials: Synthesis, Properties, Structure, and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2010; p. 648. [Google Scholar]
- Saifutdinov, B.R. On the interrelation between the distribution constant and the retention factor in liquid chromatography. Russ. J. Phys. Chem. A 2013, 87, 512–515. [Google Scholar] [CrossRef]
- Saifutdinov, B.R.; Buryak, A.K. The influence of water–acetonitrile solvent composition on thermodynamic characteristics of adsorption of aromatic heterocycles on octadecyl-bonded silica gels. Coll. J. 2019, 81, 754–761. [Google Scholar] [CrossRef]
- Saifutdinov, B.R.; Davankov, V.A.; Il’In, M.M.; Tsyurupa, M.P.; Blinnikova, Z.K. Selective adsorption of organic compounds from solutions on hyper-cross-linked polystyrenes with ultimate degrees of cross linking. Prot. Met. Phys. Chem. Surf. 2015, 51, 957–963. [Google Scholar] [CrossRef]
- Saifutdinov, B.R.; Buryak, A.K. Thermodynamics of the adsorption of isomeric dipyridyls and their derivatives from water–organic solutions on HYPERCARB™ porous graphitic carbon. Russ. J. Phys. Chem. A 2019, 93, 1796–1803. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal−organic frameworks. J. Am. Chem. Soc. 2006, 128, 1304–1315. [Google Scholar] [CrossRef]
- Dombrowski, R.J.; Lastoskie, C.M. A Two-stage Horvath-Kawazoe adsorption model for pore size distribution analysis. Stud. Surf. Sci. Catal. 2002, 144, 99–106. [Google Scholar] [CrossRef]
- Kachala, V.V.; Khemchyan, L.L.; Kashin, A.S.; Orlov, N.V.; A Grachev, A.; Zalesskiy, S.S.; Ananikov, V.P. Target-oriented analysis of gaseous, liquid, and solid chemical systems by mass spectrometry, nuclear magnetic resonance spectroscopy and electron microscopy. Russ. Chem. Rev. 2013, 82, 648–685. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds HKUST-1solv.1, HKUST-1solv.2 and HKUST-1mw are available from the authors. |






| Entry | Adsorbate | Structural Formula | Henry Constants of Adsorption K1,c, μL/m2 | |
|---|---|---|---|---|
| MeOH | MeCN | |||
| 1 | 4-Chlorophenol | 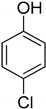 | 0.286 | 2.990 |
| 2 | 4-Chloro-2-methylphenol | 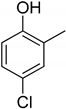 | 0.234 | 0.343 |
| 3 | 3,5-Dimethylphenol | 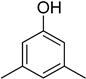 | 0.055 | 0.394 |
| 4 | 2,4-Dichloro-3,5-dimethylphenol |  | 0.215 | 0.335 |
| 5 | 2,3-Dichlorophenol |  | 0.087 | 0.766 |
| 6 | 2,4-Dichlorophenol | 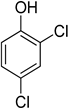 | 0.249 | 0.954 |
| 7 | 2,5-Dichlorophenol | 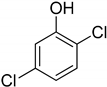 | 0.263 | 0.201 |
| 8 | 2,6-Dichlorophenol | 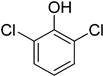 | 0.123 | 0.517 |
| 9 | 3,4-Dichlorophenol |  | 0.266 | 0.962 |
| 10 | 3,5-Dichlorophenol | 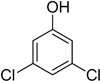 | 0.217 | 0.505 |
| 11 | 2,3,5-Trichlorophenol |  | 0.201 | 0.763 |
| 12 | 2,3,6-Trichlorophenol |  | 0.236 | 0.398 |
| 13 | 2,4,5-Trichlorophenol | 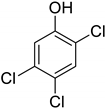 | 0.240 | 0.432 |
| 14 | 2,4,6-Trichlorophenol | 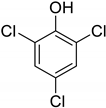 | 0.186 | 0.425 |
| 15 | 3-Nitrophenol | 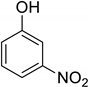 | 0.190 | 1.012 |
| 16 | 4-Nitrophenol | 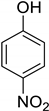 | 0.245 | 3.210 |
| 17 | 2,4-Dibromophenol | 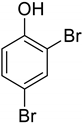 | 0.098 | 0.914 |
| 18 | Tetracycline |  | 0.518 | 0.271 |
| 19 | Tropic acid |  | 0.120 | 0.287 |
| 20 | Ibuprofen | 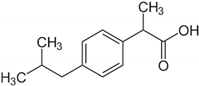 | 0.078 | 0.236 |
| 21 | Paracetamol | 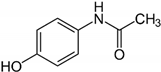 | 0.081 | 1.643 |
| 22 | Diphenylamine | 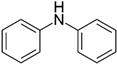 | 0.086 | 7.408 |
| 23 | Nicotin | 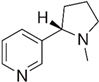 | 2.583 | 0.183 |
| 24 | Benzotriazole | 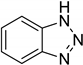 | 1.137 | 0.285 |
| Material | SBET m2/g | Vtotal, a cm3/g | Vmicro, b cm3/g | Vmeso, c cm3/g | Pore Width, nm |
|---|---|---|---|---|---|
| HKUST-1mw | 1617 | 0.767 | 0.679 | 0.088 | 0.4–0.8 |
| HKUST-1mw 20 atm press | 1613 | 0.778 | 0.695 | 0.083 | 0.4–0.8 |
| HKUST-1solv.1 | 1648 | 0.816 | 0.714 | 0.102 | 0.6–0.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isaeva, V.I.; Saifutdinov, B.R.; Chernyshev, V.V.; Vergun, V.V.; Kapustin, G.I.; Kurnysheva, Y.P.; Ilyin, M.M.; Kustov, L.M. Impact of the Preparation Procedure on the Performance of the Microporous HKUST-1 Metal-Organic Framework in the Liquid-Phase Separation of Aromatic Compounds. Molecules 2020, 25, 2648. https://doi.org/10.3390/molecules25112648
Isaeva VI, Saifutdinov BR, Chernyshev VV, Vergun VV, Kapustin GI, Kurnysheva YP, Ilyin MM, Kustov LM. Impact of the Preparation Procedure on the Performance of the Microporous HKUST-1 Metal-Organic Framework in the Liquid-Phase Separation of Aromatic Compounds. Molecules. 2020; 25(11):2648. https://doi.org/10.3390/molecules25112648
Chicago/Turabian StyleIsaeva, Vera I., Bulat R. Saifutdinov, Vladimir V. Chernyshev, Vadim V. Vergun, Gennady I. Kapustin, Yulia P. Kurnysheva, Mikhail M. Ilyin, and Leonid M. Kustov. 2020. "Impact of the Preparation Procedure on the Performance of the Microporous HKUST-1 Metal-Organic Framework in the Liquid-Phase Separation of Aromatic Compounds" Molecules 25, no. 11: 2648. https://doi.org/10.3390/molecules25112648
APA StyleIsaeva, V. I., Saifutdinov, B. R., Chernyshev, V. V., Vergun, V. V., Kapustin, G. I., Kurnysheva, Y. P., Ilyin, M. M., & Kustov, L. M. (2020). Impact of the Preparation Procedure on the Performance of the Microporous HKUST-1 Metal-Organic Framework in the Liquid-Phase Separation of Aromatic Compounds. Molecules, 25(11), 2648. https://doi.org/10.3390/molecules25112648






