Targeting Oxidative Stress for Disease Prevention and Therapy: Where Do We Stand, and Where Do We Go from Here
Abstract
1. Introduction
2. Oxidative Stress Biomarkers: Which One to Use?
3. Oxidative Stress Assessment: Limits and Challenges
3.1. Preanalytical Issues
- sample collection (e.g., biological sample type, collection tube anticoagulant, daytime of withdrawal),
- sample handling (e.g., temperature, time from collection to laboratory delivery, delay before assaying),
- sample processing (e.g., centrifugation, hemolyzed samples),
- sample short- and long-storage (e.g., time and temperature) and stability (e.g., freeze-thaw cycles).
3.2. Analytical Issues
3.3. Postanalytical Issues
4. Antioxidant Supplementation: Challenges in the Interpretation of Results
5. Cardiovascular Disease Drugs with Antioxidant Properties as an Example for Shared Disease Pathways and Common Pharmacological Prevention
6. Towards Genetic-Based Approaches to Target Oxidative Stress: Endogenous Perspective and Epigenetic MiRNA-Based Therapeutic Response
6.1. Endogenous Stress: Genome Instability, Antioxidants vs. Oxidation Therapy
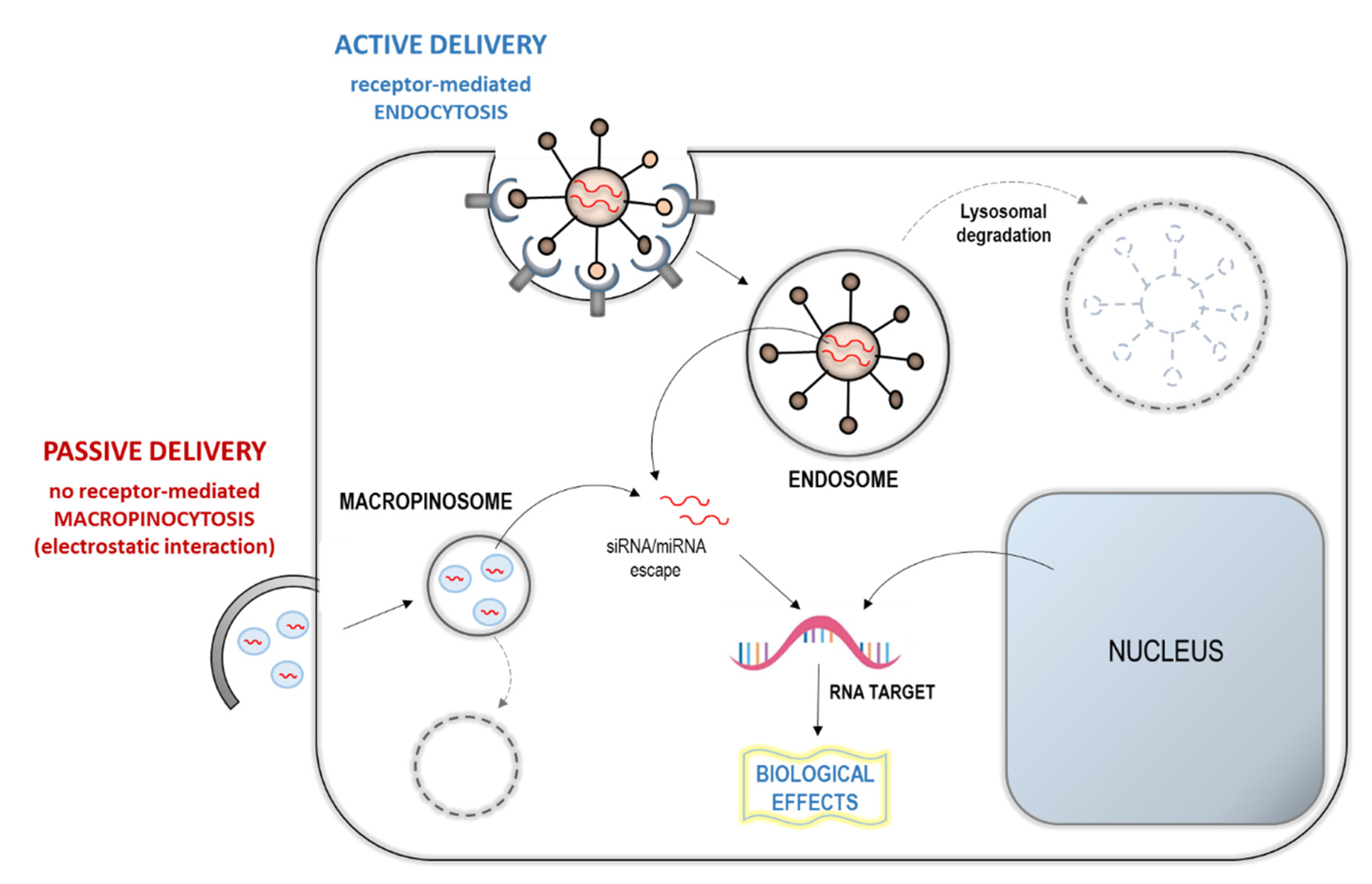
6.2. Epigenetic MiRNA-Based Approach
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Masoudkabir, F.; Sarrafzadegan, N.; Gotay, C.; Ignaszewski, A.; Krahn, A.D.; Davis, M.K.; Franco, C.; Mani, A. Cardiovascular disease and cancer: Evidence for shared disease pathways and pharmacologic prevention. Atherosclerosis 2017, 263, 343–351. [Google Scholar] [CrossRef]
- Kowalska, M.; Wize, K.; Prendecki, M.; Lianeri, M.; Kozubski, W.; Dorszewska, J. Genetic variants and oxidative stress in Alzheimer’s disease [published online ahead of print, 2020 Feb 24]. Curr. Alzheimer Res. 2020. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Ann. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Barančík, M.; Grešová, L.; Barteková, M.; Dovinová, I. Nrf2 as a key player of redox regulation in cardiovascular diseases. Physiol. Res. 2016, 65, S1–S10. [Google Scholar] [CrossRef]
- Gaggini, M.; Sabatino, L.; Vassalle, C. Conventional and innovative methods to assess oxidative stress biomarkers in the clinical cardiovascular setting. Biotechniques 2020. [Google Scholar] [CrossRef]
- Pingitore, A.; Lima, G.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef]
- Sporn, M.B.; Liby, K.T. NRF2 and cancer: The good, the bad and the importance of context. Nat. Rev. Cancer 2012, 12, 564–571. [Google Scholar] [CrossRef]
- Vassalle, C. Oxidative stress and cardiovascular risk prediction: The long way towards a radical perspective. Int. J. Cardiol. 2018, 273, 252–253. [Google Scholar]
- Sánchez-Rodríguez, M.A.; Mendoza-Núñez, V.M. Oxidative Stress Indexes for Diagnosis of Health or Disease in Humans. Oxid. Med. Cell. Longev. 2019, 2019, 4128152. [Google Scholar] [CrossRef]
- Cutler, R.G. Oxidative stress profiling: Part I. Its potential importance in the optimization of human health. Ann. N. Y. Acad. Sci. 2005, 1055, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Cutler, R.G.; Plummer, J.; Chowdhury, K.; Heward, C. Oxidative stress profiling: Part II. Theory, technology, and practice. Ann. N. Y. Acad. Sci. 2005, 1055, 136–158. [Google Scholar] [CrossRef] [PubMed]
- Condezo-Hoyos, L.; Rubio, M.; Arribas, S.M.; España-Caparrós, G.; Rodríguez-Rodríguez, P.; Mujica-Pacheco, E.; González, M.C. A plasma oxidative stress global index in early stages of chronic venous insufficiency. J. Vasc. Surg. 2013, 57, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, C.; Piaggi, P.; Weltman, N.; Prontera, C.; Garbella, E.; Menicucci, D.; Lubrano, V.; Piarulli, A.; Castagnini, C.; Passera, M.; et al. Innovative approach to interpret the variability of biomarkers after ultra-endurance exercise: The multifactorial analysis. Biomark. Med. 2014, 8, 881–891. [Google Scholar] [CrossRef]
- Xuan, Y.; Gào, X.; Holleczek, B.; Brenner, H.; Schöttker, B. With urinary biomarkers of oxidative stress: Results from a large cohort study. Int. J. Cardiol. 2018, 273, 223–229. [Google Scholar] [CrossRef]
- Abbasi, A.; Corpeleijn, E.; Postmus, D.; Gansevoort, R.T.; de Jong, P.E.; Gans, R.O.; Struck, J.; Schulte, J.; Hillege, H.L.; van der, H.P.; et al. Peroxiredoxin 4, a novel circulating biomarker for oxidative stress and the risk of incident cardiovascular disease and all-cause mortality. J. Am. Heart Assoc. 2012, 1, e002956. [Google Scholar] [CrossRef]
- Gerrits, E.G.; Alkhalaf, A.; Landman, G.W.D.; Hateren, K.J.J.; Groenier, K.H.; Struck, J. Serum Peroxiredoxin 4: A Marker of Oxidative Stress Associated with Mortality in Type 2 Diabetes (ZODIAC-28). PLoS ONE 2014, 9, e89719. [Google Scholar] [CrossRef]
- Ialongo, C. Preanalytic of total antioxidant capacity assays performed in serum, plasma, urine and saliva. Clin. Biochem. 2017, 50, 356–363. [Google Scholar] [CrossRef]
- Engelmann, M.D.; Bobier, R.T.; Hiatt, T.; Cheng, I.F. Variability of the Fenton reaction characteristics of the EDTA, DTPA, and citrate complexes of iron. Biometals 2003, 16, 519–527. [Google Scholar] [CrossRef]
- Vassalle, C.; Boni, C.; Di Cecco, P.; Ndreu, R.; Zucchelli, G.C. Automation and validation of a fast method for the assessment of in vivo oxidative stress levels. Clin. Chem. Lab. Med. 2006, 44, 1372–1375. [Google Scholar] [CrossRef]
- Jansen, E.H.J.M.; Beekhof, P.K.; Viezeliene, D.; Muzakova, V.; Skalicky, J. Long term stability of cancer biomarkers of oxidative stress, redox status, homocysteine, CRP and liver enzymes in human serum. Biomark. Med. 2015, 9, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, P. Environmental risk factors and their footprints in vivo—A proposal for the classification of oxidative stress biomarkers. Redox Biol. 2020, 101442. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; Lodovici, M. Circulating Oxidative Stress Biomarkers in Clinical Studies on Type 2 Diabetes and Its Complications. Oxid. Med. Cell. Longev. 2019, 5953685. [Google Scholar] [CrossRef] [PubMed]
- García-Blanco, A.; Baquero, M.; Vento, M.; Gil, E.; Bataller, L.; Cháfer-Pericás, C. Potential oxidative stress biomarkers of mild cognitive impairment due to Alzheimer disease. J. Neurol. Sci. 2017, 3, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Carioca, A.A.F.; Verde, S.M.M.L.; Luzia, L.; Rondo, P.R.D.C.; Latorre, M.R.D.; Ellery, T.H.D.P.; Damasceno, N.R.T. Association of oxidative stress biomarkers with adiposity and clinical staging in women with breast cancer. Eur. J. Clin. Nutr. 2015, 69, 1256–1261. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde [MDA] and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Seljeskog, E.; Hervig, T.; Mansoor, M.A. A novel HPLC method for the measurement of thiobarbituric acid reactive substances (TBARS). A comparison with a commercially available kit. Clin. Biochem. 2006, 39, 947–954. [Google Scholar] [CrossRef]
- Ekström, T.; Garberg, P.; Egestad, B.; Högberg, J. Recovery of malondialdehyde in urine as a 2,4-dinitrophenylhydrazine derivative analyzed with high-performance liquid chromatography. Chem. Biol. Interact. 1988, 66, 177–187. [Google Scholar] [CrossRef]
- Ruan, E.D.; Aalhus, J.; Juárez, M. A rapid, sensitive and solvent-less method for determination of malonaldehyde in meat by stir bar sorptive extraction coupled thermal desorption and gas chromatography/mass spectrometry with in-situ derivatization. Rapid Commun. Mass Spectrom. 2014, 28, 2723–2728. [Google Scholar] [CrossRef]
- Sobsey, C.A.; Han, J.; Lin, K.; Swardfager, W.; Levitt, A.; Borchers, C.H. Development and evaluation of a liquid chromatography-mass spectrometry method for rapid, accurate quantitation of malondialdehyde in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1029, 205–212. [Google Scholar] [CrossRef]
- Spickett, C.M.; Wiswedel, I.; Siems, W.; Zarkovic, K.; Zarkovic, N. Advances in methods for the determination of biologically relevant lipid peroxidation products. Free Radic. Res. 2010, 44, 1172–1202. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, M.; Li, C.; Jiang, X.; Su, Y.; Zhang, Y. Benefits of Vitamins in the Treatment of Parkinson’s Disease. Oxid. Med. Cell. Longev. 2019, 2019, 9426867. [Google Scholar] [CrossRef] [PubMed]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Antioxidants in Cardiovascular Therapy: Panacea or False Hope? Front. Cardiovasc. Med. 2015, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative stress in alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2020, 49, 102294. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; De Vasconcelos, A.S.; Vilhena, T.D.C.; Da Silva, T.L.; Barbosa, A.D.S.; Gomes, A.R.Q.; Dolabela, M.F.; Percario, S. Oxidative Stress in Alzheimer’s Disease: Should We Keep Trying Antioxidant Therapies? Cell. Mol. Neurobiol. 2015, 35, 595–614. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Role of Vitamin E in the Treatment of Alzheimer’s Disease: Evidence from Animal Models. Int. J. Mol. Sci. 2017, 18, 2504. [Google Scholar] [CrossRef]
- Browne, D.; Mc Guinness, B.; Woodside, J.V.; Mc Kay, G.J. Vitamin E and Alzheimer’s disease: What do we know so far? Clin. Interv. Aging 2019, 14, 1303–1317. [Google Scholar] [CrossRef]
- Katsiki, N.; Manes, C. Is there a role for supplemented antioxidants in the prevention of atherosclerosis? Clin. Nutr. 2009, 28, 3–9. [Google Scholar] [CrossRef]
- Enstrom, J.E. Vitamin C intake and mortality among a sample of the United States population. Epidemiology 1992, 3, 194–202. [Google Scholar] [CrossRef]
- Kritharides, L.; Stocker, R. The use of antioxidant supplements in coronary heart disease. Atherosclerosis 2002, 164, 211–219. [Google Scholar] [CrossRef]
- Abudu, N.; Miller, J.J.; Attaelmannan, M.; Levinson, S.S. Vitamins in human arteriosclerosis with emphasis on vitamin C and vitamin E. Clin. Chim. Acta 2004, 339, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A. Vitamin E and the risk of prostate cancer: The selenium and vitamin E cancer prevention trial (SELECT). J. Am. Med. Assoc. 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Chatzianagnostou, K.; Del Turco, S.; Pingitore, A.; Sabatino, L.; Vassalle, C. The Mediterranean Lifestyle as a Non-Pharmacological and Natural Antioxidant for Healthy Aging. Antioxidants 2015, 4, 719–736. [Google Scholar] [CrossRef] [PubMed]
- David, J.A.; Rifkin, W.J.; Rabbani, P.S.; Ceradini, D.J. The Nrf2/Keap1/ARE Pathway and Oxidative Stress as a Thepareutic target in Type II Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 04826724. [Google Scholar] [CrossRef]
- Bhakkiyalakshmi, E.; Sireesh, D.; Rajaguru, P.; Paulmurugan, R.; Ramkumar, K.M. The emerging role of redox-sensitive nrf2-keap1 pathway in diabetes. Pharmacol. Res. 2015, 91, 104–114. [Google Scholar] [CrossRef]
- Asgharzadeh, F.; Hashemzehi, M.; Marjaneh, R.M.; Hassanian, S.M.; Ferns, G.A.; Khazaei, M.; Avan, A. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers as therapeutic options in the treatment of renal cancer: A meta-analysis. Life Sci. 2019, 242, 117181. [Google Scholar] [CrossRef]
- Wassmann, S.; Laufs, U.; Bäumer, A.T.; Müller, K.; Konkol, C.; Sauer, H.; Böhm, M.; Nickenig, G. Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells, involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol. Pharmacol. 2001, 59, 646–654. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243, (published correction appears in Circ Res. 2018, 123, e20. [Google Scholar] [CrossRef]
- Carrizzo, A.; Forte, M.; Lembo, M.; Formisano, L.; Puca, A.A.; Vecchione, C. Rac-1 as a new therapeutic target in cerebro- and cardio-vascular diseases. Curr. Drug Targets 2014, 15, 1231–1246. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kostantinou, A.; Kougias, M.; Kazazis, C. Statins and cancer. Anticancer Agents Med. Chem. 2014, 14, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Göbel, A.; Rauner, M.; Hofbauer, L.C.; Rachner, T.D. Cholesterol and beyond—The role of the mevalonate pathway in cancer biology. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188351. [Google Scholar] [CrossRef] [PubMed]
- Saeedi Saravi, S.S.; Saeedi Saravi, S.S.; Arefidoust, A.; Dehpour, A.R. The beneficial effects of HMG-CoA reductase inhibitors in the processes of neurodegeneration. Metab. Brain Dis. 2017, 32, 949–965. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Lindahl, T. Repair and Genetic Consequences of Endogenous DNA Base Damage in Mammalian Cells. Ann. Rev. Genet. 2004, 38, 445–476. [Google Scholar] [CrossRef]
- Sagai, M.; Bocci, V. Mechanisms of Action Involved in Ozone Therapy: Is healing induced via a mild oxidative stress? Med. Gas Res. 2011, 1, 29. [Google Scholar] [CrossRef]
- Bowie, A.; O’Neill, L.A.J. Oxidative Stress and Nuclear Factor-kB Activation. A reassessment of the evidence in the light of recent discoveries. Biochem. Pharmacol. 2000, 59, 3–23. [Google Scholar] [CrossRef]
- Whiteside, S.T.; Israel, A. IkB proteins: Structure, function and regulation. Cancer Biol. 1997, 8, 75–82. [Google Scholar] [CrossRef]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef]
- Schmidt, K.N.; Amstad, P.; Cerutti, P.; Baeuerle, P.A. The roles of hydrogen peroxide and superoxide as messengers in the activation of transcription factor N F-KB. Chem. Biol. 1995, 2, 13–22. [Google Scholar] [CrossRef]
- Qiang, M. Role of Nrf2 in Oxidative Stress and Toxicity. Ann. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar]
- Hu, L.; Magesh, S.; Chen, L.; Wang, L.; Lewis, T.A.; Chen, Y.; Shen, J. Discovery of a small-molecule inhibitor and cellular probe of Keap1-Nrf2 protein-protein interaction. Bioorg. Med. Chem. Lett. 2013, 23, 3039–3043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Su, Z.Y.; Khor, T.O.; Shu, L.; Kong, A.N. Sulforaphane enhances Nrf2 expression in prostate cancer TRAMP C1 cells through epigenetic regulation. Biochem. Pharmacol. 2013, 85, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Pergola, P.E.; Raskin, P.; Toto, R.D.; Meyer, C.J.; Huff, J.W.; Grossman, E.B.; Krauth, M.; Ruiz, S.; Audhya, P.; Christ-Schmidt, H.; et al. Bardoxolone Methyl and Kidney Function in CKD with Type 2 Diabetes. N. Engl. J. Med. 2011, 365, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Kebede, A.F.; Schneider, R.; Daujat, S. Novel types and sites of histone modifications emerge as players in the transcriptional regulation contest. FEBS J. 2015, 282, 1658–1674. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharm. 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Baker, J.R.; Vuppusetty, C.; Colley, T.; Papaioannou, A.I.; Fenwick, P.; Donnelly, L.; Ito, K.; Barnes, P.J. Oxidative stress dependent microRNA-34a activation via PI3Kα reduces the expression of sirtuin-1 and sirtuin-6 in epithelial cells. Sci. Rep. 2016, 6, 35871. [Google Scholar] [CrossRef]
- Büssing, I.; Slack, F.J.; Großhans, H. Let-7 microRNAs in development, stem cells and cancer. Trends Mol. Med. 2008, 14, 400–409. [Google Scholar] [CrossRef]
- Zhang, W.C. microRNAs Tune Oxidative Stress in Cancer Therapeutic Tolerance and Resistance. Int. J. Mol. Sci. 2019, 20, 6094. [Google Scholar] [CrossRef]
- He, J.; Jiang, B. Interplay between Reactive Oxygen Species and MicroRNAs in Cancer. Curr. Pharmacol. Rep. 2016, 2, 82–90. [Google Scholar] [CrossRef]
- Engedal, N.; Žerovnik, E.; Rudov, A.; Galli, F.; Olivieri, F.; Procopio, A.D.; Rippo, M.R.; Monsurrò, V.; Betti, M.; Albertini, M.C. From Oxidative Stress Damage to Pathways, Networks, and Autophagy via MicroRNAs. Oxid. Med. Cell. Longev. 2018, 2018, 4968321. [Google Scholar] [CrossRef]
- De Guire, V.; Robitaille, R.; Tetreault, N.; Guerin, R.; Menard, C.; Bambace, N.; Sapieha, P. Circulating miRNAs as sensitive and specific biomarkers for the diagnosis and monitoring of human diseases: Promises and challenges. Clin. Biochem. 2013, 46, 846–860. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, E.; Purcell, A.L.; Levin, A.A. Developing microRNA therapeutics. Circ. Res. 2012, 110, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.C.; Rossi, J.J. RNA-based therapeutics: Current progress and future prospects. Chem. Biol. 2012, 19, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, A.; Pirtoli, L.; Giordano, A.; Dotta, F. Crosstalk between MicroRNA and Oxidative Stress in Physiology and Pathology. Int. J. Mol. Sci. 2020, 21, 1270. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.P.; Lau, N.C.; Garrett-Engele, P.; Grimson, A.; Schelter, J.M.; Castle, J.; Bartel, D.P.; Linsley, P.S.; Johnson, J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005, 433, 769–773. [Google Scholar] [CrossRef]
- Nussinov, R.; Tsai, C.J.; Jang, H. A New View of Pathway-Driven Drug Resistance in Tumor Proliferation. Trends Pharmacol. Sci. 2017, 38, 427–437. [Google Scholar] [CrossRef]
- Haussecker, D. Current issues of RNAi therapeutics delivery and development. J. Control. Release 2014, 195, 49–54. [Google Scholar] [CrossRef]
- Peer, D.; Lieberman, J. Special delivery: Targeted therapy with small RNAs. Gene Ther. 2011, 18, 1127–1133. [Google Scholar] [CrossRef]
- Fuchs-Tarlovsky, V. Role of antioxidants in cancer therapy. Nutrition 2013, 29, 15–21. [Google Scholar] [CrossRef]


| Steps in OxS Assesment | Main Points and Advices |
|---|---|
| Biomarker selection | select the most possible adequate analyte/s (single versus panel) for the population/setting investigated |
| consider distribution volume/metabolism/clearance of the biomarker/s | |
| Test selection | select the best assay/method for the population/setting investigated |
| Population | select the appropriate population (general population, patients) according to: |
| clinical setting (screening, diagnosis, prognosis, monitoring, treatment) | |
| athophysiology (diseases risk, diagnosis, stage) | |
| Sample collection | select biological sample (e.g., blood, urine, saliva) |
| select anticoagulant and addition of stabilizers | |
| consider subject posture | |
| consider circadian rhythm (withdrawal time) | |
| fasting status | |
| Sample transport and processin | sample handling (prompt transport, temperature, time) |
| centrifugation modalities (rpm, temperature) | |
| prompt aliquot preparation for tests non immediately assayed | |
| Sample storage | at −20 °C, best −80 °C |
| avoid freeze-thaw cycles | |
| consider possible sample alterations with long storage time | |
| Sample testing | evaluate additional steps (e.g., deproteinization, extraction/derivatization) |
| assay specificity/sensibility | |
| evaluate presence of hemolysis, high lipid content | |
| consider assay/method agreement | |
| select “one spot” versus serial assessment | |
| Result interpretation | availability of reference values/cut-off |
| knowledge of assay/method limitations | |
| knowledge of variability due to additive determinants (e.g., genetic, physiological factors, lifestyle, intra/inter variability) | |
| awareness of different measurement units that can complicate result interpretation | |
| Antioxidant supplementation | antioxidant dose |
| antioxidant type | |
| single versus multi-antioxidant approach | |
| supplementation time | |
| interaction/synergism between antioxidant | |
| interference of dietary antioxidants | |
| higher requirement of vitamin intake (e.g., smokers, inactive people, etc) | |
| supplementation able to give a sufficient blood concentration to be effective | |
| avoid too high concentration, to exclude possible pro-oxidant effects | |
| initiation time according to the stage of disease (is antioxidant supplementation more effective to reverse mild damage?) | |
| redox homeostasis as target of supplementation | |
| selection of subjects/patients with increased oxidative stress to be supplemented | |
| Antioxidant drugs | drugs that selectively target oxidative stress pathways increasing ROS production and cancer cellular death |
| common drugs with antioxidant properties (e.g., statins, B-blockers, ACE-inhibitors, ARB in the cardiovascular field) | |
| also potentially effective for other disease prevention/treatment | |
| epigenetic miRNA-based approaches |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassalle, C.; Maltinti, M.; Sabatino, L. Targeting Oxidative Stress for Disease Prevention and Therapy: Where Do We Stand, and Where Do We Go from Here. Molecules 2020, 25, 2653. https://doi.org/10.3390/molecules25112653
Vassalle C, Maltinti M, Sabatino L. Targeting Oxidative Stress for Disease Prevention and Therapy: Where Do We Stand, and Where Do We Go from Here. Molecules. 2020; 25(11):2653. https://doi.org/10.3390/molecules25112653
Chicago/Turabian StyleVassalle, Cristina, Maristella Maltinti, and Laura Sabatino. 2020. "Targeting Oxidative Stress for Disease Prevention and Therapy: Where Do We Stand, and Where Do We Go from Here" Molecules 25, no. 11: 2653. https://doi.org/10.3390/molecules25112653
APA StyleVassalle, C., Maltinti, M., & Sabatino, L. (2020). Targeting Oxidative Stress for Disease Prevention and Therapy: Where Do We Stand, and Where Do We Go from Here. Molecules, 25(11), 2653. https://doi.org/10.3390/molecules25112653








