Conversion of the Propane–Butane Fraction into Arenes on MFI Zeolites Modified by Zinc Oxide and Activated by Low-Temperature Plasma
Abstract
1. Introduction
2. Results and Discussion
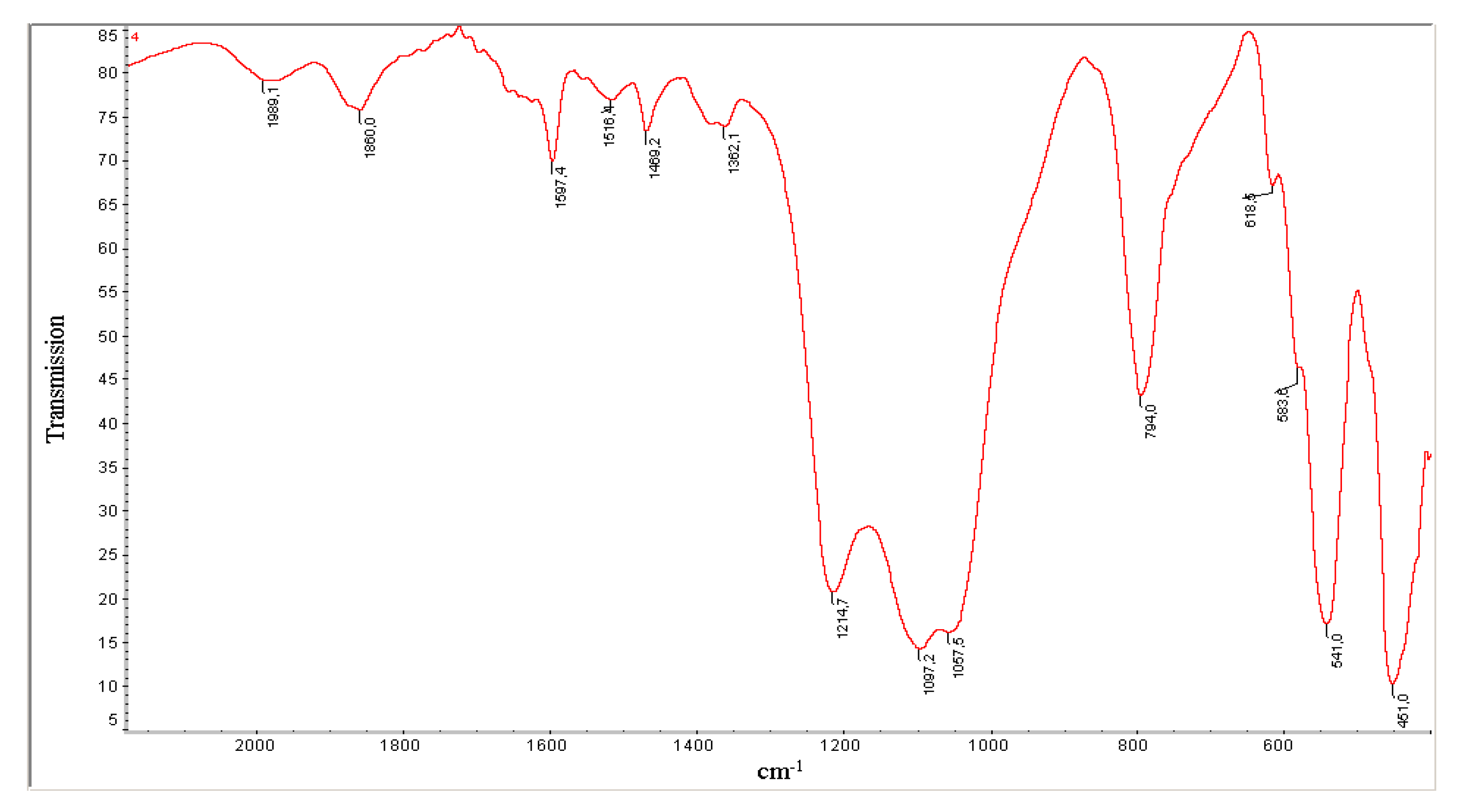
2.1. Synthesis and Characterization of Catalysts
2.2. Catalytic Activity
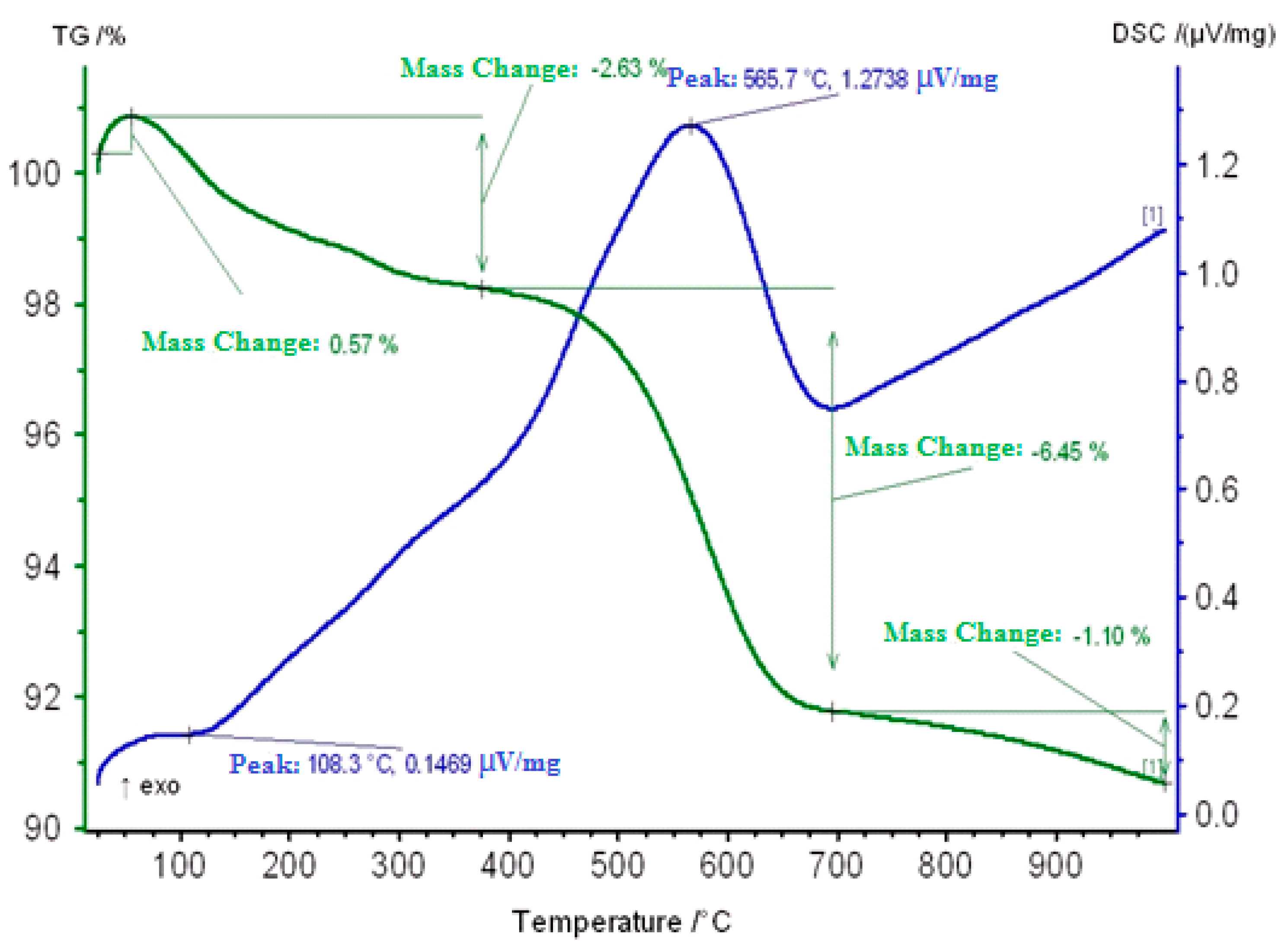
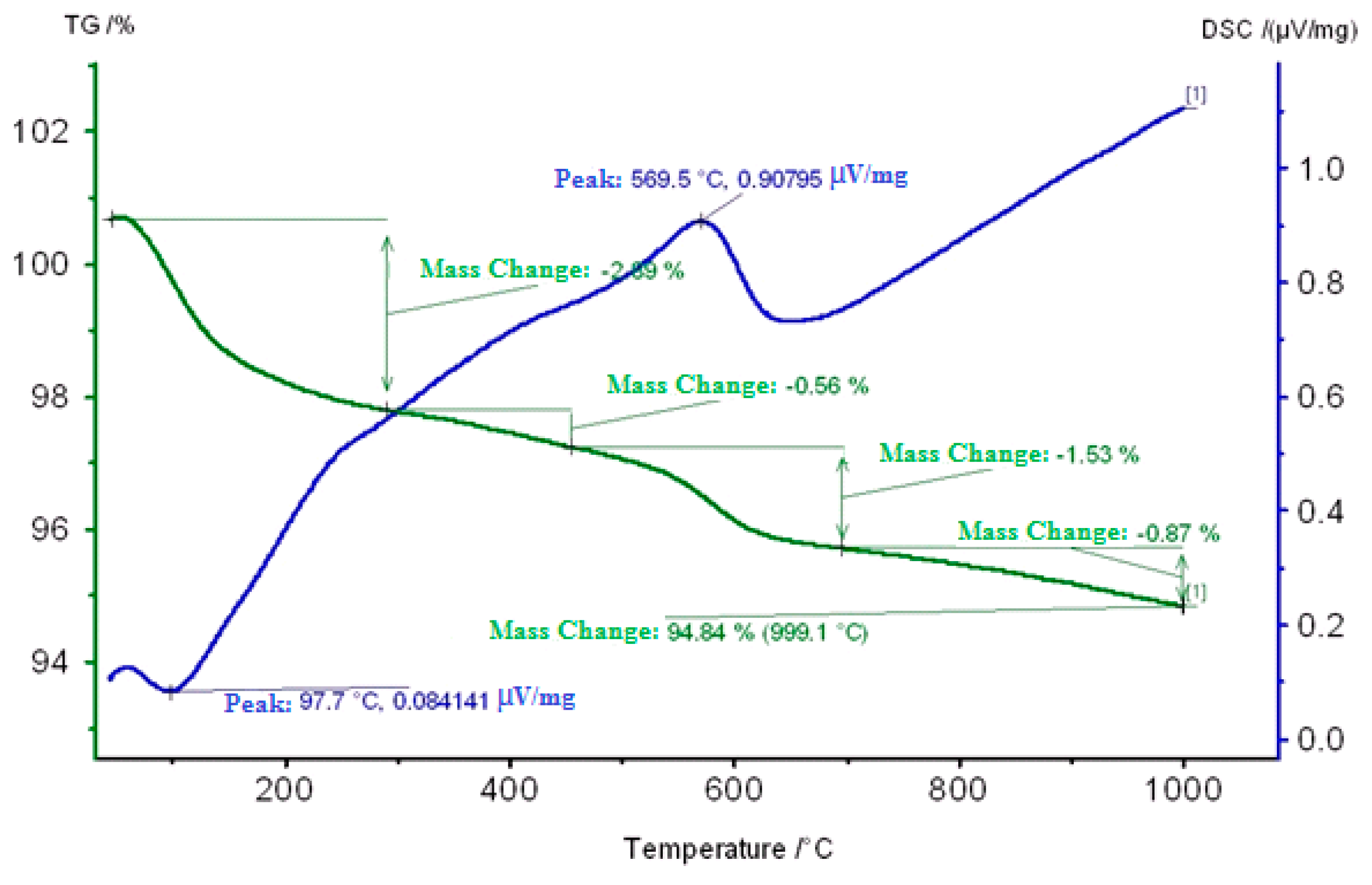
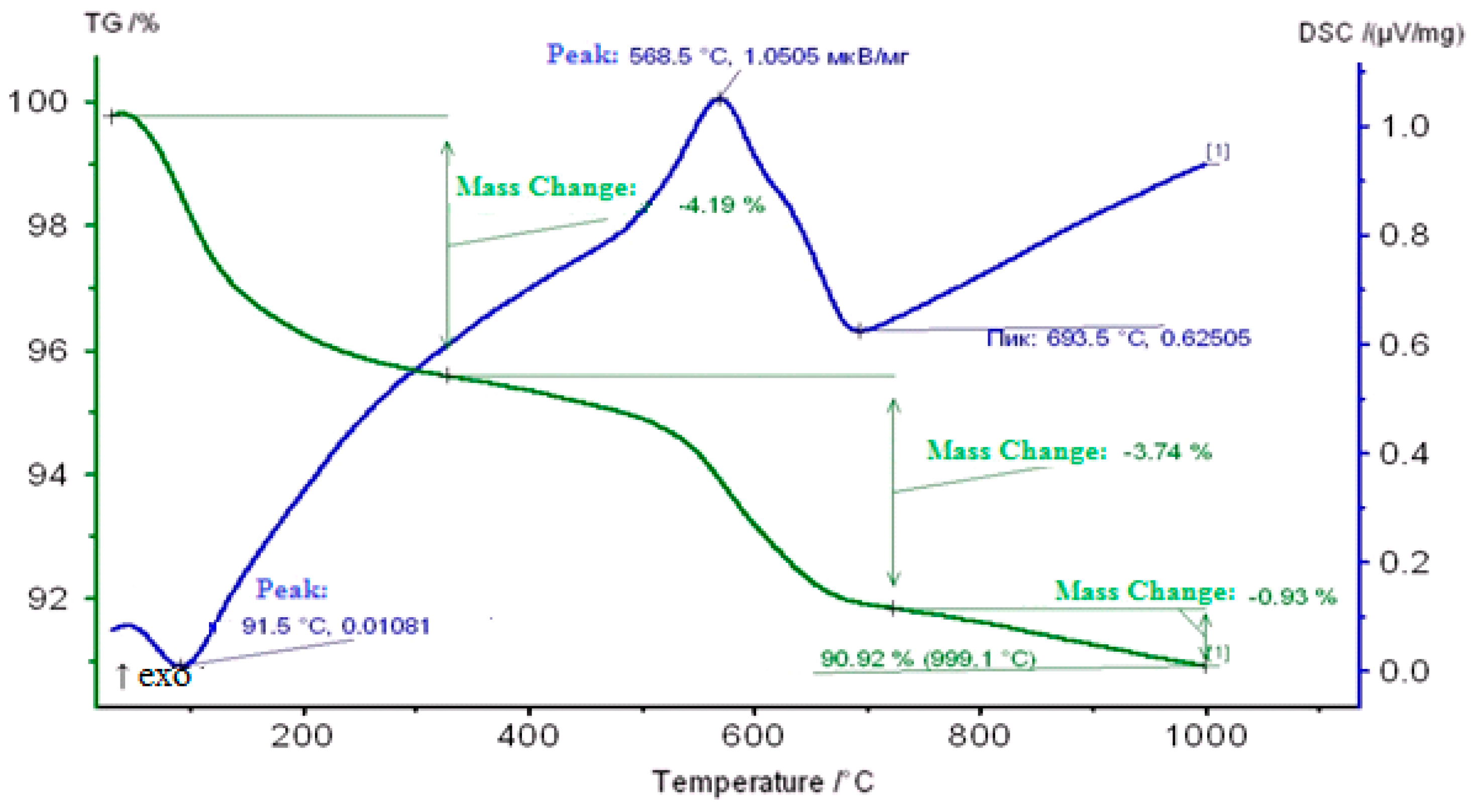
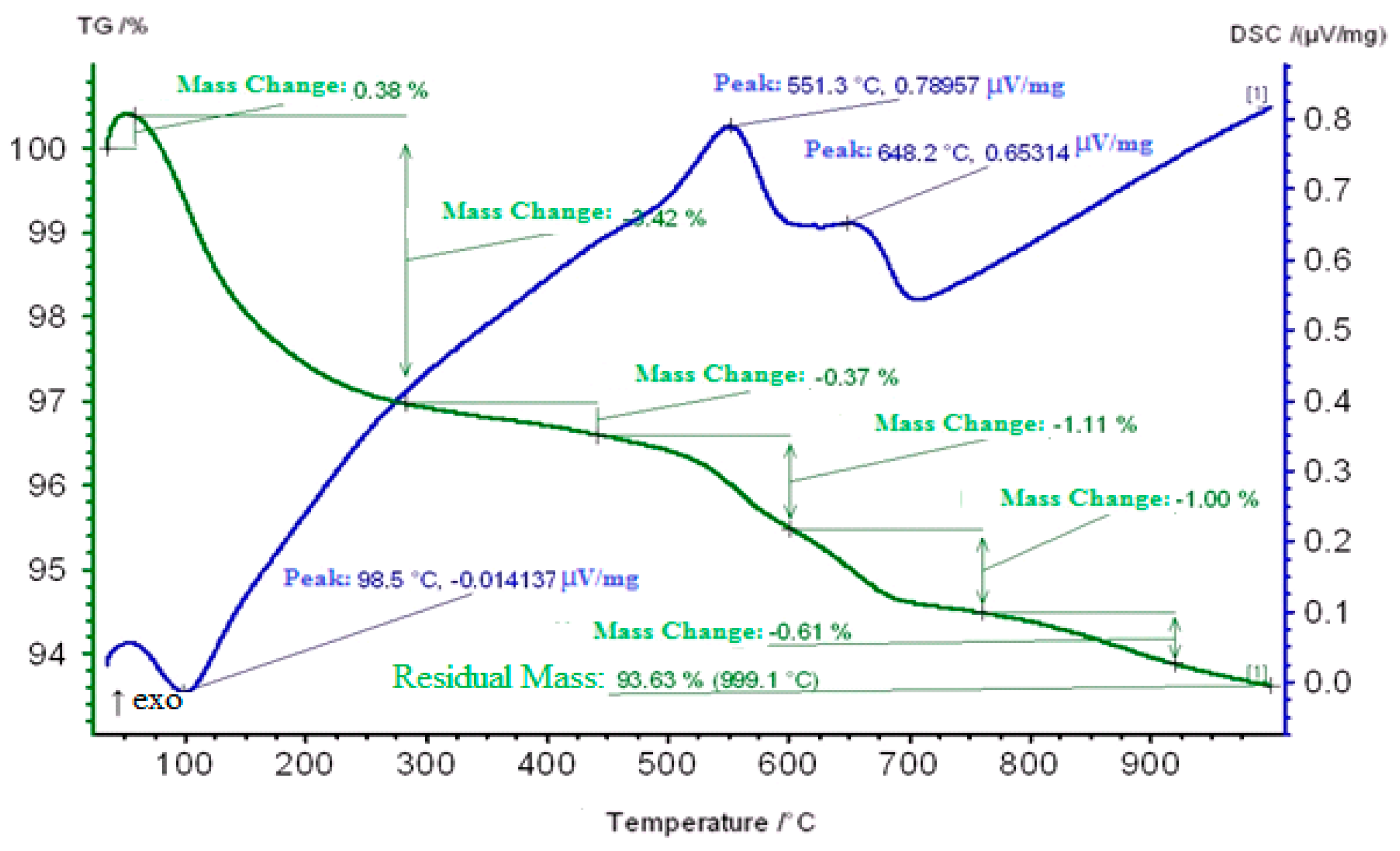
2.3. Thermogravimetric Study of Coked Zeolite-Containing Catalysts
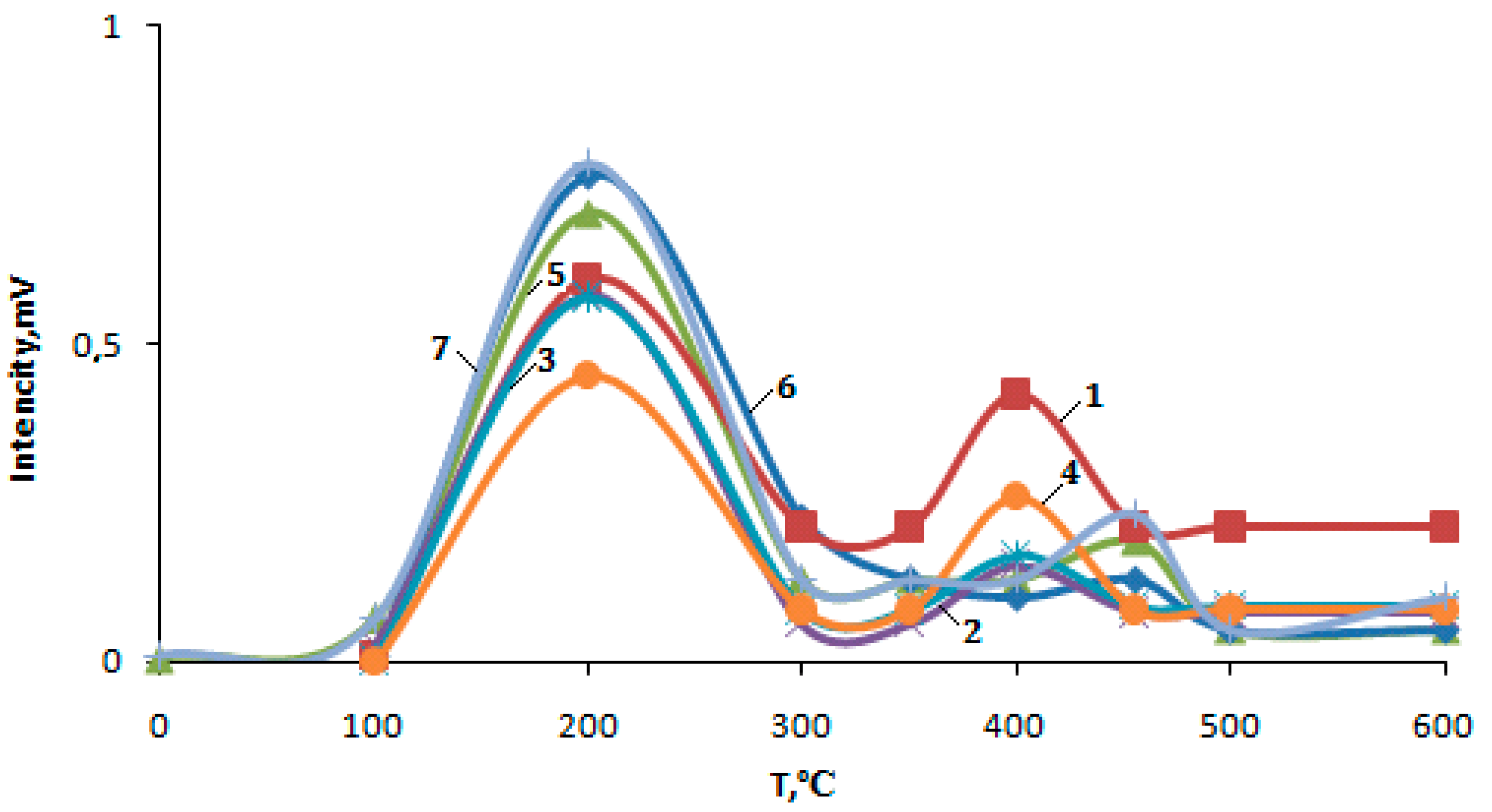
2.4. Study of the Acidic Properties of Microporous Zeolite Catalysts
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Activation of the H-ZKE-XM Zeolite Catalysts Modified by 1–5% ZnO, by Low Temperature Plasma
3.3. Characterization Techniques
3.4. Acid Properties of Catalysts
3.5. Catalytic Activity Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Berndt, H.; Lietz, G.; Volter, J. Zinc promoted H-ZSM-5 catalysts for conversion of propane to aromatics. II. Nature of the active sites and their activation. Appl. Catal. A Gen. 1996, 146, 365–379. [Google Scholar] [CrossRef]
- Trofimova, A.S.; Erofeev, V.I.; Koval, L.M. The Preparation of the lower olefins from C3-C4 Alkanes on ZSM-5 Zeolites modified by Lithium. Russ. J. Phys. Chem. 2002, 76, 922–925. [Google Scholar]
- Guo, J.; Lou, H.; Zhao, H.; Zheng, L.; Zheng, X. Degydrogenation and aromatization of propane over rhenium-modified HZSM-5 catalyst. J. Mol. Catal. A Chem. 2005, 239, 222–227. [Google Scholar] [CrossRef]
- Caeiro, G.; Carvalho, R.H.; Wang, X.; Wang, X.; Lemos, M.A.N.D.A.; Lemos, F.; Guisnet, M.; Ribeiro, F.R. Activation of C2-C4 alkanes over acid bifunctional zeolite catalysts. J. Mol. Catal. A Chem. 2006, 255, 131–158. [Google Scholar] [CrossRef]
- Rodrigues, V.D.O.; Eon, J.-G.; Faro, A.C., Jr. Correlations between dispersion, acidity, reducibility and propane aromatization activity of gallium species supported on HZSM5 zeolites. J. Phys. Chem. C 2010, 114, 4557–4567. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Arzumanov, S.S.; Stepanov, A.G.; Freude, D. Propane aromatization on Zn-modified zeolite BEA studied by solid—State NMR in situ. J. Phys. Chem. C 2010, 114, 12681–12688. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, J.; Wang, X.; Zhang, Q.; Xie, H.; Han, Y.; Tan, Y. A highly efficient Ga/ZSM-5 catalyst prepared by formic acid impregnation and in situ treatment for propane aromatization. Catal. Sci. Technol. 2015, 5, 4081–4090. [Google Scholar] [CrossRef]
- Pidko, E.A.; Santen, R.A.V. Activation of light alkanes over zinc species stabilized in ZSM-5: A comprehensive DFT study. J. Phys. Chem. C 2007, 111, 2643–2655. [Google Scholar] [CrossRef]
- Asaftei, I.V.; Lungu, N.C.; Birsa, M.L.; Sarbu, L.G.; Ignat, M.; Sandu, I.G. Conversion of light hydrocarbons from petroleum refining processes over Zn-HZSM-5 (nitrate) and Zn-HZSM-5 (acetate) catalyst a comparative study. Framework 2016, 67, 1523–1528. [Google Scholar]
- Niu, X.; Gao, J.; Miao, Q.; Dong, M.; Wang, G.; Fan, W.; Qin, Z.; Wang, J. Influence of preparation method on the Niu performance of Zn-containing HZSM-5 catalysts in methanol-to-aromatics. Microporous Mesoporous Mater. 2014, 197, 252–261. [Google Scholar] [CrossRef]
- Makarfi, Y.I.; Yakimova, M.S.; Lermontov, A.S.; Erofeev, V.I.; Koval, L.M.; Tretiyakov, V.F. Conversion of bioethanol over zeolites. Chem. Eng. J. 2009, 154, 396–400. [Google Scholar] [CrossRef]
- Erofeev, V.I.; Khasanov, V.V.; Dzhalilova, S.N.; Reschetilowski, V.P.; Syskina, A.A.; Bogdankova, L.A. Acidic and Catalytic Properties of Zeolite Modified by Zinc in the Conversion Process of Lower C3-C4 Alkanes. Catalysts 2019, 9, 421. [Google Scholar] [CrossRef]
- Erofeev, V.I.; Koval, L.M. Ru Patent, No 2313487, 27 December 2007.
- Breck, D.W. Zeolite Molecular Sieves; Publisher World: Moscow, Russia, 1976. [Google Scholar]
- Framework Type MFI Database of Zeolite Structures. Structure Commission of the International Zeolite Association (IZA-SC). Available online: http://europe.iza-structure.org/IZA-SC/framework.php?STC=MFI (accessed on 1 July 2007).
- Choi, S.-W.; Kim, W.-G.; So, J.-S.; Sievers, C.; Sholl, D.S.; Nair, S.; Jones, C.W.; Moore, J.S.; Liu, Y.; Dixit, R.S.; et al. Propane dehydrogenation catalyzed by gallosilicate MFI zeolites with perturbed acidity. J. Catal. 2017, 345, 113–123. [Google Scholar] [CrossRef]
- Liu, R.-L.; Zhu, H.-Q.; Wu, Z.-W.; Qin, Z.-F.; Fan, W.-B.; Wang, J.-G. Aromatization of propane over Ga-modified ZSM-5 Catalysts. J. Fuel Chem. Technol. 2015, 43, 961–969. [Google Scholar] [CrossRef]
- Bhan, A.; Delgass, W.N. Propane aromatization over HZSM-5 and Ga/HZSM-5 catalysts. Catal. Rev. Sci. Eng. 2008, 50, 19–151. [Google Scholar] [CrossRef]
- Wan, H.; Pallavi, C. Catalytic conversion of propane to BTX over Ga. Zn. Mo and Re impregnated ZSM-5 catalysys. J. Anal. Appl. Pyrolysis 2016, 121, 369–375. [Google Scholar] [CrossRef]
- Rodrigues, V.D.O.; Faro Junior, A.C. On catalyst activation and reaction mechanisms in propane aromatization on Ga/HZSM5 catalysts. Appl. Catal. A Gen. 2012, 435–436, 68–77. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, J.; Wang, P.; Zhang, Z.; Zhang, Q.; Xie, H.; Yang, G.; Han, Y.; Tan, Y. Mechanistic insight to acidity effects of Ga/HZSM-5 on its activity for propane aromatization. RSC Adv. 2015, 112, 92222–92233. [Google Scholar] [CrossRef]
- Rodrigues, V.D.O.; Vasconcellos, F.J., Jr.; Faro Junior, A.C. Mechanistic studies through H-D ehchange reactions: Propane aromatization in HZSM5 catalysts. J. Catal. 2016, 344, 252–262. [Google Scholar] [CrossRef]
- Erofeev, M.V.; Ripenko, V.S.; Shulepov, M.A.; Tarasenko, V.F. Generators of diffuse plasma at atmospheric pressure. Instrum. Exp. Tech. 2017, 60, 287–289. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds not available from the authors. |


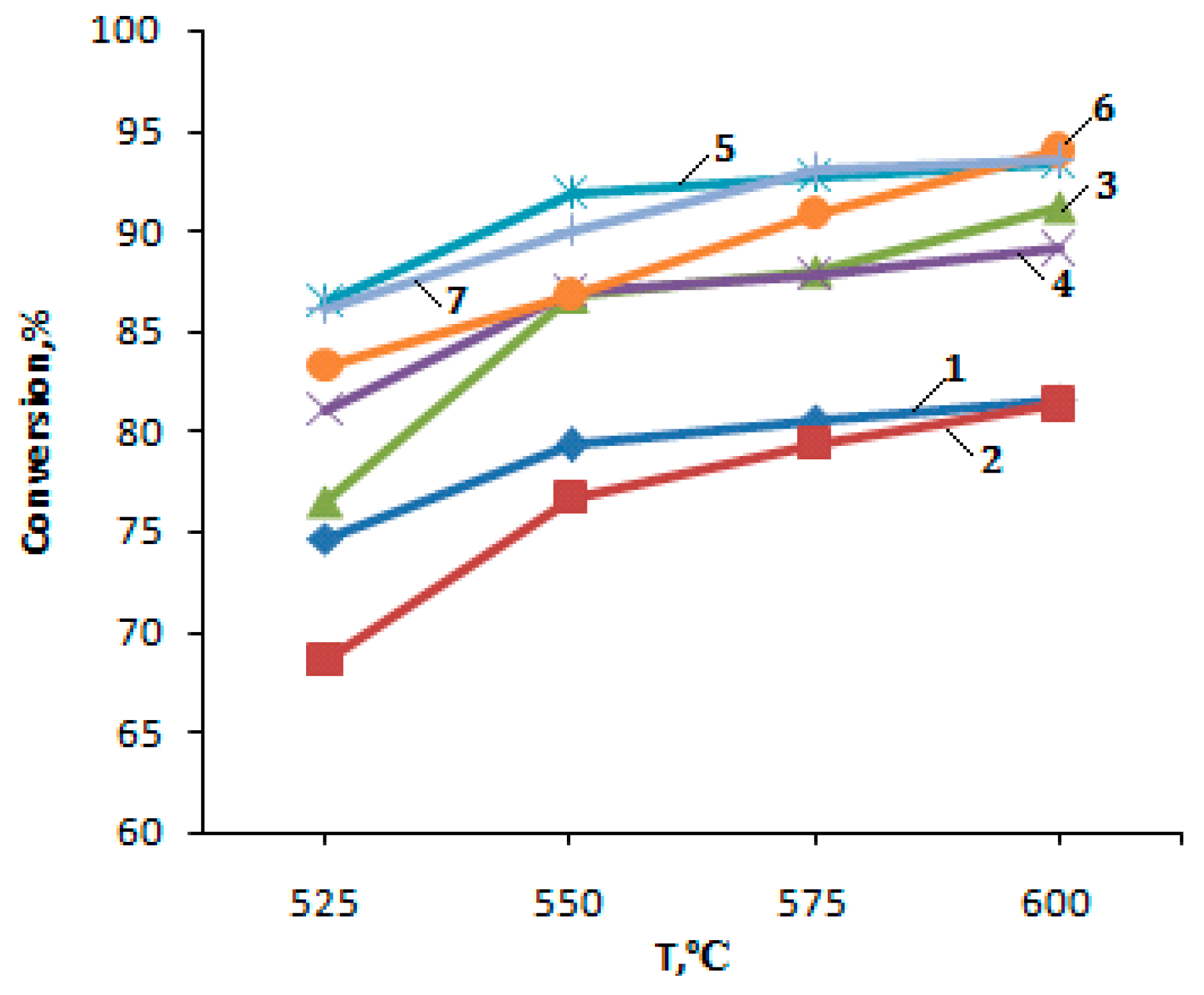


| Temperature °C | Conversion % | Gas Phase % | Gas Phase Output % | Liquid Phase % | Liquid Phase Output % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alkanes C1-C4 | Alkenes C2-C3 | C6H6 | C7H8 | C8H10 | C9 | C10+ | ||||
| H-ZKE-XM | ||||||||||
| 525 | 74.6 | 49.2 | 93.4 | 6.6 | 50.8 | 11.9 | 35.6 | 33.0 | 4.4 | 15.1 |
| 550 | 79.3 | 47.8 | 90.3 | 9.7 | 52.2 | 11.4 | 35.4 | 30.0 | 5.4 | 13.1 |
| 575 | 80.6 | 45.6 | 89.3 | 10.7 | 54.4 | 13.7 | 37.5 | 26.9 | 6.2 | 15.7 |
| 600 | 81.6 | 44 | 87.5 | 12.7 | 56.0 | 14.7 | 38.1 | 26.2 | 4.7 | 16.3 |
| 1% ZnO/H-ZKE-XM | ||||||||||
| 525 | 68.6 | 50.7 | 94.7 | 5.3 | 49.3 | 15.8 | 40.0 | 28.6 | 2.0 | 13.6 |
| 550 | 76.7 | 40.1 | 94.1 | 5.9 | 59.9 | 19.9 | 43.4 | 25.0 | 3.1 | 12.6 |
| 575 | 79.4 | 37.3 | 93.2 | 6.8 | 62.7 | 19.0 | 41.6 | 20.0 | 3.1 | 16.3 |
| 600 | 81.3 | 36.3 | 91.7 | 8.3 | 63.7 | 20.6 | 42.2 | 19.2 | 3.0 | 15.0 |
| 3% ZnO/H-ZKE-XM | ||||||||||
| 525 | 76.5 | 47.1 | 97.0 | 3.0 | 52.9 | 16.7 | 44.5 | 24.8 | 1.1 | 12.9 |
| 550 | 86.7 | 39.8 | 96.5 | 3.5 | 60.2 | 19.9 | 42.4 | 21.4 | 0.7 | 15.6 |
| 575 | 88.0 | 37.0 | 95.4 | 4.6 | 63.0 | 21.1 | 40.5 | 16.8 | 0.4 | 21.2 |
| 600 | 91.2 | 35.6 | 90.8 | 9.2 | 64.4 | 21.5 | 37.2 | 15.6 | 0.4 | 25.3 |
| 5% ZnO/H-ZKE-XM | ||||||||||
| 525 | 81.1 | 45.9 | 97.2 | 2.8 | 54.1 | 15.3 | 42.7 | 25.0 | 0.8 | 16.2 |
| 550 | 87.0 | 41.9 | 96.4 | 3.6 | 58.1 | 19.9 | 42.5 | 20.0 | 0.5 | 17.1 |
| 575 | 87.8 | 40.4 | 94.4 | 5.6 | 59.6 | 22.0 | 39.0 | 16.3 | 0.5 | 21.2 |
| 600 | 89.1 | 37.9 | 88.5 | 11.5 | 62.1 | 24.0 | 38.3 | 15.2 | 0.4 | 22.1 |
| 1% ZnO(plasma)/H-ZKE-XM | ||||||||||
| 525 | 86.5 | 45.5 | 95.6 | 4.4 | 54.5 | 18.6 | 43.2 | 20.0 | 1.3 | 16.9 |
| 550 | 91.9 | 38.5 | 94.6 | 5.4 | 61.5 | 21.6 | 41.5 | 17.3 | 0.9 | 18.7 |
| 575 | 92.7 | 36.4 | 93.0 | 7.0 | 63.5 | 23.3 | 40.2 | 13.7 | 0.8 | 22.0 |
| 600 | 93.4 | 35.1 | 88.8 | 11.2 | 64.9 | 26.0 | 38.0 | 12.3 | 0.5 | 23.2 |
| 3% ZnO (plasma)/H-ZKE-XM | ||||||||||
| 525 | 83.2 | 44.6 | 96.0 | 4.0 | 55.4 | 19.4 | 43.4 | 17.5 | 1.6 | 18.1 |
| 550 | 86.8 | 38.1 | 94.7 | 5.3 | 61.9 | 21.7 | 43.2 | 15.3 | 1.1 | 18.7 |
| 575 | 90.8 | 36.1 | 92.8 | 7.2 | 63.9 | 22.3 | 41.6 | 14.6 | 0.6 | 20.9 |
| 600 | 94.0 | 34.5 | 89.9 | 10.1 | 65.5 | 23.6 | 40.4 | 13.4 | 0.5 | 22.1 |
| 5% ZnO (plasma)/H-ZKE-XM | ||||||||||
| 525 | 86.1 | 47.1 | 96.9 | 3.1 | 52.9 | 18.2 | 44.4 | 20.4 | 0.5 | 16.5 |
| 550 | 90.0 | 41.6 | 96.2 | 3.8 | 58.4 | 22.5 | 41.5 | 16.4 | 0.4 | 19.2 |
| 575 | 93.0 | 38.3 | 94.2 | 5.8 | 61.7 | 24.4 | 39.0 | 14.2 | 0.4 | 22.0 |
| 600 | 93.6 | 36.4 | 88.8 | 11.2 | 63.6 | 24.2 | 40.0 | 13.1 | 0.4 | 22.3 |
| Catalyst | Tmax. °C | Concentration of Acid Sites. µmol/g | |||
|---|---|---|---|---|---|
| TI | TII | CI | CII | Ctotal | |
| H-ZKE-XM | 185 | 405 | 600 | 421 | 1021 |
| 1% ZnO/99% H-ZKE-XM | 185 | 405 | 626 | 151 | 777 |
| 3% ZnO/97% H-ZKE-XM | 190 | 410 | 572 | 167 | 739 |
| 5% ZnO/95% H-ZKE-XM | 190 | 410 | 450 | 258 | 708 |
| 1% ZnO/99% H-ZKE-XM; plasma | 210 | 455 | 707 | 201 | 908 |
| 3% ZnO/97% H-ZKE-XM; plasma | 215 | 465 | 764 | 220 | 984 |
| 5% ZnO/95% H-ZKE-XM; plasma | 220 | 465 | 783 | 232 | 1015 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erofeev, V.I.; Dzhalilova, S.N.; Erofeev, M.V.; Ripenko, V.S.; Reschetilowski, V.P. Conversion of the Propane–Butane Fraction into Arenes on MFI Zeolites Modified by Zinc Oxide and Activated by Low-Temperature Plasma. Molecules 2020, 25, 2704. https://doi.org/10.3390/molecules25112704
Erofeev VI, Dzhalilova SN, Erofeev MV, Ripenko VS, Reschetilowski VP. Conversion of the Propane–Butane Fraction into Arenes on MFI Zeolites Modified by Zinc Oxide and Activated by Low-Temperature Plasma. Molecules. 2020; 25(11):2704. https://doi.org/10.3390/molecules25112704
Chicago/Turabian StyleErofeev, Vladimir I., Sofiya N. Dzhalilova, Mikhail V. Erofeev, Vasilii S. Ripenko, and Vladimir P. Reschetilowski. 2020. "Conversion of the Propane–Butane Fraction into Arenes on MFI Zeolites Modified by Zinc Oxide and Activated by Low-Temperature Plasma" Molecules 25, no. 11: 2704. https://doi.org/10.3390/molecules25112704
APA StyleErofeev, V. I., Dzhalilova, S. N., Erofeev, M. V., Ripenko, V. S., & Reschetilowski, V. P. (2020). Conversion of the Propane–Butane Fraction into Arenes on MFI Zeolites Modified by Zinc Oxide and Activated by Low-Temperature Plasma. Molecules, 25(11), 2704. https://doi.org/10.3390/molecules25112704







