Effect of Addition of Pectins from Jujubes (Ziziphus jujuba Mill.) on Vitamin C Production during Heterolactic Fermentation
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction of Pectins
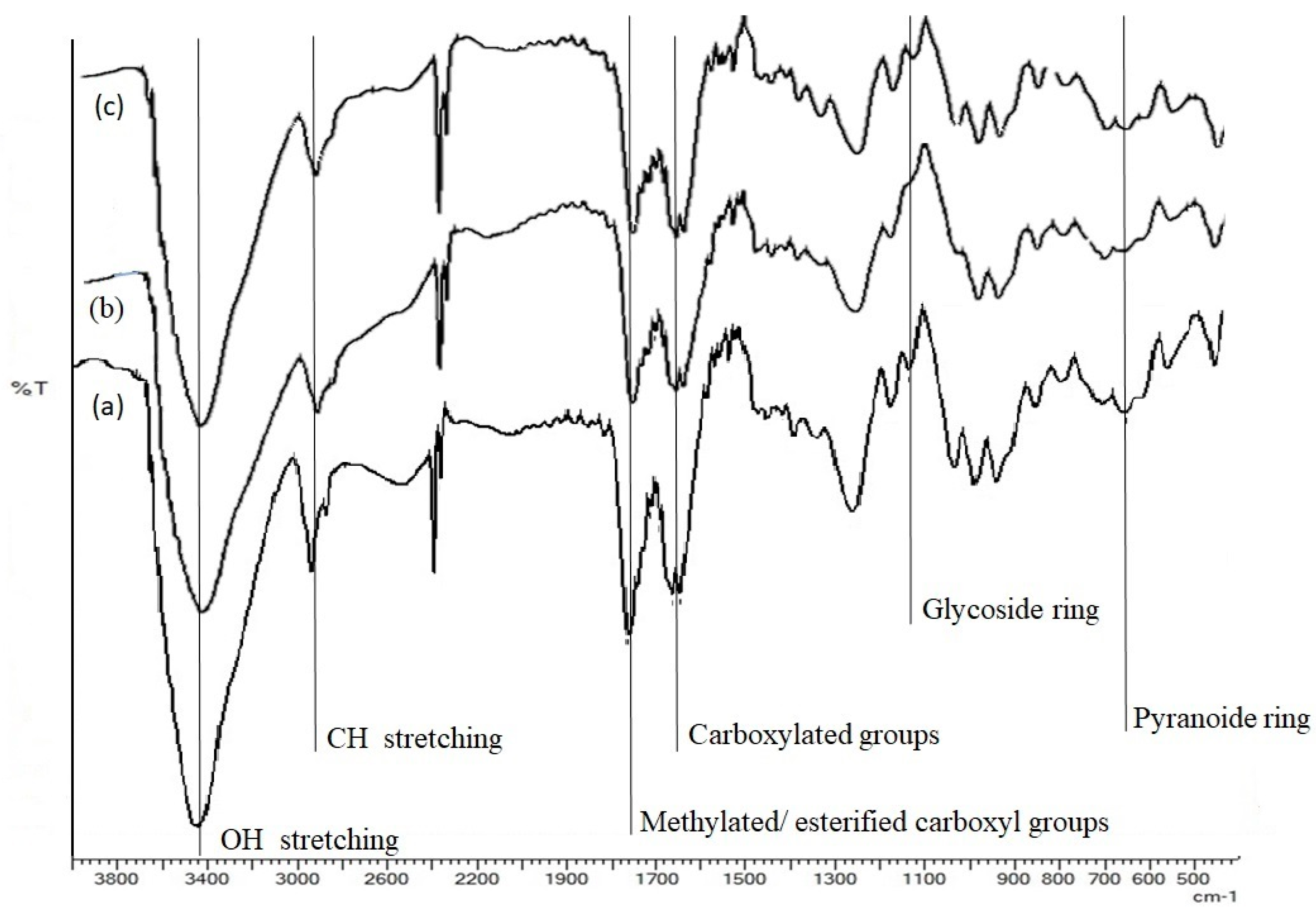
2.2. FTIR Spectroscopic Analysis
2.3. Scanning Electron Microscopy
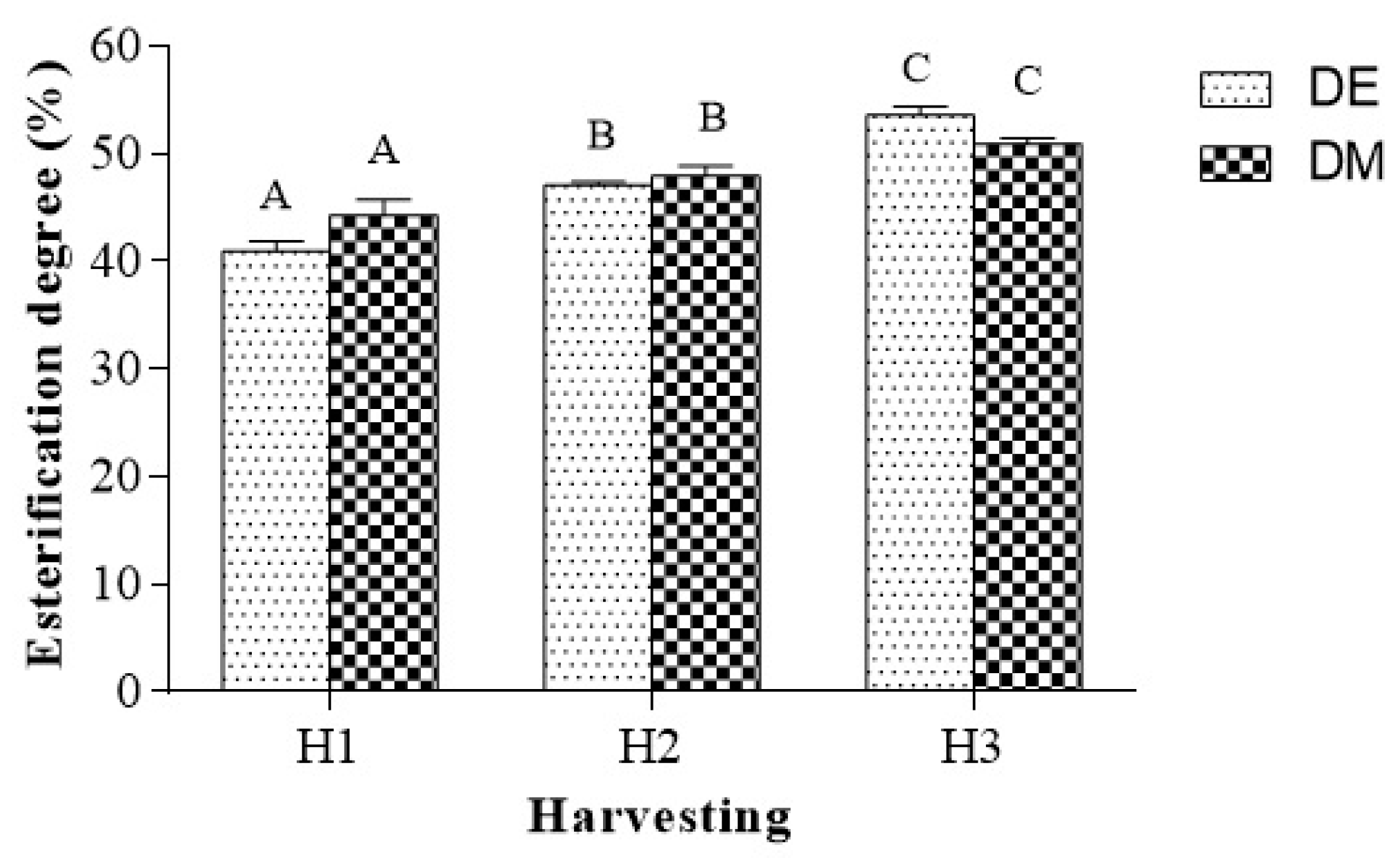
2.4. Determination of Esterification Degree
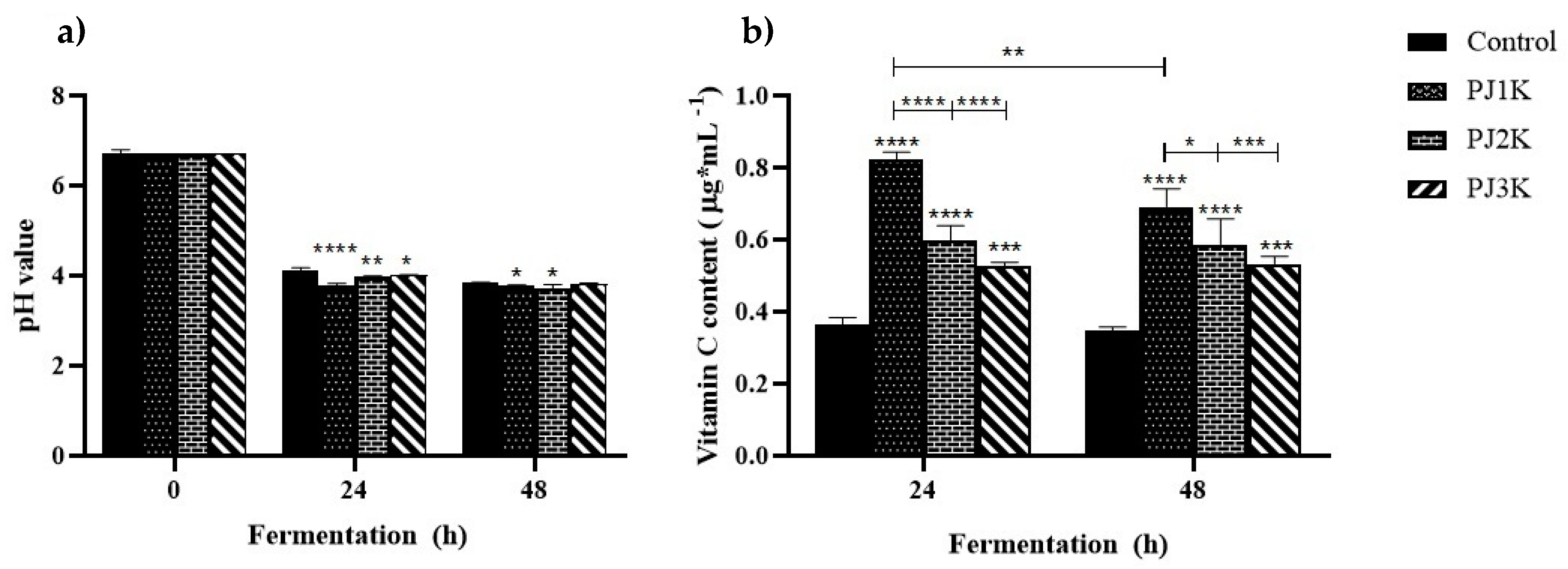
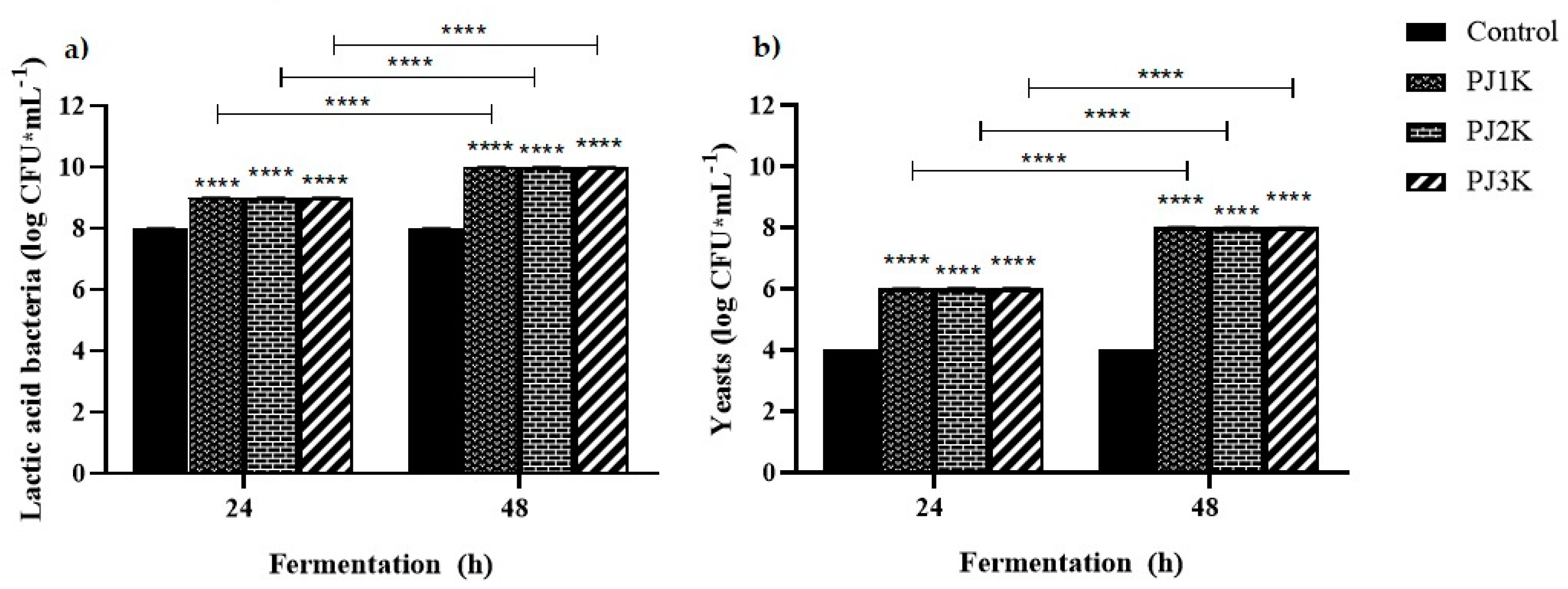
2.5. Evolution of pH and Kefir Weight during Heterolactic Fermentation
2.6. Vitamin C Content during Heterolactic Fermentation
3. Materials and Methods
3.1. Standards, Solvents, and Reagents
3.2. Milk Samples
3.3. Kefir Grains
3.4. Plant Materials
3.5. Extraction of Pectins
3.6. FTIR Spectroscopic Analysis
3.7. Scanning Electron Microscopy
3.8. Determination of Esterification Degree
3.8.1. Titration Method
3.8.2. Instrumental FTIR Method
3.9. Heterolactic Fermentation Procedure
3.10. Vitamin C Extraction and HPLC Conditions
3.11. Bacterial and Yeast Counts
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| H1 | jujube at the first harvesting |
| H2 | jujube at second harvesting |
| H3 | jujube at the third harvesting |
| PJ | pectins from jujubes |
| PJ1 | pectins from jujubes of first harvesting |
| PJ2 | pectins from jujubes of second harvesting |
| PJ3 | pectins from jujubes of third harvesting |
| PJ1K | kefir supplemented with pectin from jujube of first harvesting |
| PJ2K | kefir supplemented with pectin from jujube of second harvesting |
| PJ3K | kefir supplemented with pectin from jujube of third harvesting |
| FTIR | Fourier Transform Infrared Spectroscopy |
| DE | esterification degree determined by titration method |
| DM | esterification degree determined by instrumental FTIR method |
| SEM | Scanning Electron Microscopy |
| WDS | Wavelength Dispersive X-Ray Spectroscopy |
| HM | high-methoxyl |
| LM | low-methoxyl |
References
- Inbaraj, B.S.; Chen, B.H. Polysaccharide Composition in Chinese Dates (Jujubes) and Health Benefits. In Chinese Dates—A Traditional Functional Food, 1st ed.; Li, D., Ye, X., Jiang, Y., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 167–197. ISBN 978-1-4987-0358-1. [Google Scholar]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation. Molecules 2019, 25, 63. [Google Scholar] [CrossRef] [PubMed]
- Plastina, P.; Bonofiglio, D.; Vizza, D.; Fazio, A.; Rovito, D.; Giordano, C.; Barone, I.; Catalano, S.; Gabriele, B. Identification of bioactive constituents of Ziziphus jujube fruit extracts exerting antiproliferative and apoptotic effects in human breast cancer cells. J. Ethnopharmacol. 2012, 140, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Fazio, A.; La Torre, C.; Caroleo, M.C.; Caputo, P.; Plastina, P.; Cione, E. Isolation and Purification of Glucans from an Italian Cultivar of Ziziphus jujuba Mill. and In Vitro Effect on Skin Repair. Molecules 2020, 25, 968. [Google Scholar] [CrossRef] [PubMed]
- Plastina, P.; Gabriele, B. Phytochemical Constituents and Health Benefits of Jujubes. In Chinese Dates—A Traditional Functional Food, 1st ed.; Li, D., Ye, X., Jiang, Y., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 155–175. ISBN 978-1-4987-0358-1. [Google Scholar]
- Li, J.; Fan, L.; Ding, S.; Ding, X. Nutritional composition of five cultivars of Chinese jujube. Food Chem. 2007, 103, 454–460. [Google Scholar] [CrossRef]
- Naqash, F.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gani, A. Emerging concepts in the nutraceutical and functional properties of pectin-A Review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Thiraviam, V.; Mahejibin, K. Role of Pectin in Food Processing and Food Packaging. In Pectins-Extraction, Purification, Characterization and Applications, 22nd ed.; Masuelli, A., Ed.; IntechOpen Ltd.: London, UK, 2020; pp. 1237–1257. ISBN 978-1-78984-072-8. [Google Scholar]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017, 93, 127. [Google Scholar] [CrossRef]
- Montanuci, F.D.; Pimentel, T.C.; Garcia, S.; Prudencio, S.H. Effect of starter culture and inulin addition on microbial viability, texture, and chemical characteristics of whole or skim milk Kefir. Ciência Tecnol. Alime. 2012, 32, 850–861. [Google Scholar] [CrossRef]
- Oh, N.S.; Lee, H.A.; Myung, J.H.; Lee, J.Y.; Joung, J.Y.; Shin, Y.K. Effect of different commercial oligosaccharides on the fermentation properties in kefir during fermentation. Korean J. Food Sci. Anim. Resour. 2013, 33, 325–330. [Google Scholar] [CrossRef][Green Version]
- Satir, G.; Guzel-Seydim, Z.B. How kefir fermentation can affect product composition? Small Ruminant Res. 2016, 134, 1–7. [Google Scholar] [CrossRef]
- Garrote, G.L.; Abraham, A.G.; De Antoni, G.L. Chemical and microbiological characterisation of kefir grains. J. Dairy Res. 2001, 68, 639–652. [Google Scholar] [CrossRef]
- Glibowski, P.; Kowaslka, A. Rheological, texture and sensory properties of kefir with high performance and native inulin. J. Food Eng. 2012, 111, 299–304. [Google Scholar] [CrossRef]
- Glibowski, P.; Zielińska, E. Physicochemical and sensory properties of kefir containing inulin and oligofructose. Int. J. Dairy Technol. 2015, 68, 602–607. [Google Scholar] [CrossRef]
- Piermaria, J.A.; de la Canal, M.L.; Abraham, A.G. Gelling properties of kefiran, a food-grade polysaccharide obtained from kefir grain. Food Hydrocoll. 2008, 22, 1520–1527. [Google Scholar] [CrossRef]
- Mayo, B.; Aleksandrzak-Piekarczyk, T.; Fernández, M.; Kowalczyk, M.; Álvarez-Martín, P.; Bardowski, J. Updates in the metabolism of lactic acid bacteria. In Biotechnology of lactic acid bacteria. Novel applications; Mozzi, F., Raya, R.R., Vignolo, G.M., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 3–33. ISBN 0813815835. [Google Scholar]
- Heller, K.J. Probiotic bacteria in fermented foods: Product characteristics and starter organisms. Am. J. Clin. Nutr. 2001, 73, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Rosa, D.D.; Dias, M.M.S.; Grześkowiak, Ł.M.; Reis, S.A.; Conceição, L.L.; Peluzio, M.D.C.G. Milk kefir: Nutritional, microbiological and health benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef]
- Simova, E.; Beshkova, D.; Angelov, A.; Hristozova, T.; Frengova, G.; Spasov, Z. Lactic acid bacteria and yeasts in kefir grains and kefir made from them. J. Ind. Microbiol. Biot. 2002, 28, 1–6. [Google Scholar] [CrossRef]
- Lopitz-Otsoa, F.; Rementeria, A.; Elguezabal, N.; Garaizar, J. Kefir: A symbiotic yeasts-bacteria community with alleged healthy capabilities. Rev. Iberoam. Micol. 2006, 23, 67–74. [Google Scholar] [CrossRef]
- Di Matteo, A.; Sacco, A.; Anacleria, M.; Pezzotti, M.; Delledonne, M.; Ferrarini, A.; Frusciante, L.; Barone, A. The ascorbic acid content of tomato fruits is associated with the expression of genes involved in pectin degradation. BMC Plant. Biol. 2010, 10, 1–11. [Google Scholar] [CrossRef]
- Hancock, R.D.; Viola, R. Improving the Nutritional Value of Crops through Enhancement of L-Ascorbic Acid (Vitamin C) Content: Rationale and Biotechnological Opportunities. J. Agric. Food Chem. 2005, 53, 5248–5257. [Google Scholar] [CrossRef]
- Goncu, B.; Celikel, A.; Guler-Akin, M.B.; Akin, M.S. Some properties of kefir enriched with apple and lemon fiber. Mljekarstvo 2017, 67, 208–216. [Google Scholar] [CrossRef]
- Demir, C.; Kinik, O.; Yerlikaya, O.; Acu, M. Effect of apple and lemon dietary fibers on some characteristics of low fat kefir. Milchwiss.-Milk Sci. Int. 2012, 67, 406–410. [Google Scholar]
- Pinheiro, E.R.; Silva, I.M.D.A.; Gonzaga, L.V.; Amante, E.R.; Teófilo, R.F.; Ferreira, M.M.C.; Amboni, R.D.M.C. Optimization of extraction of high-ester pectin from passion fruit peel (Passiflora edulis Flavicarpa) with citric acid by using response surface methodology. Bioresour. Technol. 2008, 99, 5561–5566. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, F.; Yang, H.; Chen, Y.; Zhang, L.; An, H. Effects of ripening stage and cultivar on physicochemical properties and pectin nanostructures of jujubes. Carbohydr. Polym. 2012, 89, 1180–1188. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Zdunek, A. Use of FT-IR spectra and PCA to the bulk characterization of cell wall residues of fruits and vegetables along a fraction process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, R.; Fang, Q.; Yuan, Y.; Cao, J.; Jiang, W. Analyses of microstructure and cell wall polysaccharides of flesh tissues provide insights into cultivar difference in mealy patterns developed in apple fruit. Food Chem. 2020, 321, 126707. [Google Scholar] [CrossRef]
- Chylińska, M.; Szymanska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman characterization of non-cellulosic polysaccharides fractions isolated from plant cell wall. Carbohydr. Polym. 2016, 154, 48–54. [Google Scholar] [CrossRef]
- Deng, L.Z.; Pan, Z.; Zhang, Q.; Liu, Z.L.; Zhang, Y.; Meng, J.S.; Gao, Z.J.; Xiao, H.W. Effects of ripening stage on physicochemical properties, drying kinetics, pectin polysaccharides contents and nanostructure of apricots. Carbohydr. Polym. 2019, 222, 114980. [Google Scholar] [CrossRef]
- Geerkens, C.H.; Nagel, A.; Just, K.M.; Miller-Rostek, P.; Kammerer, D.R.; Schweiggert, R.M.; Carle, R. Mango pectin quality as influenced by cultivar, ripeness, peel particle size, blanching, drying, and irradiation. Food Hydrocoll. 2015, 51, 241–251. [Google Scholar] [CrossRef]
- Liu, L.; Fishman, M.L.; Hicks, K.B. Pectin in controlled drug delivery—A review. Cellulose 2007, 14, 15–24. [Google Scholar] [CrossRef]
- Delgado-Fernandez, P.; Corzo, N.; Lizasoain, S.; Olano, A.; Moreno, E.J. Fermentative properties of starter culture during manufacture of kefir with new prebiotics derived from lactulose. Int. Dairy J. 2019, 93, 22–29. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.; Wyffels, J.T.; Seydim, A.C.; Greene, A.K. Turkish kefir and kefir grains: Microbial enumeration and electron microscobic observation. Int. J. Dairy Technol. 2005, 58, 25–29. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Shimizu, H.; Shioya, S. Enhanced kefiran production by mixed culture of Lactobacillus kefiranofaciens and Saccharomyces cerevisiae. J. Biotechnol. 2003, 100, 45–53. [Google Scholar] [CrossRef]
- Rice, A.L.; Sacco, L.; Hyder, A.; Black, R.E. Malnutrition as an underlying cause of childhood deaths associated with infectious diseases in developing countries. Bull. World Health Organ. 2000, 78, 1207–1221. [Google Scholar]
- Hertzler, S.R.; Clancy, S.M. Kefir improves lactose digestion and tolerance in adults with lactose maldigestion. J. Am. Diet. Assoc. 2003, 103, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Narvhusa, J.A.; Gadaga, T.H. The role of interaction between yeasts and lactic acid bacteria in African fermented milks: A review. Int. J. Food Microbiol. 2003, 86, 51–60. [Google Scholar] [CrossRef]
- Farnworth, E.R.; Mainville, I. Kefir-A fermented milk product. In Handbook of Fermented Functional Foods, 2nd ed.; Farnworth, E.R., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 89–129. ISBN 978-1420053265. [Google Scholar]
- Ozer, B.H.; Kirmaci, H.A. Functional milks and dairy beverages. Int. J. Dairy Technol. 2010, 63, 1–15. [Google Scholar] [CrossRef]
- Bosch, A.; Golowczyc, M.A.; Abraham, A.G.; Garrote, G.L.; De Antoni, G.L.; Yantorno, O. Rapid discrimination of lactobacilli isolated from kefir grains by FT-IR spectroscopy. Int. J. Food Microbiol. 2006, 111, 280–287. [Google Scholar] [CrossRef]
- Zajsek, K.; Gorsek, A. Modelling of batch kefir fermentation kinetics for ethanol production by mixed natural microflora. Food Bioprod. Process. 2010, 88, 55–60. [Google Scholar] [CrossRef]
- Fazio, A.; La Torre, C.; Dalena, F.; Plastina, P. Screening of glucan and pectin contents in broad bean (Vicia faba L.) pods during maturation. Eur. Food Res. Technol. 2020, 246, 333–347. [Google Scholar] [CrossRef]
- Fazio, A.; Caroleo, M.C.; Cione, E.; Plastina, P. Novel acrylic polymers for food packaging: Synthesis and antioxidant properties. Food Packag. Shelf Life 2017, 11, 84–90. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Samples | N | C | O |
|---|---|---|---|
| PJ1 | 1.24 ± 0.02 | 46.17 ± 0.09 | 7.98 ± 0.88 |
| PJ2 | 1.65 ± 0.18 | 63.61 ±.0.94 | 12.93 ± 0.97 |
| PJ3 | 1.92 ± 0.14 | 69.87 ± 0.84 | 12.76 ± 0.76 |
| Samples | 0 h | 24 h | 48 h |
|---|---|---|---|
| Control | 1.01 ± 0.01 | 1.65 ± 0.01 | 1.95 ± 0.01 |
| PJ1 | 1.01 ± 0.01 | 2.60 ± 0.03 | 2.10 ± 0.04 |
| PJ2 | 1.04 ± 0.06 | 1.60 ± 0.02 | 1.46 ± 0.05 |
| PJ3 | 1.01 ± 0.01 | 1.40 ± 0.01 | 1.51 ± 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazio, A.; La Torre, C.; Caroleo, M.C.; Caputo, P.; Cannataro, R.; Plastina, P.; Cione, E. Effect of Addition of Pectins from Jujubes (Ziziphus jujuba Mill.) on Vitamin C Production during Heterolactic Fermentation. Molecules 2020, 25, 2706. https://doi.org/10.3390/molecules25112706
Fazio A, La Torre C, Caroleo MC, Caputo P, Cannataro R, Plastina P, Cione E. Effect of Addition of Pectins from Jujubes (Ziziphus jujuba Mill.) on Vitamin C Production during Heterolactic Fermentation. Molecules. 2020; 25(11):2706. https://doi.org/10.3390/molecules25112706
Chicago/Turabian StyleFazio, Alessia, Chiara La Torre, Maria Cristina Caroleo, Paolino Caputo, Roberto Cannataro, Pierluigi Plastina, and Erika Cione. 2020. "Effect of Addition of Pectins from Jujubes (Ziziphus jujuba Mill.) on Vitamin C Production during Heterolactic Fermentation" Molecules 25, no. 11: 2706. https://doi.org/10.3390/molecules25112706
APA StyleFazio, A., La Torre, C., Caroleo, M. C., Caputo, P., Cannataro, R., Plastina, P., & Cione, E. (2020). Effect of Addition of Pectins from Jujubes (Ziziphus jujuba Mill.) on Vitamin C Production during Heterolactic Fermentation. Molecules, 25(11), 2706. https://doi.org/10.3390/molecules25112706










