Dehydrozingerone, a Curcumin Analog, as a Potential Anti-Prostate Cancer Inhibitor In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
2.1. DZG Inhibited Cell Proliferation of Castration-Resistant Prostate Cancer, PLS10 Cells
2.2. DZG Induced Cell Cycle Arrest in G1 Phase
2.3. In Vivo Antitumor Activity
2.4. In vivo Pharmacokinetics and Tissue Distribution
3. Discussion
4. Materials and Methods
4.1. Reagents
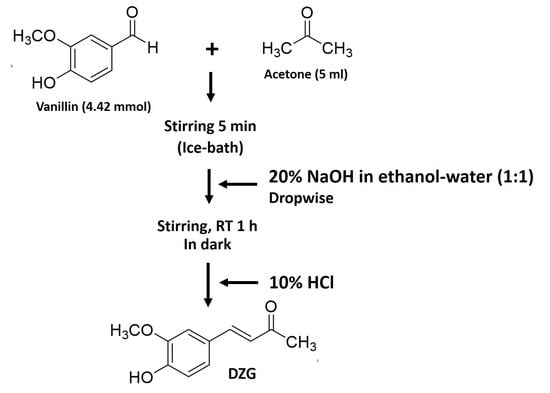
4.2. Synthesis of Dehydrozingerone (DZG)
4.3. Cell Culture
4.4. Animals
4.5. Cell Viability Assay
4.6. Cell Cycle Analysis
4.7. Western Blot Analysis
4.8. In Vivo Antitumor Activity
4.9. Immunohistochemistry
4.10. Terminal Deoxynucleotidyl Transferase dUTP Nick end Labeling (TUNEL) Assay
4.11. In Vivo Pharmacokinetics and Tissue Distributions
4.12. Serum and Tissue Concentrations of Cur and DZG
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virani, S.; Bilheem, S.; Chansaard, W.; Chitapanarux, I.; Daoprasert, K.; Khuanchana, S.; Leklob, A.; Pongnikorn, D.; Rozek, L.S.; Siriarechakul, S.; et al. National and Subnational Population-Based Incidence of Cancer in Thailand: Assessing Cancers with the Highest Burdens. Cancers (Basel) 2017, 9, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, I.; Day, T.K.; Tilley, W.D.; Selth, L.A. Androgen receptor signaling in castration-resistant prostate cancer: A lesson in persistence. Endocr.Relat. Cancer 2016, 23, 179–197. [Google Scholar] [CrossRef]
- Chang, Y.T.; Lin, T.P.; Campbell, M.; Pan, C.C.; Lee, S.H.; Lee, H.C.; Yang, M.H.; Kung, H.J.; Chang, P.C. REST is a crucial regulator for acquiring EMT-like and stemness phenotypes in hormone-refractory prostate cancer. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, W.; Madan, E.; Yee, M.; Zhang, H. Progress of molecular targeted therapies for prostate cancers. Biochim. Biophys. Acta 2012, 1825, 140–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Punfa, W.; Suzuki, S.; Pitchakarn, P.; Yodkeeree, S.; Naiki, T.; Takahashi, S.; Limtrakul, P. Curcumin-loaded PLGA nanoparticles conjugated with anti-P-glycoprotein antibody to overcome multidrug resistance. Asian Pac. J. Cancer Prev. 2014, 15, 9249–9258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shetty, D.; Kim, Y.J.; Shim, H.; Snyder, J.P. Eliminating the heart from the curcumin molecule: Monocarbonyl curcumin mimics (MACs). Molecules 2014, 20, 249–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, P.C.; Damu, A.G.; Cherng, C.Y.; Jeng, J.F.; Teng, C.M.; Lee, E.J.; Wu, T.S. Isolation of a natural antioxidant, dehydrozingerone from Zingiber officinale and synthesis of its analogues for recognition of effective antioxidant and antityrosinase agents. Arch. Pharmacal Res. 2005, 28, 518–528. [Google Scholar] [CrossRef]
- Hampannavar, G.A.; Karpoormath, R.; Palkar, M.B.; Shaikh, M.S. An appraisal on recent medicinal perspective of curcumin degradant: Dehydrozingerone (DZG). Bioorgan. Med. Chem. 2016, 24, 501–520. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, N.; Yamagami, C.; Tokuda, H.; Konoshima, T.; Okuda, Y.; Okuda, M.; Mukainaka, T.; Nishino, H.; Saito, Y. Inhibitory effects of dehydrozingerone and related compounds on 12-O-tetradecanoylphorbol-13-acetate induced Epstein-Barr virus early antigen activation. Cancer Lett. 1998, 134, 37–42. [Google Scholar] [CrossRef]
- Yogosawa, S.; Yamada, Y.; Yasuda, S.; Sun, Q.; Takizawa, K.; Sakai, T. Dehydrozingerone, a structural analogue of curcumin, induces cell-cycle arrest at the G2/M phase and accumulates intracellular ROS in HT-29 human colon cancer cells. J. Nat. Prod. 2012, 75, 2088–2093. [Google Scholar] [CrossRef]
- Baliga, M.S.; Haniadka, R.; Pereira, M.M.; Thilakchand, K.R.; Rao, S.; Arora, R. Radioprotective effects of Zingiber officinale Roscoe (ginger): Past, present and future. Food Funct. 2012, 3, 714–723. [Google Scholar] [CrossRef]
- Choi, J.G.; Kim, S.Y.; Jeong, M.; Oh, M.S. Pharmacotherapeutic potential of ginger and its compounds in age-related neurological disorders. Pharmacol. Ther. 2018, 182, 56–69. [Google Scholar] [CrossRef]
- Pavelyev, R.S.; Bondar, O.V.; Nguyen, T.N.T.; Ziganshina, A.A.; Al Farroukh, M.; Karwt, R.; Alekbaeva, G.D.; Pugachev, M.V.; Yamaleeva, Z.R.; Kataeva, O.N.; et al. Synthesis and in vitro antitumor activity of novel alkenyl derivatives of pyridoxine, bioisosteric analogs of feruloyl methane. Bioorgan. Med. Chem. 2018, 26, 5824–5837. [Google Scholar] [CrossRef]
- Tatsuzaki, J.; Bastow, K.F.; Nakagawa-Goto, K.; Nakamura, S.; Itokawa, H.; Lee, K.H. Dehydrozingerone, chalcone, and isoeugenol analogues as in vitro anticancer agents. J. Nat. Prod. 2006, 69, 1445–1449. [Google Scholar] [CrossRef] [Green Version]
- Mapoung, S.; Suzuki, S.; Fuji, S.; Naiki-Ito, A.; Kato, H.; Yodkeeree, S.; Ovatlarnporn, C.; Takahashi, S.; Limtrakul Dejkriengkraikul, P. Cyclohexanone curcumin analogs inhibit the progression of castration-resistant prostate cancer in vitro and in vivo. Cancer Sci. 2019, 110, 596–607. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Castillo, M.; Bonilla-Moreno, R.; Aleman-Lazarini, L.; Meraz-Rios, M.A.; Orozco, L.; Cedillo-Barron, L.; Cordova, E.J.; Villegas-Sepulveda, N. A Subpopulation of the K562 Cells Are Killed by Curcumin Treatment after G2/M Arrest and Mitotic Catastrophe. PLoS ONE 2016, 11, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Sha, J.; Li, J.; Wang, W.; Pan, L.; Cheng, J.; Li, L.; Zhao, H.; Lin, W. Curcumin induces G0/G1 arrest and apoptosis in hormone independent prostate cancer DU-145 cells by down regulating Notch signaling. Biomed. Pharmacother. 2016, 84, 177–184. [Google Scholar] [CrossRef]
- Parihar, V.K.; Dhawan, J.; Kumar, S.; Manjula, S.N.; Subramanian, G.; Unnikrishnan, M.K.; Rao, C.M. Free radical scavenging and radioprotective activity of dehydrozingerone against whole body gamma irradiation in Swiss albino mice. Chem. Biol. Interact. 2007, 170, 49–58. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Guha, S.; Krishnan, S.; Diagaradjane, P.; Gelovani, J.; Aggarwal, B.B. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007, 67, 3853–3861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaaf, C.; Shan, B.; Buchfelder, M.; Losa, M.; Kreutzer, J.; Rachinger, W.; Stalla, G.K.; Schilling, T.; Arzt, E.; Perone, M.J.; et al. Curcumin acts as anti-tumorigenic and hormone-suppressive agent in murine and human pituitary tumour cells in vitro and in vivo. Endocr. Relat. Cancer 2009, 16, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Banerjee, S.; Stafford, L.J.; Xia, C.; Liu, M.; Aggarwal, B.B. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene 2002, 21, 8852–8861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, S.; Ganapathy, S.; Chen, Q.; Srivastava, R.K. Curcumin sensitizes TRAIL-resistant xenografts: Molecular mechanisms of apoptosis, metastasis and angiogenesis. Mol. Cancer 2008, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, B.B.; Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef]
- Ning, X.; Guo, Y.; Ma, X.; Zhu, R.; Tian, C.; Wang, X.; Ma, Z.; Zhang, Z.; Liu, J. Synthesis and neuroprotective effect of E-3,4-dihydroxy styryl aralkyl ketones derivatives against oxidative stress and inflammation. Bioorganic Med. Chem. Lett. 2013, 23, 3700–3703. [Google Scholar] [CrossRef]
- Nakanishi, H.; Takeuchi, S.; Kato, K.; Shimizu, S.; Kobayashi, K.; Tatematsu, M.; Shirai, T. Establishment and characterization of three androgen-independent, metastatic carcinoma cell lines from 3,2′-dimethyl-4-aminobiphenyl-induced prostatic tumors in F344 rats. Jpn. J. Cancer Res. 1996, 87, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Naiki-Ito, A.; Kuno, T.; Punfa, W.; Long, N.; Kato, H.; Inaguma, S.; Komiya, M.; Shirai, T.; Takahashi, S. Establishment of a syngeneic orthotopic model of prostate cancer in immunocompetent rats. J. Toxicol. Pathol. 2015, 28, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Burton, J.B.; Priceman, S.J.; Sung, J.L.; Brakenhielm, E.; An, D.S.; Pytowski, B.; Alitalo, K.; Wu, L. Suppression of prostate cancer nodal and systemic metastasis by blockade of the lymphangiogenic axis. Cancer Res. 2008, 68, 7828–7837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.Z.; Ming-Tatt, L.; Lajis, N.H.; Sulaiman, M.R.; Israf, D.A.; Tham, C.L. Development and validation of a bioanalytical method for quantification of 2,6-bis-(4-hydroxy-3-methoxybenzylidene)-cyclohexanone (BHMC) in rat plasma. Molecules 2012, 17, 14555–14564. [Google Scholar] [CrossRef] [PubMed]






| Treatment Groups | Body Weights | Organ Weights | |
|---|---|---|---|
| Livers | Kidneys | ||
| Control | 26.75 ± 1.83 | 1.59 ± 0.20 | 0.40 ± 0.05 |
| Cur (30 mg/kg) | 26.71 ± 1.26 | 1.67 ± 0.10 | 0.40 ± 0.03 |
| DZG (30 mg/kg) | 27.12 ± 1.00 | 1.60 ± 0.09 | 0.39 ± 0.03 |
| Tissue | 30 min | 60 min | 180 min | |||
|---|---|---|---|---|---|---|
| Cur (µg/g) | DZG (µg/g) | Cur (µg/g) | DZG (µg/g) | Cur (µg/g) | DZG (µg/g) | |
| Liver | 2.60 ± 0.78 | 0.92 ± 0.67 | ND | 0.41 ± 0.11 | ND | ND |
| Kidneys | 1.01 ± 0.55 | 5.92 ± 3.80 | 0.53 ± 0.19 | 9.75 ± 4.08 | ND | 0.86 ± 0.22 |
| Lung | 1.15 ± 1.30 | 1.41 ± 0.74 | ND | 0.72 ± 0.28 | ND | ND |
Sample Availability: Samples are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mapoung, S.; Suzuki, S.; Fuji, S.; Naiki-Ito, A.; Kato, H.; Yodkeeree, S.; Sakorn, N.; Ovatlarnporn, C.; Takahashi, S.; Limtrakul, P. Dehydrozingerone, a Curcumin Analog, as a Potential Anti-Prostate Cancer Inhibitor In Vitro and In Vivo. Molecules 2020, 25, 2737. https://doi.org/10.3390/molecules25122737
Mapoung S, Suzuki S, Fuji S, Naiki-Ito A, Kato H, Yodkeeree S, Sakorn N, Ovatlarnporn C, Takahashi S, Limtrakul P. Dehydrozingerone, a Curcumin Analog, as a Potential Anti-Prostate Cancer Inhibitor In Vitro and In Vivo. Molecules. 2020; 25(12):2737. https://doi.org/10.3390/molecules25122737
Chicago/Turabian StyleMapoung, Sariya, Shugo Suzuki, Satoshi Fuji, Aya Naiki-Ito, Hiroyuki Kato, Supachai Yodkeeree, Natee Sakorn, Chitchamai Ovatlarnporn, Satoru Takahashi, and Pornngarm Limtrakul (Dejkriengkraikul). 2020. "Dehydrozingerone, a Curcumin Analog, as a Potential Anti-Prostate Cancer Inhibitor In Vitro and In Vivo" Molecules 25, no. 12: 2737. https://doi.org/10.3390/molecules25122737
APA StyleMapoung, S., Suzuki, S., Fuji, S., Naiki-Ito, A., Kato, H., Yodkeeree, S., Sakorn, N., Ovatlarnporn, C., Takahashi, S., & Limtrakul, P. (2020). Dehydrozingerone, a Curcumin Analog, as a Potential Anti-Prostate Cancer Inhibitor In Vitro and In Vivo. Molecules, 25(12), 2737. https://doi.org/10.3390/molecules25122737