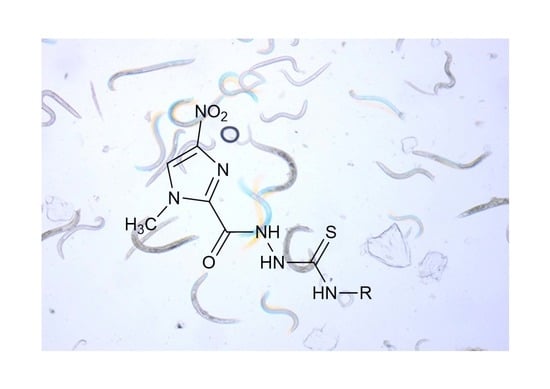
Synthesis and Anthelmintic Activity of New Thiosemicarbazide Derivatives—A Preliminary Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Anthelmintic Activity
3. Methods and Materials
3.1. The Procedure for the Synthesis of 1-[(1-Methyl-4-nitroimidazol-2-yl)carbonyl]-4-substituted-thiosemicarbazide
3.2. Anthelmintic Activity Assay
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Soil-Transmitted Helminth Infections. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 24 May 2020).
- World Health Organization. 2030 Targets for Soil-Transmitted Helminthiases Control Programmes. License: CC BY-NC-SA 3.0 IGO. Available online: https://apps.who.int/iris/handle/10665/330611 (accessed on 5 May 2020).
- Pullan, R.L.; Smith, J.L.; Jasrasaria, R.; Brooker, S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit. Vectors 2014, 7, 37. [Google Scholar] [CrossRef]
- Boatin, B.A.; Basáñez, M.G.; Prichard, R.K.; Awadzi, K.; Barakat, R.M.; García, H.H.; Gazzinelli, A.; Grant, W.N.; McCarthy, J.S.; N’Goran, E.K.; et al. A research agenda for helminth diseases of humans: Towards control and elimination. PLoS Negl. Trop. Dis. 2012, 6, e1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotez, P.J.; Brindley, P.J.; Bethony, J.M.; King, C.H.; Pearce, E.J.; Jacobson, J. Helminth infections: The great neglected tropical diseases. J. Clin. Investig. 2008, 118, 1311–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Guo, C.; Hansell, E.; Doyle, P.S.; Caffrey, C.R.; Holler, T.P.; McKerrow, J.H.; Cohen, F.E. Synthesis and structure−activity relationship study of potent trypanocidal thiosemicarbazone inhibitors of the trypanosomal cysteine protease cruzain. J. Med. Chem. 2002, 45, 2695–2707. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, D.C.; Mackey, Z.; Hansell, E.; Doyle, P.; Gut, I.; Caffrey, C.R.; Lehrnan, J.; Rosenthal, P.J.; Mc Kerrow, J.H.; Chibale, K. Synthesis and structure-activity relationship of parasiticidal thiosemicarbazone cysteine protease inhibitors against Plasmodium falciparum, Trypanosoma brucei and Trypanosoma crusi. J. Med. Chem. 2004, 47, 3212–3219. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.R.; Revankar, G.R.; Tolman, R.L. In vitro and in vivo activity of certain thiosemicarbazones against Trypanosoma cruzi. J. Med. Chem. 1974, 17, 760–761. [Google Scholar] [CrossRef]
- Klayman, D.L.; Bartosevich, J.F.; Griffin, T.S.; Mason, C.J.; Scovill, J.P. 2-Acetylpyridine thiosemicarbazones. 1. A new class of potential antimalarial agents. J. Med. Chem. 1979, 22, 855–862. [Google Scholar] [CrossRef]
- Siwek, A.; Staczek, P.; Wujec, M.; Bielawski, K.; Bielawska, A.; Paneth, P. Cytotoxic effect and molecular docking of 4-ethoxycarbonylmethyl-1-(piperidin-4-ylcarbonyl)-thiosemicarbazide–a novel topoisomerase II inhibitor. J. Mol. Model. 2013, 19, 1319–1324. [Google Scholar] [CrossRef] [Green Version]
- Coşkun, G.P.; Djikic, T.; Hayal, T.B.; Türkel, N.; Yelekçi, K.; Şahin, F.; Küçükgüzel, Ş.G. Synthesis, molecular docking and anticancer activity of diflunisal derivatives as cyclooxygenase enzyme inhibitors. Molecules 2018, 23, 1969. [Google Scholar] [CrossRef] [Green Version]
- Kaproń, B.; Czarnomysy, R.; Paneth, A.; Wujec, M.; Bielawski, K.; Bielawska, A.; Swiątek, Ł.; Rajtar, B.; Polz-Dacewicz, B.; Plech, T. Dual antibacterial and anticancer activity of 4-benzoyl-1-dichlorobenzoylthiosemicarbazide derivatives. Anti-Cancer Agents Med. Chem. 2018, 18, 529–540. [Google Scholar] [CrossRef]
- Umadevi, P.; Deepti, K.; Srinath, I.; Vijayalakshmi, G.; Tarakaramji, M. Synthesis and in-vitro antibacterial activity of some new urea, thiourea and thiosemicarbazide derivatives. Int. J. Pharm. Pharm. Sci. 2012, 4, 379–383. [Google Scholar]
- Paneth, A.; Stączek, P.; Plech, T.; Strzelczyk, A.; Janowska, D.; Stefańska, J.; Dziko, K.; Wujec, M.; Kosiek, S.; Paneth, P. Synthesis and antibacterial activity of 1,4-dibenzoylthiosemicarbazide derivatives. Biomed. Pharmacother. 2017, 88, 1235–1242. [Google Scholar] [CrossRef]
- Pitucha, M.; Karczmarzyk, Z.; Swatko-Ossor, M.; Wysocki, W.; Woś, M.; Chudzik, K.; Ginalska, G.; Fruziński, A. Synthesis, in vitro screening and docking studies of new thiosemicarbazide derivatives as antitubercular agent. Molecules 2019, 24, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paneth, A.; Stączek, P.; Plech, T.; Strzelczyk, A.; Dzitko, K.; Wujec, M.; Kuśmierz, E.; Kosikowska, U.; Grzegorczyk, A.; Paneth, P. Biological evaluation and molecular modelling study of thiosemicarbazide derivatives as bacterial type IIA topoisomerases inhibitors. J. Enzyme Inhib. Med. Chem. 2016, 31, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Gopalakrishnan, M.; Sureshkumar, P.; Thanusu, J.; Kanagarajan, V. Unusual formation of N-hydroxy-3,3-dimethyl-2,6-diarylpiperidin-4-one and its thiosemicarbazide derivative–synthesis and antimicrobial activity. Pharm. Chem. J. 2008, 42, 271–276. [Google Scholar] [CrossRef]
- Siddiqui, N.; Singh, O. Antibacterial activity of some 4-N-substituted thiosemicarbazides and thiosemicarbazones. Indian J. Pharm. Sci. 2003, 65, 423–425. [Google Scholar]
- Siwek, A.; Stefanska, J.; Dzitko, K.; Ruszczak, A. Antifungal effect of 4-arylthiosemicarbazides against Candida species. Search for molecular basis of antifungal activity of thiosemicarbazide derivatives. J. Mol. Model. 2012, 18, 4159–4170. [Google Scholar] [CrossRef] [Green Version]
- Wujec, M.; Kędzierska, E.; Kusmierz, E.; Plech, T.; Wróbel, A.; Paneth, A.; Orzelska, J.; Fidecka, S.; Paneth, P. Pharmacological and structure-activity relationship evaluation of 4-aryl-1-diphenylacetyl(thio)semicarbazides. Molecules 2014, 19, 4745–4759. [Google Scholar] [CrossRef] [Green Version]
- Paneth, A.; Węglińska, L.; Bekier, A.; Stefaniszyn, E.; Wujec, M.; Trotsko, N.; Hawrył, A.; Hawrył, M.; Dzitko, K. Discovery of potent and selective halogen-substituted imidazole-thiosemicarbazides for inhibition of Toxoplasma gondii growth in vitro via structure-based design. Molecules 2019, 24, 1618. [Google Scholar] [CrossRef] [Green Version]
- Paneth, A.; Węglińska, L.; Bekier, A.; Stefaniszyn, E.; Wujec, M.; Trotsko, N.; Dzitko, K. Systematic identification of thiosemicarbazides for inhibition of Toxoplasma gondii growth in vitro. Molecules 2019, 24, 614. [Google Scholar] [CrossRef] [Green Version]
- Naganagowda, G.; Padmashali, B. Synthesis, antimicrobial, and anthelmintic activities of some new 3-chlorobenzothiophene-2-carbonylchloride derivatives. Phosph. Sulfur Silicon 2010, 185, 1691–1700. [Google Scholar] [CrossRef]
- Kraouti, N.; Caujolle, R.; Labidalle, S.; Payard, M.; Loiseau, P.M.; Bories, C.; Gayral, P. Synthesis and nemotocidal activities of new analogs of pyrantel. Eur. J. Med. Chem. 1995, 30, 509–513. [Google Scholar] [CrossRef]
- Kenshi, S.; Moriya, Y.; Ishii, N. Effect of 5-fluorodeoxyuridine on reproduction and ageing in a strain of free-living nematodes, Rhabditidae tokai. Tokai J. Experim. Clin. 1979, 4, 159–164. [Google Scholar]
- Moeller, D.; Wenffen, W.; Wolff, H.R. 5-Nitrofurfural acetals. Orientating test for anthelmintic properties. Arch. Exp. Veterinarmed. 1968, 22, 133–137. [Google Scholar]
Sample Availability: Samples of the compounds 1–12 are available from the authors. |



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziduch, K.; Kołodziej, P.; Paneth, A.; Bogucka-Kocka, A.; Wujec, M. Synthesis and Anthelmintic Activity of New Thiosemicarbazide Derivatives—A Preliminary Study. Molecules 2020, 25, 2770. https://doi.org/10.3390/molecules25122770
Dziduch K, Kołodziej P, Paneth A, Bogucka-Kocka A, Wujec M. Synthesis and Anthelmintic Activity of New Thiosemicarbazide Derivatives—A Preliminary Study. Molecules. 2020; 25(12):2770. https://doi.org/10.3390/molecules25122770
Chicago/Turabian StyleDziduch, Katarzyna, Przemysław Kołodziej, Agata Paneth, Anna Bogucka-Kocka, and Monika Wujec. 2020. "Synthesis and Anthelmintic Activity of New Thiosemicarbazide Derivatives—A Preliminary Study" Molecules 25, no. 12: 2770. https://doi.org/10.3390/molecules25122770
APA StyleDziduch, K., Kołodziej, P., Paneth, A., Bogucka-Kocka, A., & Wujec, M. (2020). Synthesis and Anthelmintic Activity of New Thiosemicarbazide Derivatives—A Preliminary Study. Molecules, 25(12), 2770. https://doi.org/10.3390/molecules25122770