Protective Evaluation of Compounds Extracted from Root of Rhodiola rosea L. against Methylglyoxal-Induced Toxicity in a Neuronal Cell Line
Abstract
:1. Introduction
2. Results
2.1. Acetylcholinesterase Inhibitory Activity
2.2. Neuroprotective Activity
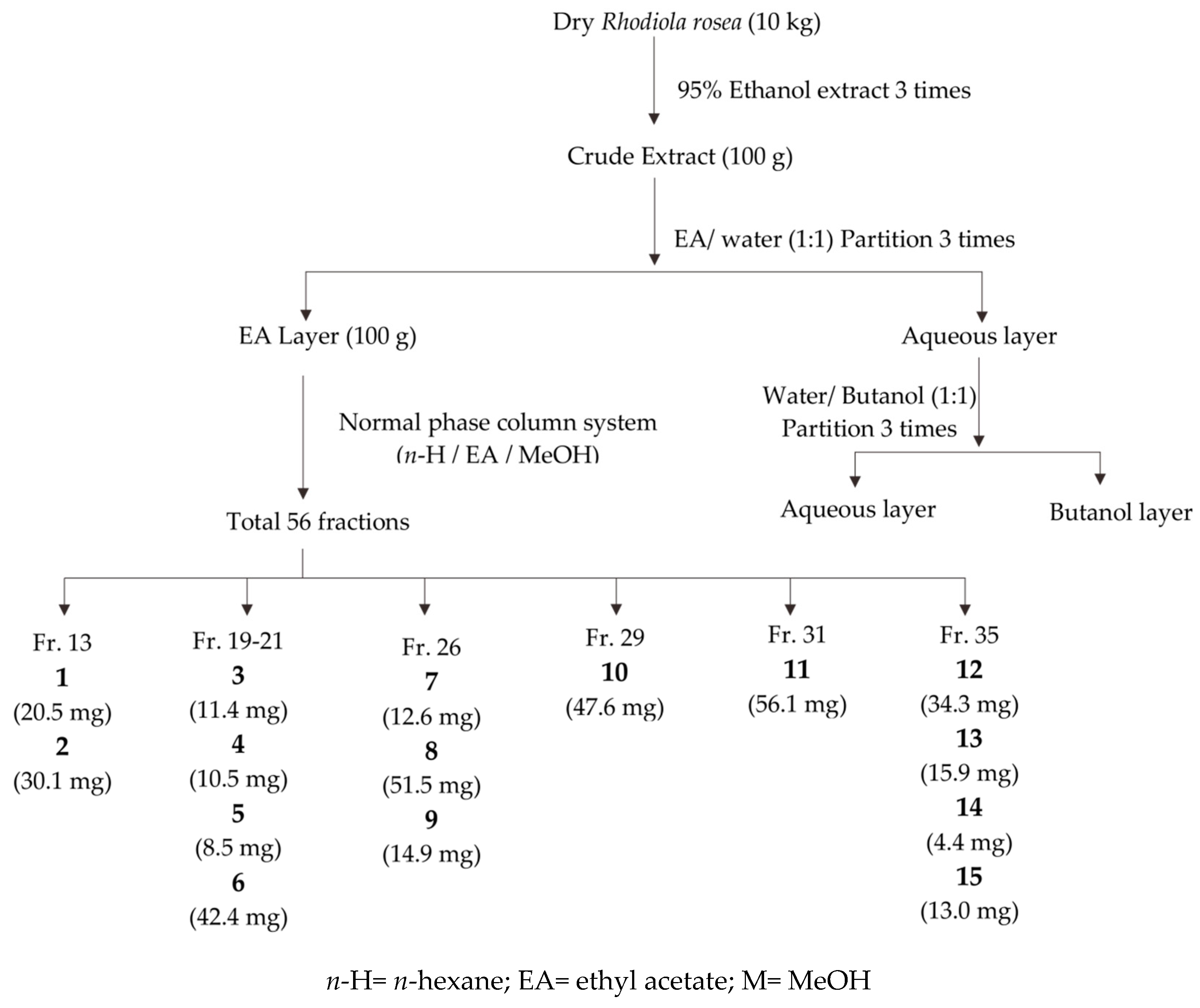
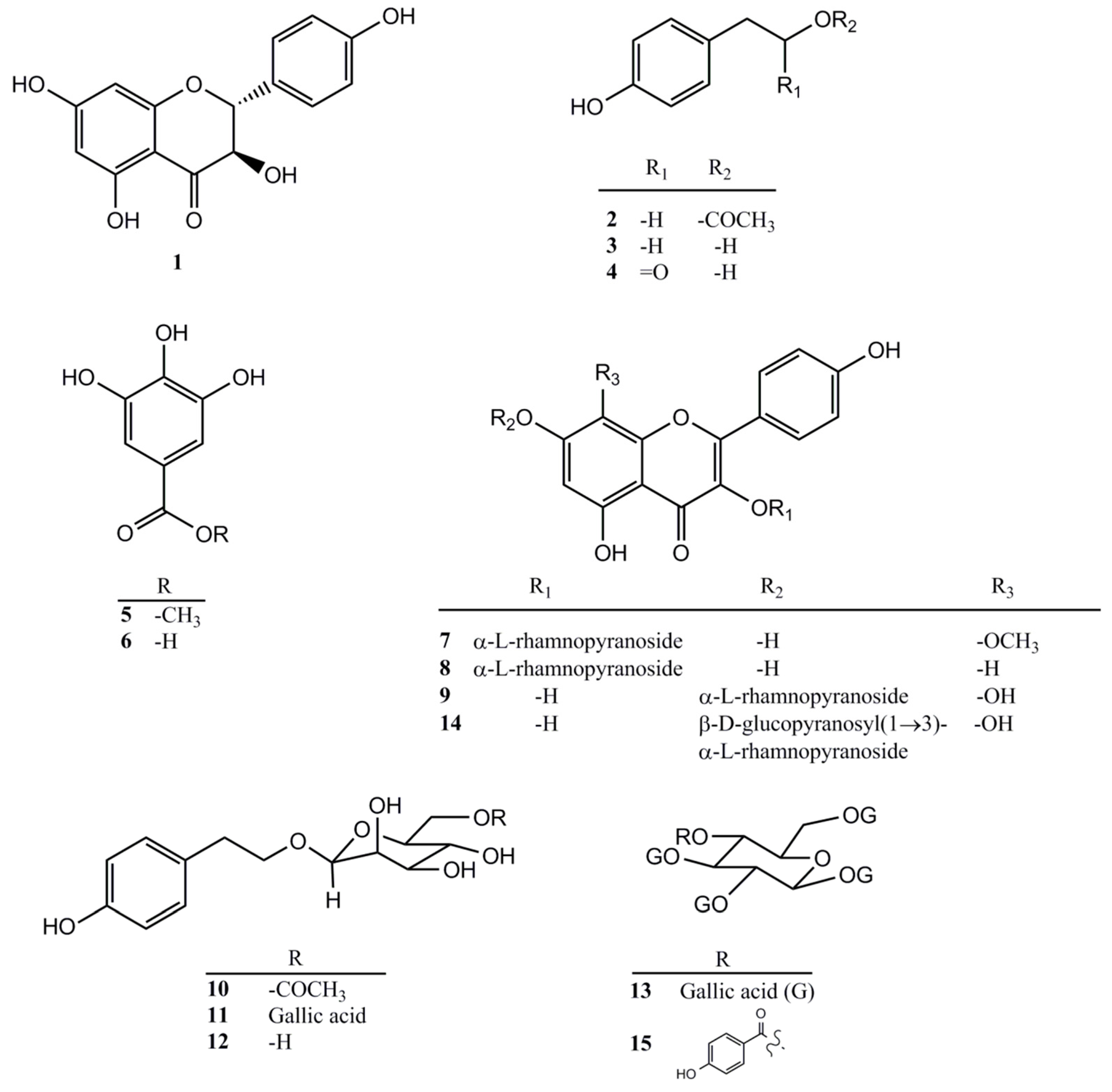
2.3. Compounds Isolated from the Ethyl Acetate Layer of R. rosea Root
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Materials
4.3. Extraction and Isolation of Compounds
4.4. Acetylcholinesterase Inhibitory Activity Assay
4.5. Cell Line and Culture
4.6. Cell Treatment
4.7. Assessment of Cell Viability
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, Its vascular complications, and other age-related diseases. Physiol. Rev. 2019, 100, 407–461. [Google Scholar] [CrossRef] [PubMed]
- Wetzels, S.; Vanmierlo, T.; Scheijen, J.L.J.M.; Horssen, J.V.; Amor, S.; Somers, V.; Schalkwijk, C.G.; Hendriks, J.J.A.; Wouters, K. Methylglyoxal-derived advanced glycation endproducts accumulate in multiple sclerosis lesions. Front. Immunol. 2019, 10, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, J.R.; Narayanasamy, P. Neuroprotection through flavonoid: Enhancement of the glyoxalase pathway. Redox Biology 2018, 14, 465–473. [Google Scholar] [CrossRef]
- Hansen, F.; Pandolfo, P.; Galland, F.; Torres, F.V.; Dutra, M.F.; Batassini, C.; Guerra, M.C.; Leite, M.C.; Gonçalves, C.A. Methylglyoxal can mediate behavioral and neurochemical alterations in rat brain. Physiol. Behav. 2016, 164, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.J.; Lee, Y.; Kim, A.H.; Lee, J. Methylglyoxal causes cell death in neural progenitor cells and impairs adult hippocampal neurogenesis. Neurotox. Res. 2016, 29, 419–431. [Google Scholar] [CrossRef]
- Lin, C.Y.; Sheu, J.J.; Tsai, I.S.; Wang, S.T.; Yang, L.Y.; Chang, H.W.; Lee, H.M.; Kao, S.H.; Lee, C.K.; Chenn, C.H.; et al. Elevated IgM against Nε-(Carboxyethyl)lysine-modified Apolipoprotein A1 peptide 141–147 in Taiwanese with Alzheimer’s disease. Clin. Biochem. 2018, 56, 75–82. [Google Scholar] [CrossRef]
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015, 9, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Khanna, K.; Mishra, K.P.; Ganju, L.; Singh, S.B. Golden root: A wholesome treat of immunity. Biomed. Pharmacother. 2017, 87, 496–502. [Google Scholar] [CrossRef]
- Palumbo, D.R.; Occhiuto, F.; Spadaro, F.; Circosta, C. Rhodiola rosea extract protects human cortical neurons against glutamate and hydrogen peroxide-induced cell death through reduction in the accumulation of intracellular calcium. Phytother. Res. 2012, 26, 878–883. [Google Scholar] [CrossRef]
- Pu, W.I.; Zhang, M.Y.; Bai, R.Y.; Sun, L.K.; Li, W.H.; Yu, Y.L.; Zhang, Y.; Song, L.; Wang, J.X.; Peng, Y.F.; et al. Anti-inflammatory effects of Rhodiola rosea L.: A review. Biomed. Pharmacother. 2020, 121, 1057–1063. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Simoneau, A.R.; Jafari, M.; Zi, X. Rhodiola rosea extracts and salidroside decrease the growth of bladder cancer cell lines via inhibition of the mTOR pathway and induction of autophagy. Mol. Carcinogen. 2012, 51, 257–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Liu, A.; Hou, R.; Zhang, J.; Jia, X.; Jiang, W.; Chen, J. Salidroside protects cardiomyocyte against hypoxia-induced death: A HIF-1α-activated and VEGF-mediated pathway. Eur. J. Pharm. 2009, 607, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.; Nalini, G.; Chidambaranathan, N. Neuroprotective effect of Rhodiola rosea Linn against MPTP induced cognitive impairment and oxidative stress. Ann. Neurosci. 2013, 20, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Braidy, N.; Orhan, I.E.; Badiee, A.; Daglia, M.; Nabavi, S.M.; Rhodiola rosea, L.; Rhodiola rosea L. and Alzheimer’s Disease: From Farm to Pharmacy. Phytother. Res. 2016, 30, 532–539. [Google Scholar] [CrossRef]
- Zhou, T.; Zheng, J.; Zhou, Y.; Li, S.; Gan, R.Y.; Li, H.B.; Gan, R.Y. Chemical components and bioactivities of Rhodiola rosea. Int. J. Tradit. Nat. Med. 2015, 5, 23–51. [Google Scholar]
- Lima, B.R.; Lima, J.M.; Maciel, J.B.; Valentim, C.Q.; Nunomura, R.C.S.; Lima, E.S.; Koolen, H.H.F.; Souza, A.D.L.; Pinheiro, M.B.; Cass, Q.B.; et al. Synthesis and inhibition evaluation of new benzyltetrahydroprotoberberine alkaloids designed as acetylcholinesterase inhibitors. Front. Chem. 2019, 7, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Santos, T.C.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; Paes, A.M.A. Naturally occurring acetylcholinesterase inhibitors and their potential use for Alzheimer’s disease therapy. Front. Pharmacol. 2018, 9, 1192–1205. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Jung, J.C.; Jang, S.; Kim, J.; Ali, Z.; khan, I.A.; Oh, S. Anti-Inflammatory and neuroprotective effects of constituents isolated from Rhodiola rosea. Evid-Based Complement. Altern. Med. 2013, 2013, 514049–514057. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.K.; Jalewa, J.; Hölscher, C. Neuroprotective and anti-apoptotic effects of liraglutide on SH-SY5Y cells exposed to methylglyoxal stress. J. Neurochem. 2014, 128, 459–471. [Google Scholar] [CrossRef]
- Huang, S.M.; Chuang, H.C.; Wu, C.H.; Yen, G.C. Cytoprotective effects of phenolic acids on methylglyoxal-induced apoptosis in Neuro-2A cells. Mol. Nutr. Food Res. 2008, 52, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Thibault, C.S.L.; Arseneault, M.; Longpre, F.; Ramassamy, C. Tyrosol and hydroxytyrosol two main components of olive oil, protect N2a cells against Amyloid-β-Induced toxicity. Involvement of the NF-κB signaling. Curr. Alzheimer Res. 2011, 8, 543–551. [Google Scholar] [CrossRef]
- Wang, Y.M.; Ming, W.Z.; Liang, H.; Wang, Y.J.; Zhang, Y.H.; Meng, D.L. Isoquinolines from national herb Corydalis tomentella and neuroprotective effect against lipopolysaccharide-induced BV2 microglia cells. Bioorganic Chem. 2020, 95, 103489–103495. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Liu, W.Y.; Liou, S.S.; Liu, I.M. The protective effects of moscatilin against methylglyoxal-induced neurotoxicity via the regulation of p38/JNK MAPK pathways in PC12 neuron-like cells. Food Chem. Toxicol. 2020, 140, 111369–111379. [Google Scholar] [CrossRef] [PubMed]
- Cásedas, G.; Les, F.; Choya-Foces, C.; Hugo, M.; López, V. The metabolite Urolithin-A ameliorates oxidative stress in Neuro-2a cells, becoming a potential neuroprotective agent. Antioxidants 2020, 9, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, J.; Rajamma, U.; Jana, N.; Mohanakumar, K.P. Quercetin improves the activity of the ubiquitin-proteasomal system in 150Q mutated huntingtin-expressing cells but exerts detrimental effects on neuronal survivability. J. Neurosci. Res. 2015, 93, 1581–1591. [Google Scholar] [CrossRef]
- Liao, C.R.; Kuo, Y.H.; Ho, Y.L.; Wang, C.Y.; Yang, C.S.; Lin, C.W.; Chang, Y.S. Studies on cytotoxic constituents from the leaves of Elaeagnus oldhamii maxim. in non-small cell lung cancer A549 cells. Molecules 2014, 19, 9515–9534. [Google Scholar] [CrossRef] [Green Version]
- Mateos, R.; Espartero, J.L.; Trujillo, M.; Rıos, J.J.; Camacho, M.L.; Alcudia, F.; Cert, A. Determination of phenols, flavones, and lignans in virgin olive oils by solid-phase extraction and high-performance liquid chromatography with diode array ultraviolet detection. J. Agric. Food Chem. 2001, 49, 2185–2190. [Google Scholar] [CrossRef]
- Yu, H.L.; Xu, J.H.; Wang, Y.X.; Lu, W.Y.; Lin, G.Q. Assembly of a three-dimensional array of glycoconjugates by combinatorial biocatalysis in nonaqueous media. J. Comb. Chem. 2008, 10, 79–87. [Google Scholar] [CrossRef]
- Guney, T.; Kohles, S.A.; Thompson, V.L.; Phillips, G.J.; Kraus, G.A. Heterocycles from wine: Synthesis and biological evaluation of salidrosides. Tetrahedron 2015, 17, 3115–3119. [Google Scholar] [CrossRef]
- Sudjaroen, Y.; Hull, W.E.; Erben, G.; Würtele, G.; Changbumrung, S.; Ulrich, C.M.; Owen, R.W. Isolation and characterization of ellagitannins as the major polyphenolic components of longan (Dimocarpus longan Lour) seeds. Phytochemistry 2012, 77, 226–237. [Google Scholar] [CrossRef] [PubMed]
- An, J.P.; Dang, L.H.; Ha, T.K.Q.; Pham, H.T.T.; Lee, B.W.; Lee, C.H.; Oh, W.K. Flavone glycosides from Sicyos angulatus and their inhibitory effects on hepatic lipid accumulation. Phytochemistry 2019, 157, 53–63. [Google Scholar] [CrossRef]
- Jeong, H.J.; Ryu, Y.B.; Park, S., J.; Kim, J.H.; Kwon, H.J.; Kim, J.H.; Park, K.H.; Rho, M.C.; Lee, W.S. Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorg. Med. Chem. 2009, 17, 6816–6823. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Mabry, T.J.; Matlin, S.A. Flavonoids of the flowers of Silybum marianum. Phytochemistry 1989, 28, 1751–1753. [Google Scholar] [CrossRef]
- Nonaka, G.; Nishimura, H.; Nishioka, I. Tannins and related compounds. IV. Seven new phenol glucoside gallates from Quercus stenophylla Makino. Chem. Pharm. Bull. 2011, 30, 2061–2067. [Google Scholar] [CrossRef] [Green Version]
- Mageed, W.M.A.; Bayoumi, S.A.H.; Chen, C.; Vavricka, C.J.; Li, L.; Malik, A.; Dai, H.; Song, F.; Wang, L.; Zhang, J.; et al. Benzophenone C-glucosides and gallotannins from mango tree stem bark with broad-spectrum anti-viral activity. Bioorg. Med. Chem. 2014, 22, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Yue, L.; Dang, X.; Chen, F.; Gong, Y.; Lin, X.; Luo, Y. Rosenroot (Rhodiola): Potential applications in Aging-related diseases. Aging Dis. 2019, 10, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Ma, H.; Dasilva, N.A.; Rose, K.N.; Johnson, S.L.; Zhang, L.; Wan, C.; Dain, J.A.; Seeram, N.P. Development of a neuroprotective potential algorithm for medicinal plants. Neurochem. Int. 2016, 100, 164–177. [Google Scholar] [CrossRef]
- Hillhouse, B.; Ming, D.S.; French, C.; Towers, G.H. Acetylcholine esterase inhibitors in Rhodiola rosea. Pharm. Biol. 2004, 42, 68–72. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, G.; Gao, X.; Wang, Y.; Yao, W. Acetylcholinesterase inhibitory-active components of Rhodiola rosea L. Food Chem. 2007, 105, 24–27. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Sun, Y.; Lin, X.; Chen, B.; Tan, T.; Cao, G.; Wang, Z. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur. J. Pharmacol. 2007, 564, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Wang, B.; Feng, D.; Zhang, W.; Lu, F.; Lai, J.; Huang, L.; Nie, T.; Yang, Q. Salidroside protects against 6-hydroxydopamine-induced cytotoxicity by attenuating ER stress. Neurosci. Bull. 2016, 32, 61–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J.; Li, W.; Wang, H.; Yan, W.; Zhou, Y.; Wang, G.; Cui, J.; Wang, F. Antitumor effects of a purified polysaccharide from Rhodiola rosea and its action mechanism. Carbohydr. Polym. 2012, 90, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.Z.; Lu, A.X.; Zhang, K.F.; Li, B.J. Anticancer effect of salidroside on A549 lung cancer cells through inhibition of oxidative stress and phospho-p38 expression. Oncol. Lett. 2014, 7, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yao, Y.; Wang, H.; Guo, Y.; Zhang, H.; Chen, L. Effects of salidroside on glioma formation and growth inhibition together with improvement of tumor microenvironment. Chin. J. Cancer Res. 2013, 25, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Napolitano, M.; Tedesco, I.; Moccia, S.; Milito, A.; Russo, G.L. Neuroprotective role of natural polyphenols. Curr. Top. Med. Chem. 2016, 1943–1950. [Google Scholar] [CrossRef]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 2001–2039. [Google Scholar] [CrossRef] [Green Version]
- Atochin, D.N.; Chernysheva, G.A.; Smolyakova, V.I.; Osipenko, A.N.; Logvinov, S.V.; Zhdankina, A.A.; Sysolyatin, S.V.; Kryukov, Y.A.; Anfinogenova, Y.; Plotnikova, T.M.; et al. Neuroprotective effects of p-tyrosol after the global cerebral ischemia in rats. Phytomedicine 2016, 23, 784–792. [Google Scholar] [CrossRef]
- Park, W.; Chang, M.S.; Kim, H.; Choi, H.Y.; Yang, W.M.; Kim, D.R.; Park, E.H.; Park, S.K. Cytotoxic effect of gallic acid on testicular cell lines with increasing H2O2 level in GC-1 spg cells. Toxicol. In Vitro 2008, 22, 159–163. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Tsai, Y.F.; Yang, D.J.; Ngo, T.H.; Shih, C.H.; Wu, Y.F.; Lee, C.K.; Phraekanjanavichid, V.; Yen, S.F.; Kao, S.H.; Lee, H.M.; et al. Ganglioside Hp-s1 analogue inhibits amyloidogenic toxicity in Alzheimer’s disease model cells. ACS Chem. Neurosci. 2019, 10, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Ting, L.L.; Lu, H.T.; Yen, S.F.; Ngo, T.H.; Tu, F.Y.; Tsai, I.S.; Tsai, Y.H.; Chang, F.Y.; Li, X.Z.; Li, S.; et al. Expression of AHI1 rescues amyloidogenic pathology in Alzheimer’s disease model cells. Mol. Neurobiol. 2019, 56, 7572–7582. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–15 are available from the authors. |



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-H.; Safwan, S.; Cheng, M.-C.; Liao, T.-Y.; Cheng, L.-C.; Chen, T.-A.; Kuo, Y.-H.; Lin, Y.-F.; Lee, C.-K. Protective Evaluation of Compounds Extracted from Root of Rhodiola rosea L. against Methylglyoxal-Induced Toxicity in a Neuronal Cell Line. Molecules 2020, 25, 2801. https://doi.org/10.3390/molecules25122801
Wang C-H, Safwan S, Cheng M-C, Liao T-Y, Cheng L-C, Chen T-A, Kuo Y-H, Lin Y-F, Lee C-K. Protective Evaluation of Compounds Extracted from Root of Rhodiola rosea L. against Methylglyoxal-Induced Toxicity in a Neuronal Cell Line. Molecules. 2020; 25(12):2801. https://doi.org/10.3390/molecules25122801
Chicago/Turabian StyleWang, Cheng-Hao, Safwan Safwan, Min-Chi Cheng, Te-Yu Liao, Lin-Chen Cheng, Ting-An Chen, Yueh-Hsiung Kuo, Yung-Feng Lin, and Ching-Kuo Lee. 2020. "Protective Evaluation of Compounds Extracted from Root of Rhodiola rosea L. against Methylglyoxal-Induced Toxicity in a Neuronal Cell Line" Molecules 25, no. 12: 2801. https://doi.org/10.3390/molecules25122801





