Empowering the Medicinal Applications of Bisphosphonates by Unveiling their Synthesis Details
Abstract
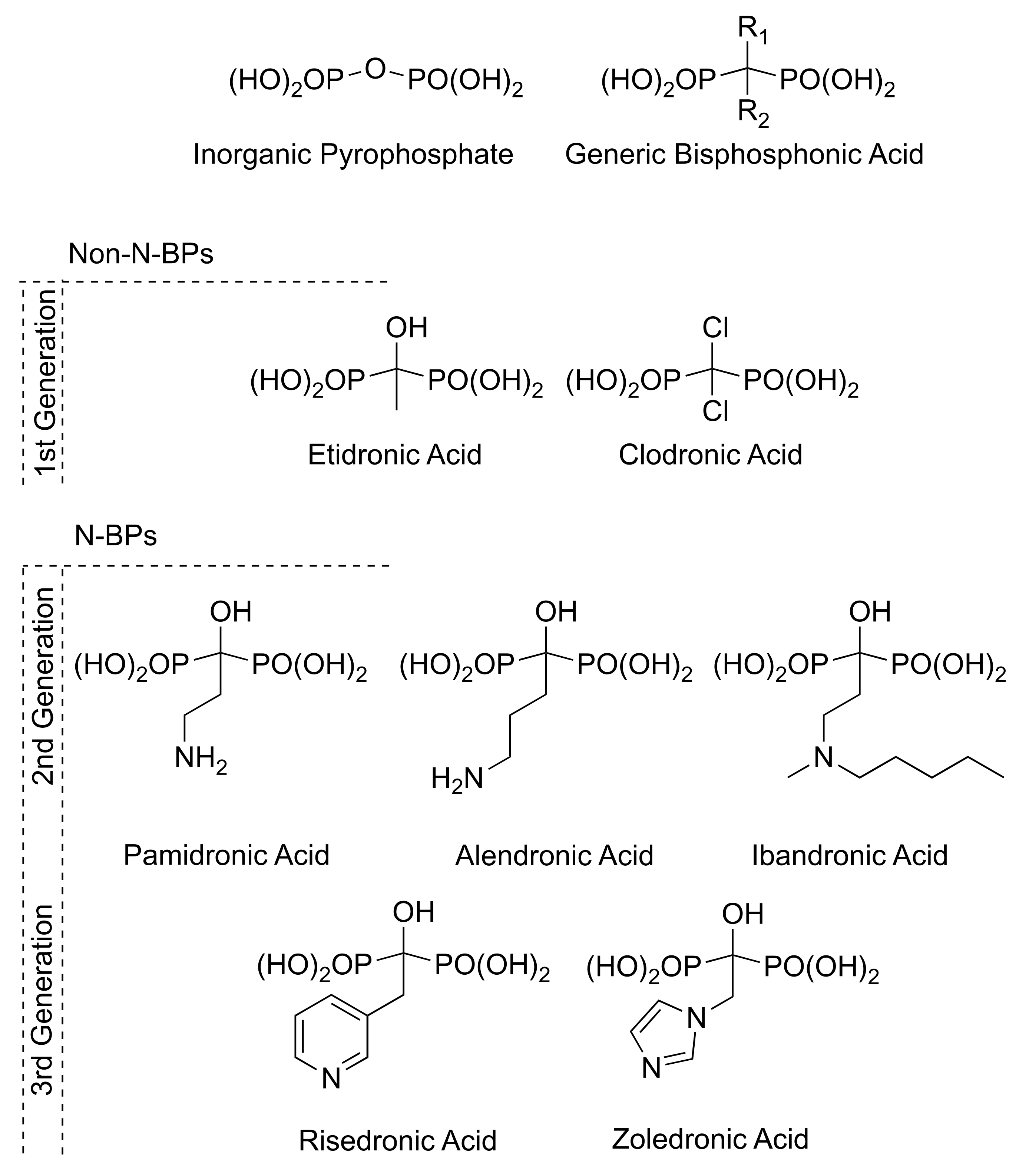
:1. Introduction
2. Bisphosphonate Applications
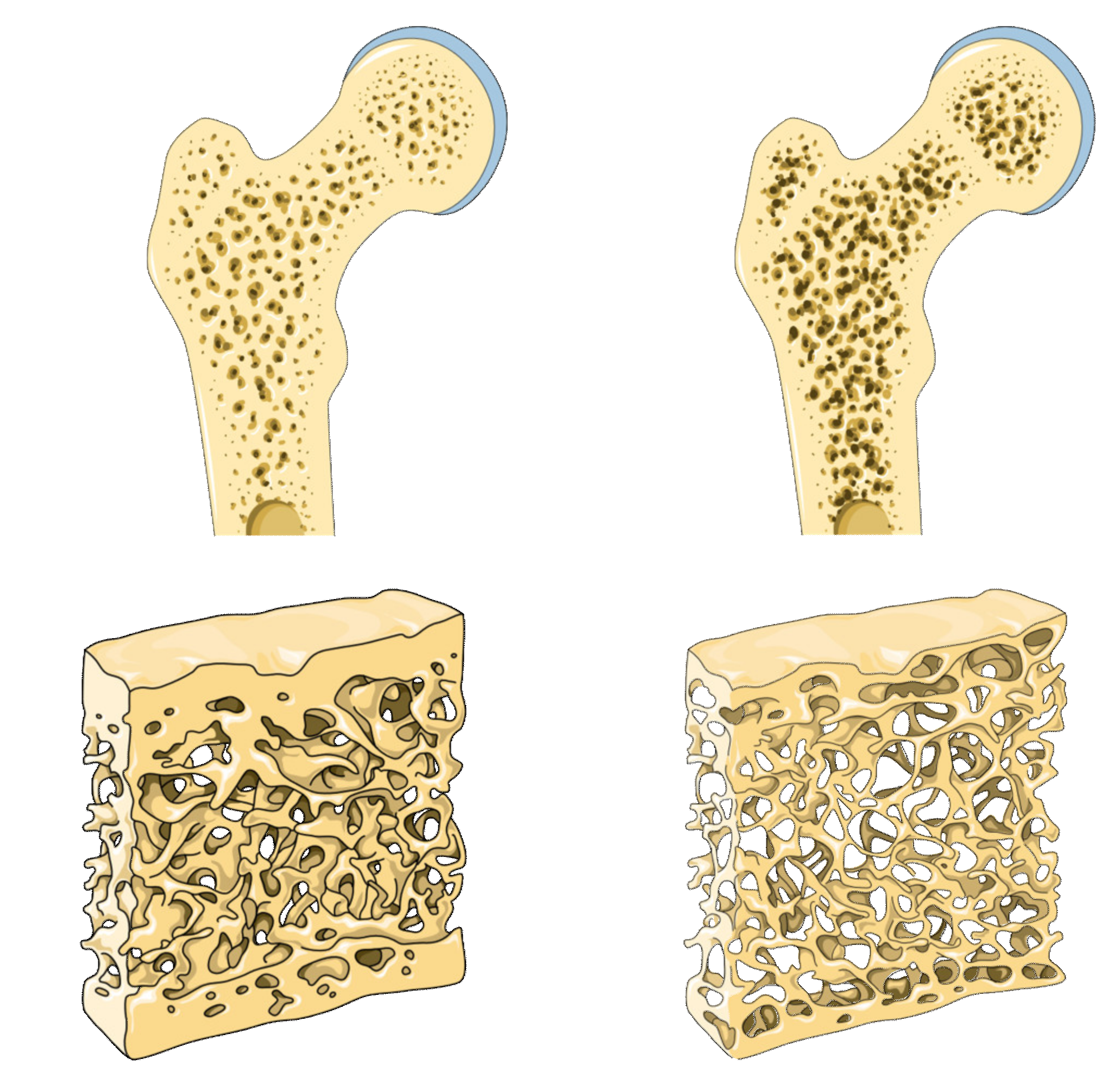
2.1. Worldwide use in Osteoporosis Treatment
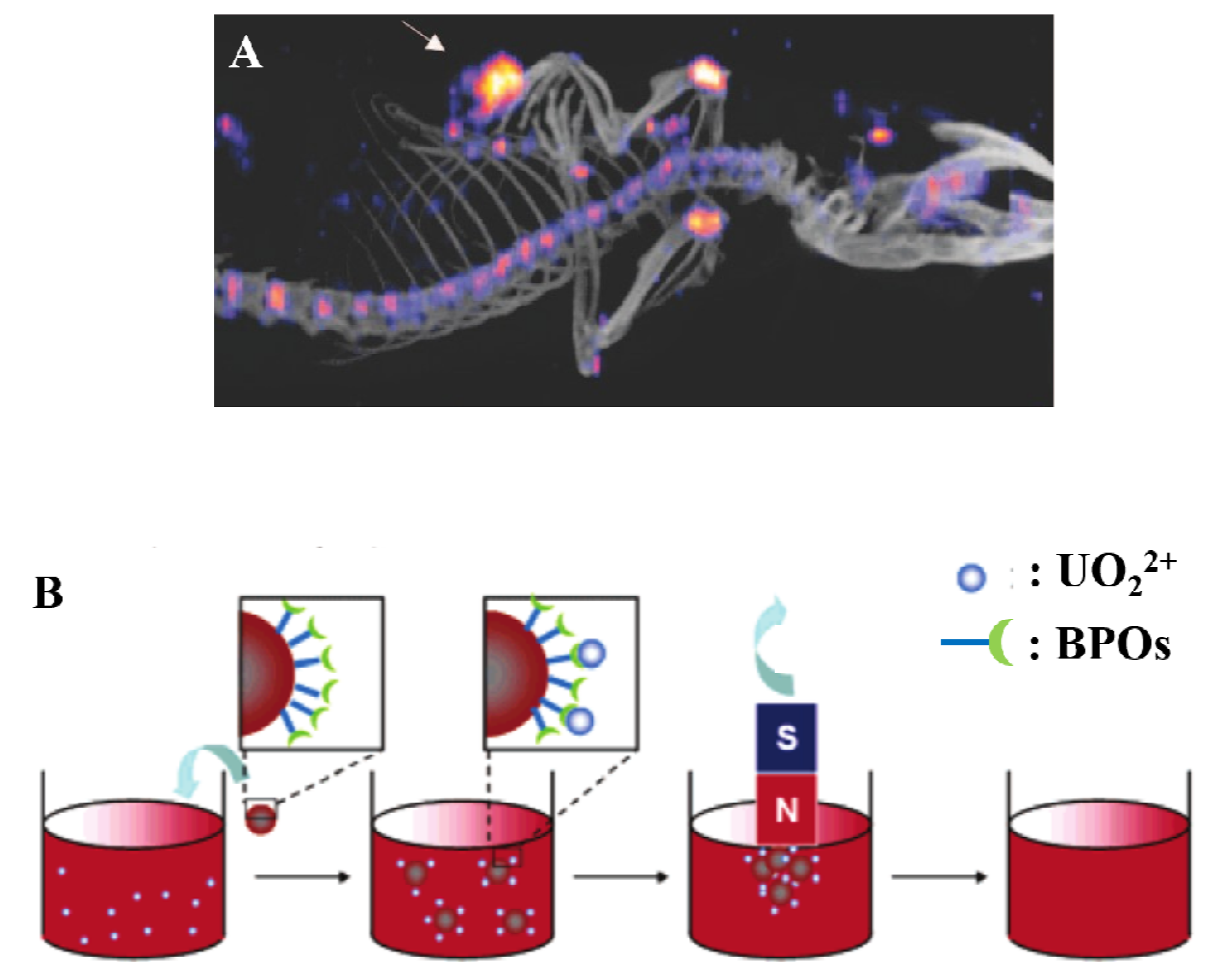
2.2. Other Pharmaceutical Uses
2.3. Seeking Novel Uses for BPs
3. Synthesis of Bisphosphonates
3.1. Solvent Selection
3.1.1. Chlorobenzene
3.1.2. Methanesulfonic Acid (MSA)
3.1.3. Sulfolane
3.1.4. Polyalkylene Glycols
3.1.5. Ionic Liquids (ILs)
3.1.6. Other Solvents
3.1.7. General Remarks
3.2. Selection of the Molar Ratios of the P-Reactants
3.2.1. Synthesis in MSA
3.2.2. Synthesis in MSA vs. Sulfolane
3.2.3. Synthesis in MSA vs. Sulfolane vs. Ionic Liquids vs. Sulfolane & Ionic Liquids
3.2.4. General Remarks
3.3. Innovative Processes
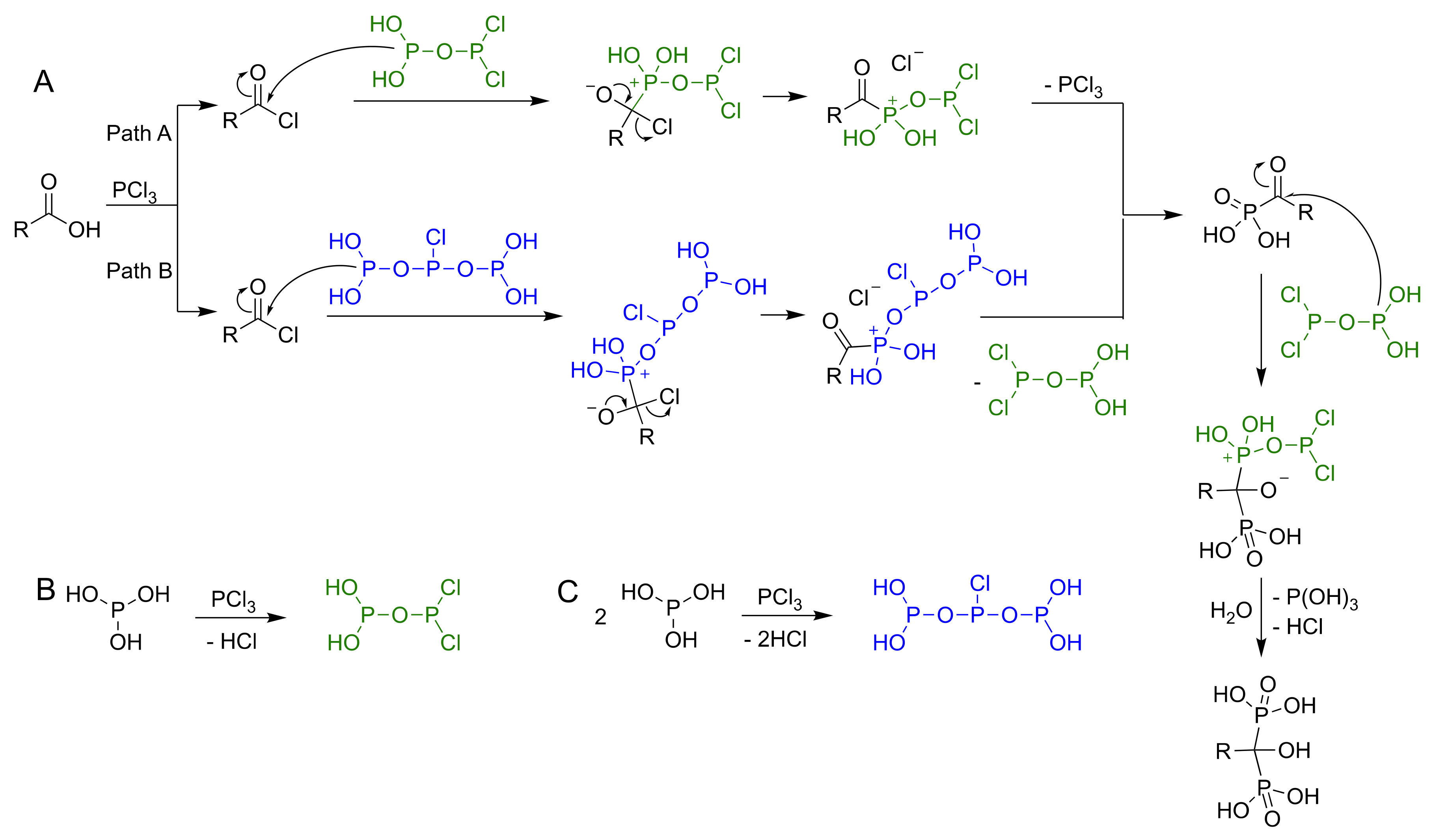
3.4. A Mechanistic Overview of the Synthesis
3.5. The Challenge of Reaction Workup to Achieve Pure Bisphosphonates
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMP | Adenosine Monophosphate |
| API | Active Pharmaceutical Ingredients |
| ATP | Adenosine triphosphate |
| bmim | 1-Butyl-3-methylimidazolium |
| BP | Bisphosphonates |
| DIC | Diclofenac |
| DMEU | N5N′- dimethylethyleneurea |
| H3PO3 | Phosphorous acid |
| HAP | Hydroxyapatite |
| IL | Ionic liquids |
| IV | Intravenous |
| MSA | Methanesulfonic acid |
| MWAS | Microwave-assisted synthesis |
| N-BPs | Nitrogen-containing BPs |
| non-N-BPs | Non-Nitrogen-containing BPs |
| PCl3 | Phosphorous trichloride |
| PEG | Polyethylene glycol |
| PPi | Inorganic pyrophosphate |
| RCOOH | Carboxylic acid |
| SPECT/CT | Single-photon emission computed tomography |
| THF | Tetrahydrofuran |
| TMSBr | Trimethylbromosilane |
References
- U.S. Food and Drug Administration. Drugs@FDA: FDA Approved Drug Products. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm (accessed on 2 June 2020).
- Russell, R.; Rogers, M. Bisphosphonates: from the laboratory to the clinic and back again. Bone 1999, 25, 97–106. [Google Scholar] [CrossRef]
- Keglevich, G.; Grün, A.; Aradi, K.; Garadnay, S.; Greiner, I. Optimized synthesis of N-heterocyclic dronic acids; closing a black-box era. Tetrahedron Lett. 2011, 52, 2744–2746. [Google Scholar] [CrossRef]
- Keglevich, G.; Grün, A.; Garadnay, S.; Greiner, I. Rational synthesis of dronic acid derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 2116–2124. [Google Scholar] [CrossRef]
- R Hudson, H.; J Wardle, N.; WA Bligh, S.; Greiner, I.; Grun, A.; Keglevich, G. N-Heterocyclic dronic acids: applications and synthesis. Mini Rev. Med. Chem. 2012, 12, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Nagy, D.I.; Grün, A.; Garadnay, S.; Greiner, I.; Keglevich, G. Synthesis of hydroxymethylenebisphosphonic acid derivatives in different solvents. Molecules 2016, 21, 1046. [Google Scholar] [CrossRef] [Green Version]
- Slater, C.S.; Savelski, M. A method to characterize the greenness of solvents used in pharmaceutical manufacture. J. Environ. Sci. Health Part A 2007, 42, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.T.; Shonnard, D.R. Green engineering: environmentally conscious design of chemical processes and products. Am. Inst. Chem. Eng. J. 2001, 47, 1906–1910. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Curzons, A.D.; Constable, D.J.; Cunningham, V.L. Cradle-to-gate life cycle inventory and assessment of pharmaceutical compounds. Int. J. Life Cycle Assess. 2004, 9, 114–121. [Google Scholar] [CrossRef]
- Nagy, D.I.; Grün, A.; Garadnay, S.; Greiner, I.; Keglevich, G. Investigation of the effect of medium in the preparation of alendronate: till now the best synthesis in the presence of an ionic liquid additive. Heteroat. Chem. 2017, 28, e21370. [Google Scholar] [CrossRef]
- Kovács, R.; Grün, A.; Garadnay, S.; Greiner, I.; Keglevich, G. “Greener” synthesis of bisphosphonic/dronic acid derivatives. Green Process. Synth. 2014, 3, 111–116. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor 25 years on: the rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Li, J.; Simmons, E.M.; Eastgate, M.D. A data-driven strategy for predicting greenness scores, rationally comparing synthetic routes and benchmarking PMI outcomes for the synthesis of molecules in the pharmaceutical industry. Green Chem. 2017, 19, 127–139. [Google Scholar] [CrossRef]
- Petroianu, G. Pharmacist Theodor Salzer (1833–1900) and the discovery of bisphosphonates. Die Pharm. Int. J. Pharm. Sci. 2011, 66, 804–807. [Google Scholar]
- Russell, R.G.G. Bisphosphonates: the first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Fleish, H.; Neuman, W.F. Mechanisms of calcification: role of collagen, polyphosphates, and phosphatase. Am. J. Physiol. Leg. Content 1961, 200, 1296–1300. [Google Scholar] [CrossRef]
- Fleisch, H.; Russell, R.; Bisaz, S.; Mühlbauer, R.; Williams, D. The inhibitory effect of phosphonates on the formation of calcium phosphate crystals in vitro and on aortic and kidney calcification in vivo. Eur. J. Clin. Investig. 1970, 1, 12–18. [Google Scholar] [CrossRef]
- Fleisch, H.; Graham, R.; Russell, G.; Francis, M.D. Diphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture and in vivo. Science 1969, 165, 1262–1264. [Google Scholar] [CrossRef]
- Vasikaran, S.D. Bisphosphonates: an overview with special reference to alendronate. Ann. Clin. Biochem. 2001, 38, 608–623. [Google Scholar] [CrossRef] [Green Version]
- Teitelbaum, S.L. Bone Resorption by Osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Rogers, M.J.; Crockett, J.C.; Coxon, F.P.; Mönkkönen, J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone 2011, 49, 34–41. [Google Scholar] [CrossRef]
- Ebetino, F.H.; Hogan, A.-M.L.; Sun, S.; Tsoumpra, M.K.; Duan, X.; Triffitt, J.T.; Kwaasi, A.A.; Dunford, J.E.; Barnett, B.L.; Oppermann, U. The relationship between the chemistry and biological activity of the bisphosphonates. Bone 2011, 49, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Seibel, M.J.; Robins, S.P.; Bilezikian, J.P. Dynamics of Bone and Cartilage Metabolism: Principles and Clinical Applications, 2nd ed.; Seibel, M.J., Robins, S.P., Bilezikian, J.P., Eds.; Academic Press: Burlington, MA, USA, 2006; p. 920. [Google Scholar]
- Ralston, S.H. Bone structure and metabolism. Medicine 2013, 41, 581–585. [Google Scholar] [CrossRef]
- Manolagas, S.C.; Jilka, R.L. Bone Marrow, Cytokines, and Bone Remodeling—Emerging Insights into the Pathophysiology of Osteoporosis. New Engl. J. Med. 1995, 332, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Holroyd, C.; Cooper, C.; Dennison, E. Epidemiology of osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Seibel, M.J. Biochemical Markers of Bone Turnover Part I: Biochemistry and Variability. Clin. Biochemist. Rev./Aust. Assoc. Clin. Biochemists. 2005, 26, 97–122. [Google Scholar]
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: medical management, epidemiology and economic burden. Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riggs, B.L.; Melton, L.J. The worldwide problem of osteoporosis: Insights afforded by epidemiology. Bone 1995, 17, S505–S511. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. Bone Health and Osteoporosis; A Report of the Surgeon General; Office of the Surgeon General (US): Rockville, MD, USA, 2004. [Google Scholar]
- SMART-Servier Medical ART: Anatomy and the Human Body. By Les Laboratoires Servier. Available online: https://smart.servier.com/ (accessed on 21 May 2020).
- Baron, R.; Ferrari, S.; Russell, R.G.G. Denosumab and bisphosphonates: Different mechanisms of action and effects. Bone 2011, 48, 677–692. [Google Scholar] [CrossRef]
- FDA Database of Approved Drug Products. AREDIA(R) (Pamidronate disodium). Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020927 (accessed on 2 June 2020).
- FDA Database of Approved Drug Products. Available online: https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases (accessed on 2 June 2020).
- FDA Database of Approved Drug Products. FOSAMAX(R) (Alendronate sodium). Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020560 (accessed on 2 June 2020).
- European Medicines Agency: Science, Medicines, Health. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/bisphosphonates (accessed on 12 June 2020).
- FDA Database of Approved Drug Products. BONIVA(R) (Ibandronate sodium). Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021455 (accessed on 2 June 2020).
- FDA Database of Approved Drug Products. ACTONEL(R) (Risedronate sodium). Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020835 (accessed on 2 June 2020).
- FDA Database of Approved Drug Products. ZOMETA(R) (Zoledronic acid). Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021223 (accessed on 2 June 2020).
- FDA Database of Approved Drug Products. RECLAST(R) (Zoledronic acid). Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021817 (accessed on 2 June 2020).
- Hechmati, G.; Cure, S.; Gouepo, A.; Hoefeler, H.; Lorusso, V.; Lüftner, D.; Duran, I.; Garzon-Rodriguez, C.; Ashcroft, J.; Wei, R. Cost of skeletal-related events in European patients with solid tumours and bone metastases: data from a prospective multinational observational study. J. Med Econ. 2013, 16, 691–700. [Google Scholar] [CrossRef]
- European Medicines Agency: Science, Medicines, Health. Questions and answers on the review of bisphosphonates and atypical stress fractures. 13 July 2011 (EMA/288359/2011 Rev.1). Available online: https://www.ema.europa.eu/en/medicines/human/referrals/bisphosphonates (accessed on 17 June 2020).
- FDA, U.S. Food and Drug Administration. USP Therapeutic Categories Model Guidelines. Available online: https://www.fda.gov/regulatory-information/fdaaa-implementation-chart/usp-therapeutic-categories-model-guidelines (accessed on 2 June 2020).
- Watts, N.B. Treatment of osteoporosis with bisphosphonates. Rheum. Dis. Clin. North Am. 2001, 27, 197–214. [Google Scholar] [CrossRef]
- Iqbal, M.M. Osteoporosis: epidemiology, diagnosis, and treatment. South. Med. J. 2000, 93, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Berenson, J.R.; Hillner, B.E.; Kyle, R.A.; Anderson, K.; Lipton, A.; Yee, G.C.; Biermann, J.S. American Society of Clinical Oncology clinical practice guidelines: the role of bisphosphonates in multiple myeloma. J. Clin. Oncol. 2002, 20, 3719–3736. [Google Scholar] [CrossRef] [PubMed]
- Hillner, B.E.; Ingle, J.N.; Berenson, J.R.; Janjan, N.A.; Albain, K.S.; Lipton, A.; Yee, G.; Biermann, J.S.; Chlebowski, R.T.; Pfister, D.G. American Society of Clinical Oncology guideline on the role of bisphosphonates in breast cancer. J. Clin. Oncol. 2000, 18, 1378–1391. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.T.; Kumar, T.P.; Veena, K. Formulation and evaluation of alendronate sodium gel for the treatment of bone resorptive lesions in periodontitis. Drug Deliv. 2005, 12, 217–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirabayashi, H.; Takahashi, T.; Fujisaki, J.; Masunaga, T.; Sato, S.; Hiroi, J.; Tokunaga, Y.; Kimura, S.; Hata, T. Bone-specific delivery and sustained release of diclofenac, a non-steroidal anti-inflammatory drug, via bisphosphonic prodrug based on the Osteotropic Drug Delivery System (ODDS). J. Control. Release 2001, 70, 183–191. [Google Scholar] [CrossRef]
- Bhushan, K.R.; Misra, P.; Liu, F.; Mathur, S.; Lenkinski, R.E.; Frangioni, J.V. Detection of breast cancer microcalcifications using a dual-modality SPECT/NIR fluorescent probe. J. Am. Chem. Soc. 2008, 130, 17648–17649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fellner, M.; Baum, R.P.; Kubíček, V.; Hermann, P.; Lukeš, I.; Prasad, V.; Rösch, F. PET/CT imaging of osteoblastic bone metastases with 68Ga-bisphosphonates: first human study. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 834. [Google Scholar] [CrossRef] [Green Version]
- Kubíček, V.; Rudovský, J.; Kotek, J.; Hermann, P.; Vander Elst, L.; Muller, R.N.; Kolar, Z.I.; Wolterbeek, H.T.; Peters, J.A.; Lukeš, I. A Bisphosphonate Monoamide Analogue of DOTA: A Potential Agent for Bone Targeting. J. Am. Chem. Soc. 2005, 127, 16477–16485. [Google Scholar] [CrossRef]
- Vitha, T.; Kubíček, V.; Hermann, P.; Elst, L.V.; Muller, R.N.; Kolar, Z.I.; Wolterbeek, H.T.; Breeman, W.A.P.; Lukeš, I.; Peters, J.A. Lanthanide(III) Complexes of Bis(phosphonate) Monoamide Analogues of DOTA: Bone-Seeking Agents for Imaging and Therapy. J. Med. Chem. 2008, 51, 677–683. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Z.; Gao, J.; Xu, K.; Gu, H.; Zhang, B.; Zhang, X.; Xu, B. A biocompatible method of decorporation: bisphosphonate-modified magnetite nanoparticles to remove uranyl ions from blood. J. Am. Chem. Soc. 2006, 128, 13358–13359. [Google Scholar] [CrossRef]
- Kieczykowski, G.R.; Jobson, R.B.; Melillo, D.G.; Reinhold, D.F.; Grenda, V.J.; Shinkai, I. Preparation of (4-amino-1-hydroxybutylidene) bisphosphonic acid sodium salt, MK-217 (alendronate sodium). An improved procedure for the preparation of 1-hydroxy-1, 1-bisphosphonic acids. J. Org. Chem. 1995, 60, 8310–8312. [Google Scholar] [CrossRef]
- Widler, L.; Jaeggi, K.A.; Glatt, M.; Müller, K.; Bachmann, R.; Bisping, M.; Born, A.-R.; Cortesi, R.; Guiglia, G.; Jeker, H. Highly potent geminal bisphosphonates. From pamidronate disodium (Aredia) to zoledronic acid (Zometa). J. Med. Chem. 2002, 45, 3721–3738. [Google Scholar] [CrossRef] [PubMed]
- Lecouvey, M.; Leroux, Y. Synthesis of 1-hydroxy-1, 1-bisphosphonates. Heteroat. Chem. 2000, 11, 556–561. [Google Scholar] [CrossRef]
- Chmielewska, E.; Kafarski, P. Synthetic procedures leading towards aminobisphosphonates. Molecules 2016, 21, 1474. [Google Scholar] [CrossRef] [Green Version]
- Romanenko, V.D.; Kukhar, V.P. 1-Amino-1, 1-bisphosphonates-Fundamental Syntheses and New Developments. ChemInform 2012, 43. [Google Scholar] [CrossRef]
- Balasubramaniam, R.; Polsani, P.R.; Tammireddy, G.N. Improved Process for the Preparation of Risedronate Sodium Hemipentahydrate. Patent WO2009034580A1, 19 March 2009. [Google Scholar]
- Deshpande, P.B.; Luthra, P.K. Process for the preparation of biphosphonic derivatives. U.S. Patent No. 7439385, 21 October 2008. [Google Scholar]
- Senthilkumar, U.P.; Arulmoli, T.; Lakshmipathi, V.S.; Rao, S.M. An improved process for the preparation of bisphosphonic acid. Patent WO2008035131A1, 27 March 2008. [Google Scholar]
- Lidor-Hadas, R.; Harel, Z.; Lifshitz-Liron, R.; Kovalevski-Ishai, E. Novel process for making bisphosphonic acids using diluents other than halogenated hydrocarbons. U.S. Patent No. 20060128960A1, 15 June 2006. [Google Scholar]
- Dabak, K.; Ozarslan, A.E.; Sahbaz, F.; Aslan, T. Process for the preparation of 4-amino-1-hydroxybutylidene-1, 1-biphosphonic acid. Canada Patent No. CA2445428C, 14 November 2002. [Google Scholar]
- Dembkowski, L.; Rynkiewicz, R.; Rachoń, J.; Makowiec, S.; Przychodzeń, W.; Witt, D. Process for the preparation of [1-hydroxy-2-(3-pyridinyl) ethylidene] bisphosphonic acid and hemipentahydrate monosodium salt thereof. U.S. Patent No. 20090281320A1, 12 November 2009. [Google Scholar]
- Szulc, M.; Slisewski, T.; Dembkowski, L.; Jastrzębska, B.; Rachoń, J.; Makowiec, S. A process for the synthesis of 1-hydroxy-3-(n-methylpentylamino) propylidene bisphosphonic acid monosodium salt, monohydrate. Patent WO2011016738A1, 10 February 2011. [Google Scholar]
- Blum, H.; Worms, K.H.; Henkel AG & Co KGaA. Process for the production of ω-amino-1-hydroxyalkylidene-1, 1-bisphosphonic acid. U.S. Patent No. 4407761A, 4 October 1983. [Google Scholar]
- Patel, V.M.; Chitturi, T.R.; Thennati, R. Process for preparation of bisphosphonic acid compounds. U.S. Patent No. 7411087, 8 December 2008. [Google Scholar]
- Wieczorek, M.; Stawinski, T.; Wieczorek, M. A process for the preparation of risedronic acid. European Patent EP1243592A2, 25 September 2002. [Google Scholar]
- Blum, H.; Worms, K.H.; Henkel AG & Co KGaA. Process for the production of 3-amino-1-hydroxypropane-1, 1-diphosphonic acid. U.S. Patent No. 4327039A, 27 April 1982. [Google Scholar]
- Dembkowski, L.; Krzyzanowski, M.; Rynkiewicz, R.; Szramka, R.; Roznerski, Z.; Zyla, D.; Rachon, J.; Makowiec, S.; Zaklady Farmaceutyczne Polpharma SA. Process for the Preparation of [1-Hydroxy-2-(1H-imidazol-1-yl)-ethylidene] bisphosphonic Acid. Patent WO2010050830A1, 6 May 2010. [Google Scholar]
- Kovács, R.; Grün, A.; Németh, O.; Garadnay, S.; Greiner, I.; Keglevich, G. The synthesis of pamidronic derivatives in different solvents: An optimization and a mechanistic study. Heteroat. Chem. 2014, 25, 186–193. [Google Scholar] [CrossRef]
- Kieczykowski, G.R.; Melillo, D.G.; Jobson, R.B.; Merck and Co Inc. Crystalline 4-amino-1-hydroxybutylidene-1, 1-bisphosphonic acid monosodium trihydrate, process therefor and compositions and use thereof. European Patent EP0402152A2, 12 December 1990. [Google Scholar]
- Singh, G.P.; Jadhav, H.S.; Maddireddy, N.V.; Srivastava, D. Process for the production of 4-amino-1-hydroxybutylidene-1,1-bisphosphonic acid or salts thereof. Patent WO2007010556A1, 25 January 2007. [Google Scholar]
- Danda, S.R.; Garimella, N.K.; Divvela, S.R.V.; Dandala, R.; Meenakshisunderam, S.; Aurobindo Pharma Ltd. Process for the preparation of biphosphonic acids. U.S. Patent No. 7528280B2, 5 May 2009. [Google Scholar]
- Mandava, V.N.B.R.; Setty, R.K.S.; Manne, N.; Reddy’s Laboratories Ltd. Reddy’s Laboratories Inc. Process for preparing bisphosphonic acids. U.S. Patent No. 20070142636A1, 21 June 2007. [Google Scholar]
- Kubela, R.; Tao, Y. Process for the Production of 4-Amino-1-hydroxybutylidene-1, 1-bisphosphonic Acid or Salts Thereof. U.S. Patent No. 5908959A, 1 June 1999. [Google Scholar]
- De Ferra, L.; Turchetta, S.; Massardo, P.; Casellato, P.; Chemi SpA. Preparation of biphosphonic acids and salts thereof. U.S. Patent No. 7332603, 19 February 2008. [Google Scholar]
- Nagy, D.I.; Grun, A.; Pavela, O.; Garadnay, S.; Greiner, I.; Keglevich, G. Efficient synthesis of Ibandronate in the presence of an ionic liquid. Lett. Drug Des. Discov. 2018, 15, 713–720. [Google Scholar] [CrossRef]
- Gore, V.G.; Shukla, V.K.; Ghadge, M.M.; Avadhut, R.M. Novel process for the preparation of bisphosphonic acids. Patent WO2008004000A1, 10 January 2008. [Google Scholar]
- Rahul, S.; Kumar, J.A.; Venkateswaran, S.C.; Lalit, W. Process for the preparation of pure risedronic acid or salts. U.S. Patent No. 8076483B2, 13 December 2011. [Google Scholar]
- Nagy, D.I.; Grün, A.; Sinkovicz, J.; Garadnay, S.; Greiner, I.; Keglevich, G. A Study on the Synthesis of Risedronic Acid: The Role of an Ionic Liquid Additive. Lett. Drug Des. Discov. 2019, 16, 238–244. [Google Scholar] [CrossRef]
- Mustafa, A.; Hülya, K.; Karliga, B. Process for the preparation of 3-pyridyl-1-hydroxyethylidene-1,1-biphosphonic acid and hydrated forms thereof. Patent WO2007068678A1, 21 June 2007. [Google Scholar]
- Nagy, D.I.; Grün, A.; Lévay, K.; Garadnay, S.; Greiner, I.; Keglevich, G. Efficient syntheses of zoledronic acid as an active ingredient of a drug against osteoporosis. Synth. Commun. 2018, 48, 663–671. [Google Scholar] [CrossRef]
- Kas, M.; Benes, M.; Pis, J. Process for making Zoledronic acid. European Patent EP2192126B1, 2 June 2010. [Google Scholar]
- Reddy, M.P.; Rani, V.U.; Chowdary, N.V. An improved process for the preparation of zoledronic acid. Patent WO2005063717A1, 14 July 2005. [Google Scholar]
- Kieczykowski, G.R. Process for preparing 4-amino-1-hydroxybutylidene-1, 1-bisphosphonic acid (ABP) or salts thereof. U.S. Patent No. 5019651A, 28 May 1991. [Google Scholar]
- Kumar, N.P.; Singare, D.; Pradhan, N.S.; Valgeirsson, J. Process for the Preparation of Risedronate Sodium. U.S. Patent No. 20100317859A1, 16 December 2010. [Google Scholar]
- Grün, A.; Rádai, Z.; Sőregi-Nagy, D.I.; Greiner, I.; Keglevich, G. Rational synthesis of α-hydroxyphosphonic derivatives including dronic acids. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 386–387. [Google Scholar] [CrossRef]
- Baptista, J.; Mendes, Z. Process for the preparation of biphosphonic acids and salts thereof. Patent WO2008056129A1, 15 May 2008. [Google Scholar]
- Ankush, T.M.; Rajiv, K.; Baburao, T.R. Novel process for preparing risedronic acid. U.S. Patent No. 20100121066A1, 13 May 2010. [Google Scholar]
- Pandey, S.C.; Haider, H.; Saxena, S.; Singh, M.K.; Thaper, R.K.; Dubey, S.K. Process for producing bisphosphonic acids and forms thereof. European Patent EP1891081A4, 27 February 2008. [Google Scholar]
- Garadnay, S.; Grün, A.; Keglevich, G.; Neu, J. Novel process for the preparation of dronic acids. Patent WO2012107787A1, 16 August 2012. [Google Scholar]
- Labriola, R.A.; Tombari, D.G.; Vechhioli, A. A crystalline form of the zoledronic acid, a process to obtain it and the pharmaceutical composition comprising it. U.S. Patent No. 8952172B2, 5 August 2010. [Google Scholar]
- Lladó, J.B.; Lista, E.P.; Miguel, M.D.C.O. Process for producing 4-amino-1-hydroxybutylidene-1, 1-bisphosphonic acid and its trihydrated monosodium salt. U.S. Patent No. 6573401B1, 3 June 2003. [Google Scholar]
- SusChem-European Technology Platform for Sustainable Chemistry. Available online: http://www.suschem.org/ (accessed on 11 November 2017).
- Colombo, M.; Peretto, I. Chemistry strategies in early drug discovery: an overview of recent trends. Drug Discov. Today 2008, 13, 677–684. [Google Scholar] [CrossRef]
- Larhed, M.; Hallberg, A. Microwave-assisted high-speed chemistry: a new technique in drug discovery. Drug Discov. Today 2001, 6, 406–416. [Google Scholar] [CrossRef]
- Vilela, S.M.; Firmino, A.D.; Mendes, R.F.; Fernandes, J.A.; Ananias, D.; Valente, A.A.; Ott, H.; Carlos, L.D.; Rocha, J.; Tomé, J.P. Lanthanide-polyphosphonate coordination polymers combining catalytic and photoluminescence properties. Chem. Commun. 2013, 49, 6400–6402. [Google Scholar] [CrossRef]
- Jhung, S.H.; Lee, J.H.; Yoon, J.W.; Serre, C.; Férey, G.; Chang, J.S. Microwave Synthesis of Chromium Terephthalate MIL-101 and Its Benzene Sorption Ability. Adv. Mater. 2007, 19, 121–124. [Google Scholar] [CrossRef]
- Kappe, C.O.; Dallinger, D. The impact of microwave synthesis on drug discovery. Nat. Rev. Drug Discov. 2005, 5, nrd1926. [Google Scholar]
- Bassyouni, F.A.; Abu-Bakr, S.M.; Rehim, M.A. Evolution of microwave irradiation and its application in green chemistry and biosciences. Res. Chem. Intermed. 2012, 38, 283–322. [Google Scholar] [CrossRef]
- Mustafa, D.A.; Kashemirov, B.A.; McKenna, C.E. Microwave-assisted synthesis of nitrogen-containing 1-hydroxymethylenebisphosphonate drugs. Tetrahedron Lett. 2011, 52, 2285–2287. [Google Scholar] [CrossRef]
- Lenin, R.; Raju, R.M.; Rao, D.V.S.; Ray, U.K. Microwave-assisted efficient synthesis of bisphosphonate libraries: a useful procedure for the preparation of bisphosphonates containing nitrogen and sulfur. Med. Chem. Res. 2013, 22, 1624–1629. [Google Scholar] [CrossRef]
- Grün, A.; Kovács, R.; Garadnay, S.; Greiner, I.; Keglevich, G. The Synthesis of Risedronic Acid and Alendronate Applying Phosphorus Oxychloride and Phosphorous Acid in Methanesulfonic Acid. Lett. Drug Des. Discov. 2015, 12, 253–258. [Google Scholar] [CrossRef]
- Rita, K.; David Illes, N.; Alajos, G.; Gyorgy Tibor, B.; Sandor, G.; Istvan, G.; Gyorgy, K. Optimized Synthesis of Etidronate. Lett. Drug Des. Discov. 2013, 10, 733–737. [Google Scholar] [CrossRef]
- Nagy, D.I.; Grün, A.; Greiner, I.; Keglevich, G. The Role of Phosphorus Trichloride and Phosphorous Acid in the Formation of -Hydroxymethylenebisphosphonic Acids from the Corresponding Carboxylic Acids—A Mechanistic Overview. Curr. Org. Chem. 2017, 21, 1567–1578. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; U.S. Food and Drug Administration (FDA); Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER). Q3C—Tables and List Guidance for Industry (3rd Revision). 2017. Available online: https://www.fda.gov/media/71737/download (accessed on 20 May 2020).



| Tradename | Formulation | Manufacturer | Refs. | |
|---|---|---|---|---|
| Pamidronate Disodium | ||||
| Generics | Aredia | IV solution | Novartis Pharmaceuticals | [33,34] |
| Pamidronate Disodium | IV solution | Areva Pharmaceuticals, Inc. | ||
| Pamidronate Disodium | IV solution | West-Ward Pharmaceuticals | ||
| Alendronate Sodium | ||||
| Generics | Fosamax | Tablet | Merck Sharp & Dohme, Ltd. | [34,35,36] |
| Fosamax | Oral solution | Merck Sharp & Dohme, Ltd. | ||
| Binosto | Effervescent tablet | ASCEND Therapeutics US, LLC. | ||
| Alendronate Sodium | Tablet | Impax Laboratories, LLC | ||
| Alendronate Sodium | Tablet | Apotex Inc. | ||
| Ibandronate Sodium/Ibandronic Acid | ||||
| Generics | Boniva | Film-coated tablet | Roche Laboratories Inc. | [34,36,37] |
| Ibandronate Sodium | Film-coated tablet | Apotex Inc. | ||
| Ibandronic Acid Bluefish | Film-coated tablet | Bluefish Pharmaceuticals AB | ||
| Risedronate Sodium | ||||
| Generics | Actonel | Film-coated tablet | Warner Chilcott Deutschland GmbH | [34,36,38] |
| Risedronate Sodium | Film-coated tablet | Teva Pharmaceutical Industries Ltd. | ||
| Sodium Risedronate Aurovitas | Film-coated tablet | Generis Farmacêutica, S.A. | ||
| Zoledronic Acid | ||||
| Generics | Zometa | IV solution | Novartis Pharmaceuticals | [34,36,39,40] |
| Reclast | IV solution | Novartis Pharmaceuticals | ||
| Zoledronic | IV solution | Gland Pharma, Ltd. | ||
| Zoledronic Acid Accord | IV solution | Accord Healthcare Ltd. | ||
| Zoledronic Acid Actavis | IV solution | Actavis Group Ptc Ehf. | ||
| Stoichiometry RCOOH:PCl3:H3PO3 | Solvent | Reaction Time (h) * | Temp. (°C) ** | Yield (%) | Ref. |
|---|---|---|---|---|---|
| Etidronate Disodium | |||||
| 1:2.2:1.1 | MSA | 28 | 75 | 36 | [11] |
| 1:1.1:2.2 | MSA | 28 | 75 | 5 | [11] |
| Pamidronic Acid/Pamidronate Sodium | |||||
| 1:2.2:2.2 | MSA | 12–16 | 75 | 27 | [72] |
| 1:1.5:1.5 | Chlorobenzene | 3 | 132 | 59 | [4] |
| 1:1.5:1.5 | Chlorobenzene | 4–7 | 100 | 65 | [70] |
| 1:2:2 | Sulfolane | 12–15 | 75 | 63 | [72] |
| Alendronic Acid/Alendronate Sodium | |||||
| 1:2:1 | MSA | 21–25 | 65 | 89 | [55] |
| 1:3:2 | MSA | 12 | 75 | 63 | [10] |
| 1:1:2 | MSA | 12 | 75 | 22 | [10] |
| 1:2.4:1.5 | MSA | 25–27 | 65 | 90 | [73] |
| 1:3.2: 0 | MSA | 12 | 75 | 57 | [4] |
| 1:1.5:1.5 | Chlorobenzene | 6 | 70–100 | 56 | [67] |
| 1:2:2 | Sulfolane | 6 | 75 | 46 | [10] |
| 1:2.5:3.5 | Sulfolane | 19–20 | 60–65 | 55 | [74] |
| 1:2:2 | Sulfolane and [bmim][BF4] | 6 | 75 | 72 | [10] |
| 1:2:2 | Sulfolane and [bmim][Cl] | 6 | 75 | 48 | [10] |
| 1:2:2 | [bmim][BF4] | 6 | 75 | 60 | [10] |
| 1:2:2 | [bmim][Cl] | 6 | 75 | 59 | [10] |
| 1:2:2 | [bmim][PF6] | 6 | 75 | 59 | [10] |
| 1:3.5:3 | Phenols | 8–11 | 65–70 | [75] | |
| 1: 3:1.5 | p-Cresol | 6–9 | 60–75 | 49 | [76] |
| 1:1.5:1 | Polyethylene glycol and toluene | 14 | 65–75 | 58 | [77] |
| 1:2:1 | Triglycerides | 8 | 70 | 57 | [64] |
| 1:3:3 | Diphenyl ether | 15–22 | 65–75 | 79 | [61] |
| 1:2:1 | Tributylammonium chloride | 5 | 60–65 | 31 | [78] |
| Ibandronate Sodium/Ibandronate Disodium | |||||
| 1:3.2:0 | MSA | 28 | 75 | 46 | [11] |
| 1:3:0 | Sulfolane | 6 | 75 | 28 | [79] |
| 1:2:2 | Sulfolane | 6 | 75 | 48 | [79] |
| 1:3:2 | Sulfolane | 6 | 75 | 61 | [79] |
| 1:3:2 | [bmim][BF4] (0.6 equiv.) *** | 6 | 75 | 75 | [79] |
| 1:3:2 | [bmim][BF4] (0.3 equiv.) *** | 6 | 75 | 87 | [79] |
| 1:3:2 | [bmim][BF4] (0.1 equiv.) *** | 6 | 75 | 90 | [79] |
| 1:2:2 | [bmim][BF4] (0.6 equiv.) *** | 6 | 75 | 60 | [79] |
| Ibandronate Sodium/Ibandronate Disodium | |||||
| 1:2:2 | [bmim][BF4] (0.3 equiv.) *** | 6 | 75 | 79 | [79] |
| 1:2:2 | [bmim][BF4] (0.1 equiv.) *** | 6 | 75 | 82 | [79] |
| 1:2:2 | [bmim][PF6] (0.6 equiv.) *** | 6 | 75 | 55 | [79] |
| 1:2:2 | [bmim][PF6] (0.3 equiv.) *** | 6 | 75 | 70 | [79] |
| 1:2:2 | [bmim][PF6] (0.1 equiv.) *** | 6 | 75 | 72 | [79] |
| 1:3:2 | Sulfolane and [bmim][BF4] (0.6 equiv.) *** | 6 | 75 | 64–68 | [79] |
| 1:3:2 | Sulfolane and [bmim][BF4] (0.3 equiv.) *** | 6 | 75 | 64–68 | [79] |
| 1:3:2 | Sulfolane and [bmim][BF4] (0.1 equiv.) *** | 6 | 75 | 64–68 | [79] |
| Risedronic Acid/Risedronate Sodium | |||||
| 1:2.1:1 | MSA | 5.5–10 | 80 | 44 | [3] |
| 1:1:2.1 | MSA | 5.5–10 | 80 | 7 | [3] |
| 1:3:3 | Chlorobenzene | 3.5–4 | 85–100 | 49 | [69] |
| 1:3.4:1.5 | Sulfolane | 5–10 | 60–70 | 70 | [68] |
| 1:3:3 | Toluene | 19–25 | 65–85 | 68 | [63] |
| 1:2:1.2 | Acetonitrile | 10–15 | 70–75 | 56 | [80] |
| 1:3:2.5 | Acetonitrile | 9 | 70–75 | 84 | [81] |
| 1:2:2 | Sulfolane | 8 | 75 | 58 | [82] |
| 1:2:2 | [bmim][BF4] | 8 | 75 | 66 | [82] |
| 1:2:2 | Sulfolane and [bmim][BF4] | 8 | 75 | 65 | [82] |
| 1:3:3 | Diphenyl ether | 15–22 | 65–75 | 77 | [61] |
| 1:3.5:2.8 | Sunflower oil | 8 | 110–115 | 28 | [83] |
| Zoledronic Acid | |||||
| 1:2:1 | MSA | 21–25 | 65 | 31 | [55] |
| 1:1.1:2 | MSA | 5.5 – 10 | 80 | 7 | [3] |
| 1:3.4:1.5 | Sulfolane | 6–10 | 60–70 | 71 | [68] |
| 1:2:2 | Sulfolane | 19 | 75 | 74 | [84] |
| 1:2:2 | [bmim][BF4] | 19 | 75 | 75 | [84] |
| 1:2:2 | Sulfolane and [bmim][BF4] | 19 | 75 | 93 | [84] |
| 1:3:3 | Polyethylene glycol & Propylene carbonate | 19–22 | 56–60 | 70 | [85] |
| 1:3:3 | Diphenyl Ether | 15–22 | 65–75 | 75 | [61] |
| 1:3:3 | Cyclohexane | 13–14 | 80 | 80 | [86] |
| Stoichiometry RCOOH:PCl3:H3PO3 | Solvent | Reaction Time (h) * | Temp. (°C) ** | Yield (%) | Ref. |
|---|---|---|---|---|---|
| Etidronate Disodium | |||||
| 1:3.2:0 | MSA | 28 | 75 | 38 | [11] |
| 1:2.2:1.1 | MSA | 28 | 75 | 36 | |
| 1:1.1:2.2 | MSA | 28 | 75 | 5 | |
| 1:0:3.2 | MSA | 28 | 75 | 0 | |
| Ibandronate Sodium | |||||
| 1:3.2:0 | MSA | 28 | 75 | 46 | [11] |
| 1:2:1 | MSA | 28 | 75 | 18 | |
| 1:1:2 | MSA | 28 | 75 | 6 | |
| 1:0:3 | MSA | 28 | 75 | 0 | |
| Risedronic Acid | |||||
| 1:3.2:0 | MSA | 8–9 | 80 | 74 | [93] |
| 1:3.1:0 | MSA | 5.5–10 | 80 | 74 | [3] |
| 1:2.1:1 | MSA | 5.5–10 | 80 | 44 | [3] |
| 1:2:1 | MSA | 21–25 | 65 | 38 | [55] |
| 1:1:2.1 | MSA | 5.5–10 | 80 | 7 | [3] |
| 1:0:3.1 | MSA | 5.5–10 | 80 | 0 | [3] |
| Zoledronic Acid | |||||
| 1:6.2:0 | MSA | 13–20 | 60–70 | 83 | [94] |
| 1:3.2:0 | MSA | 8–9 | 80 | 46 | [93] |
| 1:3.1:0 | MSA | 5.5–10 | 80 | 49 | [3] |
| 1:2.1:1 | MSA | 5.5–10 | 80 | 26 | [3] |
| 1:2:1 | MSA | 21–25 | 65 | 31 | [55] |
| 1:1.1:2 | MSA | 5.5–10 | 80 | 7 | [3] |
| 1:0:3.1 | MSA | 5.5–10 | 80 | 0 | [3] |
| Stoichiometry RCOOH:PCl3:H3PO3 | Solvent | Reaction Time (h) * | Temp. (°C) ** | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1:3.2:0 | MSA | 28 | 75 | 57 | [4] |
| 1:3.2:0 | MSA | 12–16 | 75 | 57 | [72] |
| 1:2.2:1.1 | MSA | 12–16 | 75 | 28 | [72] |
| 1:2:1 | MSA | 21–25 | 65 | 57 | [55] |
| 1:2.2:2.2 | MSA | 12–16 | 75 | 27 | [72] |
| 1:1.1:2.2 | MSA | 12–16 | 75 | 3 | [72] |
| 1:0:3.2 | MSA | 12–16 | 75 | 0 | [72] |
| 1:3.2:0 | Sulfolane | 12–15 | 75 | 0 | [72] |
| 1:3.2:1 | Sulfolane | 12–15 | 75 | 34 | [72] |
| 1:3.4:1.5 | Sulfolane | 6–10 | 60–70 | 62.7 | [68] |
| 1:2:1 | Sulfolane | 12–15 | 75 | 42 | [72] |
| 1:3.2:2 | Sulfolane | 12–15 | 75 | 55 | [72] |
| 1:2:2 | Sulfolane | 12–15 | 75 | 63 | [72] |
| 1:1:2 | Sulfolane | 12–15 | 75 | 44 | [72] |
| 1:0:3.2 | Sulfolane | 12–15 | 75 | 0 | [72] |
| Stoichiometry RCOOH:PCl3:H3PO3 | Solvent | Reaction Time (h) * | Temp. (°C) ** | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1:3.2:0 | MSA | 12 | 75 | 67 | [10] |
| 1:3.2:0 | MSA | 12 | 75 | 57 | [4] |
| 1:2:0 | MSA | 12 | 75 | 52 | [10] |
| 1:2:1 | MSA | 21–25 | 65 | 89 | [55] |
| 1:2.4:1.5 | MSA | 25–27 | 65 | 90 | [73] |
| 1:3:2 | MSA | 12 | 75 | 63 | [10] |
| 1:1:2 | MSA | 12 | 75 | 22 | [10] |
| 1:0:2 | MSA | 12 | 75 | 0 | [10] |
| 1:0:3 | MSA | 14–17 | 70–76 | 70 | [95] |
| 1:3:0 | Sulfolane | 6 | 75 | 8 | [10] |
| 1:3.4:1.5 | Sulfolane | 6–10 | 60–70 | 69 | [68] |
| 1:2:1 | Sulfolane | 6 | 75 | 37 | [10] |
| 1:3:2 | Sulfolane | 6 | 75 | 52 | [10] |
| 1:2:2 | Sulfolane | 6 | 75 | 46 | [10] |
| 1:2.5:3.5 | Sulfolane | 19–20 | 60–65 | 55 | [74] |
| 1:0:3 | Sulfolane | 6 | 75 | 0 | [10] |
| 1:3:2 | Sulfolane and [bmim][BF4] | 6 | 75 | 80 | [10] |
| 1:3:2 | Sulfolane and [bmim][Cl] | 6 | 75 | 58 | [10] |
| 1:2:2 | Sulfolane and [bmim][BF4] | 6 | 75 | 72 | [10] |
| 1:2:2 | Sulfolane and [bmim][Cl] | 6 | 75 | 48 | [10] |
| Stoichiometry RCOOH:PCl3:H3PO3 | Solvent | Reaction Time (h) * | Temp. (°C) ** | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1:6.2:0 | MSA | 13–20 | 60–70 | 83 | [94] |
| 1:3.2:0 | MSA | 8–9 | 80 | 46 | [93] |
| 1:3.1:0 | MSA | 5.5–10 | 80 | 49 | [3] |
| 1:2.1:1 | MSA | 5.5–10 | 80 | 26 | [3] |
| 1:2:1 | MSA | 21–25 | 65 | 31 | [55] |
| 1:1.1:2 | MSA | 5.5–10 | 80 | 7 | [3] |
| 1:0:3.1 | MSA | 5.5–10 | 80 | 0 | [3] |
| 1:3.2:0 | Sulfolane | 19 | 75 | 10 | [84] |
| 1:3.4:1.5 | Sulfolane | 6–10 | 60–70 | 71 | [68] |
| 1:3:1 | Sulfolane | 19 | 75 | 60 | [84] |
| 1:3:2 | Sulfolane | 19 | 75 | 71 | [84] |
| 1:2:1 | Sulfolane | 19 | 75 | 43 | [84] |
| 1:2:2 | Sulfolane | 19 | 75 | 74 | [84] |
| 1:3:3 | Sulfolane | 19 | 75 | 75 | [84] |
| 1:2:3 | Sulfolane | 19 | 75 | 63 | [84] |
| 1:2:4 | Sulfolane | 19 | 75 | 66 | [84] |
| 1:2:2 | [bmim][BF4] | 19 | 75 | 75 | [84] |
| 1:3:2 | [bmim][BF4]) | 19 | 75 | 75 | [84] |
| 1:2:2 | Sulfolane & [bmim][BF4] | 19 | 75 | 93 | [84] |
| 1:3:2 | Sulfolane & [bmim][BF4] | 19 | 75 | 91 | [84] |
| Stoichiometry RCOOH:PCl3:H3PO3 | Solvent | Reaction Time (h)* | Temp. (°C) ** | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1:3:0 | Sulfolane | 6 | 75 | 28 | [79] |
| 1:2:0 | Sulfolane | 6 | 75 | 9 | |
| 1:2:1 | Sulfolane | 6 | 75 | 30 | |
| 1:3:1 | Sulfolane | 6 | 75 | 52 | |
| 1:3:2 | Sulfolane | 6 | 75 | 61 | |
| 1:2:2 | Sulfolane | 6 | 75 | 48 | |
| 1:3:3 | Sulfolane | 6 | 75 | 76 | |
| 1:3:4 | Sulfolane | 6 | 75 | 83 | |
| 1:2:4 | Sulfolane | 6 | 75 | 73 | |
| 1:2:3 | Sulfolane | 6 | 75 | 66 | |
| 1:3:2 | [bmim][BF4] (0.6 equivalents) | 6 | 75 | 75 | |
| 1:2:2 | [bmim][BF4] (0.6 equivalents) | 6 | 75 | 60 | |
| 1:2:4 | [bmim][BF4] (0.6 equivalents) | 6 | 75 | 55 | |
| 1:3:2 | [bmim][BF4] (0.3 equivalents) | 6 | 75 | 87 | |
| 1:2:2 | [bmim][BF4] (0.3 equivalents) | 6 | 75 | 79 | |
| 1:2:4 | [bmim][BF4] (0.3 equivalents) | 6 | 75 | 57 | |
| 1:3:2 | [bmim][BF4] (0.1 equivalents) | 6 | 75 | 90 | |
| 1:2:2 | [bmim][BF4] (0.1 equivalents) | 6 | 75 | 82 | |
| 1:2:4 | [bmim][BF4] (0.1 equivalents) | 6 | 75 | 54 |
| Prepared BP | MWAS | Conventional Heating | ||||
|---|---|---|---|---|---|---|
| Reaction Time (min) | Yield (%) | Reaction Time (h) | Yield (%) | |||
| Step 1 | Step 2 | Step 1 | Step 2 | |||
| Pamidronate sodium | 3.15 | 10 | 64 | 3.5 | 6 | 72 |
| Alendronate sodium | 7 | 10 | 41 | 3.5 | 6 | 38 |
| Risedronic acid | 3.15 | 10 | 74 | - | - | - |
| Zoledronic acid | 3.45 | 10 | 70 | 3.5 | 6 | 67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, J.S.; Braga, S.S.; Almeida Paz, F.A. Empowering the Medicinal Applications of Bisphosphonates by Unveiling their Synthesis Details. Molecules 2020, 25, 2821. https://doi.org/10.3390/molecules25122821
Barbosa JS, Braga SS, Almeida Paz FA. Empowering the Medicinal Applications of Bisphosphonates by Unveiling their Synthesis Details. Molecules. 2020; 25(12):2821. https://doi.org/10.3390/molecules25122821
Chicago/Turabian StyleBarbosa, Jéssica S., Susana Santos Braga, and Filipe A. Almeida Paz. 2020. "Empowering the Medicinal Applications of Bisphosphonates by Unveiling their Synthesis Details" Molecules 25, no. 12: 2821. https://doi.org/10.3390/molecules25122821







