Morphological and Structural Properties of Amino-Functionalized Fumed Nanosilica and Its Comparison with Nanoparticles Obtained by Modified Stöber Method
Abstract
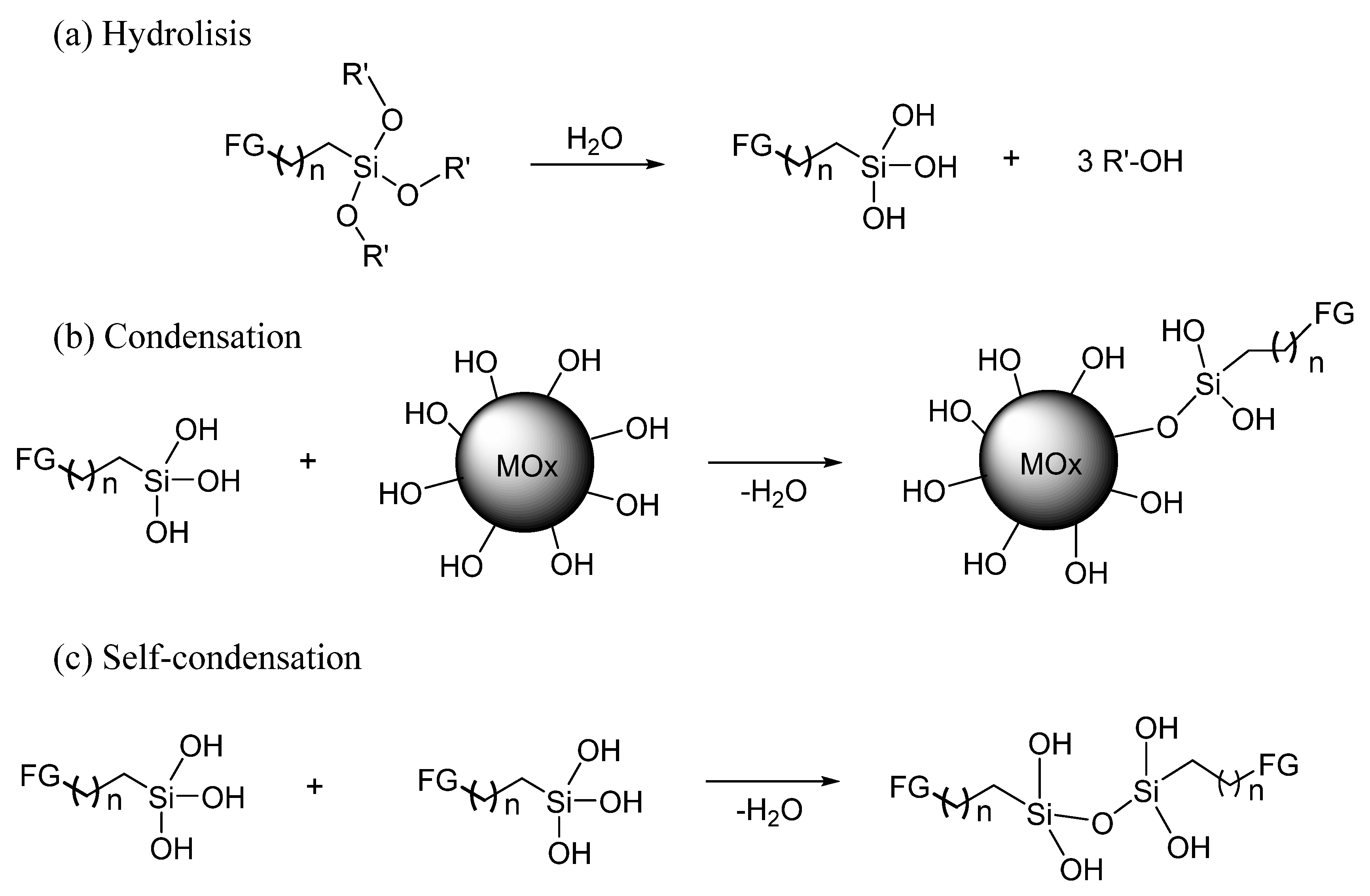
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoparticle Synthesis
2.3. Activation Reaction of the Silica NPs
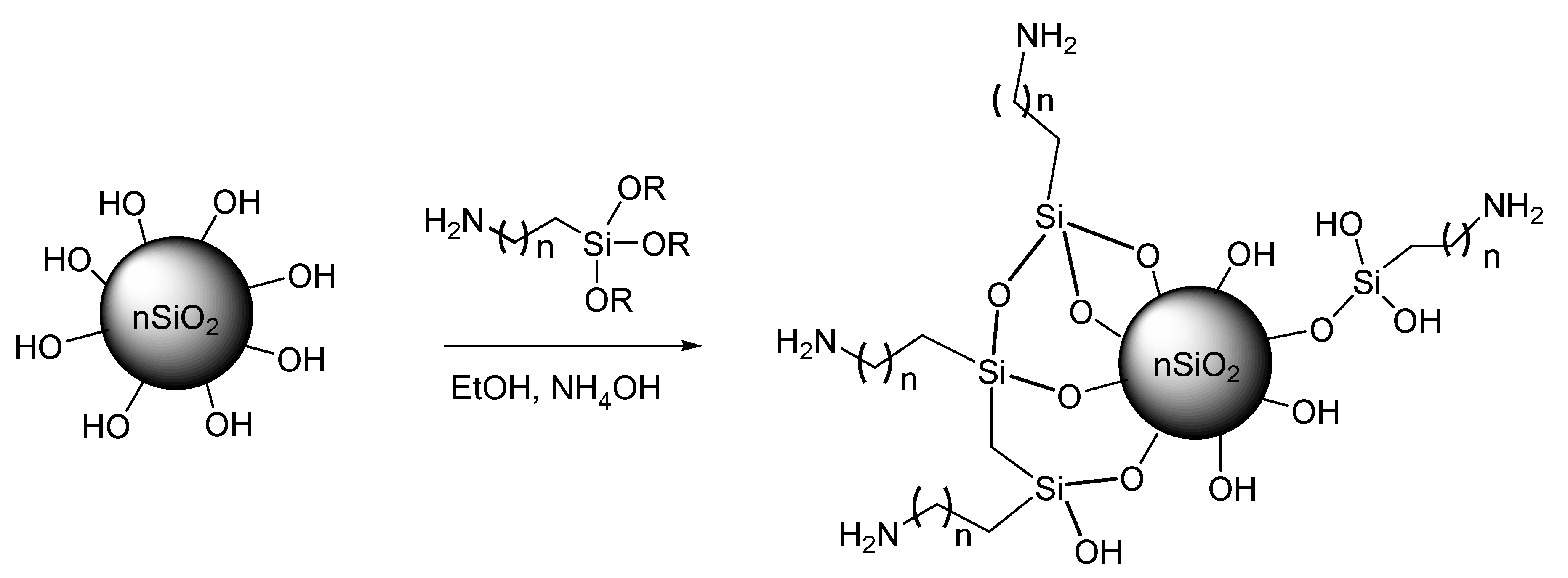
2.4. Amino-Functionalization Reaction
2.5. Determination of the Hydroxyl Group Content on the NPs Surface
2.6. NPs Characterization
3. Results and Discussion
3.1. Determination of the –OH Groups Content on the NPs Surface
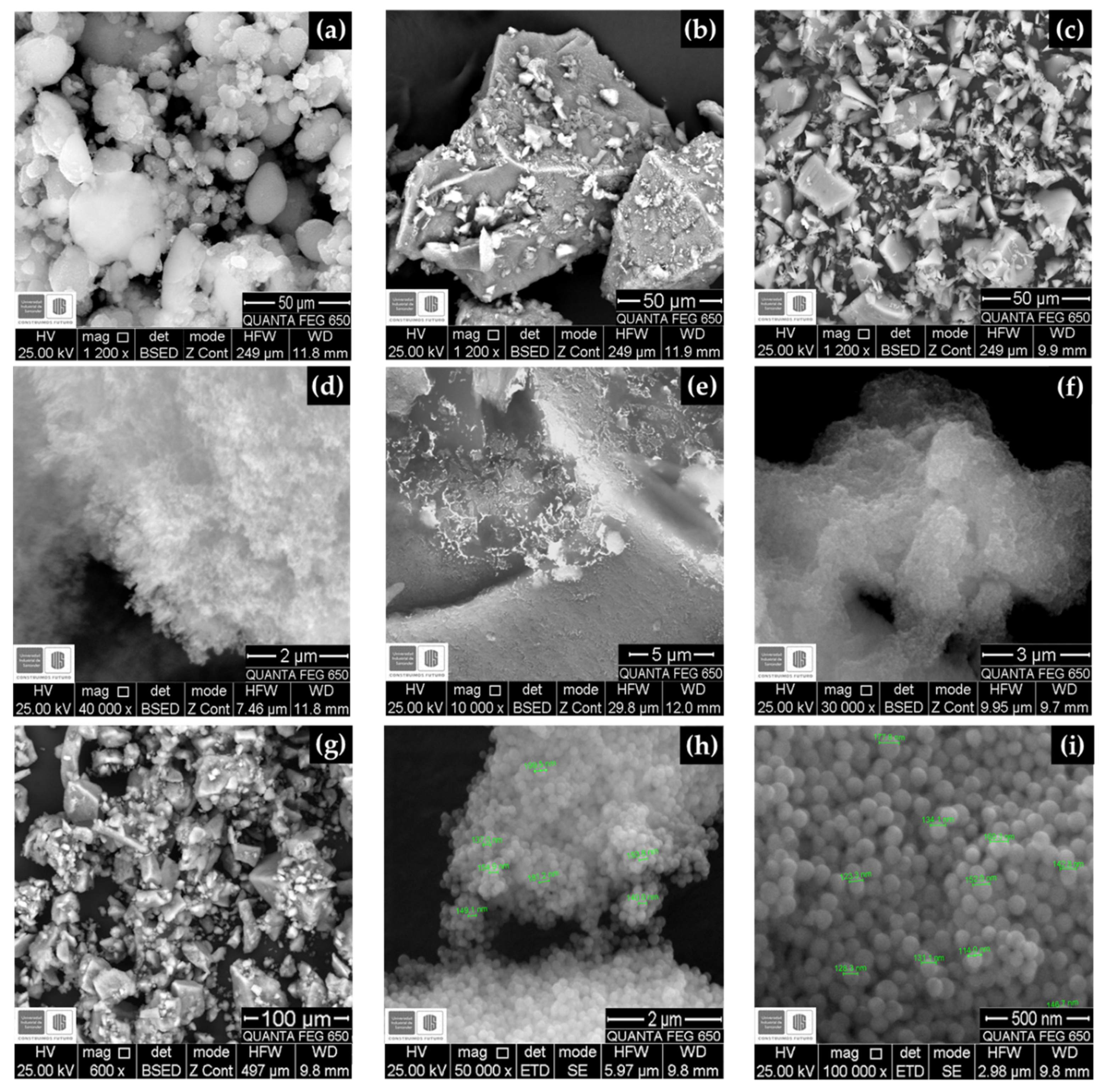
3.2. SEM—EDS Results
3.3. X-ray Diffraction (XRD)
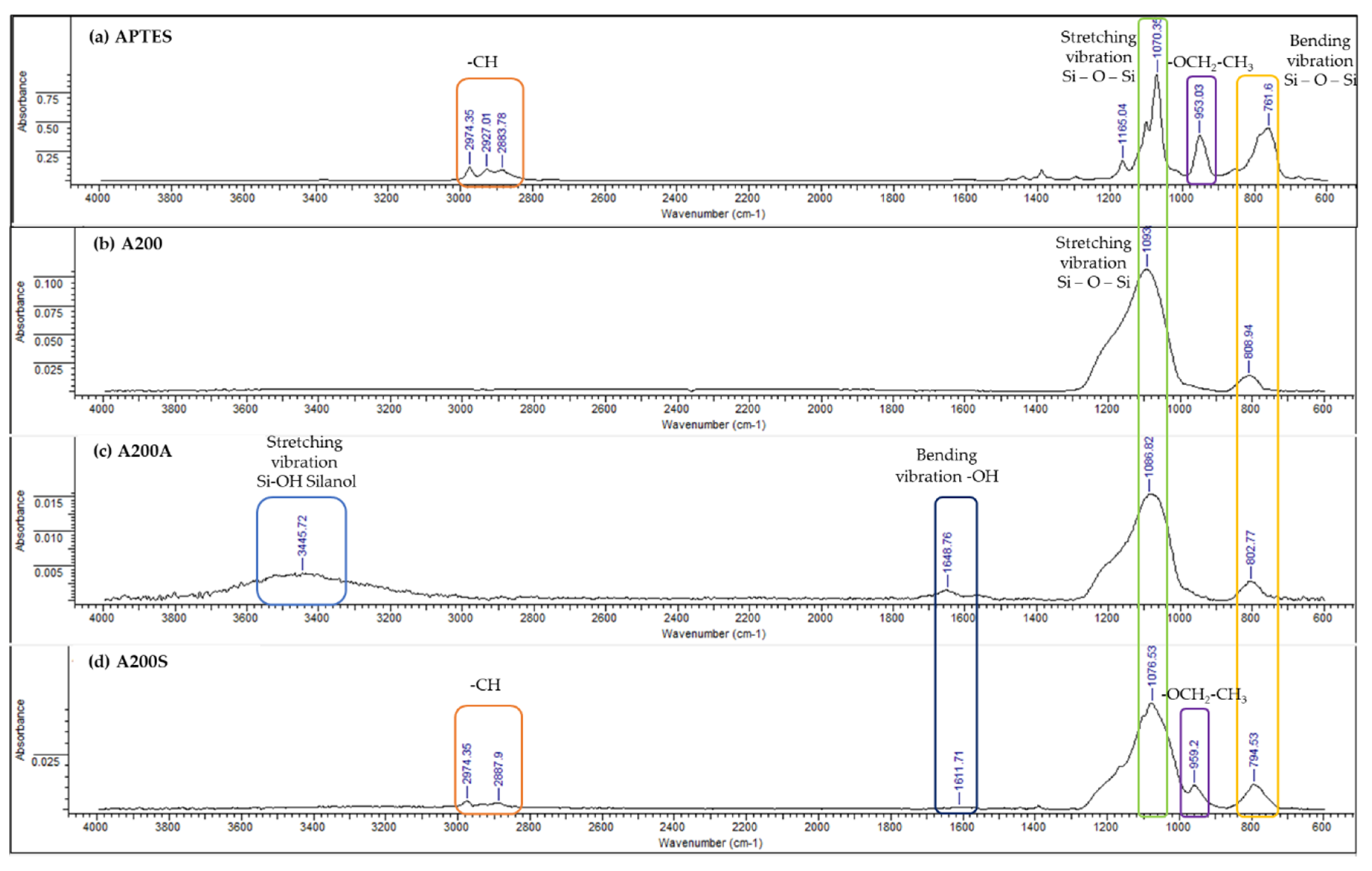
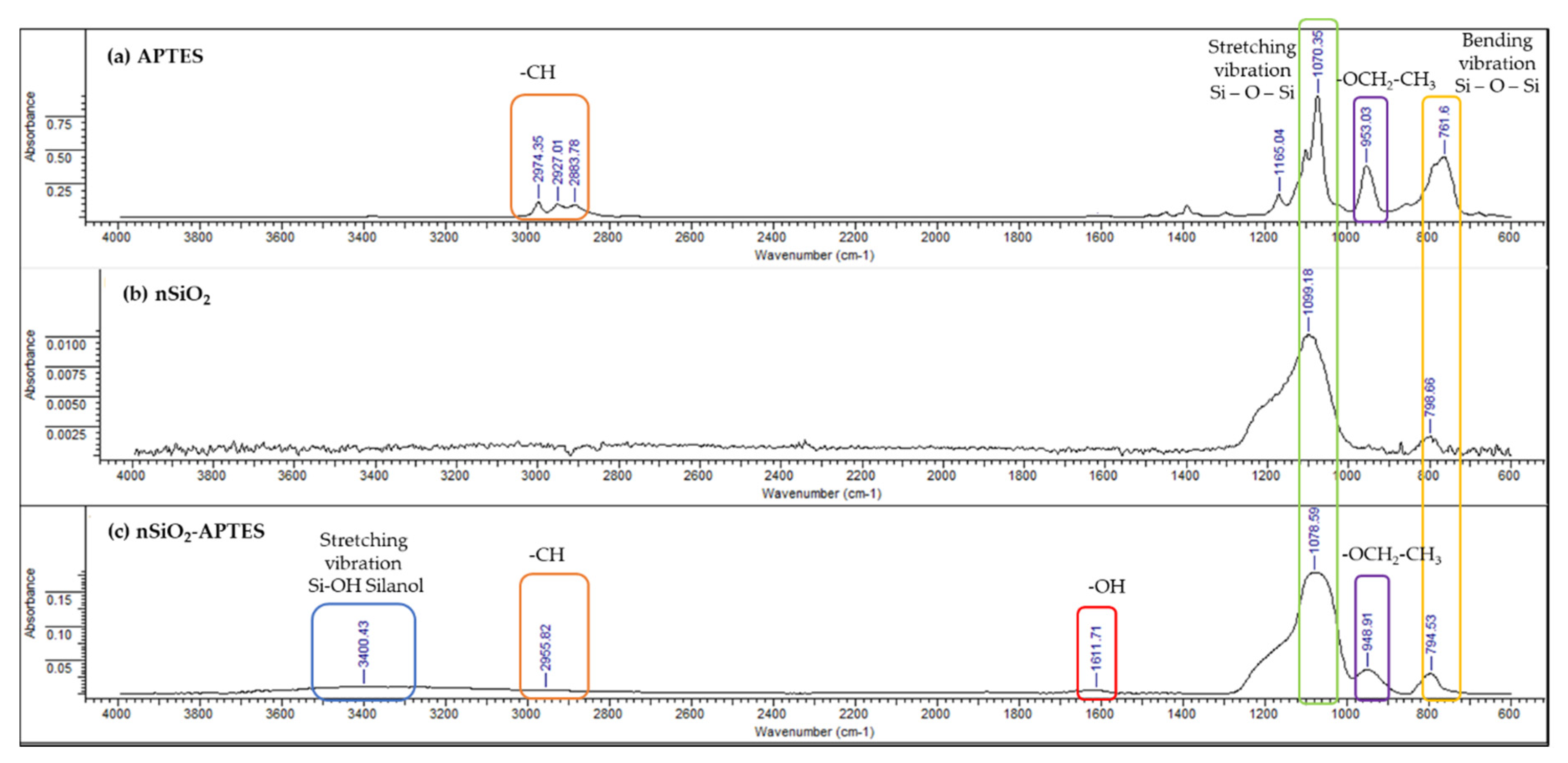
3.4. FTIR-ATR Results
3.5. Colloidal Stability (ζ-Potential)
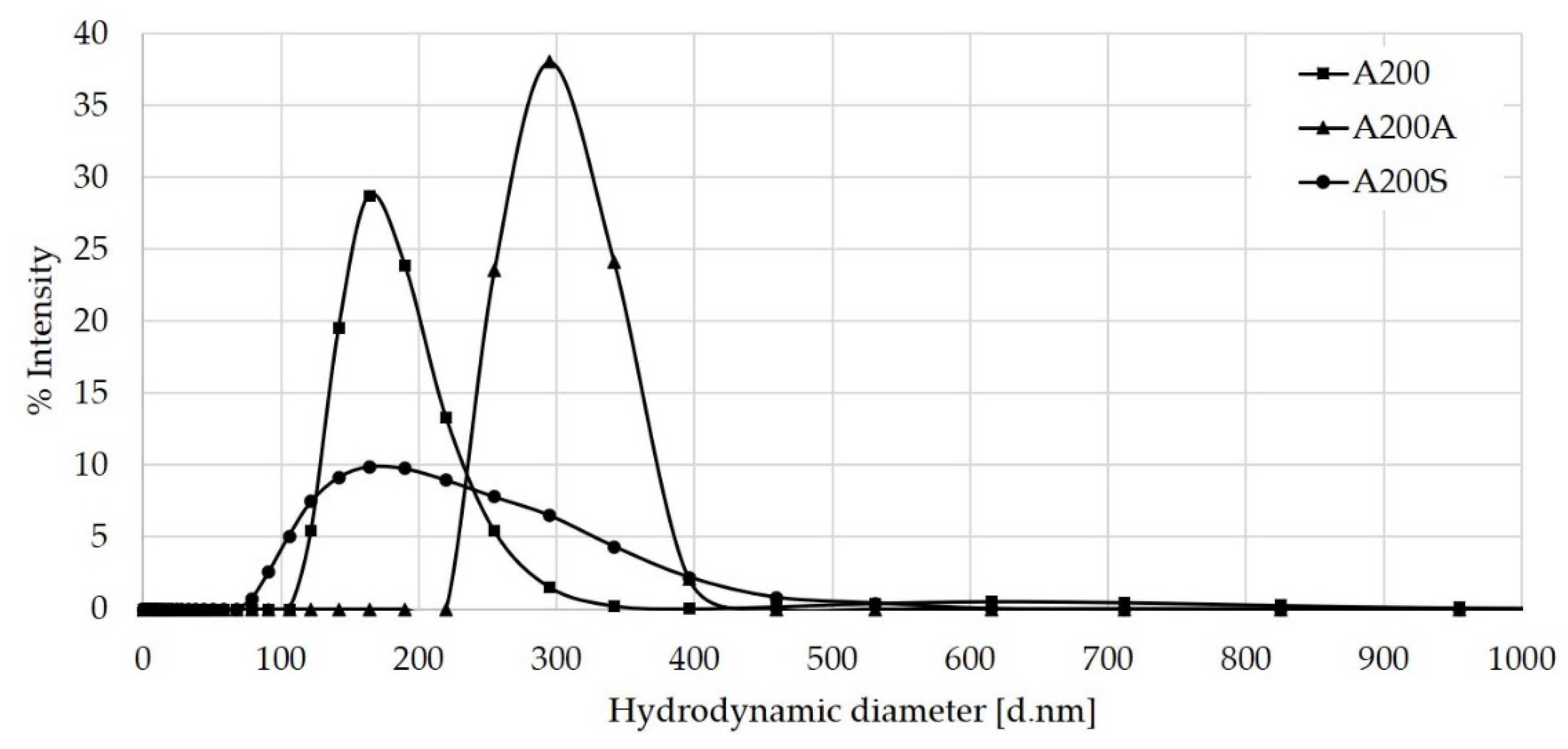
3.6. Hydrodynamic Radius by DLS Results
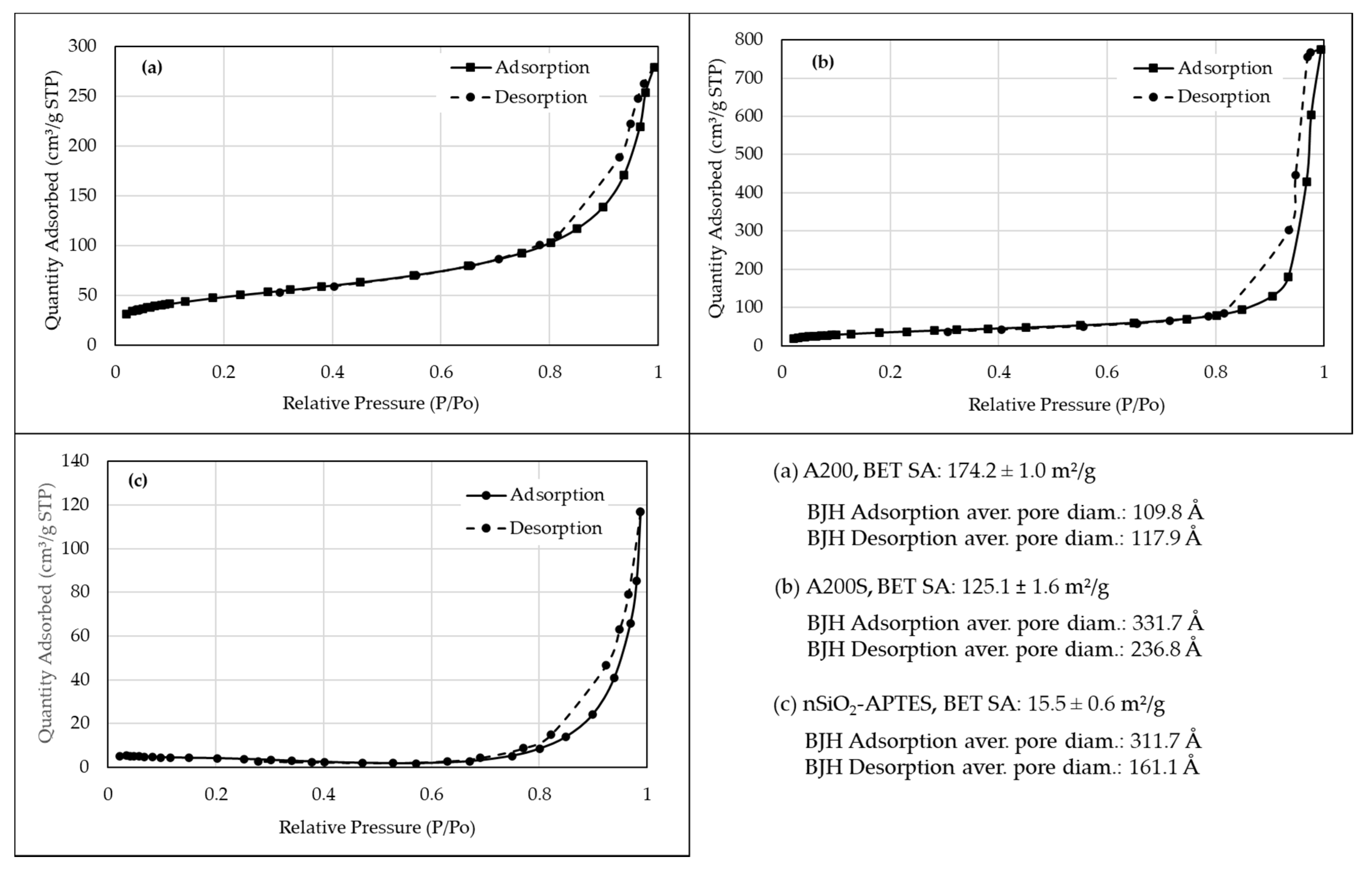
3.7. BET Isotherms
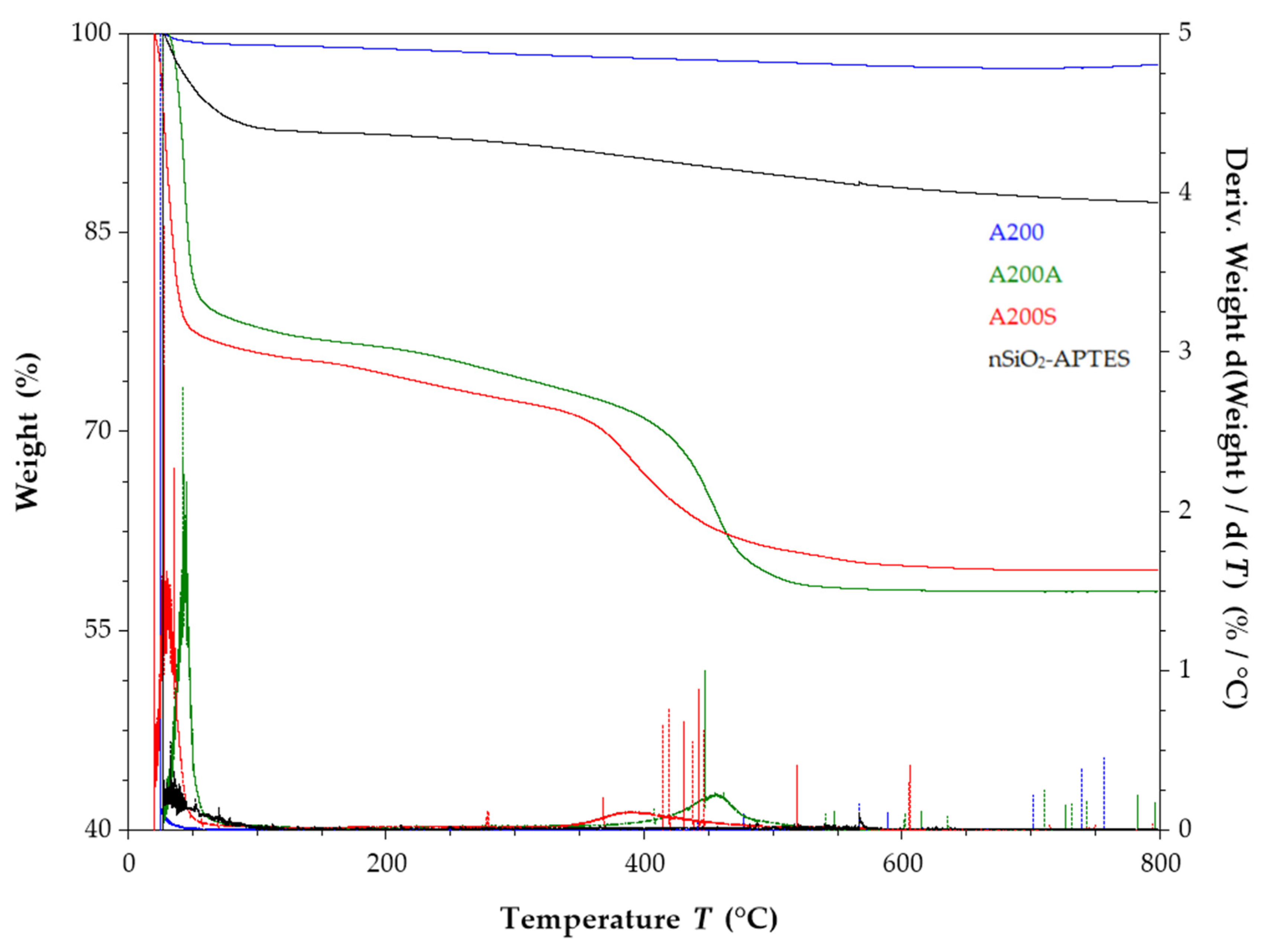
3.8. TGA Results
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bindhu, M.; Umadevi, M. Silver and Gold Nanoparticles for Sensor and Antibacterial Applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 37–45. [Google Scholar] [CrossRef]
- Alsaba, M.T.; Al Dushaishi, M.F.; Abbas, A.K. A Comprehensive Review of Nanoparticles Applications in the Oil and Gas Industry. J. Pet. Explor. Prod. Technol. 2020, 10, 1389–1399. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zhang, Y.; Chen, G.; Gai, Z. Application of Nanoparticles in Enhanced Oil Recovery: A Critical Review of Recent Progress. Energies 2017, 10, 345. [Google Scholar] [CrossRef] [Green Version]
- Bera, A.; Belhaj, H. Application of Nanotechnology by Means of Nanoparticles and Nanodispersions in Oil Recovery—A Comprehensive Review. J. Nat. Gas Sci. Eng. 2016, 34, 1284–1309. [Google Scholar] [CrossRef]
- Ponmani, S.; William, J.K.M.; Samuel, R.; Nagarajan, R.; Sangwai, J.S. Formation and Characterization of Thermal and Electrical Properties of CuO and ZnO Nanofluids in Xanthan Gum. Colloids Surf. A Physicochem. Eng. Asp. 2014. [Google Scholar] [CrossRef]
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial Applications of Nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddada, M.; Blanchard, J.; Casale, S.; Krafft, J.; Vallee, A.; Methivier, C.; Boujday, S. Optimizing the Immobilization of Gold Nanoparticles on Functionalized Silicon Surfaces: Amine- vs Thiol- Terminated Silane. Gold Bull. 2013, 46, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Milczarek, G.; Motylenko, M.; Sikorska, A.; Klapiszewski, Ł.; Wysokowski, M.; Bazhenov, V.; Piasecki, A.; Konował, E.; Ehrlich, H.; Jesionowski, T. Deposition of Silver Nanoparticles on Organically-Modified Silica in the Presence of Lignosulfonate. RSC Adv. 2014, 4, 52476–52484. [Google Scholar] [CrossRef]
- Vázquez-Velázquez, A.R.; Velasco-Soto, M.A.; Pérez-García, S.A.; Licea-Jiménez, L. Functionalization Effect on Polymer Nanocomposite Coatings Based on TiO2–SiO2 Nanoparticles with Superhydrophilic Properties. Nanomaterials 2018, 8, 369. [Google Scholar] [CrossRef] [Green Version]
- Colloidal, F.; Particles, S. Adsorption of Cu ( II ) and Ni ( II ) Ions on Functionalized Colloidal Silica Particles Model Studies for Wastewater Treatment. Ph.D. Thesis, Université de Franche-Comté, Besançon, France, 2014. [Google Scholar]
- Zienkiewicz-Strzałka, M.; Deryło-Marczewska, A.; Kozakevych, R.B. Silica Nanocomposites Based on Silver Nanoparticles-Functionalization and PH Effect. Appl. Nanosci. 2018, 8, 1649–1668. [Google Scholar] [CrossRef] [Green Version]
- Mallakpour, S.; Madani, M. A Review of Current Coupling Agents for Modification of Metal Oxide Nanoparticles. Prog. Org. Coatings 2015, 86, 194–207. [Google Scholar] [CrossRef]
- Herrera, A.P.; Ojeda, K.A.; Penaloza, A.D.; Rincón, A. Evaluation of Colloidal Stability, Kinematic Viscosity and Flash Point of B10 Diesel/Biodiesel Blends Using Nano-Structured Additives Based on Al2O3 and Oleic Acid. J. Cienc. Technol. Future 2017, 6, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Dorigato, A.; D’Amato, M.; Pegoretti, A. Thermo-Mechanical Properties of High Density Polyethylene—Fumed Silica Nanocomposites: Effect of Filler Surface Area and Treatment. J. Polym. Res. 2012, 19, 1–11. [Google Scholar] [CrossRef]
- Rodríguez-Quirós, H.A.; Casanova-Yepes, H.F. Effect of the Functionalization of Silica Nanoparticles as a Reinforcing Agent on Dental Composite Materials. Rev. Fac. Ing. 2015, 1, 36–44. [Google Scholar] [CrossRef]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and Surface Functionalization of Silica Nanoparticles for Nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Dixit, C.K.; Woolley, R.; MacCraith, B.D.; Okennedy, R.; McDonagh, C. Novel Multiparametric Approach to Elucidate the Surface Amine-Silanization Reaction Profile on Fluorescent Silica Nanoparticles. Langmuir 2010, 26, 18125–18134. [Google Scholar] [CrossRef]
- Zhang, J.F.; Chen, Y.; Facile, B.M. Functionalization of PDMS Elastomer Surfaces Using Thiolene Click Chemistry. Langmuir 2013, 29, 12432–12442. [Google Scholar] [CrossRef]
- Zhu, D.; Han, Y.; Zhang, J.; Li, X.; Feng, Y. Enhancing Rheological Properties of Hydrophobically Associative Polyacrylamide Aqueous Solutions by Hybriding with Silica Nanoparticles. J. Appl. Polym. Sci. 2014, 40876, 1–8. [Google Scholar] [CrossRef]
- Corredor, L.M.; Hemmati-Sarapardeh, A.; Husein, M.M.; Dong, M.; Maini, B.B. Rheological Behavior of Surface Modified Silica Nanoparticles Dispersed in Partially Hydrolyzed Polyacrylamide and Xanthan Gum Solutions: Experimental Measurements, Mechanistic Understanding, and Model Development. Energy Fuels 2018, 32, 10628–10638. [Google Scholar] [CrossRef]
- Mahdavian, A.R.; Ashjari, M.; Makoo, A.B. Preparation of Poly (Styrene-Methyl Methacrylate)/SiO2 Composite Nanoparticles via Emulsion Polymerization. An Investigation into the Compatiblization. Eur. Polym. J. 2007, 43, 336–344. [Google Scholar] [CrossRef]
- Wei, L.; Hu, N.; Zhang, Y. Synthesis of Polymer-Mesoporous Silica Nanocomposites. Materials (Basel) 2010, 3, 4066–4079. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Foster, E.; Wang, Y.; Nayak, S.; Cheng, V.; Ngo, V.W.; Pennell, K.D.; Bielawski, C.W.; Johnston, K.P. Effect of Grafted Copolymer Composition on Iron Oxide Nanoparticle Stability and Transport in Porous Media at High Salinity. Energy Fuels 2014, 28, 3655–3665. [Google Scholar] [CrossRef]
- Foster, E.L.; Xue, Z.; Roach, C.M.; Larsen, E.S.; Bielawski, C.W.; Johnston, K.P. Iron Oxide Nanoparticles Grafted with Sulfonated and Zwitterionic Polymers: High Stability and Low Adsorption in Extreme Aqueous Environments. ACS Macro Lett. 2014, 3, 867–871. [Google Scholar] [CrossRef]
- Ponnapati, R.; Karazincir, O.; Dao, E.; Ng, R.; Mohanty, K.K.; Krishnamoorti, R. Polymer-Functionalized Nanoparticles for Improving Waterflood Sweep Efficiency: Characterization and Transport Properties. IEC Res. Ind. Eng. Chem. Res. 2011, 50, 13030–13036. [Google Scholar] [CrossRef]
- Corredor, L.M.; Husein, M.; Maini, B. Impact of PAM-Grafted Nanoparticles on the Performance of Hydrolyzed Polyacrylamide Solutions for Heavy Oil Recovery at Different Salinities. Submitt. Ind. Eng. Chem. Res. 2019, 58, 9888–9899. [Google Scholar] [CrossRef]
- Tapia, J.F.D.; Lee, J.Y.; Ooi, R.E.H.; Foo, D.C.Y.; Tan, R.R. Optimal CO2 Allocation and Scheduling in Enhanced Oil Recovery (EOR) Operations. Appl. Energy 2016, 184, 337–345. [Google Scholar] [CrossRef]
- Corredor, L.M.; Aliabadian, E.; Husein, M.; Chen, Z.; Maini, B.; Sundararaj, U. Heavy Oil Recovery by Surface Modified Silica Nanoparticle/HPAM Nanofluids. Fuel 2019, 252, 622–634. [Google Scholar] [CrossRef]
- Prado, L.; Sriyai, M.; Ghislandi, M.; Barros, A.; Schulte, K. Surface Modification of TiO2 nanoparticles with Silane Coupling Agents. J. Braz. Chem. Soc. 2010, 21, 2238–2245. [Google Scholar] [CrossRef] [Green Version]
- Corredor, L.M.; Husein, M.M.; Maini, B.B. A Review of Polymer Nanohybrids for Oil Recovery. Adv. Colloid Interface Sci. 2019, 272, 102018. [Google Scholar] [CrossRef]
- Pham, K.N.; Fullston, D.; Surface, K.S.-C. Charge Modification of Nano-Sized Silica Colloid. Aust. J. Chem. 2007, 60, 662–666. [Google Scholar] [CrossRef]
- Rahman, I.A.; Jafarzadeh, M.; Sipaut, C.S. Synthesis of Organo-Functionalized Nanosilica via a Co-Condensation Modification Using γ-Aminopropyltriethoxysilane (APTES). Ceram. Int. 2009, 35, 1883–1888. [Google Scholar] [CrossRef]
- Uyanik, M. Synthesis and Characterization of TiO2 Nanostars. Ph.D. Thesis, Universität des Saarlandes, Saarbrücken, Germany, 2008. [Google Scholar]
- Nazir, T.; Afzal, A.; Siddiqi, H.M.; Ahmad, Z.; Dumon, M. Thermally and Mechanically Superior Hybrid Epoxy-Silica Polymer Films via Sol-Gel Method. Prog. Org. Coat. 2010, 69, 100–106. [Google Scholar] [CrossRef]
- Fattahi, N.; Ramazani, A.; Kinzhybalo, V. Imidazole-Functionalized Fe3O4/Chloro-Silane Core-Shell Nanoparticles: An Efficient Heterogeneous Organocatalyst for Esterification Reaction. Silicon 2019, 11, 1745–1754. [Google Scholar] [CrossRef]
- Hubner, E.; Allgaier, J.; Meyer, M.; Pyckhout-hintzen, W.; Richter, D. Synthesis of Polymer/Silica Hybrid Nanoparticles Using Anionic Polymerization Techniques. Macromolecules 2010, 43, 856–867. [Google Scholar] [CrossRef]
- Shen, S.; Kiong Ng, W.; Chia, L.; Dong, Y.; Tan, R. Sonochemical Synthesis of (3-Aminopropyl) Triethoxysilane-Modified Monodispersed Silica Nanoparticles for Protein Immobilization. Mater. Res. Bull. 2011, 46, 1665–1669. [Google Scholar] [CrossRef]
- Yoon, D.H.; Jang, J.W.; Cheong, I.W. Synthesis of Cationic Polyacrylamide/Silica Nanocomposites from Inverse Emulsion Polymerization and Their Flocculation Property for Papermaking. Colloids Surf. A Physicochem. Eng. Asp. 2012, 411, 18–23. [Google Scholar] [CrossRef]
- Qu, R.; Ma, X.; Wang, M.; Sun, C.; Sun, X.; Sun, S.; Zhang, Y.; Yin, P. Homogeneous Preparation of Polyamidoamine Grafted Silica Gels and Their Adsorption Properties as Au3+ Adsorbents. J. Ind. Eng. Chem. 2014, 20, 4382–4392. [Google Scholar] [CrossRef]
- Jiang, R.; Kunz, H.R.; Fenton, J.M. Composite Silica/Nafion® Membranes Prepared by Tetraethylorthosilicate Sol-Gel Reaction and Solution Casting for Direct Methanol Fuel Cells. J. Memb. Sci. 2006, 272, 116–124. [Google Scholar] [CrossRef]
- Tripathi, V.S.; Kandimalla, V.B.; Ju, H. Preparation of Ormosil and Its Applications in the Immobilizing Biomolecules. Sens. Actuators B Chem. 2006, 114, 1071–1082. [Google Scholar] [CrossRef]
- Ulu, A.; Noma, S.A.A.; Koytepe, S.; Ates, B. Magnetic Fe3O4@MCM-41 Core–Shell Nanoparticles Functionalized with Thiol Silane for Efficient l-Asparaginase Immobilization. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1035–1045. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.W.; Yoo, B.R. Advanced Silica/Polymer Composites: Materials and Applications. J. Ind. Eng. Chem. 2016, 1–12. [Google Scholar] [CrossRef]
- Cao, J.; Song, T.; Zhu, Y.; Wang, S.; Wang, X.; Lv, F.; Jiang, L.; Sun, M. Application of Amino-Functionalized Nanosilica in Improving the Thermal Stability of Acrylamide-Based Polymer for Enhanced Oil Recovery. Energy Fuels 2018, 32, 246–254. [Google Scholar] [CrossRef]
- Cao, J.; Song, T.; Wang, X.; Zhu, Y.; Wang, S.; Zhao, M.; Miao, Y.; Zhang, J. Studies on the Rheological Properties of Amphiphilic Nanosilica and a Partially Hydrolyzed Polyacrylamide Hybrid for Enhanced Oil Recovery. Chem. Eng. Sci. 2019, 206, 146–155. [Google Scholar] [CrossRef]
- Jafarzadeh, M.; Ab Rahman, I.; Sipaut, C.S. Synthesis of Silica-Polypyrrole Core-Shell Nanocomposite Using in Situ γ-Aminopropyltriethoxysilane (APTES)-Modified Nanosilica. Synth. Met. 2012, 162, 466–476. [Google Scholar] [CrossRef]
- Yoncheva, K.; Popova, M.; Szegedi, A.; Mihaly, J.; Tzankov, B.; Lambov, N. Functionalized Mesoporous Silica Nanoparticles for Oral Delivery of Budesonide. J. Solid State Chem. 2014, 211, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Cheang, T.Y.; Tang, B.; Xu, A.W.; Chang, G.Q.; Hu, Z.J.; He, W.L.; Xing, Z.H.; Xu, J.B.; Wang, M.; Wang, S.M. Promising Plasmid DNA Vector Based on APTESmodified Silica Nanoparticles. Int. J. Nanomed. 2012, 7, 1061–1067. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Zheng, K.; Zhang, Z.; Shi, W.; Jing, S.; Wang, L.; Zheng, W.; Zhao, D.; Xu, J.; Zhang, P. Adsorption and Protection of Plasmid DNA on Mesoporous Silica Nanoparticles Modified with Various Amounts of Organosilane. J. Colloid Interface Sci. 2012, 369, 317–322. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Wang, J.; Yang, Y.; Li, Y.; Yuan, Y.; Liu, C. Charge-Reversal APTES-Modified Mesoporous Silica Nanoparticles with High Drug Loading and Release Controllability. ACS Appl. Mater. Interfaces 2016, 8, 17166–17175. [Google Scholar] [CrossRef]
- Karim, A.H.; Jalil, A.A.; Triwahyono, S.; Sidik, S.M.; Kamarudin, N.H.N.; Jusoh, R.; Jusoh, N.W.C.; Hameed, B.H. Amino Modified Mesostructured Silica Nanoparticles for Efficient Adsorption of Methylene Blue. J. Colloid Interface Sci. 2012, 386, 307–314. [Google Scholar] [CrossRef]
- Gu, H.; Guo, Y.; Wong, S.Y.; He, C.; Li, X.; Shim, V.P.W. Effect of Interphase and Strain-Rate on the Tensile Properties of Polyamide 6 Reinforced with Functionalized Silica Nanoparticles. Compos. Sci. Technol. 2013, 75, 62–69. [Google Scholar] [CrossRef]
- Ruiz-Cañas, M.C.; Quintero, H.I.; Corredor, L.M.; Manrique, E.; Romero Bohórquez, A.R. New Nanohybrid Based on Hydrolyzed Polyacrylamide and Silica Nanoparticles: Morphological, Structural and Thermal Properties. Polymers (Basel) 2020, 12, 1152. [Google Scholar] [CrossRef] [PubMed]
- Stober, W.; Fink, A. Controlled Growth of Monodispersed Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Rahman, I.A.; Padavettan, V. Synthesis of Silica Nanoparticles by Sol-Gel: Size-Dependent Properties, Surface Modification, and Applications in Silica-Polymer Nanocomposites—A Review. J. Nanomater. 2012, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Lin, O.H.; Akil, H.M.; Ishak, Z.A.M. Characterization and Properties of Activated Nanosilica/Polypropylene Composites with Coupling Agents. Polym. Compos. 2009, 30, 1693–1700. [Google Scholar] [CrossRef]
- Chen, S.; Hayakawa, S.; Shirosaki, Y.; Fujii, E.; Kawabata, K.; Tsuru, K.; Osaka, A. Sol-Gel Synthesis and Microstructure Analysis of Amino-Modified Hybrid Silica Nanoparticles from Aminopropyltriethoxysilane and Tetraethoxysilane. J. Am. Ceram. Soc. 2009, 92, 2074–2082. [Google Scholar] [CrossRef]
- Kang, S.; Hong, S.I.; Choe, C.R.; Park, M.; Rim, S.; Kim, J. Preparation and Characterization of Epoxy Composites Filled with Functionalized Nanosilica Particles Obtained via Sol-Gel Process. Polymer (Guildf) 2001, 42, 879–887. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids; Academic Press: Cambridge, MA, USA, 1999. [Google Scholar]
- E2550-17. Standard Test Method for Thermal Stability by Thermogravimetry; ASTM International: West Conshohocken, PA, USA, 2017; Volume 11, pp. 1–5. [Google Scholar] [CrossRef]
- Shang, H.; Lu, Y.; Zhao, F.; Chao, C.; Zhang, B.; Zhang, H. Preparing High Surface Area Porous Carbon from Biomass by Carbonization in Molten Salt Medium. RSC Adv. 2015, 5, 75728–75734. [Google Scholar] [CrossRef]
- Kumar, R.D.; Kannan, G.K.; Kadirvelu, K.J. Populus Tree Wood: A Noble Bioresource from Western Himalayas for the Development of Various Carbon Types for the Effective Application in Environment Protection i.e., Phenol Adsorption from Wastewater. J. Bioremed. Biodegrad. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Hamid, S.; Syed, W.H.; Mohammad, G.-M. Synthesis and Characterization of Amino-Functionalized Mesoporous Silicate MCM-41 for Removal of Toxic Metal Ions. Chin. J. Chem. 2009, 27, 915–919. [Google Scholar] [CrossRef]
- Marler, B.; Oberhagemann, U.; Vortmann, S.; Gies, H. Influence of the Sorbate Type on the XRD Peak Intensities of Loaded MCM-41. Microporous Mater. 1996, 6, 375–383. [Google Scholar] [CrossRef]
- Mercier, L.; Pinnavaia, T.J. Heavy Metal Ion Adsorbents Formed by the Grafting of a Thiol Functionality to Mesoporous Silica Molecular Sieves: Factors Affecting Hg(II) Uptake. Environ. Sci. Technol. 1998, 32, 2749–2754. [Google Scholar] [CrossRef]
- Ay, F.; Aydinli, A. Comparative Investigation of Hydrogen Bonding in Silicon Based PECVD Grown Dielectrics for Optical Waveguides. Opt. Mater. 2004, 26, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Benmessaoud, A. Caracterización de Subóxidos de Silicio Obtenidos por las Técnicas de PECVD. Ph.D. Thesis, Universidad Autónoma de Barcelona, Barcelona, Spain, 2001. [Google Scholar]
- Fukuda, Y.; Zhou, W.; Furuya, K.; Suzuki, H. Photoluminescence Change of As-Prepared and Aged Porous Silicon with NaOH Treatment. J. Electrochem. Soc. 1999, 146, 2697. [Google Scholar] [CrossRef]
- Vázquez-Valerdi, D.E.; Luna-López, J.A.; García-Salgado, G.; Carrillo López, J.; Diaz-Becerril, T.; Rosendo, E.; Juárez-Santiesteban, H. Propiedades Ópticas, de Composición y Morfológicas de Películas Delgadas de SiOx Depositadas Por HFCVD. Superf. Vacío 2011, 24, 54–60. [Google Scholar]
- Vansant, E.F.; Van Der Voort, P.; Vrancken, K.C. Characterization and Chemical Modification of the Silica Surface; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Al-Oweini, R.; El-Rassy, H. Synthesis and Characterization by FTIR Spectroscopy of Silica Aerogels Prepared Using Several Si(OR)4 and R’’Si(OR’)3 Precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Kim, J.; Seidler, P.; Wan, L.S.; Fill, C. Formation, Structure, and Reactivity of Amino-Terminated Organic Films on Silicon Substrates. J. Colloid Interface Sci. 2009, 329, 114–119. [Google Scholar] [CrossRef]
- Vandenberg, E.T.; Bertilsson, L.; Liedberg, B.; Uvdal, K.; Erlandsson, R.; Elwing, H.; Lundström, I. Structure of 3-Aminopropyl Triethoxy Silane on Silicon Oxide. J. Colloid Interface Sci. 1991, 147, 103–118. [Google Scholar] [CrossRef]
- Clogston, J.; Patri, A. Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Humana Press: New York, NY, USA, 2011; Volume 697, pp. 63–70. [Google Scholar] [CrossRef] [Green Version]
- Sikora, A.; Bartczak, D.; Geißler, D.; Kestens, V.; Roebben, G.; Ramaye, Y.; Varga, Z.; Palmai, M.; Shard, A.G.; Goenaga-Infante, H.; et al. A Systematic Comparison of Different Techniques to Determine the Zeta Potential of Silica Nanoparticles in Biological Medium. Anal. Methods 2015, 7, 9835–9843. [Google Scholar] [CrossRef] [Green Version]
- Llinàs, M.C.; Sánchez-García, D. Nanopartículas de Sílice: Preparación y Aplicaciones En Biomedicina. Afinidad LXXI 2014, 565, 20–31. [Google Scholar]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials (Basel) 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, S.S. Estudio de Propiedades y Aplicaciones de Nanoestructuras de Sílice Mesoporosa y Aluminosilicatos En El Calzado. Ph.D. Thesis, Universidad de la Rioja, La Rioja, Spain, 2017. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Goeppert, A.; Meth, S.; Prakash, G.K.S.; Olah, G.A. Nanostructured Silica as a Support for Regenerable High-Capacity Organoamine-Based CO2 Sorbents. Energy Environ. Sci. 2010, 3, 1949–1960. [Google Scholar] [CrossRef]
- Evonik. Aerosil—Fumed Silica: Technical Overview; Evonik: Essen, Germany, 2015. [Google Scholar]
- Xia, K.; Ferguson, R.Z.; Losier, M.; Tchoukanova, N.; Brüning, R. Synthesis of Hybrid Silica Materials with Tunable Pore Structures and Morphology and Their Application for Heavy Metal Removal from Drinking Water Synthesis of Hybrid Silica Materials with Tunable Pore Structures and Morphology and Their Application for H. J. Hazard. Mater. 2010, 183, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Jaroniec, C.P.; Gilpin, R.K.; Jaroniec, M. Adsorption and Thermogravimetric Studies of Silica-Based Amide Bonded Phases. J. Phys. Chem. B 1997, 101, 6861–6866. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds A200S and nSiO2-APTES are available from the authors. |
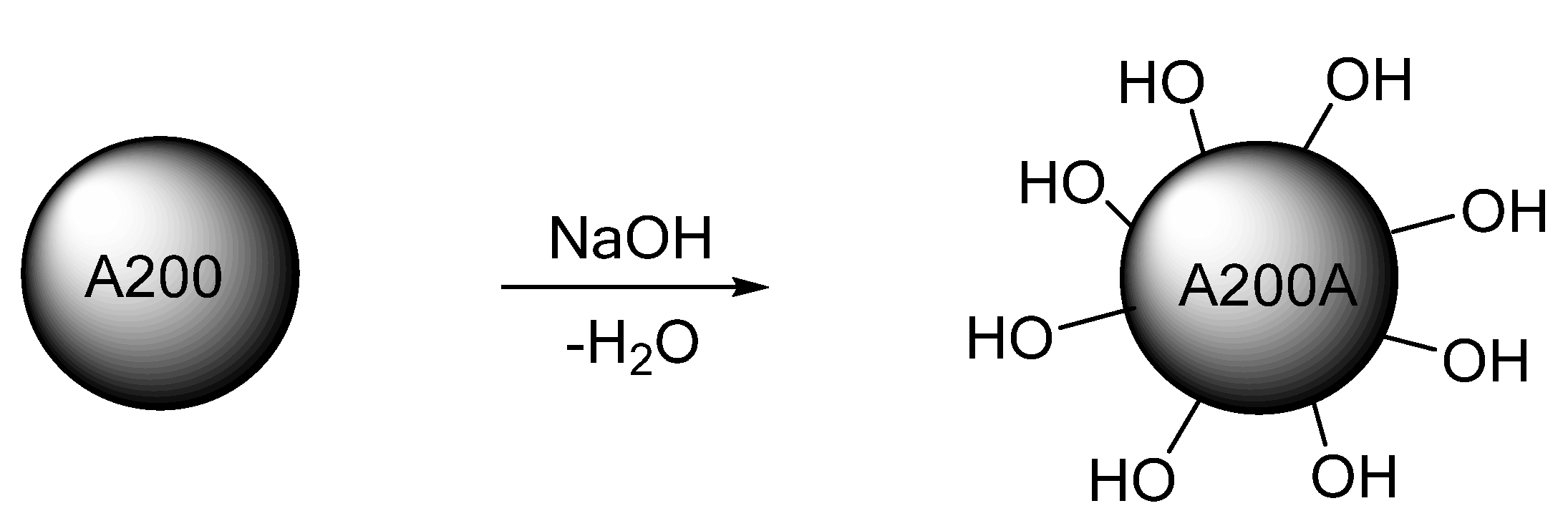
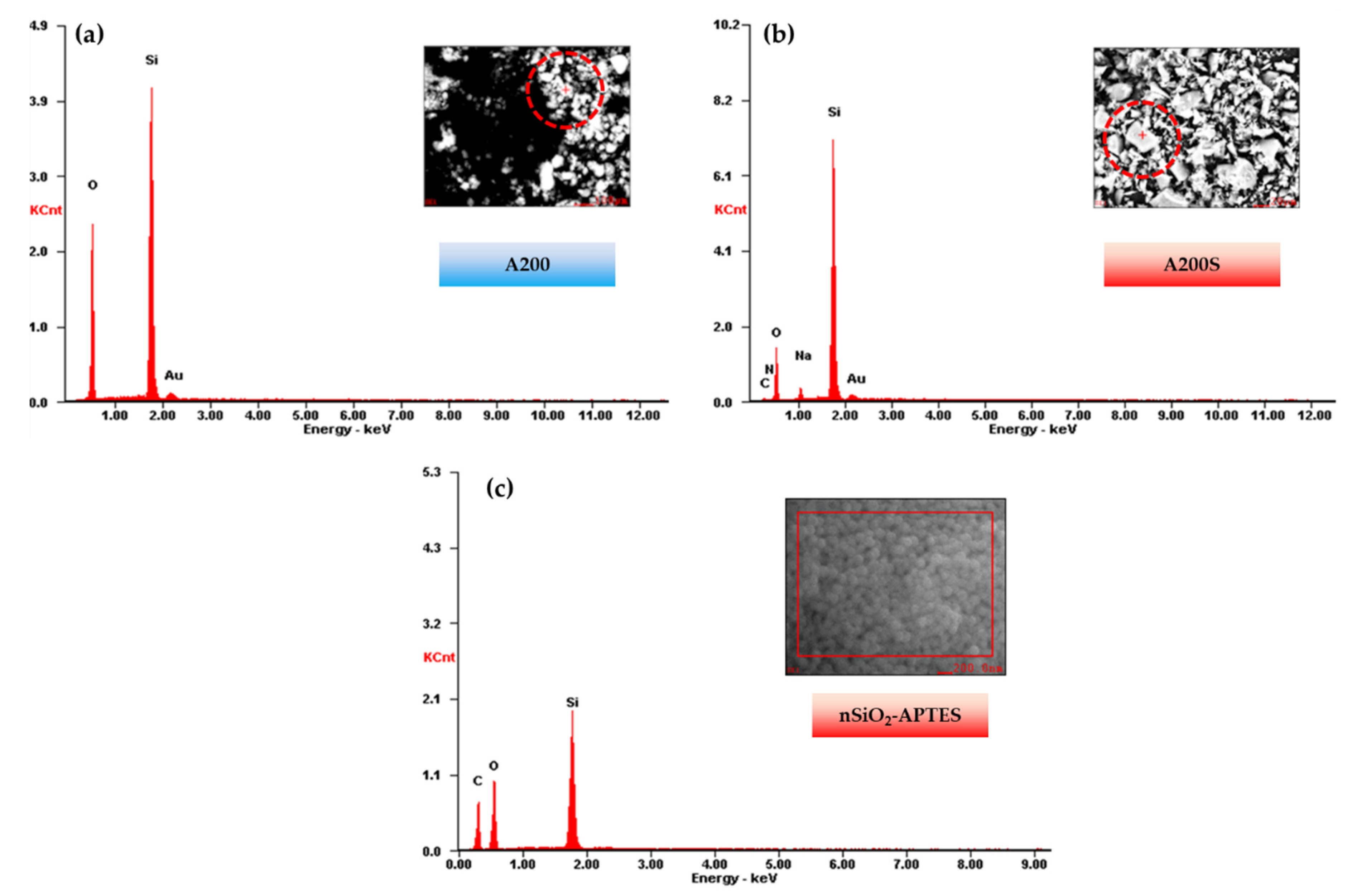




| Element | A200 | A200S | nSiO2-APTES | |||
|---|---|---|---|---|---|---|
| wt% | at% | wt% | at% | wt% | at% | |
| C | 3.44 | 6.35 | 36.29 | 47.16 | ||
| N | 1.17 | 1.85 | 0.50 | 0.50 | ||
| O | 53.80 | 65.16 | 32.69 | 45.35 | 41.51 | 40.50 |
| Si | 36.84 | 25.41 | 53.31 | 42.12 | 22.20 | 12.34 |
| Na | 3.83 | 3.70 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Cañas, M.C.; Corredor, L.M.; Quintero, H.I.; Manrique, E.; Romero Bohórquez, A.R. Morphological and Structural Properties of Amino-Functionalized Fumed Nanosilica and Its Comparison with Nanoparticles Obtained by Modified Stöber Method. Molecules 2020, 25, 2868. https://doi.org/10.3390/molecules25122868
Ruiz-Cañas MC, Corredor LM, Quintero HI, Manrique E, Romero Bohórquez AR. Morphological and Structural Properties of Amino-Functionalized Fumed Nanosilica and Its Comparison with Nanoparticles Obtained by Modified Stöber Method. Molecules. 2020; 25(12):2868. https://doi.org/10.3390/molecules25122868
Chicago/Turabian StyleRuiz-Cañas, María C., Laura M. Corredor, Henderson I. Quintero, Eduardo Manrique, and Arnold R. Romero Bohórquez. 2020. "Morphological and Structural Properties of Amino-Functionalized Fumed Nanosilica and Its Comparison with Nanoparticles Obtained by Modified Stöber Method" Molecules 25, no. 12: 2868. https://doi.org/10.3390/molecules25122868
APA StyleRuiz-Cañas, M. C., Corredor, L. M., Quintero, H. I., Manrique, E., & Romero Bohórquez, A. R. (2020). Morphological and Structural Properties of Amino-Functionalized Fumed Nanosilica and Its Comparison with Nanoparticles Obtained by Modified Stöber Method. Molecules, 25(12), 2868. https://doi.org/10.3390/molecules25122868







