Real-Time Analysis of the Stability of Oil-In-Water Pickering Emulsion by Electrochemical Impedance Spectroscopy
Abstract
1. Introduction
2. Results and Discussion
2.1. The Establishment of an Equivalent Circuit
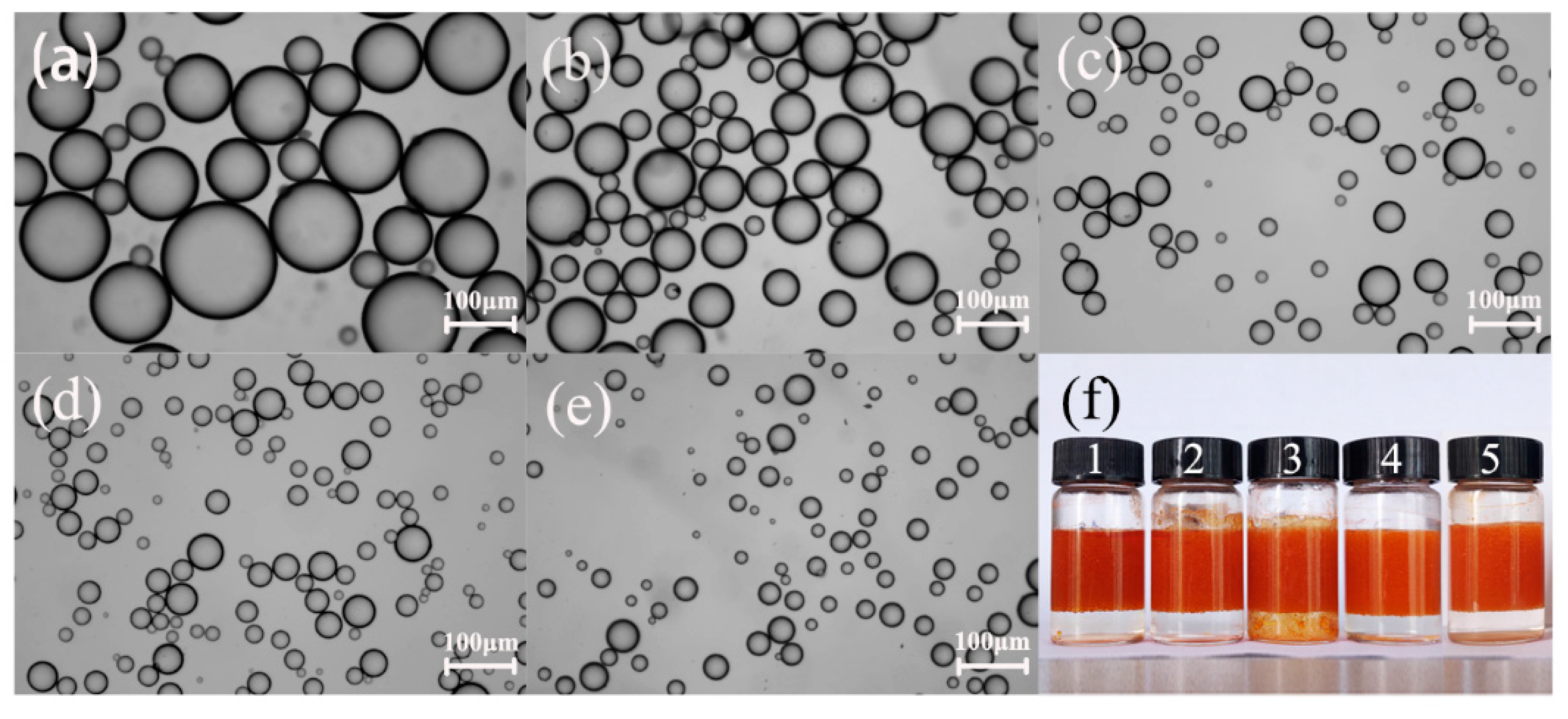
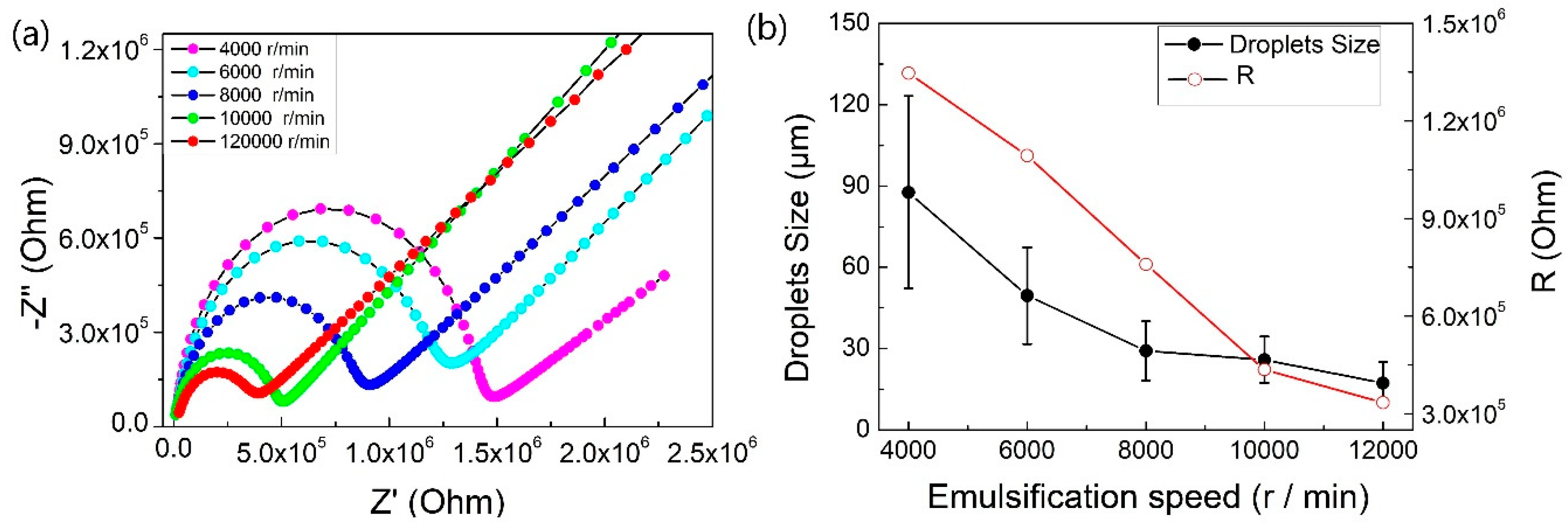
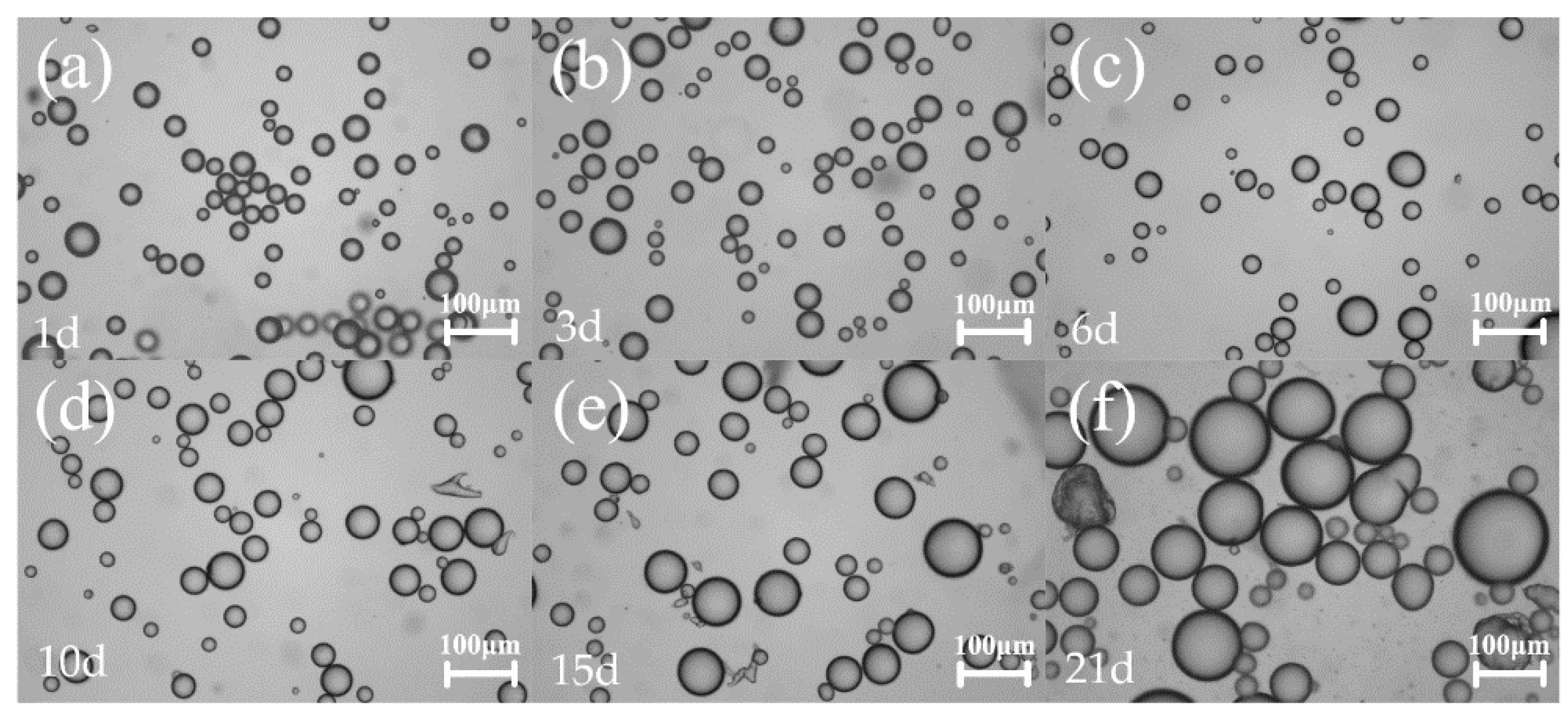
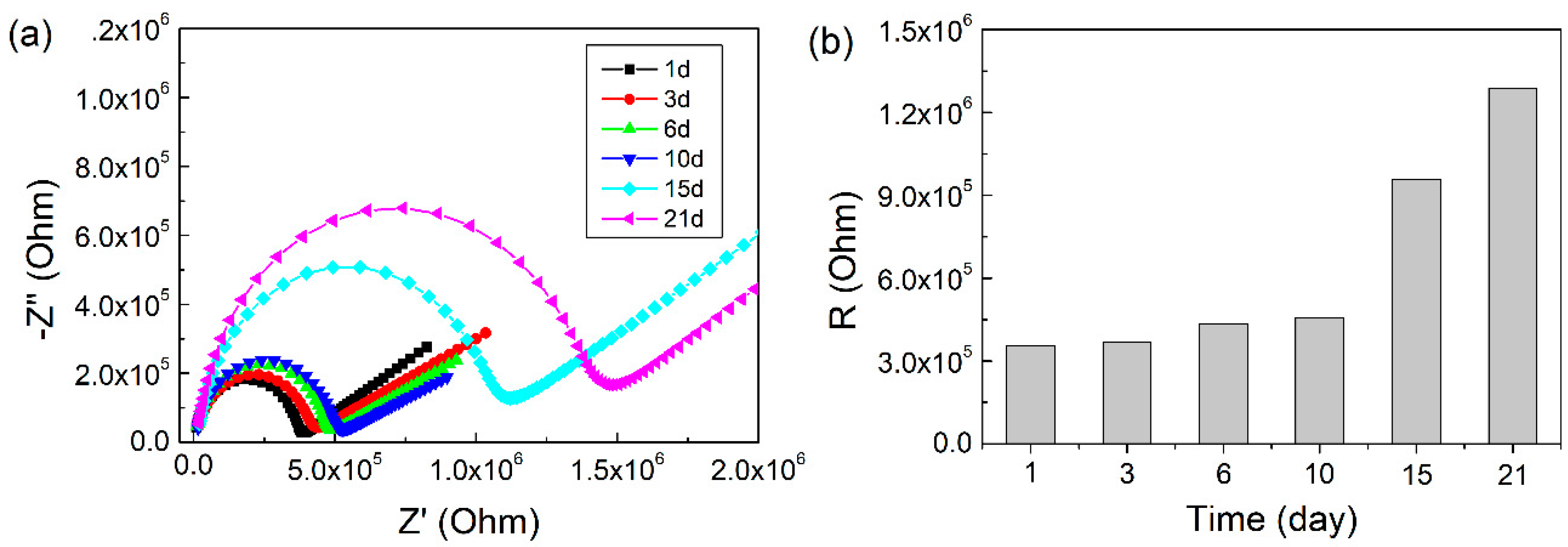
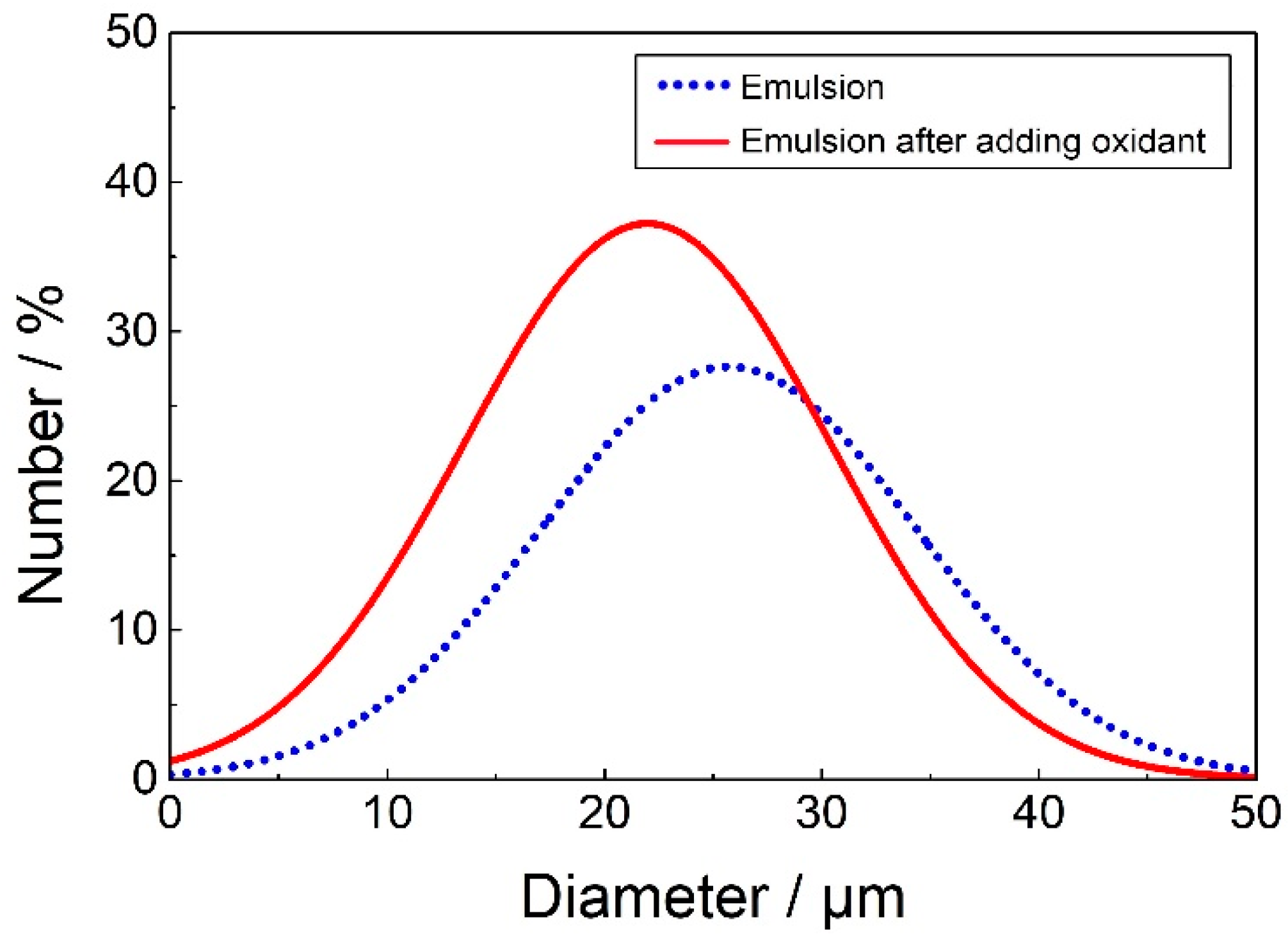
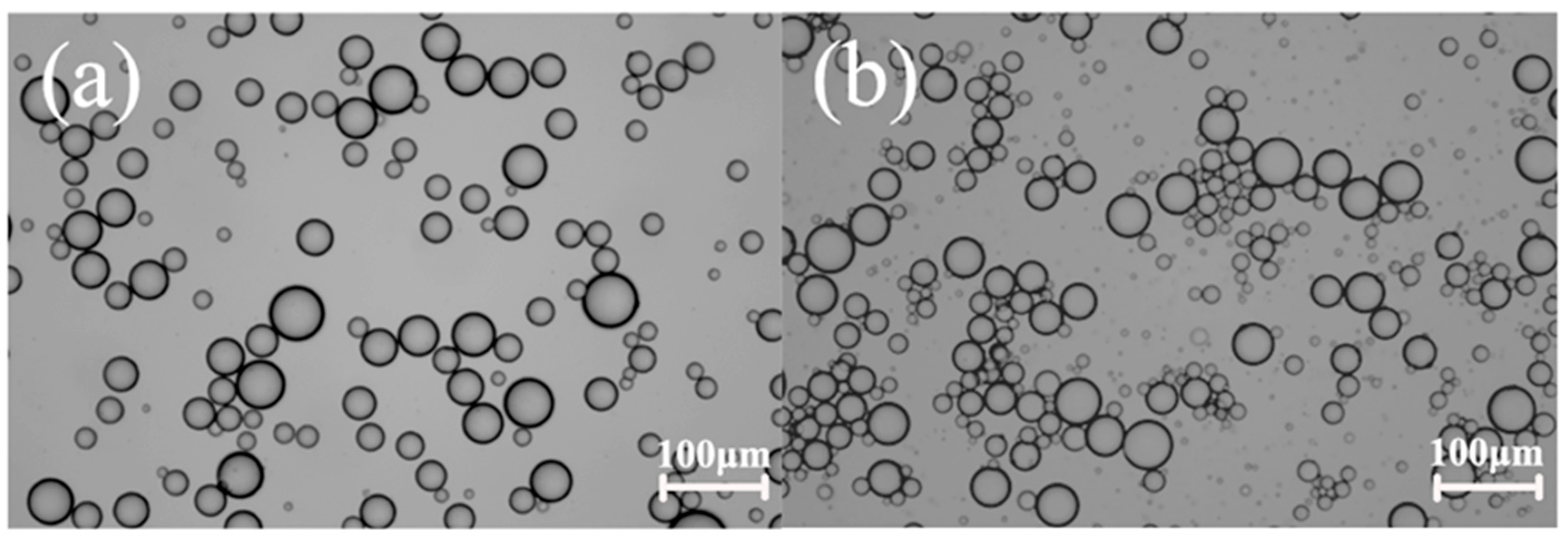
2.2. EIS Characterization of Pickering Emulsion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ramsden, W. Separation of solids in the surface-layers of solutions and suspensions. Proc. R Soc. Lond 1903, 72, 156–164. [Google Scholar]
- Pickering, S.U. Emulsions. J. Chem. Soc. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as surfactants—Similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions stabilized solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100–102, 503–546. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, Y. CO2/N2-switchable high internal phase pickering emulsion stabilized by silica nanoparticles and low-cost commercial N,N-dimethyl-N-dodecylamine. Colloid Surf. A 2019, 562, 119–126. [Google Scholar] [CrossRef]
- Lee, J.; Chang, J.Y. Pickering emulsion stabilized by microporous organic polymer particles for the fabrication of a hierarchically porous monolith. Langmuir 2018, 34, 11843–11849. [Google Scholar] [CrossRef] [PubMed]
- Briggs, N.; Raman, A.K.Y.; Barrett, L.; Brown, C.; Li, B.; Leavitt, D.; Aichele, C.P.; Crossley, S. Stable pickering emulsions using multi-walled carbon nanotubes of varying wettability. Colloid Surf. A 2018, 537, 227–235. [Google Scholar] [CrossRef]
- Pei, X.; Zhai, K.; Wang, C.; Deng, Y.; Tan, Y.; Zhang, B.; Bai, Y.; Xu, K.; Wang, P. Polymer brush graft-modified starch-based nanoparticles as pickering emulsifiers. Langmuir 2019, 35, 7222–7230. [Google Scholar] [CrossRef]
- Huang, F.; Liang, Y.; He, Y. On the pickering emulsions etabilized by calcium carbonate particles with various morphologies. Colloid Surf. A 2019, 580, 123722. [Google Scholar] [CrossRef]
- Jiang, W.-L.; Fu, Q.-J.; Yao, B.-J.; Ding, L.-G.; Liu, C.-X.; Dong, Y.-B. Smart pH-responsive polymer-tethered and Pd NP-loaded NMOF as the pickering interfacial catalyst for one-pot cascade biphasic reaction. ACS Appl Mater. Inter. 2017, 9, 36438–36446. [Google Scholar] [CrossRef]
- Lin, Q.; Xu, M.; Cui, Z.; Pei, X.; Jiang, J.; Song, B. Structure and stabilization mechanism of diesel oil-in-water emulsions stabilized solely by either positively or negatively charged nanoparticles. Colloid Surf. A 2019, 573, 30–39. [Google Scholar] [CrossRef]
- Fouilloux, S.; Malloggi, F.; Daillant, J.; Thill, A. Aging mechanism in model pickering emulsion. Soft Matter 2016, 12, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Schröder, A.; Sprakel, J.; Schroën, K.; Spaen, J.N.; Berton-Carabin, C.C. Coalescence stability of pickering emulsions produced with lipid particles: A microfluidic study. J. Food Process. Eng. 2018, 234, 63–72. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Heuzey, M.-C. Chitosan-based conventional and pickering emulsions with long-term stability. Langmuir 2016, 32, 929–936. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, G.; Li, J.; Feng, Y.; Zhang, L.; Peng, Y.; Tang, Y.; Wang, L. Eco-friendly pickering emulsion stabilized by silica nanoparticles dispersed with high-molecular-weight amphiphilic alginate derivatives. ACS Sustain. Chem. Eng. 2018, 6, 4105–4114. [Google Scholar] [CrossRef]
- de Oliveira, H.P.; de Melo, C.P. Use of electrical impedance spectroscopy as a practical method of investigating the formation of aggregates in aqueous solutions of dyes and surfactants. J. Phys. Chem. B 2011, 115, 6903–6908. [Google Scholar] [CrossRef]
- Ghasemi, S.; Darestani, M.T.; Abdollahi, Z.; Hawkett, B.S.; Gomes, V.G. Electrical impedance spectroscopy for determining critical micelle concentration of ionic emulsifiers. Colloid Surf. A 2014, 441, 195–203. [Google Scholar] [CrossRef]
- Jiang, Q.; Sun, N.; Li, Q.; Si, W.; Li, J.; Li, A.; Gao, Z.; Wang, W.; Wang, J. Redox-responsive pickering emulsions based on silica nanoparticles and electrochemical active fluorescent molecules. Langmuir 2019, 35, 5848–5854. [Google Scholar] [CrossRef]
- Westerlund, S.; Ekstam, L. Capacitor theory. IEEE Trans. Dielect. El 1994, 1, 826–839. [Google Scholar] [CrossRef]
- Pradhan, R.; Mitra, A.; Das, S. Characterization of electrode/electrolyte interface of ECIS devices. Electroanalysis 2012, 24, 2405–2414. [Google Scholar] [CrossRef]
- Bordi, F.; Cametti, C.; Gili, T. Reduction of the contribution of electrode polarization effects in the radiowave dielectric measurements of highly conductive biological cell suspensions. Bioelectrochemistry 2001, 54, 53–61. [Google Scholar] [CrossRef]
- Huang, V.M.-W.; Vivier, V.; Orazem, M.E.; Pébère, N.; Tribollet, B. The apparent constant-phase-element behavior of a disk electrode with faradaic reactions. J. Electrochem. Soc. 2007, 154, 99–107. [Google Scholar] [CrossRef]
- Dabros, T. Electrokinetic and colloid transport phenomena. Can. J. Chem. Eng. 2006, 84, 13–32. [Google Scholar]
- Shahidi, S.; Koch, C.R.; Bhattacharjee, S.; Sadrzadeh, M. Dielectric behavior of oil–water emulsions during phase separation probed by electrical impedance spectroscopy. Sens. Actuatorsb. 2017, 243, 460–464. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds FcA are available from the authors. |

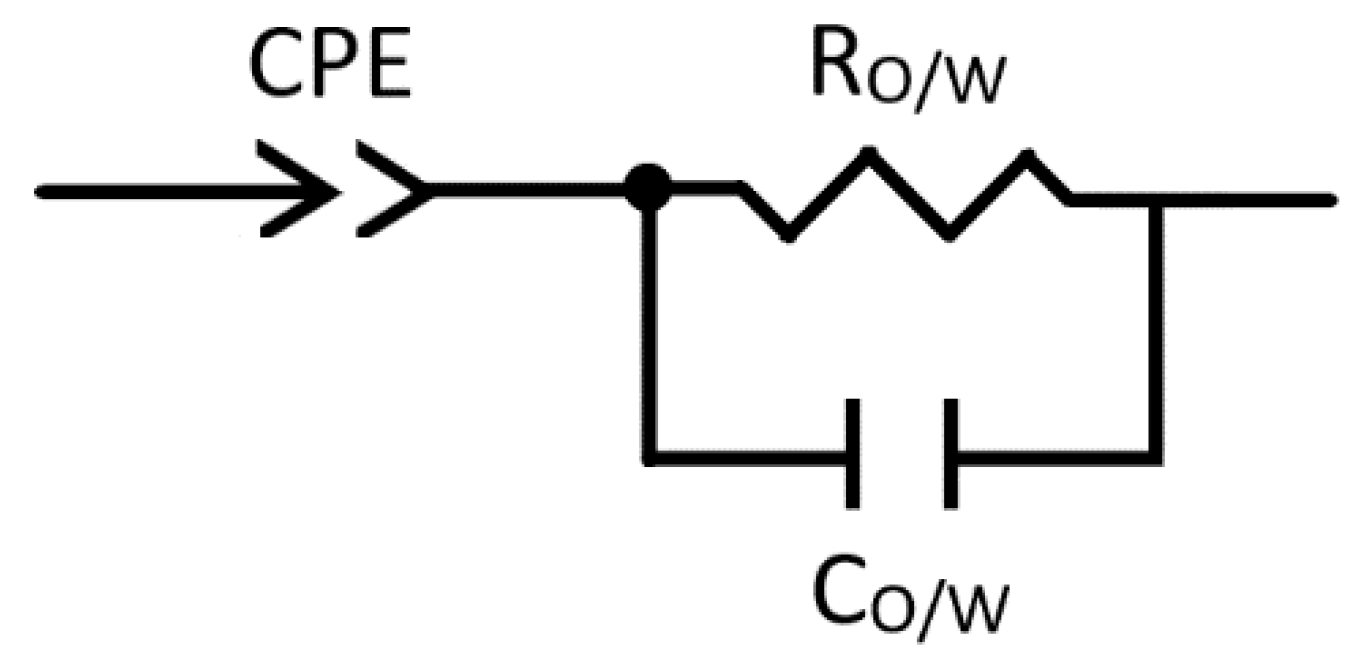
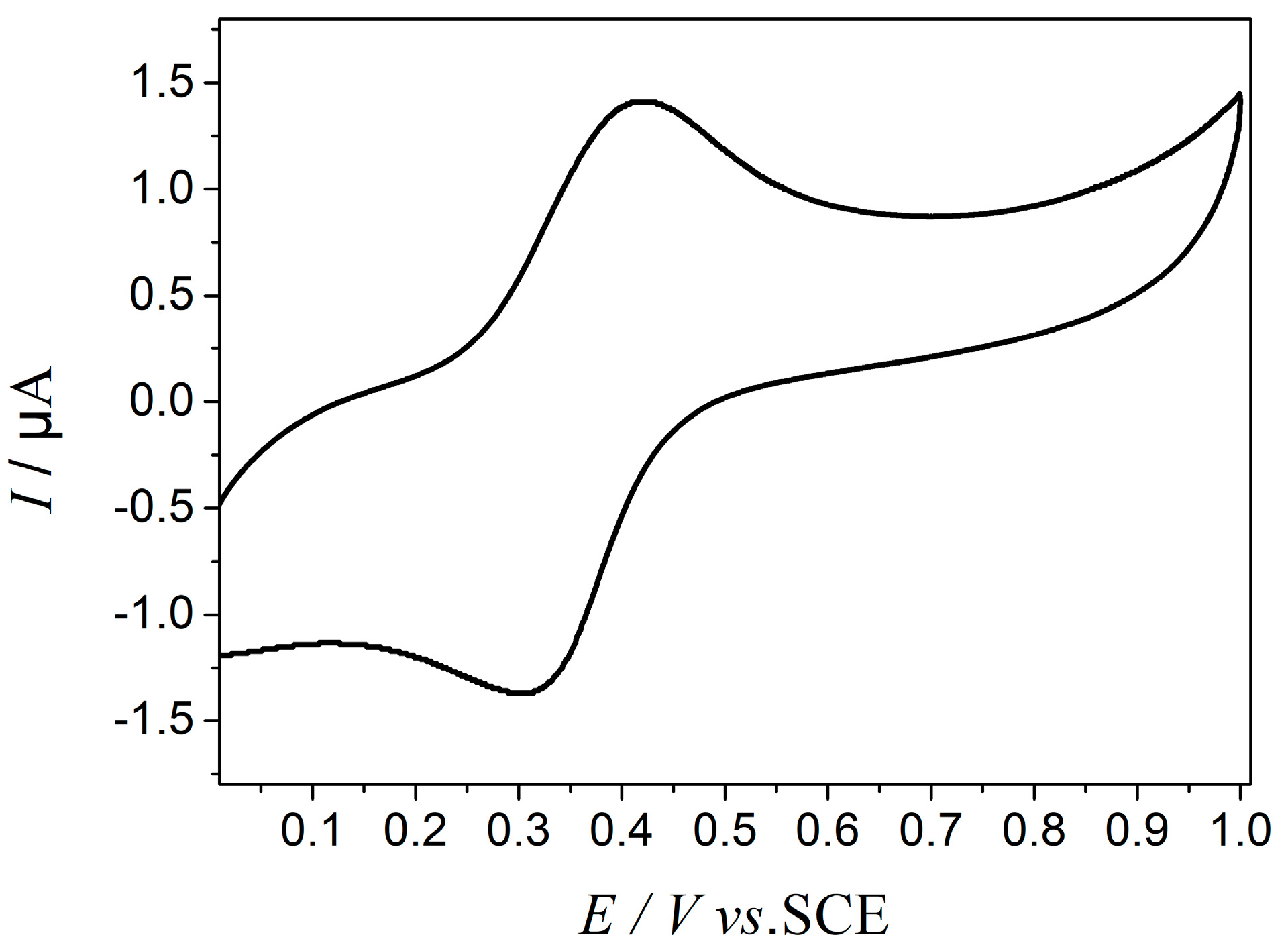


| Parameter | Fitted Value | Error % |
|---|---|---|
| R (MOhm) | 0.44 | 2.19 |
| C (pF) | 5.02 | 2.66 |
| CPE-T(μF) | 0.16 | 4.13 |
| CPE-P | 0.42 | 2.24 |
| Speed (r/min) | Average Droplet Size (μm) | R (MOhm) | C (pF) | τ (μs) |
|---|---|---|---|---|
| 4000 | 87.65 ± 35.52 | 1.35 | 3.92 | 5.28 |
| 6000 | 49.52 ± 17.88 | 1.09 | 4.00 | 4.37 |
| 8000 | 29.16 ± 10.93 | 0.76 | 4.02 | 3.05 |
| 10000 | 25.89 ± 8.57 | 0.44 | 5.02 | 2.18 |
| 12000 | 17.28 ± 7.75 | 0.33 | 5.15 | 1.72 |
| Storage Time (Day) | Average Droplet Size (μm) | R (MOhm) | C (pF) | τ (μs) |
|---|---|---|---|---|
| 1 | 25.06 ± 6.27 | 0.35 | 3.91 | 1.38 |
| 3 | 25.89 ± 7.30 | 0.37 | 3.87 | 1.43 |
| 6 | 26.28 ± 7.02 | 0.43 | 3.77 | 1.64 |
| 10 | 28.30 ± 8.93 | 0.46 | 4.46 | 2.04 |
| 15 | 42.68 ± 15.05 | 0.96 | 3.88 | 3.71 |
| 21 | 65.36 ± 22.85 | 1.29 | 3.69 | 4.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Q.; Sun, N.; Kumar, P.; Li, Q.; Liu, B.; Li, A.; Wang, W.; Gao, Z. Real-Time Analysis of the Stability of Oil-In-Water Pickering Emulsion by Electrochemical Impedance Spectroscopy. Molecules 2020, 25, 2904. https://doi.org/10.3390/molecules25122904
Jiang Q, Sun N, Kumar P, Li Q, Liu B, Li A, Wang W, Gao Z. Real-Time Analysis of the Stability of Oil-In-Water Pickering Emulsion by Electrochemical Impedance Spectroscopy. Molecules. 2020; 25(12):2904. https://doi.org/10.3390/molecules25122904
Chicago/Turabian StyleJiang, Qiuyan, Ning Sun, Parveen Kumar, Qiuhong Li, Bo Liu, Aixiang Li, Weiwei Wang, and Zengli Gao. 2020. "Real-Time Analysis of the Stability of Oil-In-Water Pickering Emulsion by Electrochemical Impedance Spectroscopy" Molecules 25, no. 12: 2904. https://doi.org/10.3390/molecules25122904
APA StyleJiang, Q., Sun, N., Kumar, P., Li, Q., Liu, B., Li, A., Wang, W., & Gao, Z. (2020). Real-Time Analysis of the Stability of Oil-In-Water Pickering Emulsion by Electrochemical Impedance Spectroscopy. Molecules, 25(12), 2904. https://doi.org/10.3390/molecules25122904






