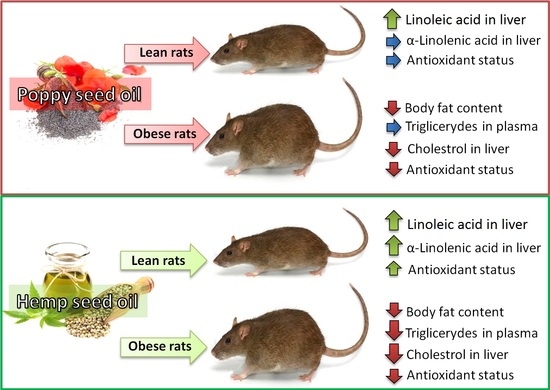
Comparative Effects of Dietary Hemp and Poppy Seed Oil on Lipid Metabolism and the Antioxidant Status in Lean and Obese Zucker Rats
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemical Composition of Oils
4.2. Animal Study
4.3. Sample Collection, Growth Parameters, and Body Composition
4.4. Liver Antioxidant Status and Lipid Content
4.5. Plasma Antioxidant Status, Lipid Profile, and Inflammatory Indicators
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Crescente, G.; Piccolella, S.; Esposito, A.; Scognamiglio, M.; Fiorentino, A.; Pacifico, S. Chemical composition and nutraceutical properties of hempseed: An ancient food with actual functional value. Phytochem. Rev. 2018, 17, 733–749. [Google Scholar]
- Bozan, B.; Temelli, F. Chemical composition and oxidative stability of flax, safflower and poppy seed and seed oils. Bioresour. Technol. 2008, 99, 6354–6359. [Google Scholar] [CrossRef]
- Abudak, M.; Kara, H.H. Fatty acid composition and some bioactive properties of edible oil extracted from different varieties of poppy (Papaver somniferum L.) seeds. Riv. Ital. Sostanze Grasse 2017, 94, 19–25. [Google Scholar]
- Rodriguez-Leyva, D.; Pierce, G.N. The cardiac and haemostatic effects of dietary hempseed. J. Nutr. Metab. 2010, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Siano, F.; Moccia, S.; Picariello, G.; Russo, G.L.; Sorrentino, G.; Di Stasio, M.; La Cara, F.; Grazia, M.V. Comparative study of chemical, biochemical characteristic and ATR-FTIR analysis of seeds, oil and flour of the edible fedora cultivar hemp (Cannabis sativa L.). Molecules 2018, 24, 83. [Google Scholar] [CrossRef] [Green Version]
- Multari, S.; Marsol-Vall, A.; Heponiemi, P.; Suomela, J.P.; Yang, B. Changes in the volatile profile, fatty acid composition and other markers of lipid oxidation of six different vegetable oils during short-term deep-frying. Food Res. Int. 2019, 122, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Ozcan, M.M.; AL-Juhaimi, F.; Babiker, E.E.; Fadimu, G.J. Changes in quality, bioactive compounds, fatty acids, tocopherols, and phenolic composition in oven- and microwave-roasted poppy seeds and oil. Lwt–Food Sci. Technol. 2019, 99, 490–496. [Google Scholar] [CrossRef]
- Schwab, U.S.; Callaway, J.C.; Erkkila, A.T.; Gynther, J.; Uusitupa, M.I.J.; Jarvinen, T. Effects of hempseed and flaxseed oils on the profile of serum lipids, serum total and lipoprotein lipid concentrations and haemostatic factor. Eur. J. Nutr. 2006, 45, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Sargolzaie, N.; Sargolzaei, J.; Mohammadizadegan, M. Effects of hempseed oil injection to rats on blood lipoproteins level. Int. J. Med. Res. Prof. 2016, 2, 239–246. [Google Scholar]
- Chan, R.S.; Woo, J. Prevention of overweight and obesity: How effective is the current public health approach. Int. J. Environ. Res. Public Health 2010, 7, 765–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, G.A.; Krauss, R.M. Overfeeding of polyunsaturated versus saturated fatty acids reduces ectopic fat. Diabetes 2014, 63, 2222–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, R.; Huang, Y.S. Gamma linolenic acid: An anti-inflammatory omega-6 fatty acid. Curr. Pharm. Biotechnol. 2006, 7, 531–534. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. Essential fatty acids: Biochemistry, physiology and pathology. Biotechnol. J. 2006, 1, 420–439. [Google Scholar] [CrossRef]
- Radzikowska, U.; Rinaldi, A.O.; Çelebi Sözener, Z.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The influence of dietary fatty acids on immune responses. Nutrients 2019, 11, 2990. [Google Scholar] [CrossRef] [Green Version]
- Lunn, J.; Theobald, H.E. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Filion, K.B.; El Khoury, F.; Bielinski, M.; Schiller, I.; Dendukuri, N.; Brophy, J.M. Omega 3 fatty acids in high-risk cardiovascular patients: A meta-analysis of randomized controlled trials. Bmc Cardiovasc. Disord. 2010, 3, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Giordano, E.; Visioli, F. Long-chain omega 3 fatty acids: Molecular bases of potential antioxidant actions. Prostaglandins Leukot. Essent. Fat. Acids 2014, 90, 1–4. [Google Scholar] [CrossRef]
- Choque, B.; Catheline, D.; Rioux, V.; Legrand, P. Linoleic acid: Between doubts and certainties. Biochimie 2014, 96, 14–21. [Google Scholar] [CrossRef]
- Yang, J.; Fernández-Galilea, M.; Martínez-Fernández, L.; González-Muniesa, P.; Pérez-Chávez, A.; Martínez, J.A.; Moreno-Aliaga, M.J. Oxidative Stress and Non-Alcoholic Fatty Liver Disease: Effects of Omega-3 Fatty Acid Supplementation. Nutrients 2019, 11, 872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.W.; Chien, Y.S.; Chen, Y.J.; Ajuwon, K.M.; Mersmann, H.M.; Ding, S.T. Role of n-3 polyunsaturated fatty acids in ameliorating the obesity-induced metabolic syndrome in animal models and humans. Int. J. Mol. Sci. 2016, 17, 1689. [Google Scholar] [CrossRef] [PubMed]
- Kummerow, F.A.; Zhou, Q.; Mahfouz, M.M.; Smiricky, M.R.; Grieshop, C.M.; Schaeffer, D.J. Trans fatty acids in hydrogenated fat inhibited the synthesis of the polyunsaturated fatty acids in the phospholipid of arterial cells. Life Sci. 2004, 22, 2707–2723. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, F.; Iggman, D.; Kullberg, J.; Cedernaes, J.; Johansson, H.E.; Larsson, A.; Johansson, L.; Ahlström, H.; Arner, P.; Dahlman, I.; et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes 2014, 63, 2356–2368. [Google Scholar] [CrossRef] [Green Version]
- Cardel, M.; Lemas, D.J.; Jackson, K.H.; Friedman, J.E.; Fernández, J.R. Higher intake of PUFAs is associated with lower total and visceral adiposity and higher lean mass in a racially diverse sample of children. J. Nutr. 2015, 145, 2146–2152. [Google Scholar] [CrossRef]
- Clarke, S.D. Nonalcoholic steatosis and steatohepatitis. I. Molecular mechanism for polyunsaturated fatty acid regulation of gene transcription. Am. J. Physiol. Gastrointest Liver Physiol. 2001, 281, 865–869. [Google Scholar] [CrossRef] [Green Version]
- Teran-Garcia, M.; Adamson, A.W.; Yu, G.; Rufo, C.; Suchankova, G.; Dreesen, T.D.; Tekle, M.; Clarke, S.D.; Gettys, T.W. Polyunsaturated fatty acid suppression of fatty acid synthase (FASN): Evidence for dietary modulation of NF-Y binding to the Fasn promoter by SREBP-1c. Biochem. J. 2007, 402, 591–600. [Google Scholar] [CrossRef] [Green Version]
- Mc Auley, M.T. Effects of obesity on cholesterol metabolism and its implications for healthy ageing. Nutr. Res. Rev. 2020, 27, 1–13. [Google Scholar] [CrossRef]
- Kamisako, T.; Tanaka, Y.; Ikeda, T.; Yamamoto, K.; Ogawa, H. Dietary fish oil regulates gene expression of cholesterol and bile acid transporters in mice. Hepatol. Res. 2012, 42, 321–326. [Google Scholar] [CrossRef]
- Smit, M.J.; Temmerman, A.M.; Wolters, H.; Kuipers, F.; Beynen, A.C.; Vonk, R.J. Dietary fish oil-induced changes in intrahepatic cholesterol transport and bile acid synthesis in rats. J. Clin Invest. 1991, 88, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris., W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; . Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 fatty acids for the management of hypertriglyceridemia: A science advisory from the american heart association. Circulation 2019, 140, 673–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasgupta, S.; Bhattacharyya, D.K. Dietary effect of gamma-linolenic acid on the lipid profile of rat fed erucic acid rich oil. J. Oleo. Sci. 2007, 56, 569–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokoła-Wysoczańska, E.; Wysoczański, T.; Wagner, J.; Czyż, K.; Bodkowski, R.; Lochyński, S.; Patkowska-Sokoła, B. Polyunsaturated fatty acids and their potential therapeutic role in cardiovascular system disorders. Nutrients 2018, 21, 1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouwer, I.A.; Wanders, A.J.; Katan, M.B. Effect of animal and industrial trans fatty acids on HDL and LDL cholesterol levels in humans—A quantitative review. PLoS ONE 2010, 2, e9434. [Google Scholar]
- Opyd, P.M.; Jurgoński, A.; Fotschki, B.; Juśkiewicz, J. Dietary hemp seeds more effectively attenuate disorders in genetically obese rats than their lipid fraction. J. Nutr. 2020, 150, 1425–1433. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Firat, O.; Makay, O.; Yeniay, L.; Gokce, G.; Yenisey, C.; Coker, A. Omega-3 fatty acids inhibit oxidative stress in a rat model of liver regeneration. Ann. Surg Treat. Res. 2017, 93, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jurgoński, A.; Fotschki, B.; Juśkiewicz, J. Disparate metabolic effects of blackcurrant seed oil in rats fed a basal and obesogenic diet. Eur. J. Nutr. 2015, 54, 991–999. [Google Scholar]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127, 838–841. [Google Scholar] [CrossRef]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Bromke, M.A.; Hochmuth, A.; Tohge, T.; Fernie, A.R.; Giavalisco, P.; Burgos, A.; Yariv, B. Liquid chromatography high-resolution mass spectrometry for fatty acid profiling. Plant J. 2015, 81, 529–536. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Diet Intake | Body Weight | Body Weight Gain | Body Lean | Body Fat | Epididymal Fat | |
|---|---|---|---|---|---|---|
| g/day | g | g | % | % | % | |
| Group 1 | ||||||
| LC (n = 8) | 15.6 ± 0.3 | 259 ± 3.4 | 113 ± 3.2 | 66.4 ± 1.53 b | 23.6 ± 1.46 c | 0.96 ± 0.07 c |
| LPO (n = 8) | 15.1 ± 0.3 | 266 ± 4.5 | 117 ± 4.1 | 68.4 ± 0.59 ab | 23.2 ± 0.67 c | 0.86 ± 0.06 c |
| LHO (n = 8) | 15.0 ± 0.3 | 264 ± 5.1 | 115 ± 5.1 | 70.9 ± 0.92 a | 21.2 ± 0.82 c | 0.80 ± 0.05 c |
| OC (n = 6) | 24.2 ± 0.8 | 379 ± 14.2 | 197 ± 6.2 | 23.4 ± 1.36 d | 69.1 ± 1.25 a | 2.10 ± 0.08 a |
| OPO (n = 6) | 23.2 ± 0.9 | 377 ± 13.7 | 187 ± 7.4 | 29.9 ± 1.20 c | 65.1 ± 1.44 b | 1.91 ± 0.05 b |
| OHO (n = 6) | 24.2 ± 0.5 | 381 ± 13.2 | 197 ± 5.4 | 31.2 ± 1.00 c | 62.6 ± 0.81 b | 1.92 ± 0.09 ab |
| Oil type (O) | ||||||
| Control (n = 14) | 19.3 | 310 | 149 | 48.0 | 43.1 | 1.45 |
| Poppy seed oil (n = 14) | 18.5 | 313 | 147 | 51.9 | 41.1 | 1.31 |
| Hemp seed oil (n = 14) | 18.9 | 314 | 150 | 53.9 | 38.9 | 1.23 |
| p value | 0.053 | NS | NS | <0.001 | <0.005 | <0.05 |
| Zucker phenotype (T) | ||||||
| Lean (n = 24) | 15.2 | 263 | 115 | 68.6 | 22.7 | 0.876 |
| Obese (n = 18) | 23.9 | 379 | 194 | 28.2 | 65.6 | 1.98 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Interaction O × T | ||||||
| p value | NS | NS | NS | NS | NS | NS |
| TC | HDL | LDL | TG | |
|---|---|---|---|---|
| mmol/L | mmol/L | mmol/L | mmol/L | |
| Group 1 | ||||
| LC (n = 8) | 3.15 ± 0.19 | 0.97 ± 0.12 c | 0.16 ± 0.02 c | 2.86 ± 0.54 b |
| LPO (n = 8) | 2.87 ± 0.21 | 0.86 ± 0.03 c | 0.22 ± 0.01 c | 2.52 ± 0.43 b |
| LHO (n = 8) | 2.81 ± 0.12 | 0.76 ± 0.09 c | 0.21 ± 0.02 c | 2.04 ± 0.29 b |
| OC (n = 6) | 5.66 ± 0.12 | 2.03 ± 0.04 a | 0.35 ± 0.03 b | 8.81 ± 1.62 a |
| OPO (n = 6) | 6.05 ± 0.32 | 1.52 ± 0.06 b | 0.54 ± 0.04 a | 9.10 ± 1.62 a |
| OHO (n = 6) | 5.47 ± 0.16 | 1.46 ± 0.02 b | 0.37 ± 0.02 b | 3.73 ± 1.08 b |
| Oil type (O) | ||||
| Control (n = 14) | 4.23 | 1.43 | 0.24 | 5.41 |
| Poppy seed oil (n = 14) | 4.34 | 1.14 | 0.36 | 5.56 |
| Hemp seed oil (n = 14) | 3.95 | 1.06 | 0.28 | 2.76 |
| p value | NS | <0.005 | <0.005 | <0.005 |
| Zucker phenotype (T) | ||||
| Lean (n = 24) | 2.95 | 0.86 | 0.19 | 2.47 |
| Obese (n = 18) | 5.73 | 1.67 | 0.42 | 7.21 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 |
| Interaction O × T | ||||
| p value | NS | NS | NS | <0.05 |
| Liver Mass | Fat Content | Cholesterol | TG | α-Linolenic Acid (omega-3) | Linoleic Acid (omega-6) | Oleic Acid (omega-9) | |
|---|---|---|---|---|---|---|---|
| g/100 g BW | % | mg/g liver | mg/g liver | mg/g liver | |||
| Group 1 | |||||||
| LC (n = 8) | 4.80 ± 0.03 | 7.36 ± 0.50 | 1.15 ± 0.02 d | 6.62 ± 0.72 | 0.07 ± 0.01 b | 8.03 ± 0.55 b | 8.51 ± 0.43 |
| LPO (n = 8) | 4.82 ± 0.14 | 7.04 ± 0.28 | 1.26 ± 0.05 d | 5.81 ± 0.82 | 0.28 ± 0.02 b | 21.49 ± 1.11 a | 9.56 ± 0.27 |
| LHO (n = 8) | 4.91 ± 0.06 | 7.35 ± 0.43 | 1.17 ± 0.05 d | 4.30 ± 0.52 | 2.13 ± 0.11 a | 21.13 ± 0.63 a | 8.64 ± 0.18 |
| OC (n = 6) | 5.53 ± 0.45 | 23.3 ± 1.98 | 1.87 ± 0.02 a | 25.7 ± 2.37 | 0.12 ± 0.01 b | 6.36 ± 0.45 b | 5.84 ± 0.39 |
| OPO (n = 6) | 5.92 ± 0.46 | 23.2 ± 2.87 | 1.67 ± 0.09 b | 26.0 ± 3.35 | 0.16 ± 0.01 b | 8.74 ± 0.54 b | 5.66 ± 0.25 |
| OHO (n = 6) | 6.05 ± 0.34 | 26.0 ± 2.93 | 1.45 ± 0.02 c | 25.9 ± 1.99 | 0.45 ± 0.04 b | 7.31 ± 0.71 b | 6.08 ± 0.44 |
| Oil type (O) | |||||||
| Control (n = 14) | 5.11 | 14.22 | 1.46 | 14.81 | 0.10 | 7.20 | 7.18 |
| Poppy seed oil (n = 14) | 5.29 | 14.01 | 1.44 | 14.52 | 0.22 | 15.12 | 7.62 |
| Hemp seed oil (n = 14) | 5.40 | 15.42 | 1.29 | 13.62 | 1.29 | 14.22 | 7.37 |
| p value | NS | NS | <0.01 | NS | <0.001 | <0.001 | NS |
| Zucker phenotype (T) | |||||||
| Lean (n = 24) | 4.84 | 7.25 | 1.19 | 5.57 | 0.83 | 16.89 | 8.91 |
| Obese (n = 18) | 5.83 | 24.21 | 1.66 | 25.91 | 0.25 | 7.47 | 5.87 |
| p value | <0.05 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Interaction O × T | |||||||
| p value | NS | NS | <0.001 | NS | <0.01 | <0.001 | NS |
| Palm Oil | Poppy Seed Oil | Hemp Seed Oil | |
|---|---|---|---|
| Myristic acid (C14:0) | 0.74 ± 0.01 | - | - |
| Palmitic acid (C16:0) | 40.8 ± 0.04 | 8.35 ± 0.02 | 5.46 ± 0.01 |
| Palmitoleic acid (C16:1) | 0.12 ± 0.01 | 0.10 ± 0.00 | 0.08 ± 0.01 |
| Stearic acid (C18:0) | 4.39 ± 0.01 | 1.96 ± 0.01 | 2.26 ± 0.01 |
| Oleic acid (C18:1n9c) | 39.1 ± 0.01 | 15.2 ± 0.07 | 8.61 ± 0.05 |
| Vaccenic acid (C18:1n7c) | - | 1.38 ± 0.03 | 0.93 ± 0.02 |
| cis-9.trans-12-octadecadienoic acid (C18:2n6ct) | 0.11 ± 0.00 | - | 0.12 ± 0.00 |
| trans-9,cis-12-octadecadienoic acid (C18:2n6tc) | 0.10 ± 0.00 | - | - |
| Linoleic acid (C18:2n6c) | 9.05 ± 0.02 | 67.0 ± 0.05 | 52.8 ± 0.08 |
| Arachidonic acid (C20:0) | 0.33 ± 0.00 | 0.10 ± 0.00 | 0.83 ± 0.01 |
| γ-Linolenic acid (C18:3n6) | - | - | 4.26 ± 0.05 |
| Gondoic acid (C20:1n9c) | 0.15 ± 0.01 | 0.12 ± 0.01 | 0.38 ± 0.01 |
| α-linolenic acid (C18:3n3) | 0.16 ± 0.01 | 1.02 ± 0.01 | 17.47 ± 0.03 |
| cis-11,14-eicosadienoic acid (C20:2n6) | - | - | 1.52 ± 0.01 |
| Behenic acid (C22:0) | - | - | 0.33 ± 0.01 |
| Lignoceric acid (C24:0) | - | - | 0.13 ± 0.01 |
| Calculated fatty acid content (%) | |||
| SFAs | 46.3 ± 0.01 | 10.4 ± 0.01 | 9.02 ± 0.01 |
| MUFAs | 39.4 ± 0.01 | 16.8 ± 0.03 | 10.0 ± 0.03 |
| PUFAs | 9.21 ± 0.02 | 68.0 ± 0.02 | 76.1 ± 0.03 |
| omega-3 | 0.16 ± 0.01 | 1.02 ± 0.01 | 17.5 ± 0.02 |
| omega-6 | 9.05 ± 0.02 | 67.0 ± 0.01 | 58.6 ± 0.02 |
| omega-6/omega-3 ratio | 56.6 | 65.7 | 3.3 |
| TFAs | 0.22 ± 0.00 | - | 0.12 ± 0.00 |
| (g/100 g Diet) | Groups | |||||
|---|---|---|---|---|---|---|
| LC | LPO | LHO | OC | OPO | OHO | |
| Casein | 20 | 20 | 20 | 20 | 20 | 20 |
| DL-methionine | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Palm oil | 7 | - | - | 7 | - | - |
| Poppy seed oil | - | 7 | - | - | 7 | - |
| Hemp seed oil | - | - | 7 | - | - | 7 |
| Corn starch | 53 | 53 | 53 | 53 | 53 | 53 |
| Saccharose | 10 | 10 | 10 | 10 | 10 | 10 |
| Cellulose | 5 | 5 | 5 | 5 | 5 | 5 |
| Mineral mix 1 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Vitamin mix 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Choline chloride | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotschki, B.; Opyd, P.; Juśkiewicz, J.; Wiczkowski, W.; Jurgoński, A. Comparative Effects of Dietary Hemp and Poppy Seed Oil on Lipid Metabolism and the Antioxidant Status in Lean and Obese Zucker Rats. Molecules 2020, 25, 2921. https://doi.org/10.3390/molecules25122921
Fotschki B, Opyd P, Juśkiewicz J, Wiczkowski W, Jurgoński A. Comparative Effects of Dietary Hemp and Poppy Seed Oil on Lipid Metabolism and the Antioxidant Status in Lean and Obese Zucker Rats. Molecules. 2020; 25(12):2921. https://doi.org/10.3390/molecules25122921
Chicago/Turabian StyleFotschki, Bartosz, Paulina Opyd, Jerzy Juśkiewicz, Wiesław Wiczkowski, and Adam Jurgoński. 2020. "Comparative Effects of Dietary Hemp and Poppy Seed Oil on Lipid Metabolism and the Antioxidant Status in Lean and Obese Zucker Rats" Molecules 25, no. 12: 2921. https://doi.org/10.3390/molecules25122921
APA StyleFotschki, B., Opyd, P., Juśkiewicz, J., Wiczkowski, W., & Jurgoński, A. (2020). Comparative Effects of Dietary Hemp and Poppy Seed Oil on Lipid Metabolism and the Antioxidant Status in Lean and Obese Zucker Rats. Molecules, 25(12), 2921. https://doi.org/10.3390/molecules25122921