Properties of Dicationic Disiloxane Ionic Liquids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of ILs
2.2. Melting Points
- Increasing the steric effect of a cation and/or anion that prevents crystallization, this can be done by introducing bulk substituents into the cation and by using bulk anions;
- Violation of the symmetry of the cation.
2.3. Thermal Stability
2.4. Volatility
2.5. Viscosity
2.6. Hydrolytic Stability
3. Materials and Methods
3.1. General Information
3.2. Synthesis of ILs with a Symmetrical Linker (1, 2, 4, 5, 10, and 11)
3.3. Synthesis of ILs with an Asymmetric Linker (3, 6, and 12)
General Method
3.4. Synthesis of ILs with 2-Hydroxyethyl Group (ILs 7, 8, and 9)
General Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, W.; Sun, J. Triflimide (HNTf2) in Organic Synthesis. Chem. Rev. 2018, 118, 10349–10392. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T. Ionic Liquids as Tool to Improve Enzymatic Organic Synthesis. Chem. Rev. 2017, 117, 10567–10607. [Google Scholar] [CrossRef] [PubMed]
- Wegner, S.; Janiak, C. Metal Nanoparticles in Ionic Liquids. Top. Curr. Chem. 2017, 375, 65. [Google Scholar] [CrossRef]
- Łuczak, J.; Paszkiewicz-Gawron, M.; Krukowska, A.; Malankowska, A.; Zaleska-Medynska, A. Ionic liquids for nano- and microstructures preparation. Part 2: Application in synthesis. Adv. Colloid Interface Sci. 2016, 227, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Nozari, V.; Keskin, S.; Uzun, A. Toward Rational Design of Ionic Liquid/Metal–Organic Framework Composites: Effects of Interionic Interaction Energy. ACS Omega 2017, 2, 6613–6618. [Google Scholar] [CrossRef]
- Ye, R.; Ni, M.; Xu, Y.; Chen, H.; Li, S. Synthesis of Zn-based metal–organic frameworks in ionic liquid microemulsions at room temperature. RSC Adv. 2018, 8, 26237–26242. [Google Scholar] [CrossRef] [Green Version]
- Yamagata, M.; Tanaka, K.; Tsuruda, Y.; Sone, Y.; Fukuda, S.; Nakasuka, S.; Kono, M.; Ishikawa, M. The First Lithium-ion Battery with Ionic Liquid Electrolyte Demonstrated in Extreme Environment of Space. Electrochemistry 2015, 83, 918–924. [Google Scholar] [CrossRef] [Green Version]
- Fei, Z.; Manzanares, V.M.; Dyson, P.J. Ionic Liquids: From Synthesis to Applications in Solar Cells. Chim. Int. J. Chem. 2017, 71, 762–767. [Google Scholar] [CrossRef]
- Vedavathi, T.; Srinivas, T. Ionic Liquids—A New Era in Green Chemistry. IJECS 2017, 6, 19916–19920. [Google Scholar] [CrossRef]
- Shukla, S.K.; Khokarale, S.G.; Bui, T.Q.; Mikkola, J.-P.T. Ionic Liquids: Potential Materials for Carbon Dioxide Capture and Utilization. Front. Mater. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.G. Chemical recycling of poly (bisphenol A carbonate). Polym. Chem. 2020. [Google Scholar] [CrossRef]
- Meikandan, M.; Kumar, P.G.; Sundarraj, M.; Yogaraj, D.; Balaji, N. Numerical analysis on heat transfer characteristics of ionic liquids in a tubular heat exchanger. Int. J. Ambient. Energy 2018, 1–7. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.K.M.M.; Fox, E.B.; Khan, J.A. Enhanced thermophysical properties of NEILs as heat transfer fluids for solar thermal applications. Appl. Therm. Eng. 2017, 110, 1. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; He, L.; Ferreira, C.A.I. Ammonia absorption in ionic liquids-based mixtures in plate heat exchangers studied by a semi-empirical heat and mass transfer framework. Int. J. Heat Mass Transf. 2019, 134, 1302–1317. [Google Scholar] [CrossRef]
- Chernikova, E.; Glukhov, L.M.; Krasovskiy, V.G.; Kustov, L.M.; Vorobyeva, M.G.; Koroteev, A.A. Ionic liquids as heat transfer fluids: Comparison with known systems, possible applications, advantages and disadvantages. Russ. Chem. Rev. 2015, 84, 875–890. [Google Scholar] [CrossRef]
- Krasovskiy, V.G.; Chernikova, E.; Glukhov, L.M.; Redina, E.; Kapustin, G.I.; Koroteev, A.A.; Kustov, L.M. Hydroxyl-containing ionic liquids as heat-transfer agents. Mendeleev Commun. 2017, 27, 605–607. [Google Scholar] [CrossRef]
- Krasovskiy, V.G.; Chernikova, E.A.; Glukhov, L.M.; Kapustin, G.I.; Koroteev, A.A. Effect of Hydroxyl Groups in a Cation Structure on the Properties of Ionic Liquids. Russ. J. Phys. Chem. A 2018, 92, 2379–2385. [Google Scholar] [CrossRef]
- Krasovskiy, V.G.; Kapustin, G.I.; Glukhov, L.M.; Gorbatsevich, O.B.; Chernikova, E.A.; Koroteev, A.A.; Kustov, L.M. Dicationic disiloxane ionic liquids. Mendeleev Commun. 2020, 30, 114–116. [Google Scholar] [CrossRef]
- Krasovskiy, V.G.; Kapustin, G.I.; Glukhov, L.M.; Gorbatsevich, O.B.; Chernikova, E.A.; Koroteev, A.A.; Kustov, L.M. Dicationic disiloxane ionic liquids as heat transfer fluids in vacuum. Russ. Chem. Bull. 2020, 69, under review. [Google Scholar]
- Bryk, M.; Baglei, N.; Kurilenko, O. Polymerization of octamethylcyclotetrasiloxane catalysed by the ion exchange resin KU-2. Polym. Sci. USSR 1975, 17, 1187–1195. [Google Scholar] [CrossRef]
- Baglei, N.; Bryk, M. The octamethylcyclotetrasiloxane polymerization catalysed by the H form of kaolinite. Polym. Sci. USSR 1978, 20, 2777–2786. [Google Scholar] [CrossRef]
- Chen, B.; Zhan, X.; Yi, L.; Chen, F. Cationic Ring Opening Polymerization of Octamethylcyclotetrasiloxane Initiated by Acid Treated Bentonite. Chin. J. Chem. Eng. 2007, 15, 661–665. [Google Scholar] [CrossRef]
- Vaidya, A.A.; Kumar, V.G. Silica-alumina catalysts for polymerization of cyclic siloxanes. J. Appl. Polym. Sci. 1998, 70, 629–635. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, Y.-F.; Wang, Y.; Wang, X. Viscosity of Typical Room-Temperature Ionic Liquids: A Critical Review. J. Phys. Chem. Ref. Data 2019, 48, 033101. [Google Scholar] [CrossRef]
- Paul, A.; Muthukumar, S.; Prasad, S. Review—Room-Temperature Ionic Liquids for Electrochemical Application with Special Focus on Gas Sensors. J. Electrochem. Soc. 2020, 167, 037511. [Google Scholar] [CrossRef]
- Kempter, V.; Kirchner, B. The role of hydrogen atoms in interactions involving imidazolium-based ionic liquids. J. Mol. Struct. 2010, 972, 22–34. [Google Scholar] [CrossRef]
- Höfft, O.; Bahr, S.; Kempter, V. Investigations with Infrared Spectroscopy on Films of the Ionic Liquid [EMIM]Tf2N. Langmuir 2008, 24, 11562–11566. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, S.-J.; Wang, J.-J. Understanding the hydrogen bonds in ionic liquids and their roles in properties and reactions. Chem. Commun. 2016, 52, 6744–6764. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Ramenskaya, L.; Grishina, E.P.; Kudryakova, N. Physicochemical features of short-chain 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ionic liquids containing equilibrium water absorbed from air. J. Mol. Liq. 2018, 272, 759–765. [Google Scholar] [CrossRef]
- Rucigaj, A.; Krajnc, M.; Sebenik, U. Kinetic Study of Thermal Degradation of Polydimethylsiloxane: The Effect of Molecular Weight on Thermal Stability in Inert Atmosphere. Polym. Sci. 2017, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Camino, G.; Lomakin, S.; Lazzari, M. Polydimethylsiloxane thermal degradation Part 1. Kinetic aspects. Polymer 2001, 42, 2395–2402. [Google Scholar] [CrossRef]
- Deyko, A.; Lovelock, K.R.J.; Corfield, J.-A.; Taylor, A.W.; Gooden, P.N.; Villar-Garcia, I.J.; Licence, P.; Jones, R.; Krasovskiy, V.G.; Chernikova, E.; et al. Measuring and predicting ΔvapH298 values of ionic liquids. Phys. Chem. Chem. Phys. 2009, 11, 8544. [Google Scholar] [CrossRef] [PubMed]
- Deyko, A.; Hessey, S.; Licence, P.; Chernikova, E.; Krasovskiy, V.G.; Kustov, L.M.; Jones, R. The enthalpies of vaporisation of ionic liquids: New measurements and predictions. Phys. Chem. Chem. Phys. 2012, 14, 3181–3193. [Google Scholar] [CrossRef] [PubMed]
- Chilingarov, N.S.; Medvedev, A.A.; Deyko, G.S.; Kustov, L.M.; Chernikova, E.A.; Glukhov, L.M.; Markov, V.Y.; Ioffe, I.N.; Senyavin, V.M.; Polyakova, M.V.; et al. Mass spectrometric studies of 1-ethyl-3-methylimidazolium and 1-propyl-2,3-dimethylimidazolium bis(trifluoromethylsulfonyl)imides. Rapid Commun. Mass Spectrom. 2015, 29, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Chilingarov, N.S.; Medvedev, A.A.; Deyko, G.S.; Kustov, L.M.; Chernikova, E.; Glukhov, L.M.; Polyakova, M.V.; Ioutsi, V.A.; Markov, V.Y.; Sidorov, L.N. The evaporation study of silicon-containing ionic liquid. Chem. Phys. Lett. 2016, 657, 8–10. [Google Scholar] [CrossRef]
- Rocha, M.; Coutinho, J.A.P.; Santos, L.M.N.B.F. Vapor pressures of 1,3-dialkylimidazolium bis(trifluoromethylsulfonyl)imide ionic liquids with long alkyl chains. J. Chem. Phys. 2014, 141, 134502. [Google Scholar] [CrossRef] [Green Version]
- Ahrenberg, M.; Brinckmann, M.; Schmelzer, J.W.P.; Beck, M.; Schmidt, C.; Keßler, O.; Kragl, U.; Verevkin, S.P.; Schick, C.; Schmelzer, J.W.P. Determination of volatility of ionic liquids at the nanoscale by means of ultra-fast scanning calorimetry. Phys. Chem. Chem. Phys. 2014, 16, 2971. [Google Scholar] [CrossRef]
- Maton, C.; De Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963. [Google Scholar] [CrossRef]
- Heym, F.; Etzold, B.J.; Kern, C.; Jess, A. An improved method to measure the rate of vaporisation and thermal decomposition of high boiling organic and ionic liquids by thermogravimetrical analysis. Phys. Chem. Chem. Phys. 2010, 12, 12089. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Boeck, G.; Verevkin, S.P.; Ludwig, R. Volatile Times for the Very First Ionic Liquid: Understanding the Vapor Pressures and Enthalpies of Vaporization of Ethylammonium Nitrate. Chem. A Eur. J. 2014, 20, 11640–11645. [Google Scholar] [CrossRef] [PubMed]
- Earle, M.J.; Esperança, J.M.S.S.; Gilea, M.A.; Lop, J.N.C.; Rebelo, L.P.N.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The distillation and volatility of ionic liquids. Nature 2006, 439, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Widegren, J.A.; Wang, Y.-M.; Henderson, W.A.; Magee, J.W. Relative Volatilities of Ionic Liquids by Vacuum Distillation of Mixtures. J. Phys. Chem. B 2007, 111, 8959–8964. [Google Scholar] [CrossRef]
- Krasovskiy, V.G.; Glukhov, L.M.; Chernikova, E.A.; Kapustin, G.I.; Gorbatsevich, O.B.; Koroteev, A.A.; Kustov, L.M. Dicationic polysiloxane ionic liquids. Russ. Chem. Bull. 2017, 66, 1269–1277. [Google Scholar] [CrossRef]
- Krasovskiy, V.G.; Chernikova, E.A.; Glukhov, L.M.; Kapustin, G.I.; Koroteev, A.A.; Kustov, L.M. Synthesis and Properties of Hydroxyl-Containing Ionic Liquids. Russ. J. Org. Chem. 2018, 54, 143–145. [Google Scholar] [CrossRef]
- Krasovskiy, V.G.; Chernikova, E.A.; Glukhov, L.M.; Kapustin, G.I.; Koroteev, A.A.; Kustov, L.M. Hydroxyl-containing imidazolium ionic liquids. Russ. Chem. Bull. 2018, 67, 1621–1626. [Google Scholar] [CrossRef]
- Yu, G.; Zhao, D.; Wen, L.; Yang, S.; Chen, X. Viscosity of ionic liquids: Database, observation, and quantitative structure-property relationship analysis. AIChE J. 2011, 58, 2885–2899. [Google Scholar] [CrossRef]
- Ren, Z.; Ivanova, A.S.; Couchot-Vore, D.; Roe, S.G. Ultrafast Structure and Dynamics in Ionic Liquids: 2D-IR Spectroscopy Probes the Molecular Origin of Viscosity. J. Phys. Chem. Lett. 2014, 5, 1541–1546. [Google Scholar] [CrossRef] [Green Version]
- Bostrelli, D.V. Solution Chemistry Research Progress; Nova Science: New York, NY, USA, 2011; p. 187. [Google Scholar]
- Stark, F.; Falender, J.; Wright, A. Silicones. In Comprehensive Organometallic Chemistry; Elsevier BV: Amsterdam, The Netherlands, 1982; pp. 305–363. [Google Scholar]
- Brook, M.A. Silicon in Organic, Organometallic, and Polymer Chemistry; Wiley-Interscience: New York, NY, USA, 2000; p. 704. [Google Scholar]
- Liebau, F. Structural Chemistry of Silicates: Structure, Bonding and Classification; Springer: New York, NY, USA, 1985. [Google Scholar]
- Andriot, M.; DeGroot, J.V., Jr.; Meeks, R.; Gerlach, E.; Jungk, M.; Wolf, A.T.; Cray, S.; Easton, T.; Mountney, A.; Leadley, S.; et al. Silicones in Industrial Applications. In Silicon-Based Inorganic Polymers; De Jaeger, R., Gleria, M., Eds.; Nova Science Publishers: New York, NY, USA, 2009; pp. 1–106. [Google Scholar]
- Pauling, L. The nature of silicon-oxygen bonds. Am. Mineralog. 1980, 65, 321–323. [Google Scholar]
- Passmore, J.; Rautiainen, J.M. On The Lower Lewis Basicity of Siloxanes Compared to Ethers. Eur. J. Inorg. Chem. 2012, 2012, 6002–6010. [Google Scholar] [CrossRef]
- Ducom, G.; Laubie, B.; Ohannessian, A.; Chottier, C.; Germain, P.; Chatain, V. Hydrolysis of polydimethylsiloxane fluids in controlled aqueous solutions. Water Sci. Technol. 2013, 68, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Cypryk, M.; Apeloig, Y. Mechanism of the Acid-Catalyzed Si−O Bond Cleavage in Siloxanes and Siloxanols. A Theoretical Study. Organometallics 2002, 21, 2165–2175. [Google Scholar] [CrossRef]
- Laubie, B.; Bonnafous, E.; Desjardin, V.; Germain, P.; Fleury, E. Silicone-based surfactant degradation in aqueous media. Sci. Total. Environ. 2013, 454, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.C.M. Poly (dimethylsiloxane). In Polymer Data Handbook; Mark, J.E., Ed.; Oxford University Press: New York, NY, USA, 1999; pp. 411–435. [Google Scholar]
Sample Availability: Not available. |












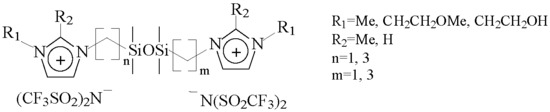
| IL | Cation Structure (Scheme 1) | Td, °C (TGA) | Tm, °C (DSC) | Volatility, mg·h−1·cm−2 at 150 °C/190 °C/220 °C ** |
|---|---|---|---|---|
| 1 | n = 1, m = 1, R1 = R2 = CH3 | 437 | 69 | 0.00/0.03/0.04 |
| 2 | n = 3, m = 3, R1 = R2 = CH3 | 419 | 59 | 0.01/0.02/0.17 |
| 3 | n = 1, m = 3, R1 = R2 = CH3 | 420 | 42 | 0.02/0.11/0.40 |
| 4 | n = 1, m = 1, R1 = CH3, R2 = H | 435 | −52 * | 0.02/0.08/0.22 |
| 5 | n = 3, m = 3, R1 = CH3, R2 = H | 402 | −58 * | 0.03/0.33/0.95 |
| 6 | n = 1, m = 3, R1 = CH3, R2 = H | 410 | −56 * | 0.08/0.59/2.44 |
| 7 | n = 1, m = 1, R1 = CH2CH2OH, R2 = H | *** | ||
| 8 | n = 3, m = 3, R1 = CH2CH2OH, R2 = H | 391 | −53 * | 0.02/0.12/0.35 |
| 9 | n = 1, m = 3, R1 = CH2CH2OH, R2 = H | *** | ||
| 10 | n = 1, m = 1, R1 = CH2CH2OCH3, R2 = CH3 | 438 | −43 * | 0.07/0.14/0.29 |
| 11 | n = 3, m = 3, R1 = CH2CH2OCH3, R2 = CH3 | 416 | −48 * | 0.02/0.22/0.94 |
| 12 | n = 1, m = 3, R1 = CH2CH2OCH3, R2 = CH3 | 421 | −47 * | 0.03/0.31/0.76 |
| IL | Viscosity at 30 °C, cSt | Parameters of the Vogel–Tamman–Fulcher equation * | ||
|---|---|---|---|---|
| a | -b | T0 | ||
| 4 | 312 | 798.0 | 1.402 | 191.5 |
| 5 | 344 | 901.3 | 1.681 | 183.3 |
| 6 | 242 | 797.4 | 1.408 | 187.5 |
| 8 | 602 | 863.0 | 1.451 | 193.4 |
| 10 | 683 | 765.7 | 1.372 | 206.0 |
| 11 | 555 | 890.9 | 1.784 | 193.3 |
| 12 | 563 | 792.7 | 1.491 | 202.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasovskiy, V.G.; Kapustin, G.I.; Gorbatsevich, O.B.; Glukhov, L.M.; Chernikova, E.A.; Koroteev, A.A.; Kustov, L.M. Properties of Dicationic Disiloxane Ionic Liquids. Molecules 2020, 25, 2949. https://doi.org/10.3390/molecules25122949
Krasovskiy VG, Kapustin GI, Gorbatsevich OB, Glukhov LM, Chernikova EA, Koroteev AA, Kustov LM. Properties of Dicationic Disiloxane Ionic Liquids. Molecules. 2020; 25(12):2949. https://doi.org/10.3390/molecules25122949
Chicago/Turabian StyleKrasovskiy, Vladimir G., Gennady I. Kapustin, Olga B. Gorbatsevich, Lev M. Glukhov, Elena A. Chernikova, Anatoly A. Koroteev, and Leonid M. Kustov. 2020. "Properties of Dicationic Disiloxane Ionic Liquids" Molecules 25, no. 12: 2949. https://doi.org/10.3390/molecules25122949
APA StyleKrasovskiy, V. G., Kapustin, G. I., Gorbatsevich, O. B., Glukhov, L. M., Chernikova, E. A., Koroteev, A. A., & Kustov, L. M. (2020). Properties of Dicationic Disiloxane Ionic Liquids. Molecules, 25(12), 2949. https://doi.org/10.3390/molecules25122949