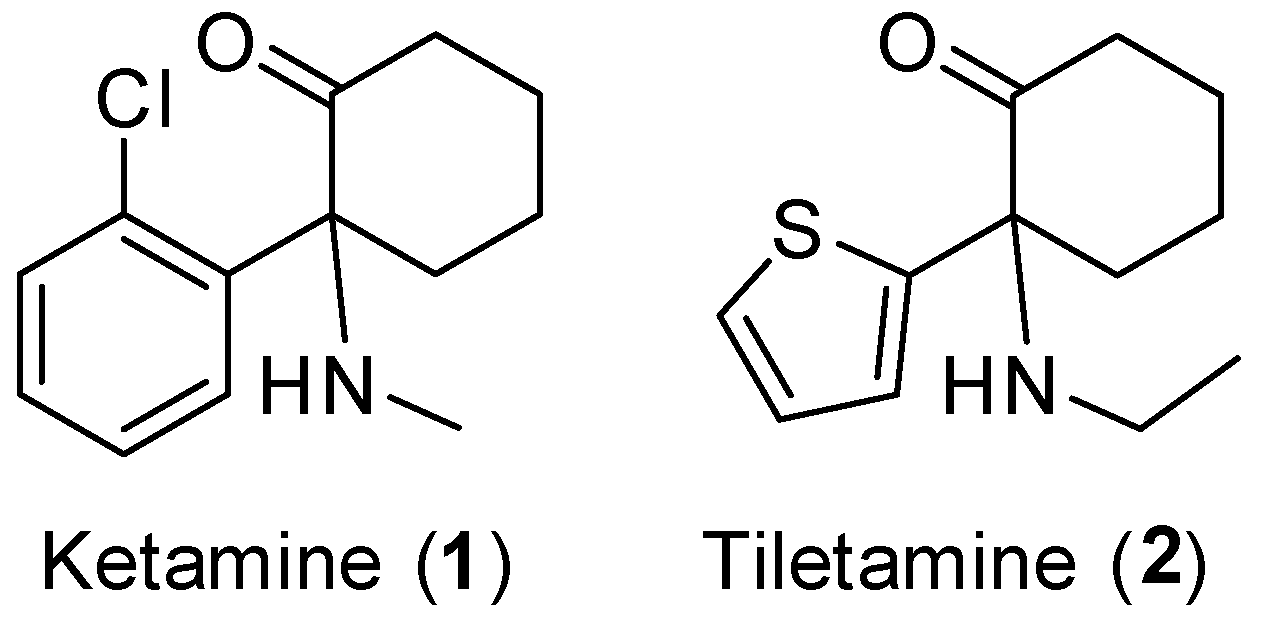
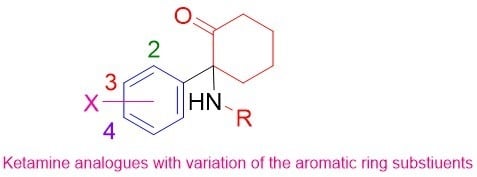
4.2. Synthesis of Ring-Substituted Norketamine Analogues. (Scheme 2)
2-Amino-2-(4-chlorophenyl)cyclohexan-1-one (22). A solution of 2-(4-chlorophenyl)cyclohexan-1-one (27d) (3.0 g, 14.4 mmol) and unsym.-dimethylhydrazine (3.46 g, 58.0 mmol), in EtOH (20 mL) was heated to 96 °C in a sealed tube for 12 h. The reaction mixture was cooled to room temperature, filtered and the solvent evaporated. The residue was purified by column chromatography on silica gel. Elution with EtOAc/hexanes (0–40%) gave 2-(2-(4-chlorophenyl)cyclohexylidene)-1,1-dimethylhydrazine (28d) (3.2 g, 90%) as a pale yellow oil. 1HNMR (CDCl3) δ 7.30–7.25 (m, 2H), 7.22–7.19 (m, 2H), 2.88–2.64 (dt, J = 13.96 Hz, 4.56 Hz, 1H), 2.47 (s, 6 H), 2.36–2.26 (m, 1H), 2.20–2.10 (m, 1 H), 2.00–1.92 (m, 1H), 1.82–1.72 (m 1H), 1.70–1.48 (m, 4H); MS m/z 251.20 (MH+).
A solution of 28d (3.2 g, 12.8 mmol) and MeI (2.20 g, 15.4 mmol), in MeCN (20 mL) was heated in a sealed tube to 40 °C for 2 h, followed by heating to 70 °C for 3 h. The reaction mixture was cooled to room temperature, diluted with Et2O (60 mL) and left overnight in the fridge for the product to crystallise out. The solid was filtered and dried under high vacuum to yield the desired salt 2-(2-(4-chlorophenyl)cyclohexylidene)-1,1,1-trimethylhydrazinium iodide (29d) (4.99 g, 99%) as pale cream solid. 1HNMR (MeOD) δ 7.32–7.29 (m, 2H), 7.25–7.23 (m, 2H), 3.82–3.79 (q, J = 4.8 Hz, 1H), 3.46 (s, 9 H), 3.20–3.10 (m, 1H), 2.78–2.68 (m, 1H) 2.30–2.08 (m, 3H), 2.20–1.78 (m, 3H); MS m/z 251.20 ((MH-MeI)+).
Sodium (0.33g, 14.5 mmol), was washed with hexane, dried, cut into small pieces and placed in EtOH (40 mL) at r.t. The solution was stirred for approximately 20 min, until the sodium disappeared. The quaternary salt 29d (5 g, 12 mmol) was added to the above solution and then it was refluxed for 1 h. The solution was cooled on ice and quenched with HCl (4 M, 40 mL). The ethanol was removed under reduced pressure, the residue was diluted with water (20 mL) and neutralised with NaOH (2 M) solution until pH 7. The aqueous layer was extracted with dichloromethane, MgSO4 dried and concentrated in vacuo. The residue was purified by column chromatography on silica gel eluting with EtOAc/hexanes (30–100%) to obtain 2-amino-2-(4-chlorophenyl)cyclohexan-1-one (22) (1.87 g, 70%) as a pale yellow oil. 1HNMR (CDCl3) δ 7.37–7.33 (m, 2H), 7.22–7.18 (m, 2H), 2.82–2.76 (m, 1H), 2.52–2.44 (m, 1H), 2.40–2.32 (m, 1H), 2.20–1.94 (m, 1H), 1.82–1.62 (m, 4H); MS m/z 224.20 (MH+).
Similarly were prepared:
2-Amino-2-phenylcyclohexan-1-one (21). Similar reaction of 2-phenylcyclohexan-1-one (27a) (2.43 g, 13.9 mmol) and unsym.-dimethylhydrazine gave 1,1-dimethyl-2-(2-phenylcyclohexylidene)hydrazine (28a) (2.62 g, 87%). 1HNMR (CDCl3) δ 7.30–7.29 (m, 3H), 7.21–7.19 (m, 2H), 3.01–2.95(dt, J = 13.84 Hz, 4.32 Hz, 1H), 2.50 (s, 6H), 2.33–2.32 (m, 1H), 2.07–1.91 (m, 2H), 1.81–1.70 (m, 2H), 1.69–1.60 (m, 1H), 1.59–1.52 (m, 2H); MS m/z 217.30 (MH+). Reaction of 28a (2.62 g, 12.1 mmol) and methyl iodide as above gave 1,1,1-trimethyl-2-(2-phenylcyclohexylidene)hydrazin-1-ium iodide (29a) (3.0 g, 70%). 1HNMR (MeOD) δ 7.35–7.30 (m, 2H), 7.26–7.20 (m, 3H), 3.82–3.78 (dd, J = 9.49 Hz, 4.64 Hz, 1H), 3.47 (s, 9H), 3.05–2.89 (m, 1H), 2.82–2.76 (m, 1H), 2.36–2.30 (m, 1H), 2.18–2.15 (m, 1H), 2.08–2.03 (m, 1H), 1.97–1.79 (m, 3 H); MS m/z 217.2 ((MH-MeI)+). Reaction of 29a (2.87 g, 8.0 mmol) with Na/EtOH as above then gave 21 (0.90 g, 60%). 1HNMR (CDCl3) δ 7.40–7.36 (m, 2H), 7.31–7.24 (m, 3H), 2.88–2.84 (m, 1H), 2.45–2.38 (m, 2H), 2.00–1.98 (m, 1H), 1.79–1.72 (m, 4H); MS m/z 190.20 (MH+).
2-Amino-2-(2-fluorophenyl)cyclohexan-1-one (30b). Similar reaction of 2-(2-fluorophenyl)cyclohexan-1-one (27b) (0.77 g, 4.0 mmol) and unsym.-dimethylhydrazine gave 2-(2-(2-fluorophenyl)cyclohexylidene)-1,1-dimethylhydrazine (28b) (0.72g, 82%) 1HNMR (CDCl3) δ 7.26–7.15 (m, 2H), 7.08–7.00 (td, J = 6.24 Hz, 1.24 Hz, 1 H), 6.98–6.95 (m, 1H), 3.86–3.74 (m, 1H), 3.10–3.04 (dt, J = 13.76 Hz, 4.40 Hz), 2.32 (s, 6 H), 2.28–2.19 (m, 2H), 2.08–1.98 (m, 2H), 1.90–1.78 (m, 2H), 1.68–1.56 (m, 2H); MS m/z 235.20 (MH+). Reaction of (28b) (0.66 g, 2.80 mmol) and methyl iodide as above gave 2-(2-(2-fluorophenyl)cyclohexylidene)-1,1,1-trimethylhydrazin-1-ium iodide (29b) (0.97 g, 94%) 1HNMR (MeOD) δ 7.31–7.24 (m, 2H), 7.15–7.11 (td, J = 7.56 Hz, 1.20 Hz, 1H), 7.07–7.02 (m, 1H), 4.01–3.97 (t, J = 8.52 Hz, 1H), 3.42 (s, 9H), 2.71–2.63 (m, 1H), 2.23–2.20 (m, 3H), 2.04–2.00 (m, 1H), 1.84–1.79 (m, 3H); MS m/z 235.30((MH-MeI)+). Reaction of 29b (0.97 g, 2.60 mmol) with Na/EtOH as above then gave 30b (0.37 g, 70%). 1HNMR (CDCl3) δ 7.53–7.48 (td, J = 7.80 Hz, 1.72 Hz, 1H), 7.33–7.28 (m, 1H), 7.22–7.18 (td, J = 7.68 Hz, 1.32 Hz, 1H), 7.07–7.02 (dd, J = 8.16 Hz, 1.24 Hz, 1H), 2.80–2.75 (m,1 H), 2.57–2.52 (m, 1H), 2.48–2.43 (m, 1H), 2.00–1.98 (m, 1H), 1.83–1.65 (m, 4H); MS m/z 208.20 (MH+).
2-Amino-2-(3-chlorophenyl)cyclohexan-1-one (30c). Similar reaction of 2-(3-chlorophenyl)cyclohexan-1-one (27c) (3.0 g, 14.4 mmol) and unsym.-dimethylhydrazine gave 2-(2-(3-chlorophenyl)cyclohexylidene)-1,1-dimethylhydrazine (28c) (3.2 g, 90%) as pale yellow oil. 1HNMR (CDCl3) δ 7.30–7.25 (m, 2H), 7.22–7.19 (m, 2H), 2.88–2.64 (dt, J = 13.96 Hz, 4.56 Hz, 1H), 2.47 (s, 6 H), 2.36–2.26 (m, 1H), 2.20–2.10 (m, 1 H), 2.00–1.92 (m, 1H), 1.82–1.72 (m 1H), 1.70–1.48 (m, 4H); MS m/z 251.20 (MH+). Reaction of 28c (3.2 g, 12.8 mmol) and methyl iodide as above gave 2-(2-(3-chlorophenyl)cyclohexylidene)-1,1,1-trimethylhydrazinium iodide (29c) (4.99 g, 99%) as a solid. 1HNMR (MeOD) δ 7.29–7.27 (m, 2H), 7.25–7.24 (m, 1H), 7.19–7.17 (m, 1H), 3.84–3.79 (q, J = 5.12 Hz, 1H), 3.46 (s, 9H), 3.20–3.15 (m, 1H), 2.70–2.69 (m, 1 H), 2.22–2.12 (m, 3H), 1.98–1.96 (m, 1H), 1.84–1.82 (m, 2H); MS m/z 251.20 ((MH-MeI)+). Treatment of 29c (3.8 g, 9.6 mmol) with Na/EtOH as above then gave 30c 1.8 g, 84%) as an yellow oil. 1H NMR (CDCl3) δ 7.29–7.28 (m, 2 H), 2.27–7.26 (m, 1 H), 7.14–7.11 (dt, J = 7.36 Hz, 1.68 Hz, 1H), 2.82–2.78 (m, 1H), 2.52–2.48 (m, 1H), 2.40–2.36 (m, 1H), 2.04–2.00 (m, 1H), 1.80–1.72 (m, 4H); MS m/z 224.20 (MH)+.
2-Amino-2-(o-tolyl)cyclohexan-1-one (30e). Similar reaction of 2-(o-tolyl)cyclohexan-1-one 27e (3.0 g, 16.0 mmol) and unsym.-dimethylhydrazine gave 1,1-dimethyl-2-(2-(o-tolyl)cyclohexylidene)hydrazine (28e) (3.65 mg, 99%). 1HNMR (CDCl3) δ 7.25–7.17 (m, 1H), 7.16–7.13 (m, 1H), 7.12–7.08 (m, 1H), 3.16–3.11 (dt, J = 10.4 Hz, 4.44 Hz, 1H), 2.34 (s, 6H), 2.26 (s, 3H), 2.12–2.02 (m, 2H), 2.00–1.82 (m, 3 H), 1.68–1.58 (m, 3H); MS m/z 231.30 (MH)+. Reaction of 28e (3.65 g, 16.0 mmol) and methyl iodide as above gave 1,1,1-trimethyl-2-(2-(o-tolyl)cyclohexylidene)hydrazin-1-ium iodide (29e) (5.1 g, 86%). 1HNMR (MeOD) δ 7.20–7.18 (m, 1H), 7.16–7.08 (m, 3H), 3.95–3.91 (m, 1H), 3.39 (s, 9H), 2.74–2.66 (m, 1H), 2.26–2.21 (m, 3H), 2.24 (s, 3H), 2.10–1.98 (m, 1H), 1.92–1.78 (m, 2H); MS m/z 231.30 ((MH-MeI)+). Reaction of 29e (5.10 g, 13.7 mmol) with Na/EtOH as above then gave 30e (2.0 g, 72%). 1HNMR (CDCl3) δ 7.56–7.54 (dd, J = 8.76 Hz, 1.12 Hz, 1H), 7.27–7.18 (m, 3H), 2.92–2.88 (m, 1H), 2.52–2.36 (m, 1H), 2.17 (s, 3H), 2.04–1.93 (m, 2H), 1.76–1.72 (m, 4H); MS m/z 204.20 (MH)+.
2-Amino-2-(m-tolyl)cyclohexan-1-one (30f). Similar reaction of 2-(m-tolyl)cyclohexan-1-one (27f) (0.89 g, 4.70 mmol) and unsym.-dimethylhydrazine gave 1,1-dimethyl-2-(2-(m-tolyl)cyclohexylidene)hydrazine (28f) (0.9 g, 90%). 1HNMR (CDCl3) δ 7.23–7.16 (m, 1H), 7.12–7.08 (m, 1H), 7.04–6.98 (m, 2H), 3.02–2.96 (dt, J = 9.52 Hz, 4.21 Hz, 1H), 2.52 (s, 6H), 2.48 (s, 3H), 2.08–1.88 (m, 2H), 1.86–1.50 (m, 6H); m/z 231.3 (MH+). Reaction of 28f (0.9 g, 3.90 mmol) and methyl iodide as above gave 1,1,1-trimethyl-2-(2-(m-tolyl)cyclohexylidene)hydrazin-1-ium iodide (29f) (1 g, 70%). 1HNMR (MeOD) δ 7.21–7.17 (t, J = 7.64 Hz, 1H), 7.07–7.04 (t, J = 6.92 Hz, 3H), 3.78–3.74 (dd, J = 9.24 Hz, 4.6 Hz, 1H), 3.48 (s, 9H), 3.02–2.96 (m, 1H), 2.86–2.74 (m, 1H), 2.40–2.30 (m, 1H), 2.36 (s, 3H), 2.18–2.00 (m, 2H), 2.00–1.76 (m, 3H); MS m/z 231.20 ((MH-MeI)+). Reaction of 29f (0.83 g, 2.23 mmol) with Na/EtOH as above then gave 30f (0.3 g, 67%). 1HNMR (CDCl3) δ 7.28–7.24 (t, J = 8.3 Hz, 1H), 7.11–7.09 (m, 1H), 7.07–7.05 (m, 2H), 2.90–2.82 (m, 1H), 2.48–2.40 (m, 2H), 2.34 (s, 3H), 2.04–1.98 (m, 1H), 1.82–1.62 (m, 4H); MS m/z 204.2 (MH)+.
2-Amino-2-(p-tolyl)cyclohexan-1-one (30g). Similar reaction of 2-(p-tolyl)cyclohexan-1-one (27g) (3 g, 16.0 mmol) and unsym.-dimethylhydrazine gave 1,1-dimethyl-2-(2-(p-tolyl)cyclohexylidene)hydrazine (28g) (3.07 g, 83%). 1H NMR (CDCl3) δ 7.18–7.16 (m, 2H), 7.14–7.10 (m, 2H), 2.99–2.94 (dt, J = 9.8 Hz, 4.28 Hz, 1H), 2.50 (s, 6H), 2.30 (s, 3H), 2.08–1.89 (m, 2H), 1.82–1.60 (m, 4H), 1.60–1.49 (m, 2 H); MS m/z 231.20 (MH)+. Reaction of 28g (3.07 g, 13.3 mmol) and methyl iodide as above gave 2-(2-(p-tolyl)cyclohexylidene)-1,1,1-trimethylhydrazinium iodide (29g) (3.72 g, 75%). 1H NMR (MeOD) δ 7.24–7.16 (m, 1H), 7.15–7.13 (m, 3H), 3.77–3.74 (m, 1H), 3.48 (s, 9H), 3.02–2.94 (m, 1H), 2.84–2.74 (m, 1H), 2.40–2.32 (m, 1H), 2.30 (s, 3H), 2.19–2.08 (m, 1H), 2.06–1.98 (m, 1H), 1.94–1.84 (m, 2H), 1.86–1.76 (m, 1H); MS m/z 231.20 ((MH-MeI)+). Reaction of 29g (3.72 g, 10.0 mmol) with Na/EtOH as above then gave 30g (1 g, 50%). 1HNMR (CDCl3) δ 7.20–7.18 (m, 2 H), 7.14–7.12 (m, 2H), 2.84 (br s, 1H), 2.35–2.30 (m, 2H), 2.34 (s, 3H), 2.20–2.00 (m, 1H), 1.80–1.70 (m, 4H); MS m/z 204.20 (MH)+.
2-Amino-2-(2-methoxyphenyl)cyclohexan-1-one (30h). Similar reaction of 2-(2-methoxyphenyl)cyclohexan-1-one (27h) (3.0 g, 14.7mmol) and unsym.-dimethylhydrazine gave 2-(2-(2-methoxyphenyl)cyclohexylidene)-1,1-dimethylhydrazine (28h) (3.0 g, 83%). 1HNMR (CDCl3) δ 7.19–7.14 (m, 2H), 6.92–6.82 (m, 2H), 3.77 (s, 3H), 3.00–2.94 (m, 1H), 2.58–2.38 (m, 1H), 2.38 (s, 4H), 2.22–2.12 (m, 1H), 2.02–1.98 (m, 2H), 1.82–1.70 (m, 2H), 1.70–1.58 (m, 2H); MS m/z 247.20 (MH+). Reaction of 28h (3.0 g, 12.2 mmol) and methyl iodide as above gave 2-(2-(2-methoxyphenyl)cyclohexylidene)-1,1,1-trimethylhydrazin-1-ium iodide (29h) (4.31 g, 91%). 1HNMR (MeOD) δ 7.24–7.17 (m, 2H), 6.94–6.88 (m, 2H), 4.01–3.97 (m, 1H), 3.82 (s, 3H), 3.38 (s, 9H), 2.70–2.62 (m, 2H), 2.22–2.14 (m, 2H), 2.08–2.00 (m, 2H), 1.90–1.74 (m, 2H); MS m/z 247.20 ((MH-MeI)+). Reaction of 29h (1.00 g, 2.60 mmol) with Na/EtOH as above then gave 30h (0.30 g, 53%). 1HNMR (CDCl3) δ 7.54–7.52 (dd, J = 7.76 Hz, 1.6 Hz, 1H), 7.32–7.27 (td, J = 7.48 Hz, 1.6 Hz, 1H), 7.06–7.01 (td, J = 7.6 Hz, 1.16 Hz, 1H), 6.90–6.87 (dd, J = 8.29 Hz, 0.92 Hz, 1H), 3.73 (s, 3H), 2.40–2.31 (m, 2H), 1.99–1.92 (m, 2H), 1.76–1.58 (m, 4H); MS m/z 220.20 (MH+).
2-Amino-2-(3-methoxyphenyl)cyclohexan-1-one (30i). Similar reaction of 2-(3-methoxyphenyl)cyclohexan-1-one (27i) (1.0 g, 4.90 mmol) and unsym.-dimethylhydrazine gave 2-(2-(3-methoxyphenyl)cyclohexylidene)-1,1-dimethylhydrazine (28i) (1.2 g, 100%). 1HNMR (CDCl3) δ 7.26–7.22 (m, 1H), 6.90–6.81 (m, 1H), 6.78–6.68 (m, 2H), 3.76 (s, 3H), 3.04–2.98 (dt, J = 13.81 Hz, 4.17 Hz, 1 H), 2.49 (s, 6H), 2.06–1.88 (m, 2H), 1.82- 1.70 (m, 2H), 1.70–1.62 (m, 2H), 1.60–1.50 (m, 2 H); MS m/z 247.20 (MH+). Reaction of 28i (1.2 g, 4.88 mmol) and methyl iodide as above gave 2-(2-(3-methoxyphenyl)cyclohexylidene)-1,1-dimethylhydrazine (29i) (1.20 g, 63%). 1HNMR (MeOD) δ 7.26–7.21 (td, J = 9.12 Hz, 1.52 Hz, 1 H), 6.85–6.79 (m, 3H), 3.78 (s, 3H), 3.49 (s, 9H), 3.04–2.98 (m, 1H), 2.83–2.77 (m, 1H), 2.70–2.58 (m, 1H), 2.40–2.32 (m, 1H), 2.20–2.10 (m, 1H), 2.10–2.00 (m, 1H), 2.00–1.80 (m, 3H); MS m/z 247.20 ((MH-MeI)+). Reaction of 29i (0.67 g, 1.72 mmol) with Na/EtOH as above then gave 30i (0.20 g, 54%).1H NMR (CDCl3) 1HNMR (CDCl3) δ 7.31–7.26 (m, 1H), 6.84–6.82 (m, 3H), 3.79 (s, 3H), 2.83–2.81 (m, 1H), 2.44–2.40 (m, 2H), 2.04–1.95 (m, 2H), 1.76–1.71 (m, 3H); MS m/z 220.20 (MH+).
2-Amino-2-(2-(trifluoromethyl)phenyl)cyclohexan-1-one (30k). Similar reaction of 2-(2-(trifluoromethyl)phenyl)cyclohexan-1-one (27k) (1.28 g, 5.30 mmol) and unsym.-dimethylhydrazine gave 1,1-dimethyl-2-(2-(2-(trifluoromethyl)phenyl)cyclohexylidene)hydrazine (28k) (1.20, 80%) 1HNMR (CDCl3) δ 7.60–7.58 (d, J = 7.92 Hz, 1H), 7.46–7.42 (m, 2H), 7.29–7.27 (m, 1H), 3.82–3.78 (dd, J = 12.25 Hz, 4.36 Hz, 1H), 3.44–3.39 (m, 1H), 2.24 (s, 6H), 2.10–1.80 (m, 5H), 1.72–1.48 (m, 3H); MS m/z 285.20 (MH+). Reaction of 28k (1.00 g, 3.50 mmol) and methyl iodide as above gave 1,1,1-trimethyl-2-(2-(2-(trifluoromethyl)phenyl)cyclohexylidene)hydrazin-1-ium iodide (29k) (1.44 g, 96%) 1HNMR (MeOD) δ 7.67–7.65 (d, J = 7.88 Hz, 1H), 7.62–7.52 (m, 2H), 7.44–7.40 (m, 1H), 4.09–4.05 (dd, J = 7.88 Hz, 4.36 Hz, 1H), 3.38 (s, 9H), 2.68–2.54 (m, 1H), 2.38–2.22 (m, 2H), 2.10–1.96 (m, 2H), 1.90–1.82 (m, 2H), 1.76–1.62 (m, 1H); MS m/z 285.20((MH-MeI)+). Reaction of 29k (1.44 g, 3.40 mmol) with Na/EtOH as above then gave 30k (0.62 g, 72%). 1HNMR (CDCl3) δ 7.98–7.96 (d, J = 8.05 Hz, 1H), 7.71–7.68 (dd, J = 7.88 Hz, 1.2 Hz, 1H), 7.61–7.57 (td, J = 7.52 Hz, 0.68 Hz, 1H), 7.52–7.42 (t, J = 7.64 Hz, 1H), 2.76–2.68 (m, 1H), 2.57–2.42 (m, 1H), 1.98–1.92 (m, 3H), 1.87–1.77 (m, 5H); MS m/z 258.20 (MH+).
2-Amino-2-(3-(trifluoromethyl)phenyl)cyclohexan-1-one (30l). Similar reaction of 2-(3-(trifluoromethyl)phenyl)cyclohexan-1-one (27l) (1.28 g, 5.30 mmol) and unsym.-dimethylhydrazine gave 1,1-dimethyl-2-(2-(3-(trifluoromethyl)phenyl)cyclohexylidene)hydrazine (28l) (1.2 g, 71%) 1HNMR (CDCl3) δ 7.54–7.50 (m,1H), 7.49–7.37 (m, 3H), 3.70–3.67 (t, J = 5.2Hz, 1H), 2.47 (s, 6H), 2.33–2.20 (m, 2H), 2.08–1.98 (m, 1H), 1.94–1.66 (m, 3H), 1.56–1.44 (m, 2H); MS m/z 285.20 (MH+). Reaction of 28l (1.07 g, 2.80 mmol) and methyl iodide as above gave 1,1,1-trimethyl-2-(2-(3-(trifluoromethyl)phenyl)cyclohexylidene)hydrazin-1-ium iodide (29l) (1.40 g, 86%) 1HNMR (MeOD) δ 7.55–7.52 (m, 4H), 3.94–3.90 (m, 1H), 3.44 (s, 9H), 2.73–2.69 (m, 1H), 2.53–2.50 (m, 1H), 2.24–2.18 (m, 3H), 2.03–1.98 (m, 1H), 1.88–1.82 (m, 2H); MS m/z 285.20((MH-MeI)+). Reaction of 29l (1.40 g, 3.30 mmol) with Na/EtOH as above then gave 30l (0.52 g, 62%). 1HNMR (CDCl3) δ 7.58–7.55 (m, 1H), 7.52–7.49 (t, J = 7.72 Hz, 2H), 7.45–7.43 (m, 1H), 2.88–2.82 (m, 1H), 2.58–2.50 (m, 1H), 2.38–2.30 (m, 1H), 2.06–1.98 (m, 1H), 1.92–1.80 (m, 3H), 1.80–1.70 (m, 1H); MS m/z 258.20 (MH+).
2-Amino-2-(4-(trifluoromethyl)phenyl)cyclohexan-1-one (30m). Similar reaction of 2-(4-(trifluoromethyl)phenyl)cyclohexan-1-one (27m) (1.28 g, 5.30 mmol) and unsym.-dimethylhydrazine gave 1,1-dimethyl-2-(2-(4-(trifluoromethyl)phenyl)cyclohexylidene)hydrazine (28m) (1.0 g, 76%) 1HNMR (CDCl3) δ 7.58–7.54 (m, 2H), 7.40–7.37 (d, J = 8.56 Hz, 2H), 3.70–3.67 (t, J = 5.16 Hz, 1H), 2.86–2.78 (m, 1H), 2.48 (s, 6H), 2.38–2.28 (m, 1H), 2.28–2.18 (m, 1H), 2.20–1.96 (m, 1H), 1.82–1.60 (m, 4H); MS m/z 285.20 (MH+). Reaction of 28m (1.00 g, 3.50 mmol) and methyl iodide as above gave 1,1,1-trimethyl-2-(2-(4-(trifluoromethyl)phenyl)cyclohexylidene)hydrazin-1-ium iodide (29m) (1.22, 82%) 1HNMR (MeOD) δ 7.65–7.59 (d, J = 13.36 Hz, 2H), 7.46–7.44 (d, J = 8.44 Hz, 2H), 4.84 (s, 9H), 3.92–3.88 (q, J = 4.88 Hz, 1H), 2.74–2.69 (m, 1H), 2.53–2.49 (m, 1H), 2.22–1.97 (m, 3H), 1.93–1.88 (m, 1H), 1.88–1.84 (m, 2H); MS m/z 285.20((MH-MeI)+). Reaction of 29m (1.22 g, 2.80 mmol) with Na/EtOH as above then gave 30m (0.48 g, 66%). 1HNMR (CDCl3) δ 7.65–7.63 (d, J = 8.25 Hz, 2H), 7.41–7.39 (d, J = 8.20 Hz, 2H), 2.88–2.80 (m, 1H), 2.58–2.50 (m, 1H), 2.40–2.32 (m, 1H), 2.04–1.98 (m, 1H), 1.88–1.72 (m, 4H); MS m/z 258.20 (MH+).
2-Amino-2-(2-(trifluoromethoxy)phenyl)cyclohexan-1-one (30n). Similar reaction 2-(2-(trifluoromethoxy)phenyl)cyclohexan-1-one (27n) (1.11 g, 5.30 mmol) and unsym.-dimethylhydrazine gave 2-(2,2-dimethylhydrazono)-1-(2-(trifluoromethoxy)phenyl)cyclohexan-1-amine (28n) (1.0 g, 78%) 1HNMR (CDCl3) δ 7.32–7.29 (m, 1H), 7.26–7.18 (m, 3H), 3.79–3.76 (dd, J = 12.40 Hz, 4.16 Hz, 1H), 2.27 (s, 6H), 2.04–1.82 (m, 5H), 1.72–1.52 (m, 3H); MS m/z 301.20 (MH+). Reaction of 28n (1.00 g, 3.30 mmol) and methyl iodide as above gave 2-(2-amino-2-(2-(trifluoromethoxy)phenyl)cyclohexylidene)-1,1,1-trimethylhydrazin-1-ium iodide (29n) (1.35g, 92%) 1HNMR (MeOD) δ 7.44–7.42 (m, 1H), 7.36–7.32 (m, 2H), 7.28–7.26 (m, 1H), 4.07–4.03 (m, 1H), 3.40 (s, 9H), 2.69–2.66 (m, 1H), 2.26–2.19 (m, 3H), 2.06–2.03 (m, 2H), 1.84–1.79 (m, 2H); MS m/z 301.20((MH-MeI)+). Reaction of 29n (1.35 g, 3.05 mmol) with Na/EtOH as above then gave 30n (0.42 g, 52%). 1HNMR (CDCl3) δ 7.69–7.67 (m, 1H), 7.39–7.34 (m, 2H), 7.29–7.26 (m, 1H), 2.77–2.71 (m, 1H), 2.58–2.52 (m, 1H), 2.45–2.38 (m, 1H), 1.82–1.77 (m, 1H), 1.82–1.62 (m, 4H); MS m/z 274.20 (MH+).
2-Amino-2-(3-(trifluoromethoxy)phenyl)cyclohexan-1-one (30o). Similar reaction of 2-(3-(trifluoromethoxy)phenyl)cyclohexan-1-one (27o) (1.62 g, 5.40 mmol) and unsym.-dimethylhydrazine gave 2-(2,2-dimethylhydrazono)-1-(3-(trifluoromethoxy)phenyl)cyclohexan-1-amine (28o) (2.0 g, 84%) 1HNMR (CDCl3) δ 7.37–7.31 (m, 1H), 7.22–7.18 (m, 1H), 7.14–7.11 (m, 1H), 7.08–7.04 (m, 1H), 3.68–3.64 (t, J = 5.0 Hz, 1H), 2.48 (s, 6H), 2.34–2.28 (m, 1H), 2.20–2.10 (m, 1H), 2.06–1.96 (m, 1H), 1.94–1.88 (m, 1H), 1.80–1.50 (m, 4H); MS m/z 301.20 (MH+). Reaction of 28o (1.62 g, 5.40 mmol) and methyl iodide as above gave 2-(2-amino-2-(3-(trifluoromethoxy)phenyl)cyclohexylidene)-1,1,1-trimethylhydrazin-1-ium iodide (29o) (2.00 g, 84%) 1HNMR (MeOD) δ 7.40–7.38 (t, J = 7.97 Hz, 1H), 7.30–7.26 (m, 1H), 7.22–7.18 (m, 1H), 7.17–7.11 (m, 1H), 3.89–3.85 (t, J = 7.73 Hz, 1H), 3.44 (s, 9H)HH, 2.52–2.44 (m, 1H), 2.24–2.14 (m, 2H), 2.04–1.96 (m, 2H), 1.92–1.78 (m, 2H), 1.70–1.60 (m, 1H); MS m/z 301.20((MH-MeI)+). Reaction of 29o (2.00 g, 4.50 mmol) with Na/EtOH as above then gave 30o (0.60 g, 50%). 1HNMR (CDCl3) δ 7.43–7.41 (td, J = 7.93 Hz, 0.96 Hz, 1H), 7.18–7.16 (m, 3H), 2.82–2.76 (m, 1H), 2.54–2.48 (m, 1H), 2.40–2.32 (m, 1H), 2.04–1.98 (m, 1H), 1.90–1.70 (m, 4H); MS m/z 274.20 (MH+).
2-Amino-2-(4-(trifluoromethoxy)phenyl)cyclohexan-1-one (30p). Similar reaction of 2-(4-(trifluoromethoxy)phenyl)cyclohexan-1-one (27p) (2.24 g, 8.68 mmol) and unsym.-dimethylhydrazine gave 2-(2,2-dimethylhydrazono)-1-(4-(trifluoromethoxy)phenyl)cyclohexan-1-amine (28p) (2.0 g, 76%) 1HNMR (CDCl3) δ 7.30–7.27 (m, 2H), 7.17–7.13 (m, 2H), 3.67–3.64 (t, J = 7.13 Hz, 1H), 2.89–2.58 (m, 1H), 2.45 (s, 6H), 2.32–2.14 (m, 2H), 2.02–1.94 (m, 1H) 1.80–1.58 (m, 4H); MS m/z 301.20 (MH+). Reaction of 28p (1.82 g, 6.10 mmol) and methyl iodide as above gave 2-(2-amino-2-(4-(trifluoromethoxy)phenyl)cyclohexylidene)-1,1,1-trimethylhydrazin-1-ium iodide (29p) (2.62 g, 98%) 1HNMR (MeOD) δ 7.36–7.34 (m, 2H), 7.22–7.20 (d, J = 7.92 Hz, 2H), 3.89–3.85 (m, 1H), 3.46 (s, 9H), 2.78–2.70 (m, 1H), 2.58–2.49 (m, 1H), 2.30–2.10 (m, 3H), 2.00–1.80 (m, 3H); MS m/z 301.20((MH-MeI)+). Reaction of 29p (2.62 g, 5.90 mmol) with Na/EtOH as above then gave 30p (0.80 g, 50%). 1HNMR (CDCl3) δ 7.32–7.29 (m, 2H), 7.23–7.21 (m, 2H), 2.84–2.78 (m, 1H), 2.52–2.49 (m, 1H), 2.40–2.34 (m, 1H), 2.06–1.98 (m, 1H), 1.88–1.62 (m, 4H); MS m/z 274.20 (MH+).