Localization of (+)-Catechin in Picea abies Phloem: Responses to Wounding and Fungal Inoculation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. ToF-SIMS Analysis of (+)-Catechins, Abietic Acid, and Stilbenes
2.3. Cryo-Sectioning and GC Analysis of (+)-Catechins and Stilbenes in Older Trees
2.4. Quantitative GC-MS Analysis of the Phloem Defense Compounds of Saplings
2.5. Phloem Microscopic Analysis
2.6. Statistical Analysis
3. Results
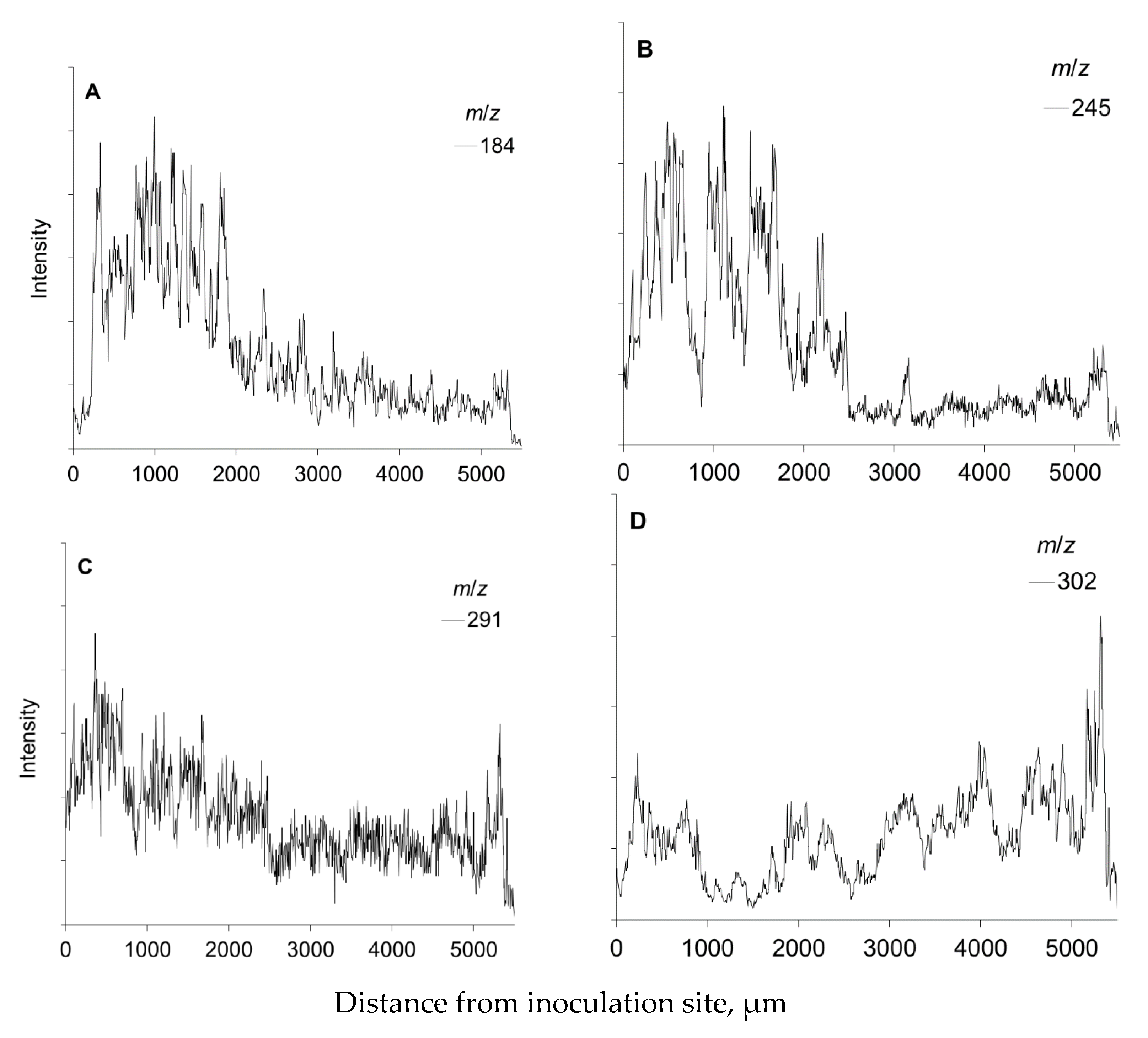
3.1. Tof-SIMS Spectrum of Norway Spruce Phloem
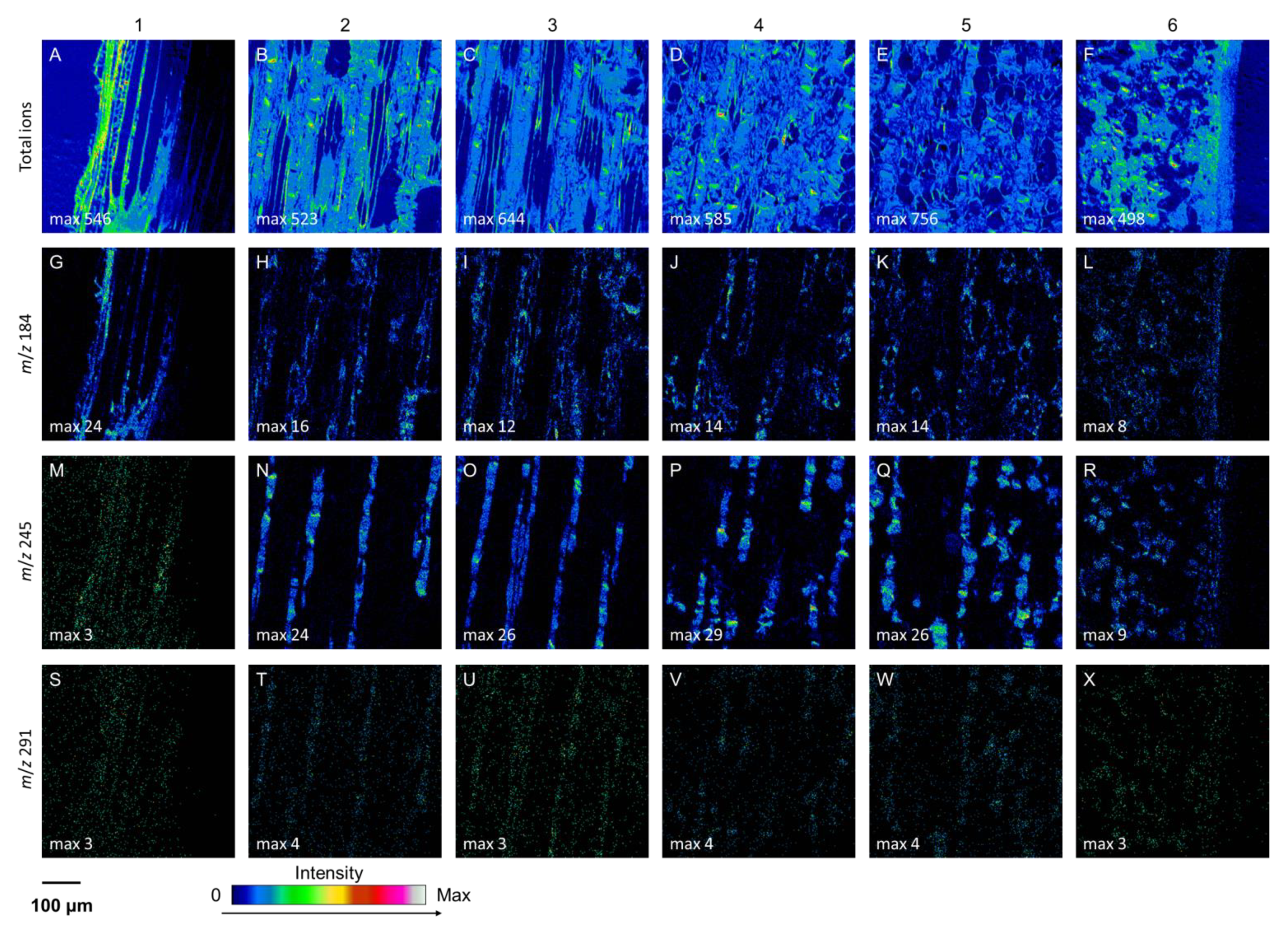
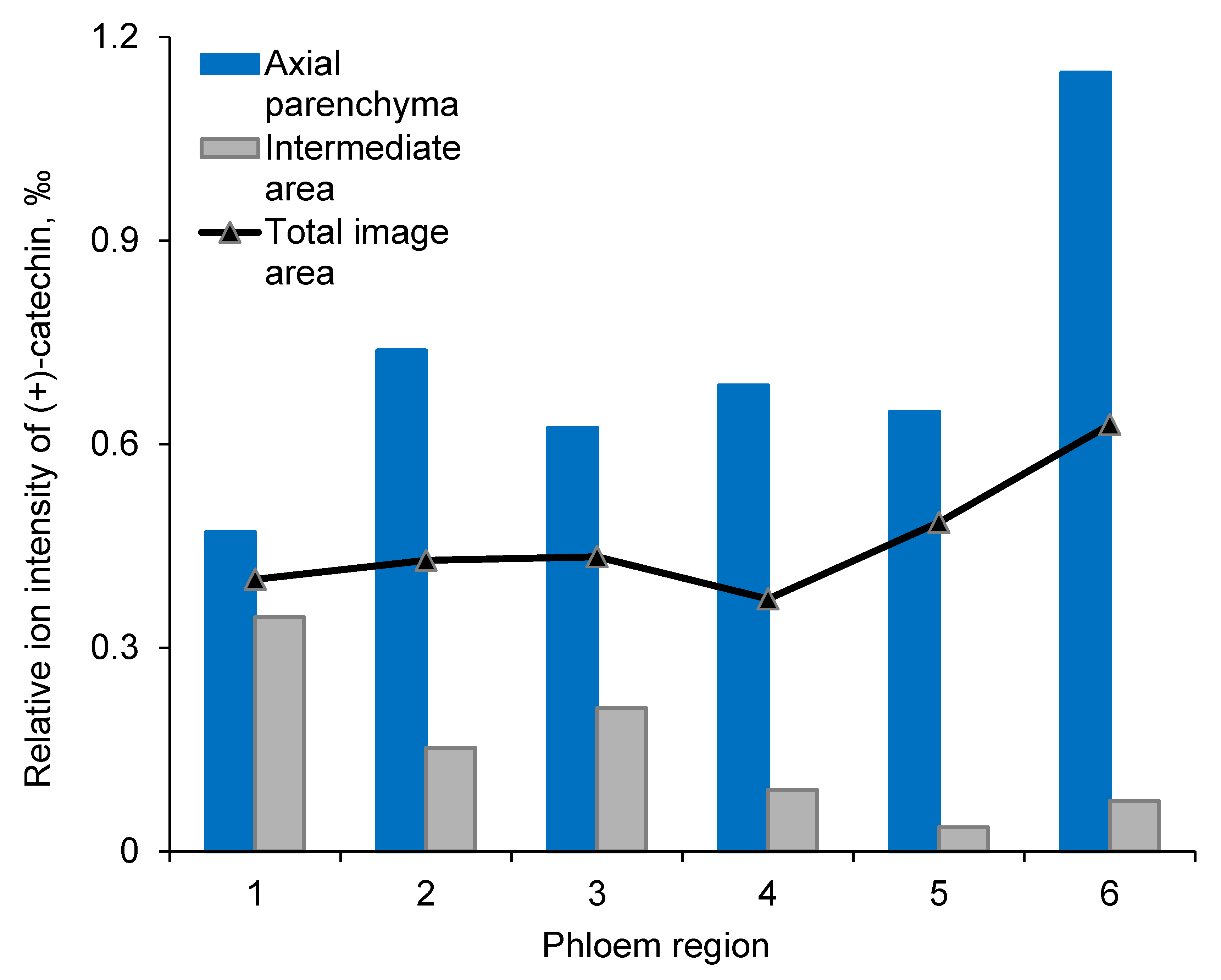
3.2. Localization and Accumulation of Constitutive (+)-Catechin within Phloem
3.3. Anatomical Changes in Phloem after Wounding and Fungal Inoculation
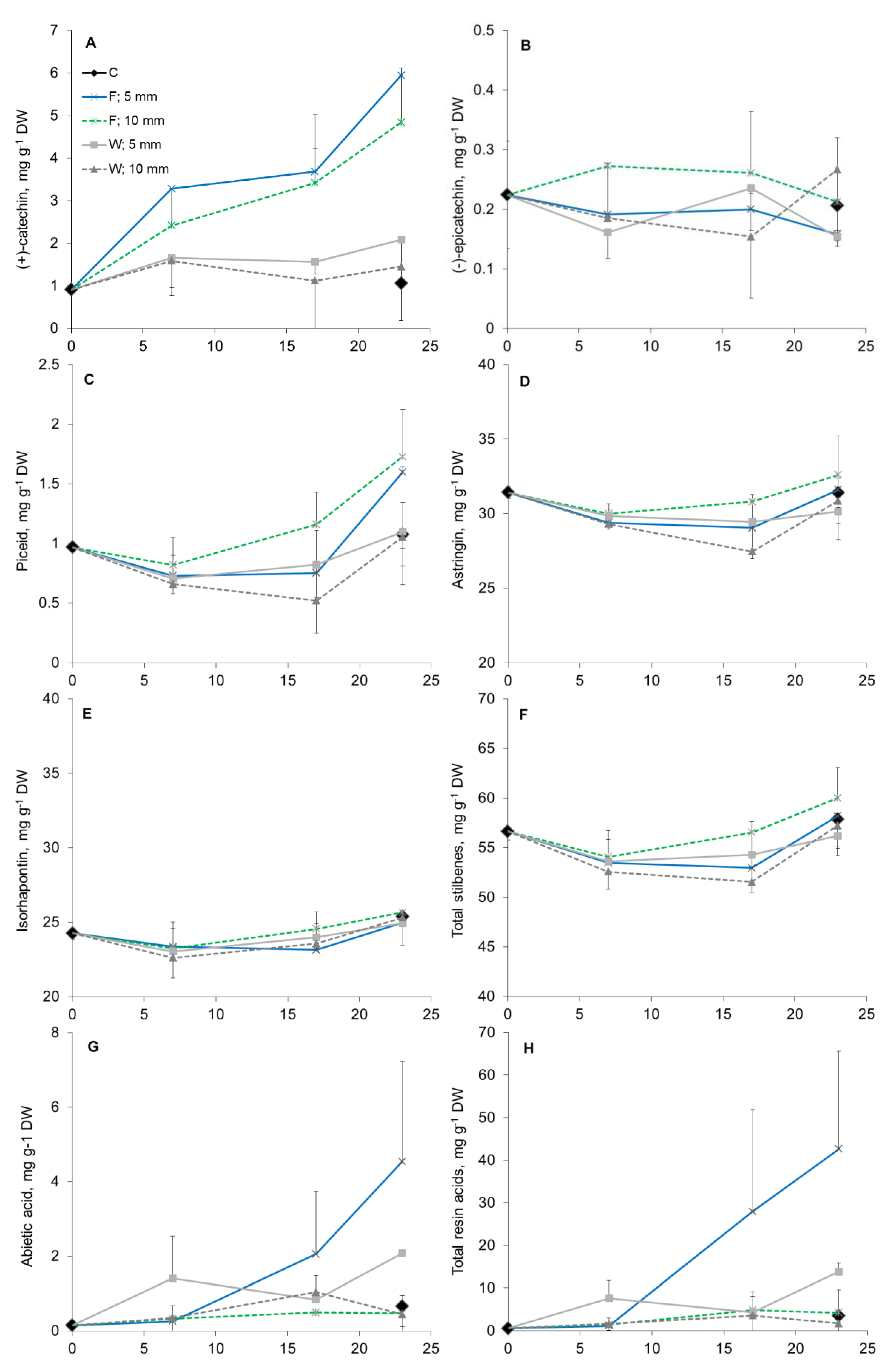
3.4. Phloem Chemical Defenses after Wounding and Fungal Inoculation
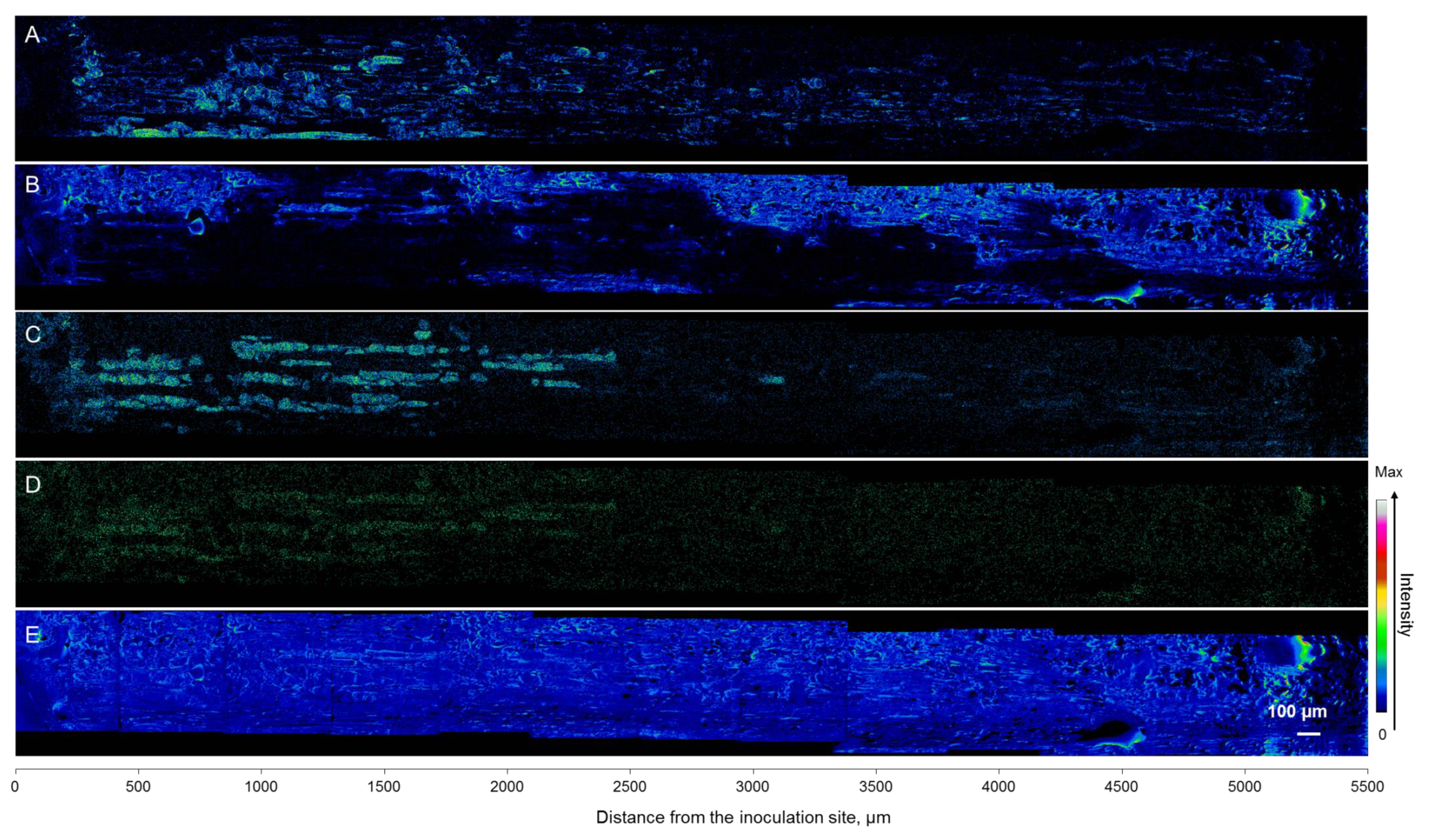
3.5. Mapping of Defense Compounds Within Phloem after Wounding and Fungal Inoculation
4. Discussion
4.1. (+)-Catechins are Localized in Living Axial Parenchyma Cells in Norway Spruce Phloem
4.2. Induced Tissue and Cell-Level Responses to Wounding and Fungal Infection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlyter, P.; Stjernquist, I.; Bärring, L.; Jönsson, A.M.; Nilsson, C. Assessment of the impacts of climate change and weather extremes on boreal forests in northern Europe, focusing on Norway spruce. Clim. Res. 2006, 31, 75–84. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005, 167, 353–376. [Google Scholar] [CrossRef] [PubMed]
- Eyles, A.; Bonello, P.; Ganley, R.; Mohammed, C. Induced resistance to pests and pathogens in trees. New Phytol. 2010, 185, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Krokene, P. Conifer defense and resistance to bark beetles. In Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2015; pp. 177–207. [Google Scholar]
- Sampedro, L.; Moreira, X.; Zas, R. Costs of constitutive and herbivore-induced chemical defences in pine trees emerge only under low nutrient availability: Costs of constitutive and induced pine tree defences. J. Ecol. 2011, 99, 818–827. [Google Scholar] [CrossRef] [Green Version]
- Arango-Velez, A.; Kayal, W.E.; Copeland, C.C.J.; Zaharia, L.I.; Lusebrink, I.; Cooke, J.E.K. Differences in defence responses of Pinus contorta and Pinus banksiana to the mountain pine beetle fungal associate Grosmannia clavigera are affected by water deficit. Plant Cell Environ. 2016, 39, 726–744. [Google Scholar] [CrossRef] [Green Version]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Matthews, B.; Netherer, S.; Katzensteiner, K.; Pennerstorfer, J.; Blackwell, E.; Henschke, P.; Hietz, P.; Rosner, S.; Jansson, P.-E.; Schume, H.; et al. Transpiration deficits increase host susceptibility to bark beetle attack: Experimental observations and practical outcomes for Ips typographus hazard assessment. Agric. For. Meteorol. 2018, 263, 69–89. [Google Scholar] [CrossRef]
- Brasier, C.M.; Mehrotra, M.D. Ophiostoma himal-ulmi sp. nov., a new species of Dutch elm disease fungus endemic to the Himalayas. Mycol. Res. 1995, 99, 205–215. [Google Scholar] [CrossRef]
- Harrington, T.C.; Fraedrich, S.; Aghayeva, D.N. Raffaelea lauricola, a new ambrosia beetle symbiont and pathogen on the Lauracea. Mycotaxon 2008, 104, 399–404. [Google Scholar]
- Hessburg, P.F.; Hansen, E.M. Infection of Douglas-fir by Leptographium wageneri. Can. J. Bot. 2000, 78, 1254–1261. [Google Scholar]
- Plattner, A.; Kim, J.J.; DiGuistini, S.; Breuil, C. Variation in pathogenicity of a mountain pine beetle–associated blue-stain fungus, Grosmannia clavigera, on young lodgepole pine in British Columbia. Can. J. Plant Pathol. 2008, 30, 457–466. [Google Scholar] [CrossRef]
- Paap, T.; de Beer, Z.W.; Migliorini, D.; Nel, W.; Wingfield, M.J. The polyphagous shot hole borer (PSHB) and its fungal symbiont Fusarium euwallaceae: A new invasion in South Africa. Australas. Plant. Pathol. 2018, 47, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Linnakoski, R.; Sugano, J.; Junttila, S.; Pulkkinen, P.; Asiegbu, F.O.; Forbes, K.M. Effects of water availability on a forestry pathosystem: Fungal strain-specific variation in disease severity. Sci. Rep. 2017, 7, 13501. [Google Scholar] [CrossRef] [Green Version]
- Netherer, S.; Matthews, B.; Katzensteiner, K.; Blackwell, E.; Henschke, P.; Hietz, P.; Pennerstorfer, J.; Rosner, S.; Kikuta, S.; Schume, H.; et al. Do water-limiting conditions predispose Norway spruce to bark beetle attack? New Phytol. 2015, 205, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Marini, L.; Økland, B.; Jönsson, A.M.; Bentz, B.; Carroll, A.; Forster, B.; Grégoire, J.-C.; Hurling, R.; Nageleisen, L.M.; Netherer, S.; et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 2017, 40, 1426–1435. [Google Scholar] [CrossRef]
- Woodward, S.; Pearce, R.B. The role of stilbenes in resistance of Sitka spruce (Picea sitchensis (Bong) Carr.) to entry of fungal pathogens. Physiol. Mol. Plant Pathol. 1988, 33, 127–149. [Google Scholar] [CrossRef]
- Krogell, J.; Holmbom, B.; Pranovich, A.; Hemming, J.; Willför, S. Extraction and chemical characterization of Norway spruce inner and outer bark. Nord. Pulp. Paper Res. J. 2012, 27, 6–17. [Google Scholar] [CrossRef]
- Latva-Mäenpää, H.; Laakso, T.; Sarjala, T.; Wähälä, K.; Saranpää, P. Variation of stilbene glucosides in bark extracts obtained from roots and stumps of Norway spruce (Picea abies [L.] Karst.). Trees 2013, 27, 131–139. [Google Scholar] [CrossRef]
- Latva-Mäenpää, H.; Laakso, T.; Sarjala, T.; Wähälä, K.; Saranpää, P. Root neck of Norway spruce as a source of bioactive lignans and stilbenes. Holzforschung 2014, 68, 1–7. [Google Scholar] [CrossRef]
- Jyske, T.; Laakso, T.; Latva-Mäenpää, H.; Tapanila, T.; Saranpää, P. Yield of stilbene glucosides from the bark of young and old Norway spruce stems. Biomass Bioenergy 2014, 71, 216–227. [Google Scholar] [CrossRef]
- Matthews, S.; Mila, I.; Scalbert, A.; Donnelly, D.M. Extractable and non-extractable proanthocyanidins in barks. Phytochemistry 1997, 45, 405–410. [Google Scholar] [CrossRef]
- Bianchi, S.; Kroslakova, I.; Janzon, R.; Mayer, I.; Saake, B.; Pichelin, F. Characterization of condensed tannins and carbohydrates in hot water bark extracts of European softwood species. Phytochemistry 2015, 120, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Salminen, J.; Karonen, M. Chemical ecology of tannins and other phenolics: We need a change in approach. Funct. Ecol. 2011, 25, 325–338. [Google Scholar] [CrossRef]
- Brignolas, F.; Lieutier, F.; Sauvard, D.; Christiansen, E.; Berryman, A.A. Phenolic predictors for Norway spruce resistance to the bark beetle Ips typographus (Coleoptera: Scolytidae) and an associated fungus, Ceratocystis polonica. Can. J. For. Res. 1998, 28, 720–728. [Google Scholar] [CrossRef]
- Evensen, P.C.; Solheim, H.; Høiland, K.; Stenersen, J. Induced resistance of Norway spruce, variation of phenolic compounds and their effects on fungal pathogens. For. Pathol. 2000, 30, 97–108. [Google Scholar] [CrossRef]
- Lieutier, F.; Brignolas, F.; Sauvard, D.; Yart, A.; Galet, C.; Brunet, M.; van de Sype, H. Intra- and inter-provenance variability in phloem phenols of Picea abies and relationship to a bark beetle-associated fungus. Tree Physiol. 2003, 23, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Arango-Velez, A.; Chakraborty, S.; Blascyk, K.; Phan, M.T.; Barsky, J.; El Kayal, W. Anatomical and Chemical Responses of Eastern White Pine (Pinus strobus L.) to Blue-Stain (Ophiostoma minus) Inoculation. Forests 2018, 9, 690. [Google Scholar] [CrossRef] [Green Version]
- Danielsson, M.; Lundén, K.; Elfstrand, M.; Hu, J.; Zhao, T.; Arnerup, J.; Ihrmark, K.; Swedjemark, G.; Borg-Karlson, A.-K.; Stenlid, J. Chemical and transcriptional responses of Norway spruce genotypes with different susceptibility to Heterobasidion spp. infection. BMC Plant Biol. 2011, 11, 154. [Google Scholar] [CrossRef] [Green Version]
- Li, S.H.; Nagy, N.E.; Hammerbacher, A.; Krokene, P.; Niu, X.M.; Gershenzon, J.; Schneider, B. Localization of phenolics in phloem parenchyma cells of Norway spruce (Picea abies). ChemBioChem 2012, 13, 2707–2713. [Google Scholar] [CrossRef] [PubMed]
- Hammerbacher, A.; Paetz, C.; Wright, L.P.; Fischer, T.C.; Bohlmann, J.; Davis, A.J.; Fenning, T.M.; Gershenzon, J.; Schmidt, A. Flavan-3-ols in Norway spruce: Biosynthesis, accumulation, and function in response to attack by the bark beetle-associated fungus Ceratocystis polonica. Plant Physiol. 2014, 164, 2107–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemesio-Gorriz, M.; Hammerbacher, A.; Ihrmark, K.; Källman, T.; Olson, Å.; Lascoux, M.; Stenlid, J.; Gershenzon, J.; Elfstrand, M. Different alleles of a gene encoding leucoanthocyanidin reductase (PaLAR3) influence resistance against the fungus Heterobasidion parviporum in Picea abies. Plant Physiol. 2016, 171, 2671–2681. [Google Scholar] [CrossRef] [PubMed]
- Wadke, N.; Kandasamy, D.; Vogel, H.; Lah, L.; Wingfield, B.D.; Paetz, C.; Wright, L.P.; Gershenzon, J.; Hammerbacher, A. The bark-beetle-associated fungus, Endoconidiophora polonica, utilizes the phenolic defense compounds of its host as a carbon source. Plant Physiol. 2016, 171, 914–931. [Google Scholar] [PubMed] [Green Version]
- Zhao, T.; Kandasamy, D.; Krokene, P.; Chen, J.; Gershenzon, J.; Hammerbacher, A. Fungal associated of the tree-killing bark beetle, Ips typographus, vary in virulence, ability to degrade conifer phenolics and influence bark beetle tunnelling behaviour. Fungal Ecol. 2018. [Google Scholar] [CrossRef]
- Jyske, T.; Kuroda, K.; Suuronen, J.P.; Pranovich, A.; Roig Juan, S.; Aoki, D.; Fukushima, K. In Planta Localization of Stilbenes within PICEA Abies Phloem. Plant Physiol. 2016, 172, 913–928. [Google Scholar] [CrossRef] [Green Version]
- Aoki, D.; Hanaya, Y.; Akit, T.; Matsushita, Y.; Yoshida, M.; Kuroda, K.; Yagami, S.; Takama, R.; Fukushima, K. Distribution of coniferin in freeze-fixed stem of Ginkgo biloba L. by cryo-TOF-SIMS/SEM. Sci. Rep. 2016, 6, 31525. [Google Scholar] [CrossRef] [Green Version]
- Aoki, D.; Matsushita, Y.; Fukushima, K. Cryo-TOF-SIMS visualization of water-soluble compounds in plants. In Advances in Plant Phenolics: From Chemistry to Human Health; Jayaprakasha, G.K., Gattuso, G., Patil, B.S., Eds.; American Chemical Society: Washington, DC, USA, 2018; pp. 137–150. [Google Scholar]
- Franceschi, V.R.; Krekling, T.; Berryman, A.A.; Christiansen, E. Specialized phloem parenchyma cells in Norway spruce (Pinaceae) bark are an important site of defense reactions. Am. J. Bot. 1998, 85, 601–615. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krokene, P.; Krekling, T.; Christiansen, E. Phloem parenchyma cells are involved in local and distant defense responses to fungal inoculation or bark-beetle attack in Norway spruce (Pinaceae). Am. J. Bot. 2000, 87, 314–326. [Google Scholar] [CrossRef]
- Metzner, R.; Schneider, H.U.; Breuer, U.; Schroeder, W.H. Imaging nutrient distributions in plant tissue using time-of-flight secondary ion mass spectrometry and scanning electron microscopy. Plant Physiol. 2008, 147, 1774–1787. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, K.; Fujiwara, T.; Imai, T.; Takama, R.; Saito, K.; Matsushita, Y.; Fukushima, K. The cryo-TOF-SIMS/SEM system for the analysis of the chemical distribution in freeze-fixed Cryptomeria japonica wood. Surf. Interface Anal. 2013, 45, 215–219. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Pallardy, S.G. Physiology of Woody Plants; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Sostarecz, A.G.; Cannon, D.M., Jr.; McQuaw, C.M.; Sun, S.; Ewing, A.G.; Winograd, N. Influence of molecular environment on the analysis of phospholipids by time-of-flight secondary ion mass spectrometry spectrometry. Langmuir 2004, 20, 4926–4932. [Google Scholar] [CrossRef] [PubMed]
- Börner, K.; Malmberg, P.; Månsson, J.E.; Nygren, H. Molecular imaging of lipids in cells and tissues. Int. J. Mass Spectrom. 2007, 260, 128–136. [Google Scholar] [CrossRef]
- Jones, E.A.; Lockyer, N.P.; Vickerman, J.C. Mass spectral analysis and imaging of tissue by ToF-SIMS—The role of buckminsterfullerene, C60+, primary ions. Int. J. Mass Spectrom. 2007, 260, 146–157. [Google Scholar] [CrossRef]
- Nygren, H.; Hagenhoff, B.; Malmberg, P.; Nilsson, M.; Richter, K. Bioimaging TOF-SIMS: High resolution 3D imaging of single cells. Microsc. Res. Techniq. 2007, 70, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.E.; Sikora, K.; Krokene, P.; Hietala, A.M.; Solheim, H.; Fossdal, C.G. Using laser micro-dissection and qRT-PCR to analyze cell type-specific gene expression in Norway spruce phloem. PeerJ 2014, 2, e362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spicer, R. Symplasmic networks in secondary vascular tissues: Parenchyma distribution and activity supporting long-distance transport. J. Exp. Bot. 2014, 65, 1829–1848. [Google Scholar] [CrossRef] [Green Version]
- Viiri, H.; Annila, A.; Kitunen, V. Induced responses in stilbenes and terpenes in fertilized Norway spruce after inoculation with blue-stain fungus Ceratocystis polonica. Trees 2001, 15, 112–122. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Schmidt, A.; Wadke, N.; Wright, L.P.; Schneider, B.; Bohlmann, J.; Brand, W.A.; Fenning, T.M.; Gershenzon, J.; Paetz, C. A common fungal associate of the spruce bark beetle metabolizes the stilbene defenses of Norway spruce. Plant Physiol. 2013, 162, 1324–1336. [Google Scholar] [CrossRef] [Green Version]
- Brignolas, F.; Lacroix, B.; Lieutier, F.; Sauvard, D.; Drouet, A.; Claudot, A.-C.; Yart, A.; Berryman, A.A.; Christiansen, E. Induced responses in phenolic metabolism in two Norway spruce clones after wounding and inoculation with Ophiostoma polonicum, a bark beetleassociated fungus. Plant Physiol. 1995, 109, 821–827. [Google Scholar] [CrossRef] [Green Version]
- Brignolas, F.; Lieutier, F.; Sauvard, D.; Yarta, A.; Drouet, A.; Claudot, A.C. Changes in soluble phenol content of Norway spruce (Picea abies L. Karst) phloem in response to wounding and inoculation with Ophiostoma polonicum. Eur. J. For. Pathol. 1995, 25, 253–265. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Ralph, S.G.; Bohlmann, J.; Fenning, T.M.; Gershenzon, J.; Schmidt, A. Biosynthesis of the major tetrahydroxystilbenes in spruce, astringin and isorhapontin, proceeds via resveratrol and is enhanced by fungal infection. Plant Physiol. 2011, 157, 876–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammerbacher, A.; Kandasamy, D.; Ullah, C.; Schmidt, A.; Wright, L.P.; Gershenzon, J. Flavanone-3-Hydroxylase Plays an Important Role in the Biosynthesis of Spruce Phenolic Defenses Against Bark Beetles and Their Fungal Associates. Front. Plant Sci. 2019, 10, 208. [Google Scholar] [CrossRef]
- Christiansen, E. Ceratocystis polonica inoculated in Norway spruce: Blue-staining in relation to inoculum density, resinosis and tree growth. Eur. J. For. Pathol. 1985, 15, 160–167. [Google Scholar] [CrossRef]
- Felhofer, M.; Prats-Mateu, B.; Bock, P.; Gierlinger, N. Antifungal stilbene impregnation: Transport and distribution on the micron-level. Tree Physiol. 2018, 38, 1526–1537. [Google Scholar] [CrossRef]
- Zhang, L.; Gellerstedt, G. 2D Heteronuclear (1H–13C) Single quantum correlation (HSQC) NMR analysis of Norway spruce bark components. In Characterization of Lignocellulosic Materials; Hu, T., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2008; pp. 3–16. ISBN 978-1-405-15880-0. [Google Scholar]
- Kemppainen, K.; Siika-aho, M.; Pattathil, S.; Giovando, S.; Kruus, K. Spruce bark as an industrial source of condensed tannins and noncellulosic sugars. Ind. Crops Prod. 2014, 52, 158–168. [Google Scholar] [CrossRef]
- Brillouet, J.M.; Romieu, C.; Schoefs, B.; Solymosi, K.; Cheynier, V.; Fulcrand, H.; Verdeil, J.L.; Conéjéro, G. The tannosome is an organelle forming condensed tannins in the chlorophyllous organs of Tracheophyta. Ann. Bot. 2013, 112, 1003–1014. [Google Scholar] [CrossRef] [Green Version]
- Kelm, M.A.; Hammerstone, J.F.; Schmitz, H.H. Identification and quantitation of flavanols and proanthocyanidins in foods: How good are the datas? Clin. Dev. Immunol. 2005, 12, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Ottaviani, J.I.; Heiss, C.; Spencer, J.P.; Kelm, M.; Schroeter, H. Recommending flavanols and procyanidins for cardiovascular health: Revisited. Mol. Aspects Med. 2018, 61, 63–75. [Google Scholar] [CrossRef]
- Hagerman, A.E. Fifty years of polyphenol-protein complexes. In Recent Advances in Polyphenol Research; Cheynier, V., Sarni-Manchado, P., Quideau, S., Eds.; Wiley-Blackwell: Oxford, UK, 2012; Volume 3, pp. 71–97. [Google Scholar]
- Andebrhan, T.; Hammerstone, J.F.; Romanczyk, L.J.; Furtek, D.B. Sensitivity of Crinipellis perniciosa to procyanidins from Theobroma cacao L. Physiol. Mol. Plant Pathol. 1995, 46, 339–348. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, J.; Zhang, C.; Rodrigues, C.J., Jr. Epicatechin and catechin may prevent coffee berry disease by inhibition of appressorial melanization of Colletotrichum kahawae. Biotechnol. Lett. 2006, 28, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Ebbole, D.J. Magnaporthe as a model for understanding host-pathogen interactions. Annu. Rev. Phytopathol. 2007, 45, 437–456. [Google Scholar] [CrossRef]
- Oliva, J.; Stenlid, J.; Martínez-Vilalta, J. The effect of fungal pathogens on the water and carbon economy of trees: Implications for drought-induced mortality. New Phytol. 2014, 203, 1028–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Hammerbacher, A.; Weinhold, A.; Reichelt, M.; Gleixner, G.; Behrendt, T.; van Dam, N.M.; Sala, A.; Gershenzon, J.; Trumbore, S.; et al. Eyes on the future - evidence for trade-offs between growth, storage and defense in Norway spruce. New Phytol. 2019, 222, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Forkelová, L.; Unsicker, S.B.; Forkel, M.; Griffith, D.W.T.; Trumbore, S.; Hartmann, H. Isotope labeling reveals contribution of newly fixed carbon to carbon storage and monoterpenes production under water deficit and carbon limitation. Environ. Exp. Bot. 2019, 162, 333–344. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






| Dependent Variable | Fixed Effect | F-Value | p-Value |
|---|---|---|---|
| (+)-catechin | Treatment (T) | 62.91 | 0.000 |
| Day (D) | 3.05 | 0.110 | |
| Location (L) | 5.95 | 0.026 | |
| T*D | 4.58 | 0.030 | |
| T*L | 0.06 | 0.814 | |
| D*L | 0.21 | 0.814 | |
| T*D*L | 1.02 | 0.381 | |
| (−)-epicatechin | T | 1.90 | 0.193 |
| D | 0.13 | 0.936 | |
| L | 1.76 | 0.206 | |
| T*D | 1.45 | 0.282 | |
| T*L | 0.48 | 0.499 | |
| D*L | 1.52 | 0.252 | |
| T*D*L | 1.12 | 0.353 | |
| Piceid | T | 12.27 | 0.002 |
| D | 12.24 | 0.000 | |
| L | 0.19 | 0.669 | |
| T*D | 2.29 | 0.126 | |
| T*L | 3.47 | 0.077 | |
| D*L | 0.01 | 0.990 | |
| T*D*L | 1.10 | 0.350 | |
| Astringin | T | 4.66 | 0.043 |
| D | 3.18 | 0.146 | |
| L | 0.45 | 0.511 | |
| T*D | 0.97 | 0.404 | |
| T*L | 2.98 | 0.103 | |
| D*L | 0.37 | 0.697 | |
| T*D*L | 1.04 | 0.378 | |
| Isorhapontin | T | 0.30 | 0.589 |
| D | 5.33 | 0.007 | |
| L | 0.31 | 0.584 | |
| T*D | 0.07 | 0.930 | |
| T*L | 0.83 | 0.373 | |
| D*L | 0.32 | 0.733 | |
| T*D*L | 0.35 | 0.709 | |
| Total stilbenes | T | 3.15 | 0.091 |
| D | 6.27 | 0.003 | |
| L | 0.36 | 0.553 | |
| T*D | 0.28 | 0.761 | |
| T*L | 2.45 | 0.133 | |
| D*L | 0.25 | 0.781 | |
| T*D*L | 0.96 | 0.401 | |
| Abietic acid | T | 1.11 | 0.307 |
| D | 1.62 | 0.287 | |
| L | 24.97 | 0.000 | |
| T*D | 2.21 | 0.153 | |
| T*L | 1.79 | 0.200 | |
| D*L | 6.26 | 0.010 | |
| T*D*L | 3.10 | 0.073 | |
| Total resin acids | T | 5.62 | 0.029 |
| D | 1.52 | 0.309 | |
| L | 19.10 | 0.000 | |
| T*D | 3.04 | 0.081 | |
| T*L | 3.40 | 0.083 | |
| D*L | 3.81 | 0.044 | |
| T*D*L | 2.11 | 0.153 |
| Resin Acid, mg g−1 DW | Days after Onset | Treatment | ||||
|---|---|---|---|---|---|---|
| Control | Wounding | Fungal Inoculation | ||||
| Location | ||||||
| 5 mm | 10 mm | 5 mm | 10 mm | |||
| Pimaric | 0 | - | ||||
| 7 | n.d. | 0.14 ± 0.05 | - | - | - | |
| 17 | n.d. | 0.25 | - | 0.51 ± 0.04 | 0.05 | |
| 23 | 0.03 ± 0.02 | 0.20 ± 0.06 | - | 0.92 ± 0.64 | 0.11 | |
| Isopimaric | 0 | 0.14 ± 0.06 | ||||
| 7 | n.d. | 1.57 ± 1.35 | 0.41 ± 0.53 | 0.22 ± 0.06 | 0.33 ± 0.36 | |
| 17 | n.d. | 0.74 ± 0.59 | 0.68 ± 0.01 | 2.01 ± 1.67 | 0.42 ± 0.09 | |
| 23 | 0.83 ± 0.76 | 2.43 ± 0.39 | 0.40 ± 0.05 | 4.21 ± 3.35 | 0.58 ± 0.72 | |
| Palustric | 0 | 0.03 | ||||
| 7 | n.d. | 0.45 ± 0.19 | 0.27 | 0.08 | - | |
| 17 | n.d. | 0.56 ± 0.76 | 0.43 ± 0.07 | 7.35 ± 6.33 | 0.90 ± 1.03 | |
| 23 | 1.19 ± 0.91 | 2.02 ± 0.91 | 0.07 | 10.58 ± 5.57 | 1.48 | |
| Levopimaric | 0 | - | ||||
| 7 | n.d. | 0.83 ± 0.09 | 0.13 | 0.18 | 0.24 | |
| 17 | n.d. | 1.02 ± 1.68 | 0.26 ± 0.03 | 17.6 ± 3.82 | 1.97 ± 2.64 | |
| 23 | 0.37 ± 0.48 | 2.13 ± 1.67 | - | 9.96 ± 0.95 | 0.41 | |
| Dehydroabietic | 0 | 0.13 ± 0.06 | ||||
| 7 | n.d. | 2.54 ± 0.72 | 0.30 ± 0.31 | 0.35 ± 0.02 | 0.20 ± 0.20 | |
| 17 | n.d. | 0.76±0.58 | 0.36±0.07 | 2.18 ± 1.79 | 0.61 ± 0.41 | |
| 23 | 0.74 ± 0.63 | 3.48 ± 0.58 | 0.60 ± 0.39 | 9.01 ± 7.93 | 1.99 ± 2.66 | |
| Neoabietic | 0 | 0.11 ± 0.07 | ||||
| 7 | n.d. | 0.64 ± 0.65 | 0.66 | 0.13 ± 0.02 | 0.41 ± 0.44 | |
| 17 | n.d. | 0.50 ± 0.39 | 0.78 ± 0.06 | 2.24 ± 1.87 | 0.41 ± 0.01 | |
| 23 | 0.58 ± 0.55 | 1.42 ± 0.30 | 0.23 ± 0.03 | 3.38 ± 1.79 | 0.12 ± 0.10 | |
| Abietic acid | 0 | 0.15 ± 0.07 | ||||
| 7 | n.d. | 1.41 ± 1.13 | 0.36 ± 0.43 | 0.26 ± 0.09 | 0.33 ± 0.35 | |
| 17 | n.d. | 0.84 ± 0.64 | 1.04 ± 0.14 | 2.06 ± 1.69 | 0.50 ± 0.02 | |
| 23 | 0.67 ± 0.55 | 2.08 ± 0.06 | 0.45 ± 0.00 | 4.54 ± 2.70 | 0.47 ± 0.48 | |
| Total resinacids | 0 | 0.50 ± 0.17 | ||||
| 7 | n.d. | 7.57 ± 4.20 | 1.60 ± 2.03 | 1.09 ± 0.02 | 1.38 ± 1.52 | |
| 17 | n.d. | 4.26 ± 3.74 | 3.56 ± 0.10 | 27.90 ± 24.01 | 4.83 ± 4.22 | |
| 23 | 3.48 ± 3.60 | 13.76 ± 2.03 | 1.71 ± 0.51 | 42.59 ± 22.92 | 4.16 ± 5.37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jyske, T.; Kuroda, K.; Keriö, S.; Pranovich, A.; Linnakoski, R.; Hayashi, N.; Aoki, D.; Fukushima, K. Localization of (+)-Catechin in Picea abies Phloem: Responses to Wounding and Fungal Inoculation. Molecules 2020, 25, 2952. https://doi.org/10.3390/molecules25122952
Jyske T, Kuroda K, Keriö S, Pranovich A, Linnakoski R, Hayashi N, Aoki D, Fukushima K. Localization of (+)-Catechin in Picea abies Phloem: Responses to Wounding and Fungal Inoculation. Molecules. 2020; 25(12):2952. https://doi.org/10.3390/molecules25122952
Chicago/Turabian StyleJyske, Tuula, Katsushi Kuroda, Susanna Keriö, Andrey Pranovich, Riikka Linnakoski, Noriko Hayashi, Dan Aoki, and Kazuhiko Fukushima. 2020. "Localization of (+)-Catechin in Picea abies Phloem: Responses to Wounding and Fungal Inoculation" Molecules 25, no. 12: 2952. https://doi.org/10.3390/molecules25122952
APA StyleJyske, T., Kuroda, K., Keriö, S., Pranovich, A., Linnakoski, R., Hayashi, N., Aoki, D., & Fukushima, K. (2020). Localization of (+)-Catechin in Picea abies Phloem: Responses to Wounding and Fungal Inoculation. Molecules, 25(12), 2952. https://doi.org/10.3390/molecules25122952





