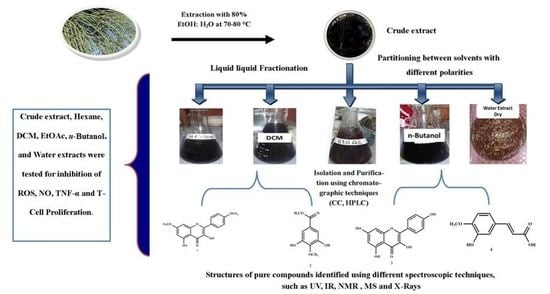
Anti-Inflammatory Principles from Tamarix aphylla L.: A Bioassay-Guided Fractionation Study
Abstract
:1. Introduction
2. Results
2.1. Reactive Oxygen Species (ROS)
2.2. Nitric Oxide (NO)
2.3. The Proinflammatory Cytokine TNF-α
2.4. The Proliferation of T-Cells
2.5. Bioassay Guided Fractionation
3. Discussion
3.1. Tamarix aphylla L. Extracts
3.2. Purified Compounds
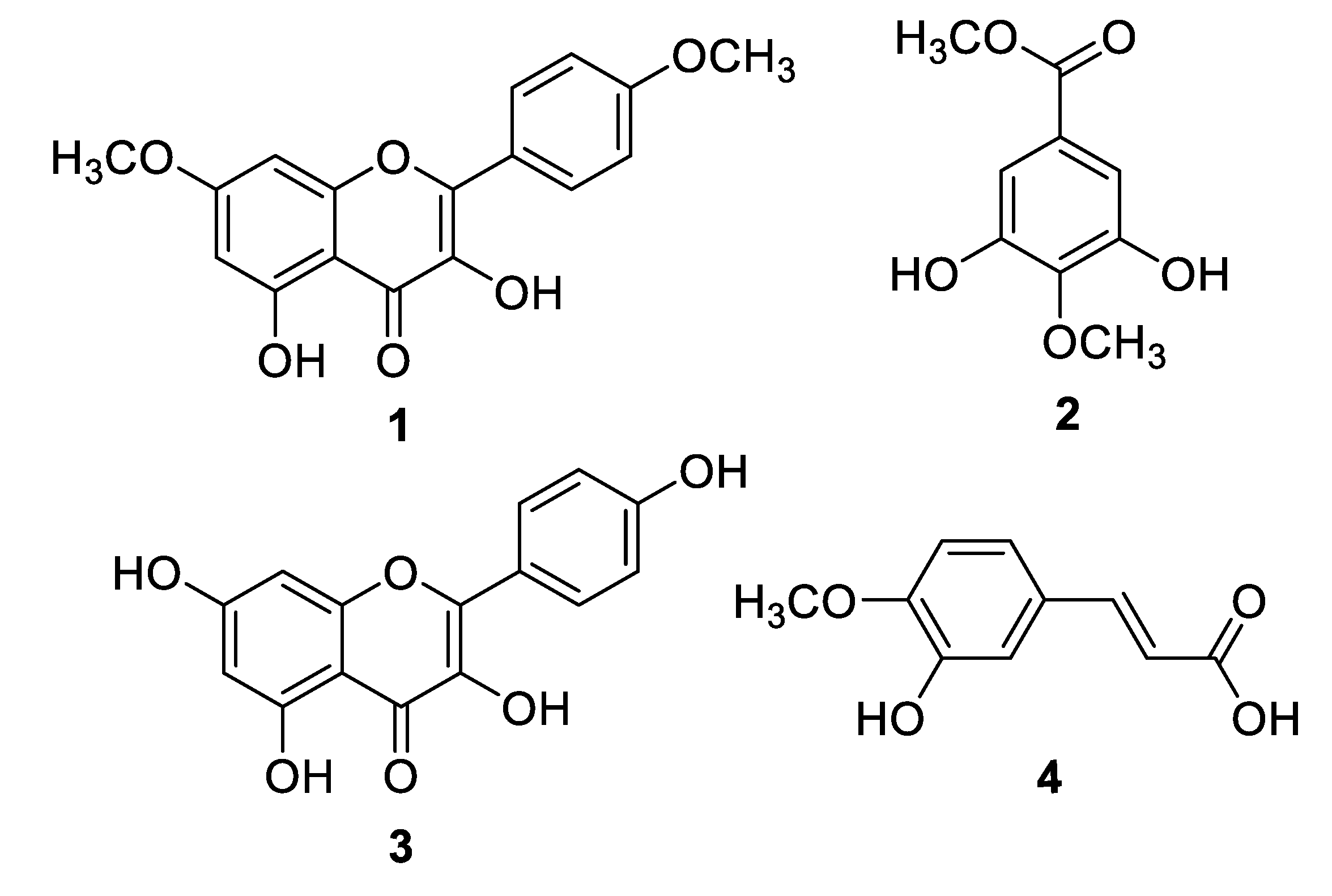
3.2.1. Kaempferol-7,4’-dimethyl ether (1)
3.2.2. Methyl 4-O-methylgallate (2)
3.2.3. Kaempferol (3)
3.2.4. Trans-Isoferulic Acid (4)
4. Materials and Methods
4.1. General Information
4.2. Plant Material
4.3. Extraction and Isolation
4.3.1. Kaempferol-7,4’-dimethyl ether (1)
4.3.2. Methyl 4-O-methylgallate (2)
4.3.3. Kaempferol (3)
4.3.4. Trans-Isoferulic Acid (4)
4.4. Anti-Inflammatory and Immunomodulatory Activities
4.4.1. Oxidative Burst Inhibition Assay
4.4.2. Nitric Oxide Inhibition Assay
4.4.3. Proinflammatory Cytokine TNF-α Production and Quantification
4.4.4. Lymphocyte Proliferation Assay
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef]
- Isailovic, N.; Daigo, K.; Mantovani, A.; Selmi, C. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015, 60, 1–11. [Google Scholar] [CrossRef]
- Florence, T.M. The role of free radicals in disease. Aust. N. Z. J. Ophthalmol. 1995, 23, 3–7. [Google Scholar] [CrossRef]
- Curcic, S.; Holzer, M.; Frei, R.; Pasterk, L.; Schicho, R.; Heinemann, A.; Marsche, G. Neutrophil effector responses are suppressed by secretory phospholipase A2 modified HDL. Biochim. Biophys. Acta (BBA)-Molecular Cell Biol. Lipids 2015, 1851, 184–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonardi, G.C.; Accardi, G.; Monastero, R.; Nicoletti, F.; Libra, M. Ageing: From inflammation to cancer. Immun Ageing 2018, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A comprehensive review of non-steroidal antiInflammatory drug use in the elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laraia, L.; Robke, L.; Waldmann, H. Bioactive compound collections: From design to target identification. Chem 2018, 4, 705–730. [Google Scholar] [CrossRef]
- Mukherjee, D.; Palit, P.; Roychoudhury, S.; Kundu, S.K.; Mandal, S.C. Role of stress in diseases and its remedial approach by herbal and natural products in stress-related disease management: Experimental studies and clinical reports. In Natural Products and Drug Discovery; Elsevier: Amsterdam, The Netherland, 2018; pp. 375–410. [Google Scholar]
- Ali, M.S.; Alam, M.S.; Ahmad, S.; Ali, M.; Ahsan, W.; Siddiqui, M.R.; Ansari, M.S.; Shamim, M.; Ali, M.D. Wound healing activity of alcoholic extract of Tamarixaphylla L. on animal models. Biomed. Pharmacol. J. 2019, 12, 41–48. [Google Scholar] [CrossRef]
- Orabi, M.A.A.; Yoshimura, M.; Amakura, Y.; Hatano, T. Ellagitannins, gallotannins, and gallo-ellagitannins from the galls of Tamarix aphylla. Fitoterapia 2015, 104, 55–63. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Kalkhorani, M.; Zaidi, S.M.A.; Farzaei, M.H.; Roja, R. The genus Tamarix: Traditional uses, phytochemistry, and pharmacology. J. Ethnopharmacol. 2020, 246, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Boulos, L. Flora of Egypt: Geraniaceae-Boraginaceae; Al Hadara Pub.: Cairo, Egypt, 2000; Volume 2. [Google Scholar]
- Marwat, S.K.; Khan, M.A.; Fazal-ur-Rehman, M.; Ahmad; Zafar, M.; Sultana, S. Salvadora persica, Tamarix aphylla and Zizyphus mauritiana-Three woody plant species mentioned in Holy Quran and Ahadith and their ethnobotanical uses in North Western part (D.I. Khan) of Pakistan. Pak. J. Nutr. 2009, 8, 542–547. [Google Scholar]
- Alzweiri, M.; Al Sarhan, A.; Mansi, K.; Hudaib, M.; Aburjai, T. Ethnopharmacological survey of medicinal herbs in Jordan, the Northern Badia region. J. Ethnopharmacol. 2011, 137, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Alhazmi, H.A.; Ansari, S.H.; Hussain, A.; Ahmad, S.; Alam, M.S.; Ali, M.S.; El-Sharkawy, K.A.; Hakeem, K.R. Tamarix aphylla (L.) Karst. Phytochemical and bioactive profile compilations of less discussed but effective naturally growing Saudi plant. In Plant and Human Health, Volume 3; Springer: Cham, Switzerland, 2019; pp. 343–352. [Google Scholar]
- Blunder, M.; Orthaber, A.; Bauer, R.; Bucar, F.; Kunert, O. Efficient identification of flavones, flavanones and their glycosides in routine analysis via off-line combination of sensitive NMR and HPLC experiments. Food Chem. 2017, 218, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Nawwar, M.A.M.; Hussein, S.A.M.; Merfort, I. NMR spectral analysis of polyphenols from Punica granatum. Phytochemistry 1994, 36, 793–798. [Google Scholar] [CrossRef]
- Ishak, M.S.; el-Sissi, H.I.; El-Sherbieny, A.E.; Nawwar, M.A. Tannins and polyphenolics of the galls of Tamarix aphylla. II. Planta Med. 1972, 21, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Lin, C.H.; Hung, S.K.; Chou, J.H.; Chi, C.W.; Fu, S.L. Fisetin inhibits lipopolysaccharide-induced macrophage activation and dendritic cell maturation. J. Agric. Food Chem. 2010, 58, 10831–10839. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Hung, S.-K.; Fu, S.-L. Immunosuppressive effects of fisetin in ovalbumin-induced asthma through inhibition of NF-κB activity. J. Agric. Food Chem. 2011, 59, 10496–10504. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Ando, S.; Toguchida, I.; Yoshikawa, M. Structural requirements of flavonoids for nitric oxide production inhibitory activity and mechanism of action. Bioorg. Med. Chem. 2003, 11, 1995–2000. [Google Scholar] [CrossRef]
- Sudsai, T.; Prabpai, S.; Kongsaeree, P.; Wattanapiromsakul, C.; Tewtrakul, S. Anti-inflammatory activity of compounds from Boesenbergia longiflora rhizomes. J. Ethnopharmacol. 2014, 154, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.J.; Le Lamer, A.; Olagnier, D.; Castillo, D.; Arevalo, J.; Valadeau, C.; Coste, A.; Pipy, B.; Bourdy, G.; Sauvian, M.; et al. Leishmanicidal compounds and potent PPARγ activators from Renealmia thyrsoidea (Ruiz & Pav.) Poepp. & Endl. J. Ethnopharmacol. 2014, 157, 149–155. [Google Scholar] [PubMed]
- Syahida, A.; Israf, D.A.; Lajis, N.H.; Khozirah, S.; Habsah, M.; Jasril; Permana, D.; Norhadiani, I. Effect of compounds isolated from natural products on IFN-c/LPS–induced nitric oxide production in RAW 264.7 macrophages. Pharm. Biol. 2006, 44, 50–59. [Google Scholar] [CrossRef]
- Sudsai, T.; Wattanapiromsakul, C.; Tewtrakul, S. Wound healing property of isolated compounds from Boesenbergia kingii rhizomes. J. Ethnopharmacol. 2016, 184, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Hoensch, H.P.; Oertel, R. The value of flavonoids for the human nutrition: Short review and perspectives. Clin. Nutr. Exp. 2015, 3, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Correa, L.B.; Padua, T.A.; Seito, L.N.; Costa, T.E.; Silva, M.A.; Candea, A.L.; Rosas, E.C.; Henriques, M.G. Anti-inflammatory effect of methyl gallate on experimental arthritis: Inhibition of neutrophil recruitment, production of inflammatory mediators, and activation of macrophages. J. Nat. Prod. 2016, 79, 1554–1566. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tang, H.; Zhang, Z.; Zhang, Y.; Qiu, C.; Zhang, L.; Huang, P.; Li, F. Kaempferol slows intervertebral disc degeneration by modifying LPS-induced osteogenesis/adipogenesis imbalance and inflammation response in BMSCs. Int. Immunopharmacol. 2017, 43, 236–242. [Google Scholar] [CrossRef]
- Boots, A.W.; Wilms, L.C.; Swennen, E.L.; Kleinjans, J.C.; Bast, A.; Haenen, G.R. In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers. Nutrition 2008, 24, 703–710. [Google Scholar] [CrossRef]
- Paul, A.T.; Gohil, V.M.; Bhutani, K.K. Modulating TNF-alpha signaling with natural products. Drug Discov. Today 2006, 11, 725–732. [Google Scholar] [CrossRef]
- Crespo, I.; Garcia-Mediavilla, M.V.; Gutierrez, B.; Sanchez-Campos, S.; Tunon, M.J.; Gonzalez-Gallego, J. A comparison of the effects of kaempferol and quercetin on cytokine-induced pro-inflammatory status of cultured human endothelial cells. Br. J. Nutr. 2008, 100, 968–976. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-C.; Chow, M.-P.; Huang, W.-C.; Lin, Y.-C.; Chang, Y.-J. Flavonoids inhibit tumor necrosis factor-α-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-κB: Structure-activity relationships. Mol. Pharmacol. 2004, 66, 683–693. [Google Scholar] [PubMed]
- De, P.; Baltas, M.; Bedos-Belval, F. Cinnamic acid derivatives as anticancer agents-a review. Curr. Med. Chem. 2011, 18, 1672–1703. [Google Scholar] [CrossRef]
- Song, F.; Li, H.; Sun, J.; Wang, S. Protective effects of cinnamic acid and cinnamic aldehyde on isoproterenol-induced acute myocardial ischemia in rats. J. Ethnopharmacol. 2013, 150, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.-T.; Wu, J.-H.; Huang, C.-Y.; Kuo, Y.-H.; Chang, S.-T. Antioxidant activities and phytochemical characteristics of extracts from Acacia confusa bark. Bioresour. Technol. 2009, 100, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Helfand, S.L.; Werkmeister, J.; Roder, J.C. Chemiluminescence response of human natural killer cells. I. The relationship between target cell binding, chemiluminescence, and cytolysis. J. Exp. Med. 1982, 156, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Grisham, M.B.; Johnson, G.G.; Lancaster, J.R., Jr. Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzym. 1996, 268, 237–246. [Google Scholar]
- Singh, U.; Tabibian, J.; Venugopal, S.K.; Devaraj, S.; Jialal, I. Development of an in vitro screening assay to test the antiinflammatory properties of dietary supplements and pharmacologic agents. Clin. Chem. 2005, 51, 2252–2256. [Google Scholar] [CrossRef]
- Froebel, K.S.; Pakker, N.G.; Aiuti, F.; Bofill, M.; Choremi-Papadopoulou, H.; Economidou, J.; Rabian, C.; Roos, M.T.L.; Ryder, L.P.; Miedema, F. Standardisation and quality assurance of lymphocyte proliferation assays for use in the assessment of immune function. J. Immunol. Meth. 1999, 227, 85–97. [Google Scholar] [CrossRef]
Sample Availability: Samples of all the compounds are available from the authors. |

| Code | ROS Inhibition (IC50 ± SD µg/mL) | Nitric Oxide (NO) Inhibition (IC50 ± SD µg/mL) | TNF-α Inhibition (IC50 ± SD µg/mL) | T-Cell Proliferation Inhibition (IC50 ± SD µg/mL) |
|---|---|---|---|---|
| EtOH: H2O | 16.7 ± 0.6 | 29.5 ± 1.7 | >100 | 13.8 ± 2.4 |
| DCM | 19.2 ± 1.8 | 14 ± 0.6 | 3.7 ± 0.3 | >100 |
| n-BuOH | 19.8 ± 3.5 | 27.2 ± 0.07 | 44.9 ± 8.9 | 8.1 ± 0.3 |
| EtOAc | 12 ± 0.6 | >50 | >100 | 43.3 ± 1.2 |
| Compound 1 | 79.5 ± 6.7 | NT | NT | NT |
| Compound 2 | 3.0 ± 0.2 | >50 | 12.6 ± 0.2 | >100 |
| Compound 3 | 2.5 ± 0.8 | 2.4 ± 0.3 | 5.5 ± 1.1 | 22.9 ± 1.5 |
| Compound 4 | >100 | NT | NT | NT |
| Ibuprofen | 11.2 ± 1.9 | - | - | - |
| L-NMMA | - | 24.2 ± 0.8 | - | - |
| Pentoxifillin | - | - | 94.8 ± 2.1 | - |
| Prednisolone | - | - | - | <0.62 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadallah, A.S.; Mujeeb-ur-Rehman; Atta-ur-Rahman; Yousuf, S.; Atia-tul-Wahab; Jabeen, A.; Swilam, M.M.; Khalifa, S.A.M.; El-Seedi, H.R.; Choudhary, M.I. Anti-Inflammatory Principles from Tamarix aphylla L.: A Bioassay-Guided Fractionation Study. Molecules 2020, 25, 2994. https://doi.org/10.3390/molecules25132994
Gadallah AS, Mujeeb-ur-Rehman, Atta-ur-Rahman, Yousuf S, Atia-tul-Wahab, Jabeen A, Swilam MM, Khalifa SAM, El-Seedi HR, Choudhary MI. Anti-Inflammatory Principles from Tamarix aphylla L.: A Bioassay-Guided Fractionation Study. Molecules. 2020; 25(13):2994. https://doi.org/10.3390/molecules25132994
Chicago/Turabian StyleGadallah, Adel S., Mujeeb-ur-Rehman, Atta-ur-Rahman, Sammer Yousuf, Atia-tul-Wahab, Almas Jabeen, Mahmoud M. Swilam, Shaden A. M. Khalifa, Hesham R. El-Seedi, and M. Iqbal Choudhary. 2020. "Anti-Inflammatory Principles from Tamarix aphylla L.: A Bioassay-Guided Fractionation Study" Molecules 25, no. 13: 2994. https://doi.org/10.3390/molecules25132994