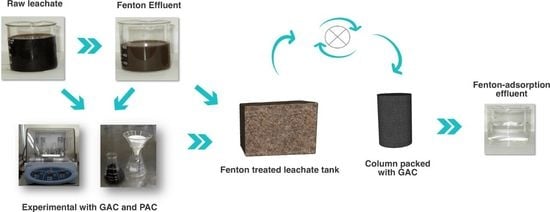
Selection of the Activated Carbon Type for the Treatment of Landfill Leachate by Fenton-Adsorption Process
Abstract
:1. Introduction
- X = mass of adsorbed solute (adsorbate) (mg),
- M = adsorbent weight (g),
- Ce = equilibrium concentration of adsorbate (mg/L),
- a = maximum number of moles adsorbed per mass of adsorbent and
- b = equilibrium constant of adsorbate in a solution after adsorption (L/mg).
- X = C0 − C,
- X/M = retained adsorbate (mg),
- C0 = initial concentration of adsorbate (mg/L),
- C = final concentration (mg/L),
- M = mass of adsorbent (g),
- k = empirical constant (y intercept of the linear equation) and
- n = constant (slope m of the linear equation).
- B = (RT)/bT,
- T = absolute temperature in Kelvin,
- R = gas constant, 8314 J/molK,
- Ce = equilibrium concentration of adsorbate (mg/L),
- bT = constant related to heat of adsorption and
- A = balance constant (L/min) corresponding to the maximum compulsory energy.
2. Results
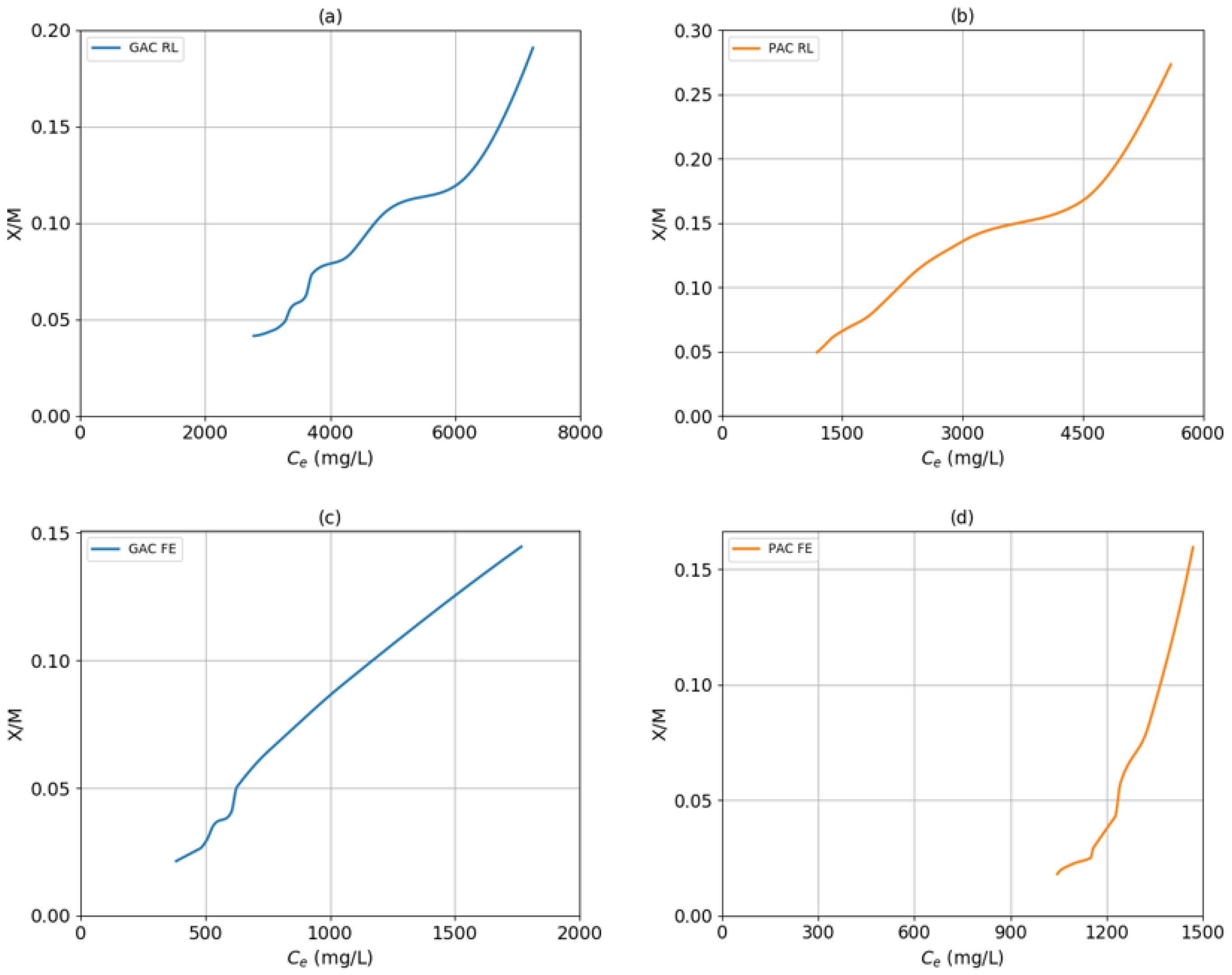
2.1. Adsorption Models with Activated Carbon
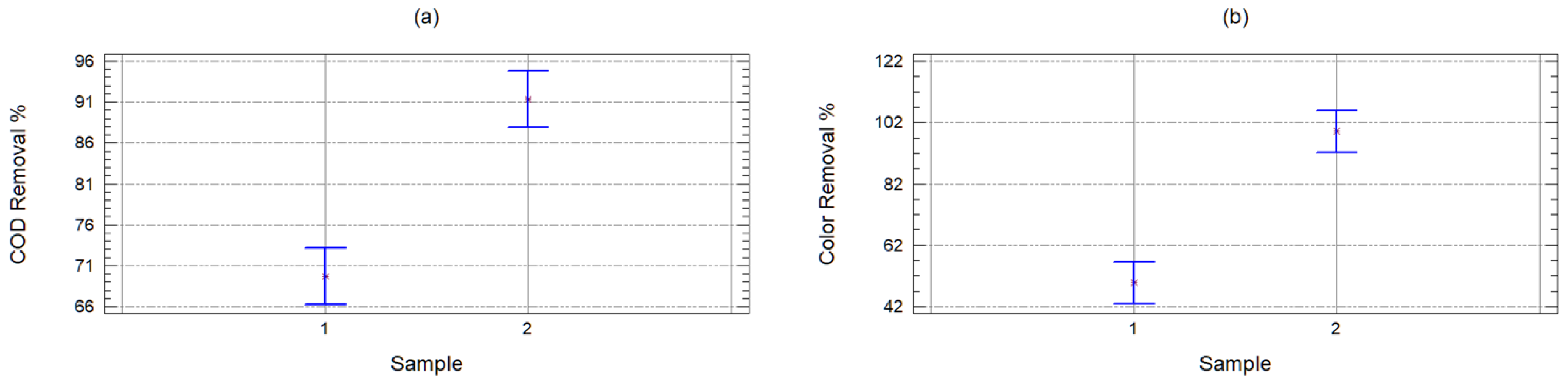
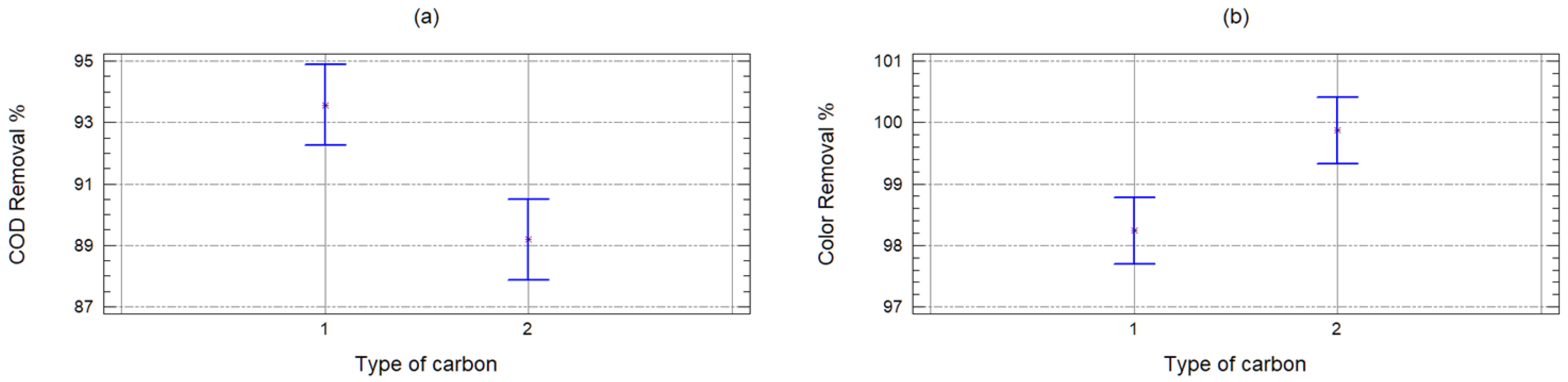
2.2. Organic Matter Removal Efficiency
2.3. Design of the Adsorption Column
3. Materials and Methods
3.1. Selection of Carbons
- Macroporous lignitic granular activated carbon (GAC). Gama L brand; raw material: lignite mineral (lignite); mesh number: 8 × 30 and surface area of 348.61 m2/g, relative density of 0.38 and cross-section of the adsorption area of 0.162 nm2.
- Powdered activated carbon (PAC) from mesoporous coconut shell. Micropol brand; raw material: coconut shell; mesh numbers: <50, <150 and <325 and holding capacity: between 0.2 and 1 kg of contaminants per kg of activated carbon.
3.2. Leachate Samples
Fenton Process
3.3. Adsorption Test
- In samples of 50 mL of leachate, amounts of 1 to 10 grams were added and stirred for one hour using stir plates.
- Subsequently, they were filtered with Whatman #40 filters to separate the carbon from the leachate, and the COD concentration and color were determined.
- yij = removal percentage (COD and color),
- μ = great mean of the response variable,
- αi = effect of GAC or PAC on the response variable,
- βj = covariable effect of the dose of coal on the response variable and
- εij = random error (due to variability in leachate composition and laboratory errors).
3.4. Determination of the Adsorption Model
- Carbon mass (M). Depending on the type of carbon used (GAC or PAC), the doses range from 1000 mg to 10,000 mg, varying from thousand to thousand.
- Final COD (Ce). For both samples (RL and FE), the COD was measured after treatment with carbon.
- Mass of the adsorbed solute (X). The ratio between the initial COD minus the final COD, divided by proportional volume.
- mg/mg ratio (X/M). Product of the mass of the adsorbed solute divided by the mass of the carbon used in the treatment.
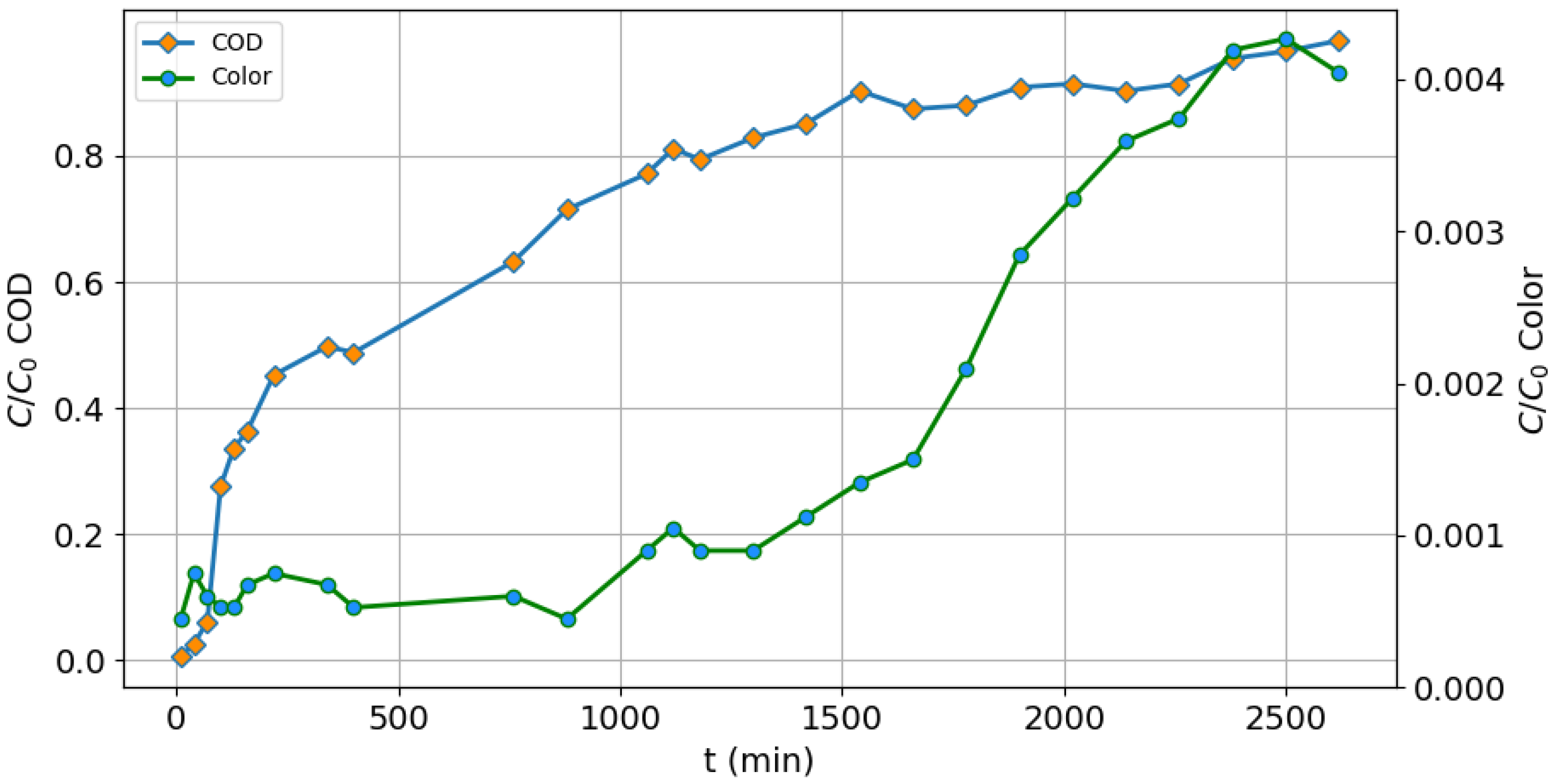
3.5. Packed Column Adsorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- San Pedro, L.; Méndez-Novelo, R.I.; Rojas-Valencia, M.N.; Barceló-Quintal, M.; Castillo-Borges, E.R.; Sauri-Riancho, M.R.; Marrufo-Gómez, J.M. Evaluation of adsorption and Fenton-adsorption processes for landfill leachate treatment. Rev. Mex. Ing. Química 2015, 14, 745–755. [Google Scholar]
- Abbas, A.; Jungsong, G.; Ping, L.; Ya, P.; Al-Rekabi, W. Review on landfill leachate treatments. J. Appl. Sci. Res. 2009, 5, 534–545. [Google Scholar] [CrossRef]
- Deng, Y.; Jung, C.; Zhao, R.; Torrens, K.; Wu, L. Adsorption of UV-quenching substances (UVQS) from landfill leachate with activated carbon. Chem. Eng. J. 2018, 350, 739–746. [Google Scholar] [CrossRef]
- Maron, S.; Prutton, C. Fundamentos de Fisicoquímica, 1st ed.; Limusa: México DF, Mexico, 2002; pp. 822–834. [Google Scholar]
- Ishak, A.R.; Hamid, F.S.; Mohamad, S.; Tay, K.S. Removal of organic matter from stabilized landfill leachate using Coagulation-Flocculation-Fenton coupled with activated charcoal adsorption. Waste Manag. Res. 2017, 35, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Maneerung, T.; Liew, J.; Dai, Y.; Kawi, S.; Chong, C.; Wang, C.H. Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: Kinetics, isotherms and thermodynamic studies. Bioresour. Technol. 2016, 200, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab. J. Chem. 2017, 10, S3381–S3393. [Google Scholar] [CrossRef] [Green Version]
- Pathania, D.; Sharma, S.; Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef] [Green Version]
- Xing, W.; Ngo, H.; Kim, H.; Guo, W.; Hagare, P. Physico-chemical processes for landfill leachate treatment: Experiments and mathematical models. Sep. Sci. Technol. 2008, 43, 347–361. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Wang, M.; Li, Z.; Gong, Y.; Zeng, E. In situ remediation of mercury-contaminated soil using thiol-functionalized graphene oxide/Fe-Mn composite. J. Hazard. Mater. 2019, 373, 783–790. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Y.; Yang, N.; Huang, B.; Kuang, Y.; Liao, Y. Adsorption behavior of isocyanate/ethylenediamine tetraacetic acid-functionalized graphene oxides for Cu2+ removal. Water Sci. Technol. 2018, 78, 2459–2468. [Google Scholar] [CrossRef]
- Wei, B.; Cheng, X.; Wang, G.; Li, H.; Song, X.; Dai, L. Graphene oxide adsorption enhanced by Attapulgite to remove Pb (II) from aqueous solution. Appl. Sci. 2019, 9, 1390. [Google Scholar] [CrossRef] [Green Version]
- Yanyan, L.; Kurniawan, T.; Albadarin, A.; Walker, G. Enhanced removal of acetaminophen from synthetic wastewater using multi-walled carbon nanotubes (MWCNTs) chemically modified with NaOH, HNO3/H2SO4, ozone, and/or chitosan. J. Mol. Liq. 2018, 251, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, S.; Igwegbe, C.; Rahdar, S.; Asadi, Z. The survey of application of the linear and nonlinear kinetic models for the adsorption of nickel (II) by modified multi-walled carbon nanotubes. Appl. Water Sci. 2019, 9, 98. [Google Scholar] [CrossRef] [Green Version]
- Tuzen, M. Development of tetraethylene pentamine functionalized multi-wall carbon nanotubes as a new adsorbent in a syringe system for removal of bisphenol A by using multivariate optimization techniques. Microchem. J. 2019, 147, 1147–1154. [Google Scholar]
- Daud, Z.; Awang, H.; Ibrahim, F.; Ab Aziz, N.; Ridzuan, M.; Ahmad, Z.; Abubakar, M.; Tajarudin, H. Chitosan ultilization in biocomposite adsorbent in Iron (Fe) removal from landfill leachate. Int. J. Integr. Eng. 2018, 10, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Aprianti, T.; Miskah, S.; Moeksin, R.; Sisnayati, S.; Nasir, S. Pb removal in pulp and paper industry leachate wastewater using activated carbon-ceramic composite adsorbent. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Palembang, South Sumatera, Indonesia, 2019; Volume 298, p. 012011. [Google Scholar]
- Reshadi, M.; Bazargan, A.; McKay, G. A review of the application of adsorbents for landfill leachate treatment: Focus on magnetic adsorption. Sci. Total Environ. 2020, 731, 138863. [Google Scholar] [CrossRef] [PubMed]
- Ben-Mansour, R.; Habib, M.A.; Bamidele, O.E.; Basha, M.; Qasem, N.A.A.; Peedikakkal, A.; Laoui, T.; Ali, M. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations–a review. Appl. Energy 2016, 161, 225–255. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef] [Green Version]
- Lima, É.C.; Adebayo, M.A.; Machado, F.M. Kinetic and equilibrium models of adsorption. In Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications, 1st ed.; Springer: Cham, Switzerland, 2005; pp. 33–69. [Google Scholar]
- Brenner, H. Adsorption Calculations and Modelling, 1st ed.; Elsevier: New York, NY, USA, 2013; pp. 16–17. [Google Scholar]
- Erdogan, F.O. Freundlich, Langmuir, Temkin and Harkins-Jura Isotherms Studies of H2 Adsorption on Porous Adsorbents. Chem. Chem. Technol. 2019, 13, 129–135. [Google Scholar] [CrossRef]
- Daud, Z.; Abubakar, M.H.; Kadir, A.A.; Latiff, A.A.A.; Awang, H.; Halim, A.A.; Marto, A. Adsorption studies of leachate on cockle shells. Int. J. Geomate 2017, 12, 46–52. [Google Scholar] [CrossRef]
- Osorio, P. Adsorción Mediante Cáscara de Huevo y de Camarón con un Lixiviado de Relleno Sanitario. Master’s Thesis, Universidad Autónoma Metropolitana, Mexico city, México, August 2010. [Google Scholar]
- Pedron, F.; Grifoni, M.; Barbafieri, M.; Petruzzelli, G.; Rosellini, I.; Franchi, E.; Bagatin, R.; Vocciante, M. Applicability of a Freundlich-like model for plant uptake at an industrial contaminated site with a high variable arsenic concentration. Environments 2017, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Balarak, D.; Mostafapour, F.K.; Azarpira, H.; Joghataei, A. Langmuir, Freundlich, Temkin and Dubinin–radushkevich isotherms studies of equilibrium sorption of ampicilin unto montmorillonite nanoparticles. J. Pharm. Res. Int. 2017, 20, 1–9. [Google Scholar] [CrossRef]
- Araújo, C.S.; Almeida, I.L.; Rezende, H.C.; Marcionilio, S.M.; Léon, J.J.; de Matos, T.N. Elucidation of mechanism involved in adsorption of Pb (II) onto lobeira fruit (Solanum lycocarpum) using Langmuir, Freundlich and Temkin isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- Chen, X. Modeling of experimental adsorption isotherm data. Information 2015, 6, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Kavci, E. Malachite green adsorption onto modified pinecone: Isotherms, kinetics and thermodynamics mechanism. Chem. Eng. Commun. 2020, 207, 1–10. [Google Scholar] [CrossRef]
- Andrade, P.R.D.; Lemus, M.R.; Pérez, C.C.E. Models of sorption isotherms for food: Uses and limitations. Vitae 2011, 18, 325–334. [Google Scholar]
- Corrêa, P.C.; Oliveira, G.H.H.; de Oliveira, A.P.L.; Goneli, A.L.D.; Botelho, F.M. Isotermas de dessorção de sementes de beterraba. Rev. Eng. Agric. Reveng 2016, 24, 15–21. [Google Scholar]
- Katal, R.; Baei, M.S.; Rahmati, H.T.; Esfandian, H. Kinetic, isotherm and thermodynamic study of nitrate adsorption from aqueous solution using modified rice husk. J. Ind. Eng. Chem. 2012, 18, 295–302. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Lee, S.; Hur, J. Heterogeneous adsorption behavior of landfill leachate on granular activated carbon revealed by fluorescence excitation emission matrix (EEM)-parallel factor analysis (PARAFAC). Chemosphere 2016, 149, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-pajooh, E.; Turcios, A.E.; Cuff, G.; Weichgrebe, D.; Rosenwinkel, K.H.; Vedenyapina, M.D.; Sharifullina, L.R. Removal of inert COD and trace metals from stabilized landfill leachate by granular activated carbon (GAC) adsorption. J. Environ. Manag. 2018, 228, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Kulikowska, D.; Bernat, K.; Parszuto, K.; Sułek, P. Efficiency and kinetics of organics removal from landfill leachate by adsorption onto powdered and granular activated carbon. Desalin. Water Treat. 2016, 57, 4458–4468. [Google Scholar] [CrossRef]
- Onn, S.; Bashir, M.; Sethupathi, S.; Amr, S.; Nguyen, T. Colour and COD removal from mature landfill leachate using electro-persulphate oxidation process. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Sruthi, T.; Gandhimathi, R.; Ramesh, S.; Nidheesh, P. Stabilized landfill leachate treatment using heterogeneous Fenton and electro-Fenton processes. Chemosphere 2018, 210, 38–43. [Google Scholar] [CrossRef]
- Mohajeri, S.; Hamidi, A.; Isa, M.; Zahed, M. Landfill leachate treatment through electro-Fenton oxidation. Pollution 2019, 5, 199–209. [Google Scholar]
- Asaithambi, P.; Govindarajan, R.; Yesuf, M.; Alemayehu, E. Removal of color, COD and determination of power consumption from landfill leachate wastewater using an electrochemical advanced oxidation processes. Sep. Purif. Technol. 2020, 233, 115935. [Google Scholar] [CrossRef]
- Li, R.; Wang, B.; Owete, O.; Dertien, J.; Lin, C.; Ahmad, H.; Chen, G. Landfill Leachate Treatment by Electrocoagulation and Fiber Filtration: Li et al. Water Environ. Res. 2017, 89, 2015–2020. [Google Scholar] [CrossRef]
- Smaoui, Y.; Mseddi, S.; Ayadi, N.; Sayadi, S.; Bouzid, J. Evaluation of influence of coagulation/flocculation and Fenton oxidation with iron on landfill leachate treatment. Environ. Prot. Eng. 2019, 45, 139–153. [Google Scholar] [CrossRef]
- Dia, O.; Drogui, P.; Buelna, G.; Dubé, R. Hybrid process, electrocoagulation-biofiltration for landfill leachate treatment. Waste Manag. 2018, 75, 391–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosli, M.; Daud, Z.; Awang, H.; Ab Aziz, N.; Ridzuan, M.; Abubakar, M.; Adnan, M.; Tajarudin, H. Adsorption efficiency and isotherms of COD and color using limestone and zeolite adsorbents. Int. J. Integr. Eng. 2018, 10, 8–13. [Google Scholar] [CrossRef]
- Daud, Z.; Rahman, S.; Awang, H.; Abubakar, M.; Ridzuan, M.; Tajarudin, H. Utilization of Waste Paper Sludge as an Alternative Adsorbent for the Adsorption of Ammonia Nitrogen and COD in Stabilized Landfill Leachate. Int. J. Integr. Eng. 2018, 10, 6–10. [Google Scholar] [CrossRef]
- Rosli, M.; Daud, Z.; Ridzuan, M.; Abd Aziz, N.; Awang, H.; Oyekanmi Adeleke, A. Equilibrium isotherm and kinetic study of the adsorption of organic pollutants of leachate by using micro peat-activated carbon composite media. Desalin. Water Treat. 2019, 160, 185–192. [Google Scholar] [CrossRef] [Green Version]
- American Public Health Association, American Water Works Association, Water Environment Federation (APHA). Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
Sample Availability: Not available. |


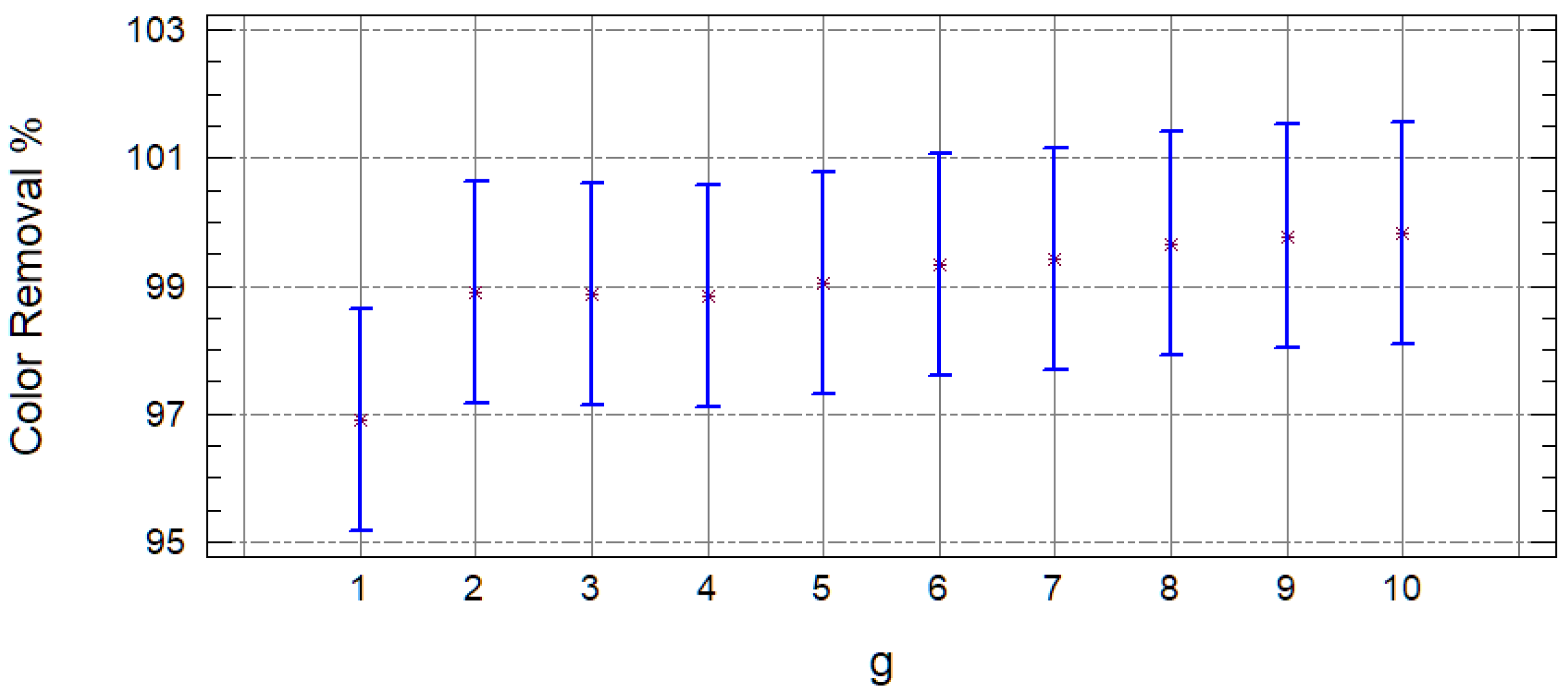
| Sample | COD (mg/L) | S.D. | % Removal | Color (Platinum Unit Pt-Co) | S.D. | % Removal |
|---|---|---|---|---|---|---|
| M1C1d1 | 7247.5 | 110 | 34.49 | 13,150 | 778 | 4.02 |
| M1C1d2 | 6147.5 | 343 | 44.41 | 12,950 | 212 | 5.37 |
| M1C1d3 | 4827.5 | 265 | 56.35 | 11,300 | 566 | 17.27 |
| M1C1d4 | 4330 | 198 | 60.85 | 11,200 | 566 | 18.00 |
| M1C1d5 | 3710 | 396 | 66.44 | 10,200 | 707 | 25.57 |
| M1C1d6 | 3610 | 304 | 67.35 | 10,850 | 495 | 20.57 |
| M1C1d7 | 3355 | 7 | 69.67 | 11,550 | 1626 | 15.22 |
| M1C1d8 | 3272.5 | 74 | 70.41 | 11,650 | 1909 | 14.43 |
| M1C1d9 | 3087.5 | 95 | 72.09 | 13,850 | 5162 | -2.33 |
| M1C1d10 | 2777.5 | 53 | 74.89 | 13,450 | 5869 | 0.45 |
| M1C2d1 | 5597.5 | 329 | 49.41 | 6400 | 283 | 53.15 |
| M1C2d2 | 4450 | 870 | 59.82 | 4000 | 283 | 70.70 |
| M1C2d3 | 2975 | 417 | 73.13 | 2435 | 658 | 82.33 |
| M1C2d4 | 2352.5 | 124 | 78.74 | 745 | 92 | 94.57 |
| M1C2d5 | 2047.5 | 95 | 81.50 | 1125 | 601 | 91.65 |
| M1C2d6 | 1817.5 | 18 | 83.57 | 711.5 | 408 | 94.71 |
| M1C2d7 | 1555 | 49 | 85.95 | 543.5 | 250 | 95.97 |
| M1C2d8 | 1370 | 0 | 87.62 | 310.5 | 23 | 97.73 |
| M1C2d9 | 1272.5 | 53 | 88.49 | 337.5 | 110 | 97.51 |
| M1C2d10 | 1185 | 49 | 89.29 | 238 | 130 | 98.23 |
| M2C1d1 | 1765.5 | 162 | 84.05 | 805 | 106 | 94.09 |
| M2C1d2 | 1047 | 52 | 90.53 | 278 | 115 | 97.99 |
| M2C1d3 | 758.5 | 40 | 93.14 | 286.5 | 13 | 97.91 |
| M2C1d4 | 626.5 | 16 | 94.34 | 299.5 | 46 | 97.80 |
| M2C1d5 | 604.5 | 8 | 94.54 | 251 | 1 | 98.16 |
| M2C1d6 | 527.5 | 4 | 95.23 | 164.5 | 28 | 98.79 |
| M2C1d7 | 506.5 | 2 | 95.42 | 147 | 115 | 98.90 |
| M2C1d8 | 477 | 4 | 95.69 | 76 | 18 | 99.44 |
| M2C1d9 | 425 | 42 | 96.16 | 51.5 | 25 | 99.62 |
| M2C1d10 | 383.5 | 54 | 96.53 | 41.5 | 16 | 99.69 |
| M2C2d1 | 1468.5 | 5 | 86.72 | 39 | 20 | 99.71 |
| M2C2d2 | 1332 | 103 | 87.96 | 27.5 | 4 | 99.80 |
| M2C2d3 | 1241.5 | 19 | 88.78 | 23.5 | 4 | 99.83 |
| M2C2d4 | 1226 | 20 | 88.92 | 17.5 | 6 | 99.87 |
| M2C2d5 | 1184.5 | 21 | 89.29 | 11 | 1 | 99.92 |
| M2C2d6 | 1157 | 38 | 89.54 | 16.5 | 8 | 99.88 |
| M2C2d7 | 1150 | 42 | 89.60 | 10 | 1 | 99.93 |
| M2C2d8 | 1091.5 | 80 | 90.13 | 16 | 13 | 99.88 |
| M2C2d9 | 1058.5 | 111 | 90.43 | 9 | 6 | 99.93 |
| M2C2d10 | 1045.5 | 121 | 90.54 | 4 | 0 | 99.97 |
| Sample | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| a (mg/g) | b (mg/L) | R2 | K (mg/g) | n | R2 | A | B | R2 | |
| GAC RL | 0.1339 | 8.55 × 10−5 | 0.9574 | 15.603 | 0.6392 | 0.9727 | 9.7786 | 231.67 | 0.9834 |
| PAC RL | 0.6859 | 5.88 × 10−5 | 0.9920 | 11.076 | 0.8737 | 0.9934 | 10.9851 | 131.56 | 0.9526 |
| GAC FE | 0.1074 | 4.39 × 10−4 | 0.9575 | 11.74 | 0.7508 | 0.9672 | 10.9272 | 44.135 | 0.9523 |
| PAC FE | 0.0079 | 6.75 × 10−4 | 0.9537 | 49.532 | 0.1531 | 0.9769 | 9.8878 | 61.686 | 0.9969 |
| Source | Sum of Squares | Degrees of Freedom | Half-Square | F Coefficient | p-Value |
|---|---|---|---|---|---|
| Sample | 4688.74 | 1 | 4688.74 | 61.36 | 0 |
| Carbon | 341.348 | 1 | 341.348 | 4.47 | 0.0436 |
| Quantity | 2196.44 | 9 | 244.049 | 3.19 | 0.0088 |
| Waste | 2139.5 | 28 | 76.4109 | ||
| Corrected total | 9366.03 | 39 |
| Source | Sum of Squares | Degrees of Freedom | Half-Square | F Coefficient | p-Value |
|---|---|---|---|---|---|
| Sample | 24,304.4 | 1 | 24,304.4 | 43.62 | 0 |
| Carbon | 14,988.9 | 1 | 14,988.9 | 26.9 | 0 |
| Quantity | 958.521 | 9 | 106.502 | 0.19 | 0.9933 |
| Waste | 15,599.8 | 28 | 557.136 | ||
| Corrected total | 55,851.6 | 39 |
| Parameter | Quantity |
|---|---|
| COD affluent (mg/L) | 1750 |
| COD effluent to 10 min (mg/L) | 11 |
| % removal of COD | 99.37 |
| Color affluent (U Pt-Co) | 13,356 |
| Color effluent (U Pt-Co) | 6 |
| % de removal de color | 99.96 |
| kg CODREMOVED/kg of carbon | 21.68 |
| Liters of leachate/kg of carbon | 62.36 |
| Treatment | COD Removal % | Color Removal % | Reference |
|---|---|---|---|
| Electro-persulfate oxidation process | 45.7 | 97.3 | [39] |
| Heterogeneous Fenton | 88.6 | - | [40] |
| Electro-Fenton | 93 | 92 | [41] |
| Photo-electro-Fenton process | 97 | 100 | [42] |
| Electrocoagulation | 94 | - | [43] |
| Coagulation/flocculation and Fenton combined treatment | 62 | - | [44] |
| Electrocoagulation and biofiltration | 63 | - | [45] |
| Adsorption with limestone and zeolite | 55 | 76 | [46] |
| Adsorption with wastepaper sludge and activated carbon | 85.9 | - | [47] |
| Micro-peat and activated carbon composite | 87 | 74 | [48] |
| Adsorption with granular activated carbon | 89 | 92 | [1] |
| Fenton-adsorption process with granular activated carbon | 99.3 | 99.9 | This study |
| Test | Mass Carbon (mg) M | Final COD (mg/L) Ce | Mass of Adsorbed Solute (mg) X | Ratio (mg/mg) X/M |
|---|---|---|---|---|
| 1 | 1000 | Ce1 | X1 | X1/M1 |
| 2 | 2000 | Ce2 | X2 | X2/M2 |
| 3 | 3000 | Ce3 | X3 | X3/M3 |
| 4 | 4000 | Ce4 | X4 | X4/M4 |
| 5 | 5000 | Ce5 | X5 | X5/M5 |
| 6 | 6000 | Ce6 | X6 | X6/M6 |
| 7 | 7000 | Ce7 | X7 | X7/M7 |
| 8 | 8000 | Ce8 | X8 | X8/M8 |
| 9 | 9000 | Ce9 | X9 | X9/M9 |
| 10 | 10,000 | Ce10 | X10 | X10/M10 |
| Characteristic | Value |
|---|---|
| Adsorbent mass (GAC) | 7144 g |
| Column height | 60 cm |
| Column diameter | 20 cm |
| Flow | 170 mL/min |
| Initial COD | 1750 mg/L |
| Initial color | 13,356 U Pt-Co |
| Volume of empty spaces | 7650 mL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
San-Pedro, L.; Méndez-Novelo, R.; Hernández-Núñez, E.; Flota-Bañuelos, M.; Medina, J.; Giacomán-Vallejos, G. Selection of the Activated Carbon Type for the Treatment of Landfill Leachate by Fenton-Adsorption Process. Molecules 2020, 25, 3023. https://doi.org/10.3390/molecules25133023
San-Pedro L, Méndez-Novelo R, Hernández-Núñez E, Flota-Bañuelos M, Medina J, Giacomán-Vallejos G. Selection of the Activated Carbon Type for the Treatment of Landfill Leachate by Fenton-Adsorption Process. Molecules. 2020; 25(13):3023. https://doi.org/10.3390/molecules25133023
Chicago/Turabian StyleSan-Pedro, Liliana, Roger Méndez-Novelo, Emanuel Hernández-Núñez, Manuel Flota-Bañuelos, Jorge Medina, and Germán Giacomán-Vallejos. 2020. "Selection of the Activated Carbon Type for the Treatment of Landfill Leachate by Fenton-Adsorption Process" Molecules 25, no. 13: 3023. https://doi.org/10.3390/molecules25133023
APA StyleSan-Pedro, L., Méndez-Novelo, R., Hernández-Núñez, E., Flota-Bañuelos, M., Medina, J., & Giacomán-Vallejos, G. (2020). Selection of the Activated Carbon Type for the Treatment of Landfill Leachate by Fenton-Adsorption Process. Molecules, 25(13), 3023. https://doi.org/10.3390/molecules25133023