Neopapillarine, an Unusual Coumarino-Alkaloid from the Root Extract of Neocryptodiscus papillaris with Cytotoxic Activity on Renal Cancer Cells
Abstract
:1. Introduction
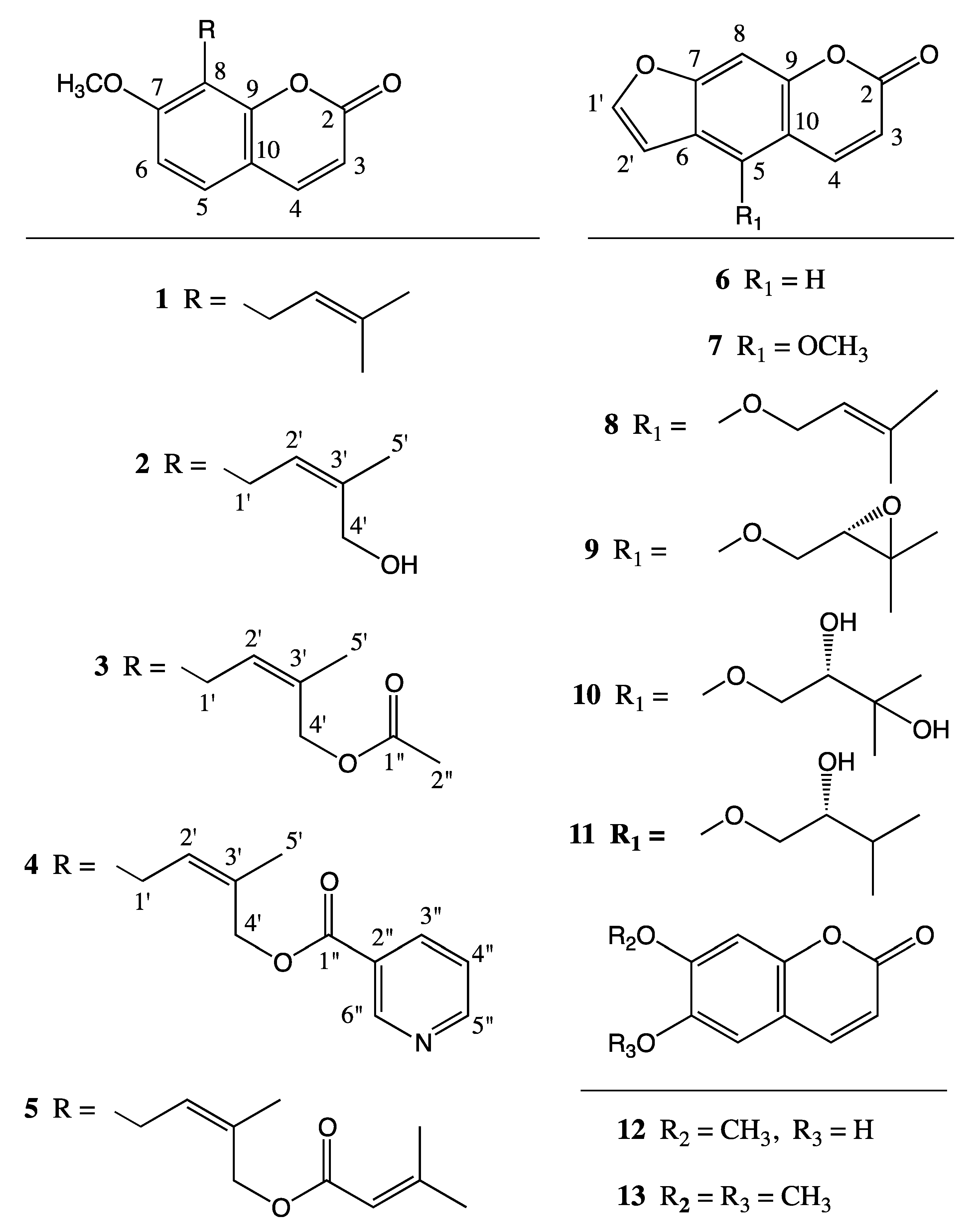
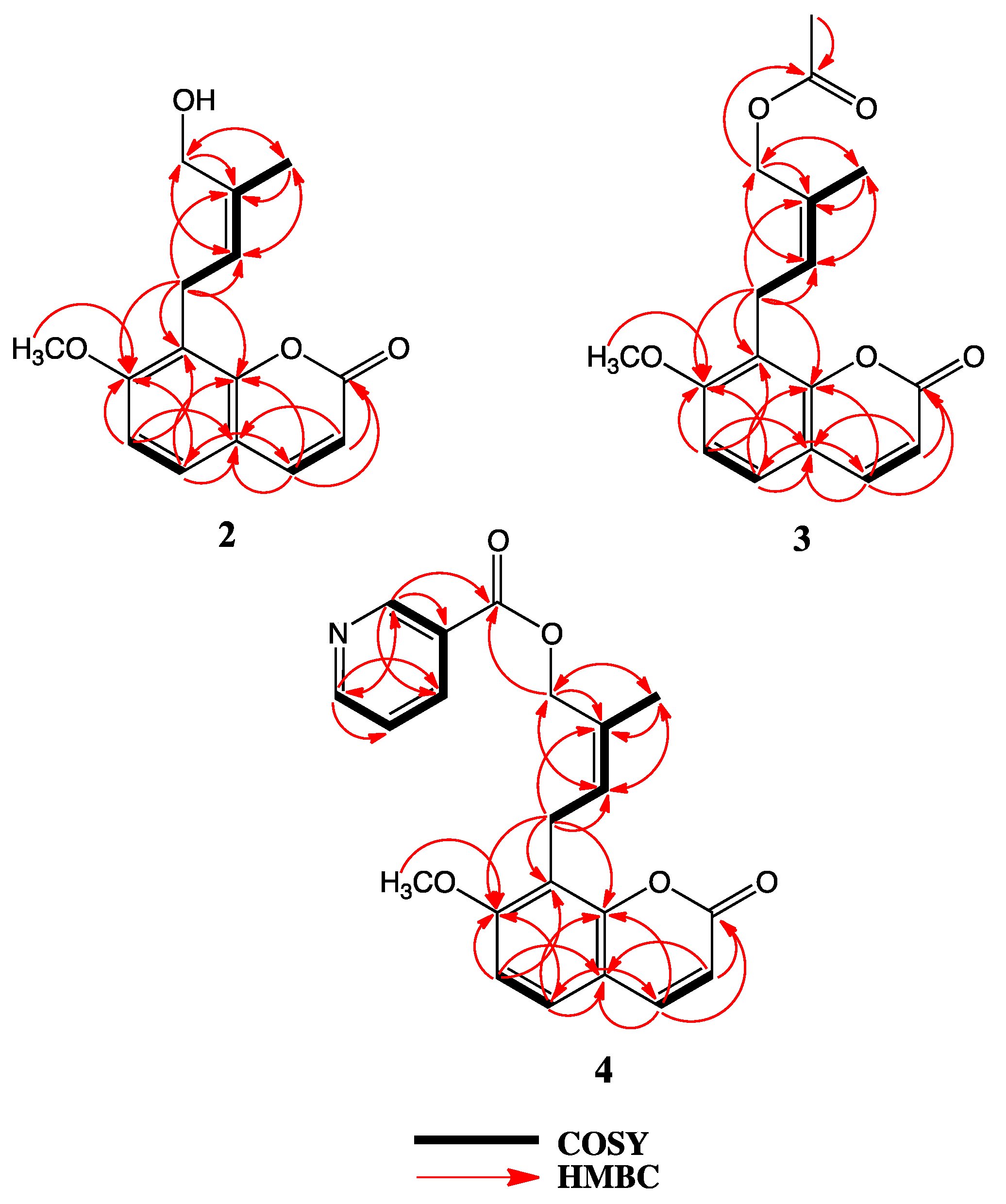
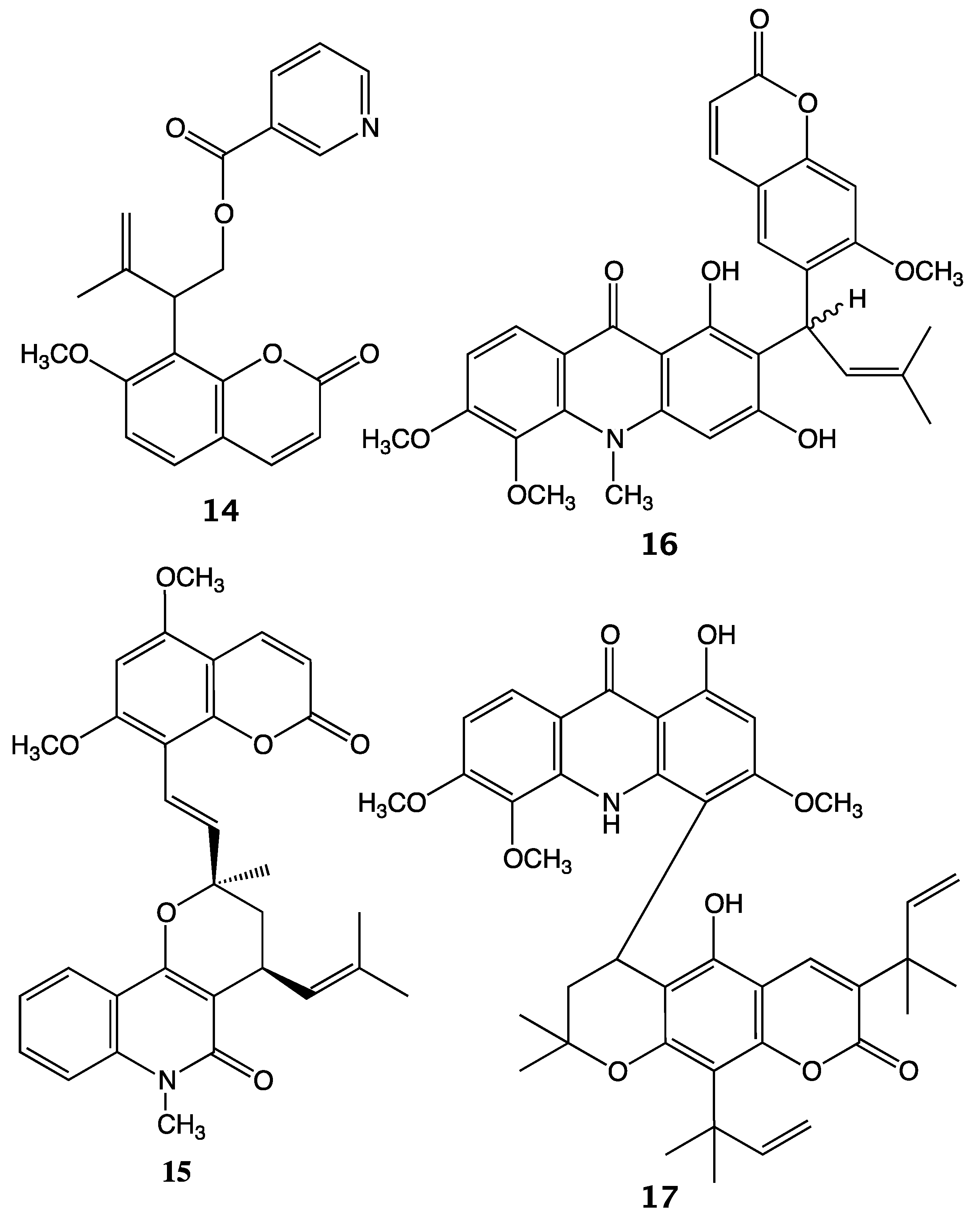
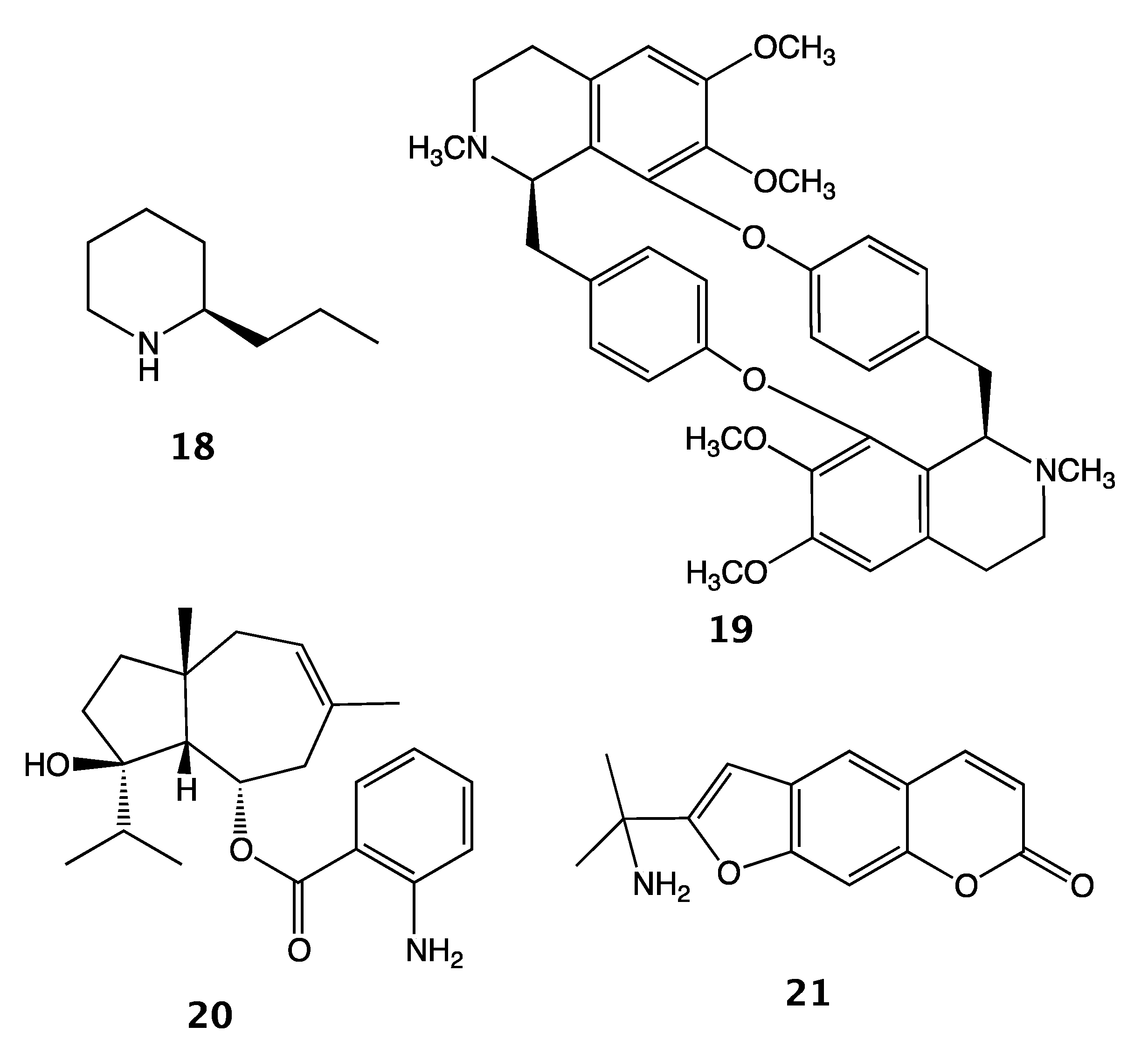
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Cytotoxicity Assay on Renal Cancer Cell Lines
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- A.A.; Yerli, S.V. Biodiversity in Turkey. In Global Biodiversity; Pullaiah, T., Ed.; Apple Academic Press, Inc.: Oakville, ON, Canada, 2019; Volume 2, pp. 397–442. [Google Scholar]
- Tosun, F.; Beutler, J.A.; Ransom, T.T.; Miski, M. Anatolicin, a highly potent and selective cytotoxic sesquiterpene coumarin, from the root extract of Heptaptera anatolica. Molecules 2019, 24, 01153. [Google Scholar] [CrossRef] [Green Version]
- Hedge, I.C.; Lamond, J.M. Neocryptodiscus . In Flora Iranica; Rechinger, K.H., Ed.; Akademische Druck-u. Verlagsanstalt: Graz, Austria, 1987; Volume 162, pp. 207–209. [Google Scholar]
- The Plant List. Available online: http://www.theplantlist.org/tpl1.1/search?q=Neocryptodiscus (accessed on 26 April 2020).
- Herrnstadt, I.; Heyn, C.C. Neocryptodiscus papillaris (Boiss.) Herrnst. & Heyn–A new combination based on Cachrys papillaris Boiss. Candollea 1997, 52, 181–184. [Google Scholar]
- Tosun, F.; Mıhoğlugil, F.; Miski, M. Cytotoxic activity of the root and fruit extracts of Neocryptodiscus papillaris (Boiss.) Herrnst. & Heyn. In Full Text Proceedings Book of I. International Congress on Medicinal and Aromatic Plants “Natural and Healthy Life”; Şeker, M., Ed.; Necmettin Erbakan Üniversitesi Kültür Yayınları: Konya, Turkey, 2018; pp. 667–670. [Google Scholar]
- Farozi, A.; Shah, S.A.; Banday, J.A. Structural and optical studies of 7-methoxy-8-(3-methylbut-2-enyl)-2-chromenone (osthol), a plant based coumarin. Optik 2016, 127, 2802–2805. [Google Scholar] [CrossRef]
- Tan, N.; Yazıcı-Tütüniş, S.; Bilgin, M.; Tan, E.; Miski, M. Antibacterial activities of prenylated coumarins from the roots of Prangos hulusii. Molecules 2017, 22, 1098–1105. [Google Scholar] [CrossRef]
- Masuda, T.; Takasugi, M.; Anetai, M. Psoralen and other linear furanocoumarins as phytoalexins in Glehnia littoralis. Phytochemistry 1997, 47, 13–16. [Google Scholar] [CrossRef]
- Thanh, P.N.; Jin, W.Y.; Song, G.Y.; Bae, K.H.; Kang, S.S. Cytotoxic coumarins from the root of Angelica dahurica. Arch. Pharm. Res. 2004, 27, 1211–1215. [Google Scholar] [CrossRef]
- Tesso, H.; König, W.A.; Kubeczka, K.-H.; Bartnik, M.; Glowniak, K. Secondary metabolites of Peucedanum tauricum fruits. Phytochemistry 2005, 66, 707–713. [Google Scholar] [CrossRef]
- Kuznetsova, G.A.; Abyshev, A.Z.; Perel’son, M.E.; Sheinker, Y.N.; Pek, G.Y. A new coumarin, pranferol, from the roots of Prangos ferulacea. Khim. Prir. Soedin. 1966, 9, 310–315. [Google Scholar]
- Intekhab, J.; Aslam, M. Constituents from Feronia limonia. Analele Universitate din Bucureşti Chimie 2009, 18, 95–101. [Google Scholar]
- Kostova, I. Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem. Anti Cancer Agents 2005, 5, 29–46. [Google Scholar] [CrossRef]
- Majnooni, M.B.; Fakhri, S.; Smeriglio, A.; Trombetta, D.; Croley, C.R.; Bhattacharyya, P.; Sobarzo-Sánchez, E.; Farzaei, M.H.; Bishayee, A. Antiangiogenic effects of coumarins against cancer: From chemistry to medicine. Molecules 2019, 24, 4278–4296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penta, S. (Ed.) Advances in Structure and Activity Relationship of Coumarin Derivatives; Academic Press-Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Hassan, M.Z.; Osman, H.; Ali, M.A.; Ahsan, M.J. Therapeutic potential of coumarins as antiviral agents. European J. Med. Chem 2016, 123, 236–255. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Pandey, A.; Manvati, S. Coumarin: An emerging antiviral agent. Heliyon 2020, 6, e03217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Hu, Y.; Shen, Y.-F.; Wang, G.-X. Evaluation on antiviral activity of coumarin derivatives against viraemia of carp virus in epithelioma cyprinid cells. Antiviral Res. 2017, 144, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Šarkanj, B.; Molnar, M.; Čačić, M.; Gille, L. 4-Methyl-7-hydroxycoumarin antifungal and antioxidant activity enhancement by substitution with thiosemicarbazide and thiazolidinone moieties. Food Chem. 2013, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Čačić, M.; Pavic, V.; Molnar, M.; Šarkanj, B.; Has-schön, E. Design and synthesis of some new 1,3,4-thiadiazines with coumarin moieties and their antioxidative and antifungal activity. Molecules 2014, 19, 1163–1177. [Google Scholar] [CrossRef] [Green Version]
- Widelski, J.; Popova, M.; Graikou, K.; Glowniak, K.; Chinou, I. Coumarins from Angelica lucida L-antibacterial activities. Molecules 2009, 14, 2729–2734. [Google Scholar] [CrossRef] [Green Version]
- Song, H.Y.; Jo, A.; Shin, J.; Lim, E.H.; Lee, Y.E.; Jeong, D.E.; Lee, M. Anti-inflammatory activites of isogosferol, a furanocoumarin isolated from Citrus junos seed shells through bioactivity-guided fractionation. Molecules 2019, 4088–4103. [Google Scholar] [CrossRef] [Green Version]
- Coppock, R.W.; Christian, R.G.; Jacobsen, B.J. Aflatoxins. In Veterinary Toxicology, Basic and Clinical Principles, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Oxford, UK, 2018; pp. 983–994. [Google Scholar]
- Lv, X.; Hou, W.J.; Zhang, B.-J.; Deng, S.; Tian, Y.; Huang, S.-S.; Zhang, H.-L.; Shu, X.-H.; Zhen, Y.-H.; Liu, K.-X.; et al. Isolation and identification of metabolites of osthole in rats. Xenobiotica 2012, 42, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Flekhter, O.B.; Medvedeva, N.I.; Karachurina, L.T.; Baltina, L.A.; Galin, F.Z.; Zarudii, F.S.; Tolstikov, G.A. Search for New Drugs-Synthesis and pharmacological activity of betulin, betulinic acid, and allobetulin esters. Pharm. Chem. J. 2005, 39, 401–404. [Google Scholar] [CrossRef]
- The tube models of compounds 2, 3 and 4 were generated using Spartan’18 Parallel Suite for Macintosh Molecular Modeling Software Package ver. 1.4.5 . Wavefunction, Inc.: Irvine, CA, USA, https://www.wavefun.com/spartan.
- Ito, C.; Furukawa, H. Three new coumarins from leaves of Murraya paniculata. Heterocycles 1987, 26, 2959–2962. [Google Scholar]
- Ishii, H.; Kobayashi, J.; Ishikawa, T. Toddacoumalone, a novel mixed dimer of coumarin and quinolone from Toddalia asiatica (L.) Lam. (T. aculeata Pers.). Tetrahedron Lett. 1991, 32, 6907–6910. [Google Scholar] [CrossRef]
- Takemura, Y.; Inoue, M.; Kawaguchi, H.; Ju-ichi, M.; Ito, C.; Furukawa, H.; Omura, M. New acrimarines from Citrus plants. Heterocycles 1992, 34, 2363–2372. [Google Scholar]
- Furukawa, H.; Ito, C.; Mizuno, T.; Ju-ichi, M.; Inoue, M.; Kajiura, I.; Omura, M. Spectrometric elucidation of acrimarines, the first naturally occurring acridone-coumarin dimers. J. Chem. Perkin Trans. I 1990, 1593–1599. [Google Scholar] [CrossRef]
- Takemura, Y.; Maki, S.; Ju-ichi, M.; Omura, M.; Ito, C.; Furukawa, H. Two novel acridone-coumarin dimers, Neoacrimarines-A and -B, from Citrus plants. Heterocycles 1993, 36, 675–680. [Google Scholar]
- Takemura, Y.; Kurozumi, T.; Ju-ichi, M.; Okano, M.; Fukamiya, N.; Ito, C.; Ono, T.; Furukawa, H. The structures of Neoacrimarines-C and -D, two new acridone-coumarin dimers from Citrus hassaku. Chem. Pharm. Bull. 1993, 41, 1757–1759. [Google Scholar] [CrossRef] [Green Version]
- Buckingham, J.; Baggaley, K.H.; Roberts, A.D.; Szabó, L.F. Dictionary of Alkaloids, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Gupta, B.D.; Banerjee, S.K.; Handa, K.L. Alkaloids and coumarins of Heracleum wallichii. Phytochemistry 1976, 15, 576. [Google Scholar] [CrossRef]
- Alkhatib, R.; Hennebelle, T.; Joha, S.; Idziorek, T.; Preudhomme, C.; Quesnel, B.; Sahpaz, S.; Bailleul, F. Activity of elaeochytrin A from Ferula elaeochytris on leukemia cell lines. Phytochemistry 2008, 69, 2979–2983. [Google Scholar] [CrossRef]
- Mukhamedova, K.S.; Akramov, S.T.; Yunusov, S.Y. Structure of prangosine. Khim. Prir. Soedin. 1967, 3, 117–121. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. JNCI J. National Cancer Ins. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| Compound 2 | Compound 3 | Compound 4 | ||||
|---|---|---|---|---|---|---|
| Pos. | δH (in ppm, m, J in Hz) | δC (in ppm, type) | δH (in ppm, m, J in Hz) | δC (in ppm, type) | δH (in ppm, m, J in Hz) | δC (in ppm, type) |
| 2 | 162.09; C | 161.24; C | 161.21; C | |||
| 3 | 6.25; d; 9.5; 1H | 112.96; CH | 6.23; d; 9.4; 1H | 113.24, CH | 6.23; d; 9.5; 1H | 127.70; CH |
| 4 | 7.65; d; 9.5; 1H | 144.32; CH | 7.61; d; 9.4; 1H | 143.81; CH | 7.61; d; 9.5; 1H | 143.82; CH |
| 5 | 7.33; d; 8.5; 1H | 126.76; CH | 7.31; d; 8.6; 1H | 126.73; CH | 7.31; d; (8.6); 1H | 126.82; CH |
| 6 | 6.86; d; 8.5; 1H | 107.80; CH | 6.82; d; 8.6; 1H | 107.46; CH | 6.83; d; 8.6; 1H | 107.49; CH |
| 7 | 160.01; C | 160.19; C | 160.19; C | |||
| 8 | 116.70; C | 116.68; C | 116.56; C | |||
| 9 | 153.39; C | 153.03; C | 153.01; C | |||
| 10 | 113.50; C | 113.11; C | 113.15; C | |||
| OCH3 | 3.94; s; 3H | 56.36; CH3 | 3.91; s; 3H | 56.18; CH3 | 3.90; s; 3H | 56.24; CH3 |
| 1′ | 3.59; br dd; 0.6, 7.9; 2H | 21.63; CH2 | 3.61; br d; 7.7; 2H | 21.73; CH2 | 3.68; br d; 7.7; 2H | 21.65; CH2 |
| 2′ | 5.22; br t; 7.9; 1H | 123.14; CH | 5.51; br t; 7.7; 1H | 126.98; CH | 5.60; br t; 7.7; 1H | 113.30; CH |
| 3′ | 136.51; C | 130.98; C | 130.54; C | |||
| 4′ | 4.41; d; 0.6; 2H | 61.21; CH2 | 4.85; br s; 2H | 63.46; CH2 | 5.16; br s; 2H | 64.53; CH2 |
| 5′ | 1.80; br d; 1.4; 3H | 21.59; CH3 | 1.72; br d; 0.9; 3H | 21.52; CH3 | 1.81; br d; 1.0; 3H | 21.85; CH3 |
| 1′′ | 171.37; C | 165.33; C | ||||
| 2′′ | 2.09; s; 3H | 21.14; CH3 | 126.82; C | |||
| 3′′ | 8.35; d t; 1.9, 7.8; 1H | 137.67; CH | ||||
| 4′′ | 7.42; br dd; 4.8, 7.8; 1H | 123.63; CH | ||||
| 5′′ | 8.78; br d; 3.5; 1H | 153.07; CH | ||||
| 6′′ | 9.24; br s; 1H | 150.71; CH | ||||
| Compounds | 1 | 2 | 3 | 4 | 5 | 8 | 9 |
|---|---|---|---|---|---|---|---|
| A498 | >1000 | >1000 | >1000 | 67 | >100 | >1000 | 720 |
| UO31 | >1000 | ≥1000 | >1000 | 20 | 20 | >1000 | 630 |
| COLO205 | 20 | NT * | NT | NT | NT | 14 | 30 |
| KM12 | >100 | NT | NT | NT | NT | >100 | 31 |
| MCF7 | 24 | NT | NT | NT | NT | >100 | >100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tosun, F.; Mıhoğlugil, F.; Beutler, J.A.A.; Eroğlu Özkan, E.; Miski, M. Neopapillarine, an Unusual Coumarino-Alkaloid from the Root Extract of Neocryptodiscus papillaris with Cytotoxic Activity on Renal Cancer Cells. Molecules 2020, 25, 3040. https://doi.org/10.3390/molecules25133040
Tosun F, Mıhoğlugil F, Beutler JAA, Eroğlu Özkan E, Miski M. Neopapillarine, an Unusual Coumarino-Alkaloid from the Root Extract of Neocryptodiscus papillaris with Cytotoxic Activity on Renal Cancer Cells. Molecules. 2020; 25(13):3040. https://doi.org/10.3390/molecules25133040
Chicago/Turabian StyleTosun, Fatma, Feyyaz Mıhoğlugil, John A. A. Beutler, Esra Eroğlu Özkan, and Mahmut Miski. 2020. "Neopapillarine, an Unusual Coumarino-Alkaloid from the Root Extract of Neocryptodiscus papillaris with Cytotoxic Activity on Renal Cancer Cells" Molecules 25, no. 13: 3040. https://doi.org/10.3390/molecules25133040









