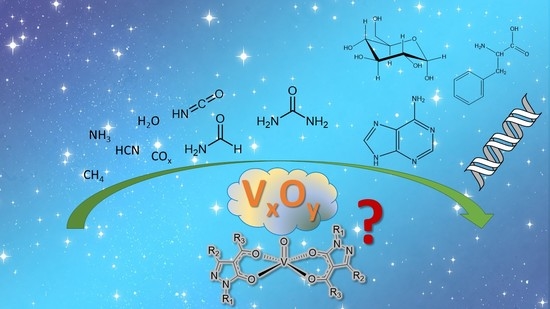
On the Capability of Oxidovanadium(IV) Derivatives to Act as All-Around Catalytic Promoters Since the Prebiotic World
Abstract
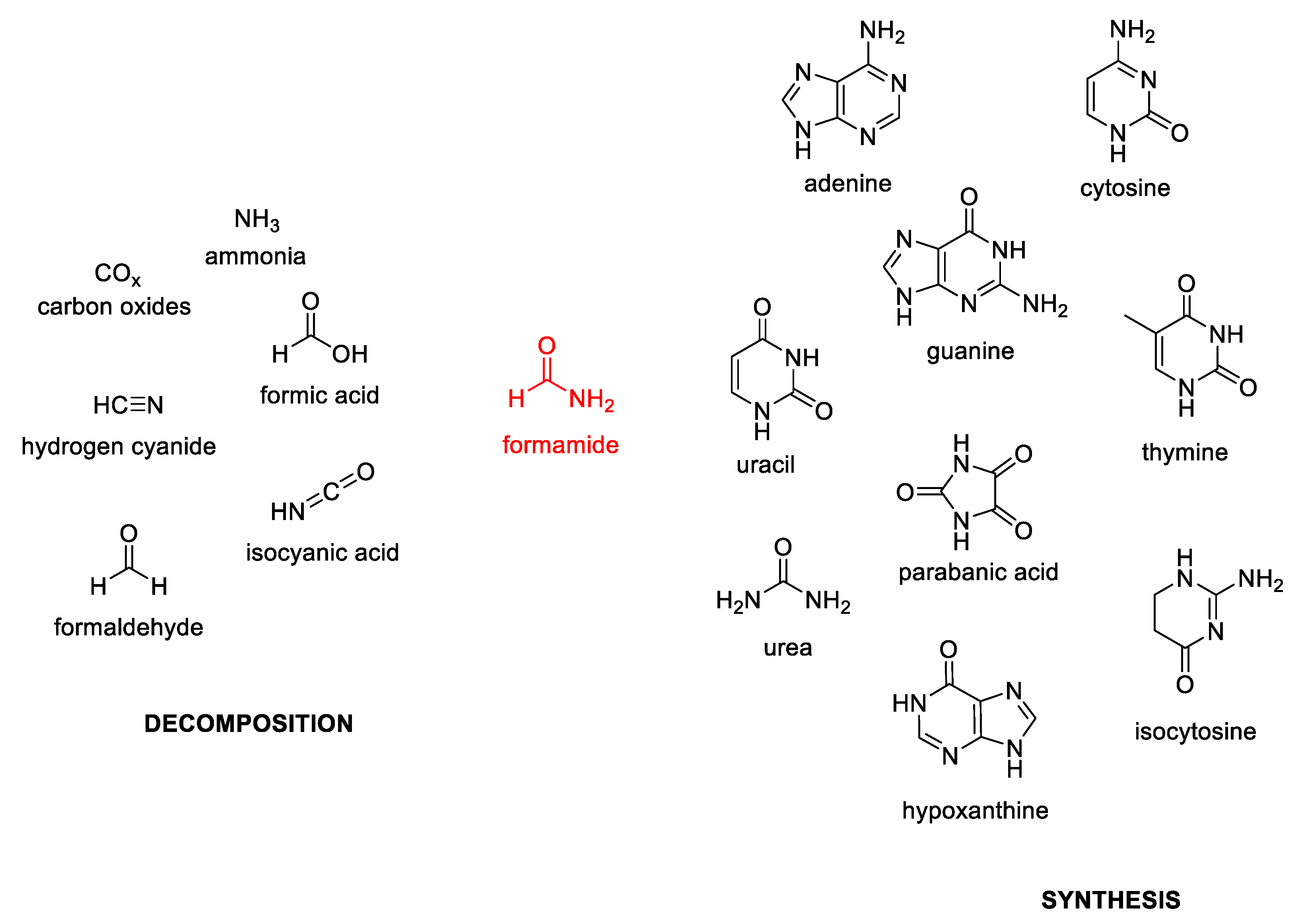
1. The Role of Oxidovanadium(IV) Derivatives or Vanadium Oxides in Prebiotic Chemistry
2. Main Relevant Functions of Vanadium in the Bioinorganic Chemistry
- The tetrahedral vanadate anion VO43− is very similar to the phosphate anion PO43− and this allows an interaction with different biological targets that are normally activated by the phosphate itself [48];
- This transition metal can expand its coordination sphere beyond tetrahedral geometry and easily change its oxidation state.
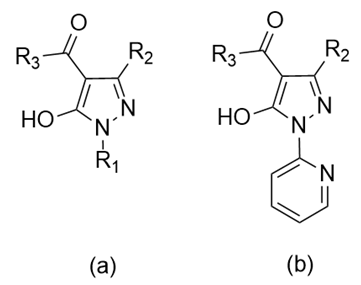
3. Structure and Biological Significance of Acylpyrazolones and Their Related Metal Complexes
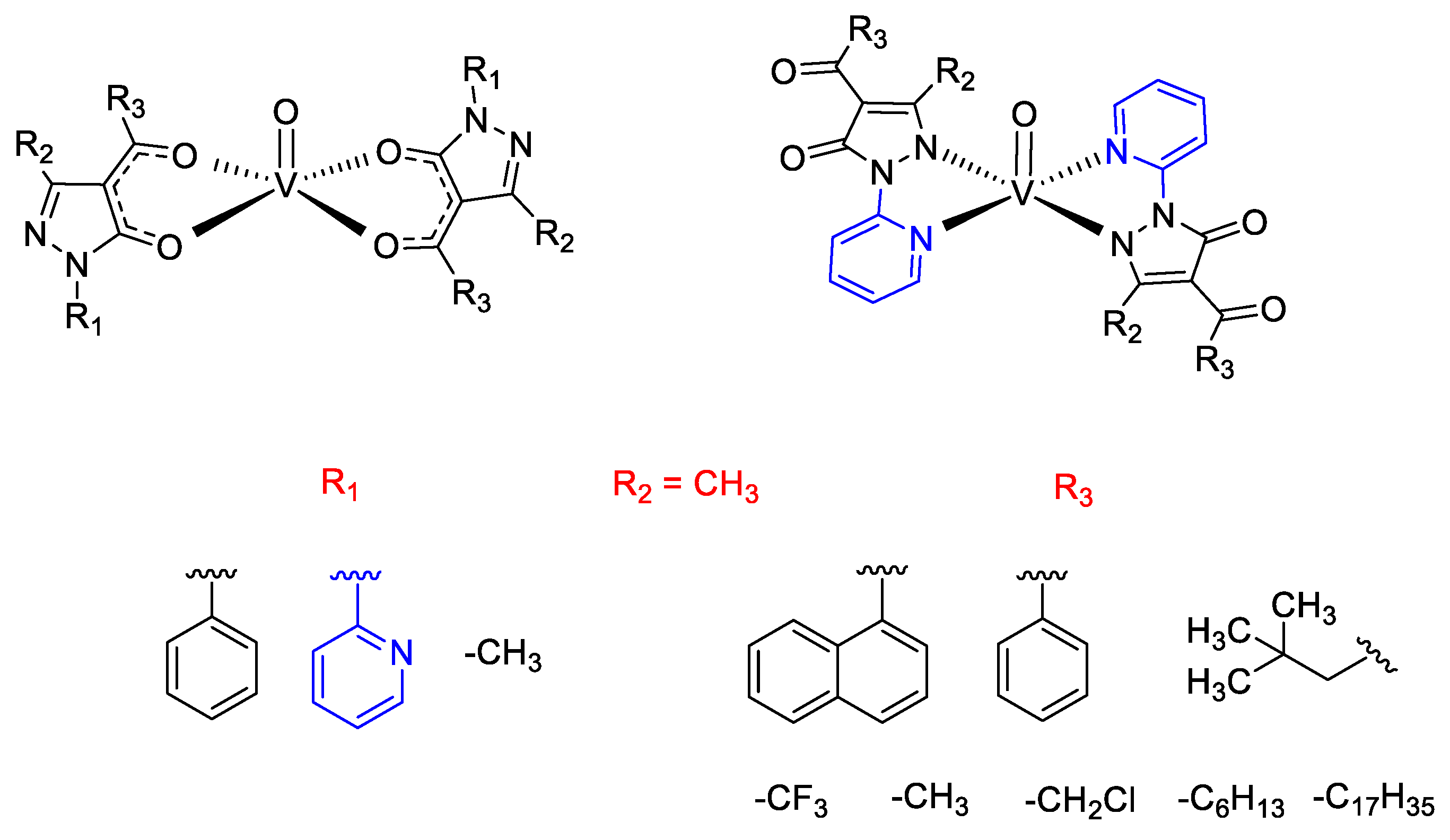
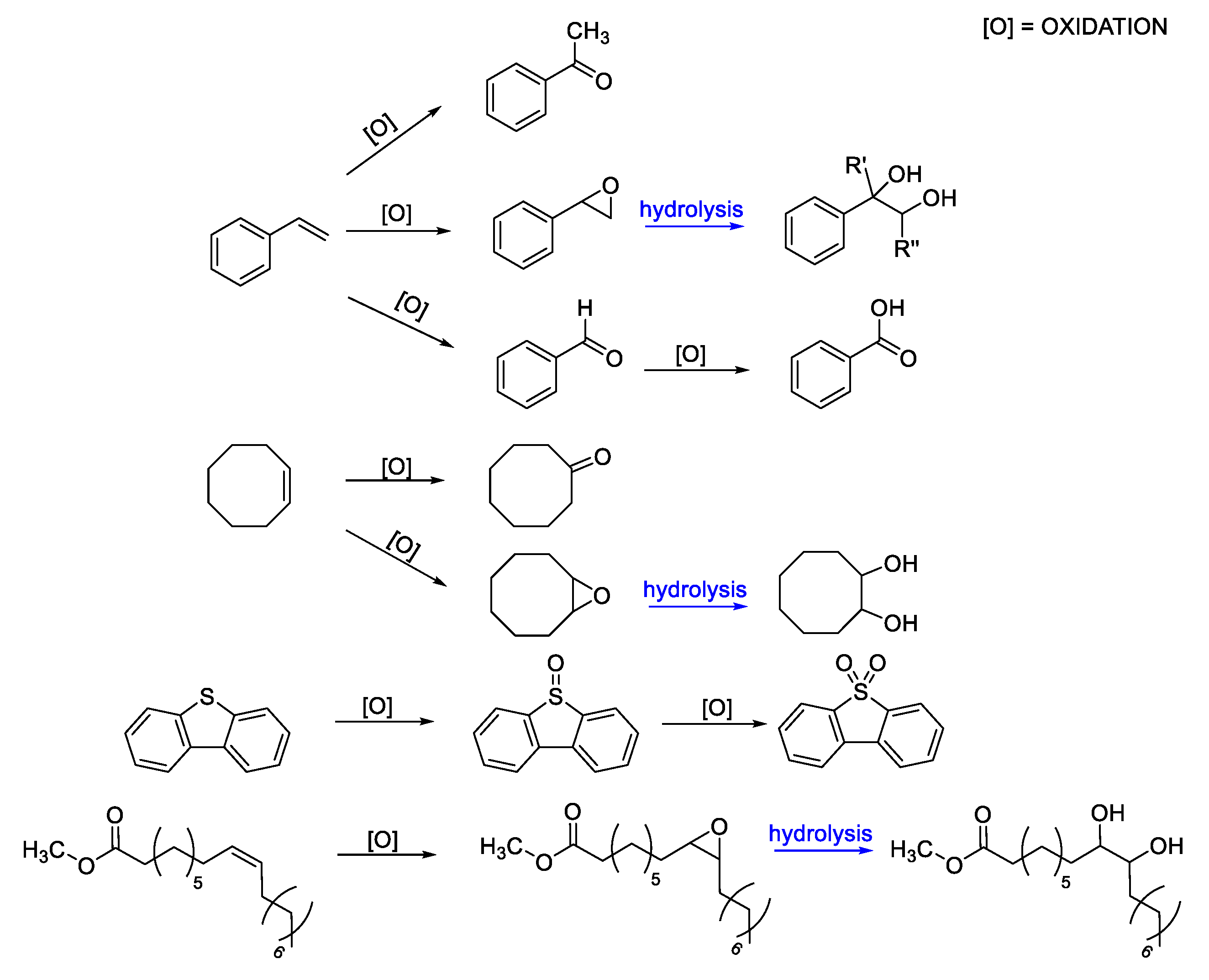
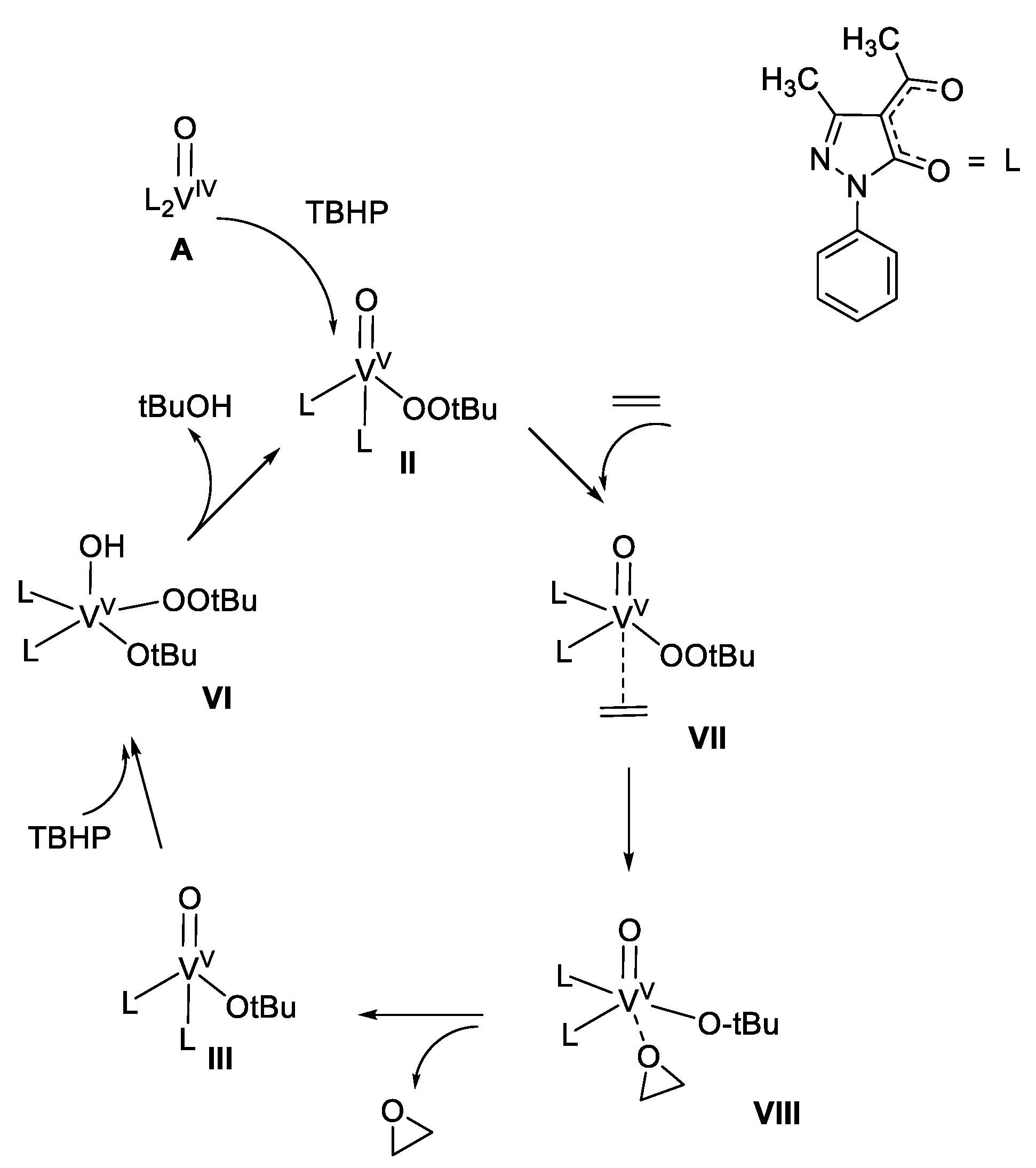
4. Selection of Some Catalytic Applications of Oxidovanadium Complexes from Our Research Group
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rehder, D. Is vanadium a more versatile target in the activity of primordial life forms than hitherto anticipated? Org. Biomol. Chem. 2008, 6, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D. The potentiality of vanadium in medicinal applications. Future Med. Chem. 2012, 4, 1823–1827. [Google Scholar] [CrossRef] [PubMed]
- Nautilus. Available online: http://nautil.us/issue/34/adaptation/the-classic-metal-behind-the-origins-of-life (accessed on 1 May 2020).
- Ghiretti-Magaldi, A.; Nuzzolo, C.; Ghiretti, F. Chemical Studies on Hemocyanins. I. Amino Acid Composition. Biochemistry 1966, 5, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Sadoc, A.; Messaoudi, S.; Furet, E.; Gautier, R.; Le Fur, E.; le Pollès, L.; Pivan, J.-Y. Structure and Stability of VO2+ in Aqueous Solution: A Car—Parrinello and Static ab Initio Study. Inorg. Chem. 2007, 46, 4835–4843. [Google Scholar] [CrossRef]
- Crans, D.C.; Zhang, B.; Gaidamauskas, E.; Keramidas, A.D.; Willsky, G.R.; Roberts, C.R. Is Vanadate Reduced by Thiols under Biological Conditions? Changing the Redox Potential of V(V)/V(IV) by Complexation in Aqueous Solution. Inorg. Chem. 2010, 49, 4245–4256. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-H.; Huang, F.; Evans, L.; Glasauer, S. Vanadium: Global (bio)geochemistry. Chem. Geol. 2015, 417, 68–89. [Google Scholar] [CrossRef]
- Williams, R.J.P. A system’s view of the evolution of life. J. R. Soc. Interface 2007, 4, 1049–1070. [Google Scholar] [CrossRef]
- Moore, E.K.; Hao, J.; Spielman, S.J.; Yee, N. The evolving redox chemistry and bioavailability of vanadium in deep time. Geobiology 2019, 18, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, M. Prebiotic complex organic molecules in space. In Astrobiology: From the Origins of Life to the Search for Extraterrestrial Intelligence; Yamagishi, A., Kakegawa, T., Usui, T., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2019; pp. 11–21. ISBN 9789811336393. [Google Scholar]
- Burton, A.S.; Stern, J.C.; Elsila, J.E.; Glavin, D.P.; Dworkin, J.P. Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem. Soc. Rev. 2012, 41, 5459–5472. [Google Scholar] [CrossRef]
- Saladino, R.; Botta, L.; Di Mauro, E. The prevailing catalytic role of meteorites in formamide prebiotic processes. Life 2018, 8, 6. [Google Scholar] [CrossRef]
- Hevesy, G.; Levi, H. Action of Slow Neutrons on Rare Earth Elements. Nature 1936, 137, 185. [Google Scholar] [CrossRef]
- Guinn, V.P. Past, present, future of Neutron Activation Analysis. J. Radioanal. Nucl. Chem. 1992, 160, 9–19. [Google Scholar] [CrossRef]
- Kemp, D.M.; Smales, A.A. The determination of vanadium in rocks and meteorites by Neutron-Activation Analysis. Anal. Chim. Acta 1960, 23, 397–410. [Google Scholar] [CrossRef]
- Nichiporuk, W.; Chodos, A.A. The Concentration of Vanadium, Chromium, Iron, Cobalt, Nickel, Copper, Zinc, and Arsenic in the Meteoritic Iron Sulfide Nodules. J. Geophys. Res. 1959, 64, 2451–2463. [Google Scholar] [CrossRef]
- Nichiporuk, W.; Bingham, E. Vanadium and copper in chondrites. Meteoritics 1970, 5, 115–130. [Google Scholar] [CrossRef]
- Biver, N.; Bockelée-Morvan, D.; Debout, V.; Crovisier, J.; Boissier, J.; Lis, D.C.; Dello Russo, N.; Moreno, R.; Colom, P.; Paubert, G.; et al. Complex organic molecules in comets C/2012 F6 (Lemmon) and C/2013 R1 (Lovejoy): Detection of ethylene glycol and. Astron. Astrophys. 2014, 566, 1–5. [Google Scholar] [CrossRef]
- Adande, G.R.; Woolf, N.J.; Ziurys, L.M. Observations of Interstellar Formamide: Availability of a Prebiotic Precursor in the Galactic Habitable Zone. Astrobiology 2013, 13, 439–453. [Google Scholar] [CrossRef]
- Bada, J.L.; Chalmers, J.H.; Cleaves, H.J. Is formamide a geochemically plausible prebiotic solvent? Phys. Chem. Chem. Phys. 2016, 18, 20085–20090. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Costanzo, G.; Negri, R.; Di Mauro, E. A Possible Prebiotic Synthesis of Purine, Adenine, Cytosine, and 4(3H)-Pyrimidinone from Formamide: Implications for the Origin of Life. Bioorg. Med. Chem. 2001, 9, 1249–1253. [Google Scholar] [CrossRef]
- Saladino, R.; Crestini, C.; Ciciriello, F.; Pino, S.; Costanzo, G.; Di, E. From formamide to RNA: The roles of formamide and water in the evolution of chemical information. Res. Microbiol. 2009, 160, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Nguyen, M.T. Decomposition pathways of formamide in the presence of vanadium and titanium monoxides. Phys. Chem. Chem. Phys. 2015, 17, 16927–16936. [Google Scholar] [CrossRef] [PubMed]
- Lépine, S.; Shara, M.M.; Rich, R.M. Discovery of an ultracool subdwarf: LSR 1425 + 7102, the first star with spectral type sdM8.0. Astrophys. J. Lett. 2003, 585, 69–72. [Google Scholar] [CrossRef][Green Version]
- Rehder, D. A possible role for extraterrestrial vanadium in the encounter of life. Coord. Chem. Rev. 2011, 255, 2227–2231. [Google Scholar] [CrossRef]
- Hazen, R.M.; Sverjensky, D.A. Mineral Surfaces, Geochemical Complexities, and the Origins of Life. Cold Spring Harb. Perspect. Biol. 2010, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Del Carpio, E.; Hernández, L.; Ciangherotti, C.; Villalobos Coa, V.; Jiménez, L.; Lubes, V.; Lubes, G. Vanadium: History, chemistry, interactions with α-amino acids and potential therapeutic applications. Coord. Chem. Rev. 2018, 372, 117–140. [Google Scholar] [CrossRef]
- Deans, R.M.; Taniguchi, M.; Chandrashaker, V.; Ptaszek, M.; Chambers, D.R.; Soares, A.R.M.; Lindsey, J.S. Complexity in structure-directed prebiotic chemistry. Unexpected compositional richness from competing reactants in tetrapyrrole formation. New J. Chem. 2016, 40, 6421–6433. [Google Scholar] [CrossRef]
- Chandrashaker, V.; Ptaszek, M.; Taniguchi, M.; Lindsey, J.S. Synthesis of diverse acyclic precursors to pyrroles for studies of prebiotic routes to tetrapyrrole macrocycles. New J. Chem. 2016, 40, 8786–8808. [Google Scholar] [CrossRef]
- Premovic, P.I.; Pavlovic, M.S.; Pavlovic, N.Z. Vanadium in ancient sedimentary rocks of marine origin. Geochim. Cosmochim. Acta 1986, 50, 1923–1931. [Google Scholar] [CrossRef]
- Marshall, C.P.; Olcott Marshall, A.; Aitken, J.B.; Lai, B.; Vogt, S.; Breuer, P.; Steemans, P.; Lay, P.A. Imaging of Vanadium in Microfossils: A New Potential Biosignature. Astrobiology 2017, 17, 1069–1076. [Google Scholar] [CrossRef]
- Tewari, B.B.; Usmanali, D.; Webster, R.W.; Kadir, I.; Tiwari, A.K.; Ramchurjee, N. Critical reviews on stability and photosensitizer potential of metal ferrocyanides: A possible prebiotic mineral; PART (II). Rev. Boliv. Quim. 2018, 35, 15–30. [Google Scholar]
- Lee, C.C.; Hu, Y.; Ribbe, M.W. Vanadium Nitrogenase Reduces CO. Science 2010, 329, 642. [Google Scholar] [CrossRef] [PubMed]
- Csotonyi, J.T.; Stackebrandt, E.; Yurkov, V. Anaerobic Respiration on Tellurate and Other Metalloids in Bacteria from Hydrothermal Vent Fields in the Eastern Pacific Ocean. Appl. Environ. Microbiol. 2006, 72, 4950–4956. [Google Scholar] [CrossRef]
- Mehta, M.P.; Butterfield, D.A.; Baross, J.A. Phylogenetic Diversity of Nitrogenase (nifH) Genes in Deep-Sea and Hydrothermal Vent Environments of the Juan de Fuca Ridge. Appl. Environ. Microbiol. 2003, 69, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Sabbioni, E.; Pozzi, G.; Pintar, A.; Casella, L.; Garattini, S. Cellular retention, cytotoxicity and morphological transformation by vanadium(IV) and vanadium(V) in BALB / 3T3 cell lines. Carcinogenesis 1991, 12, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Patra, B.; Mahapatra, S.; Banerjee, P.; Tiwari, A.; Chatterjee, M. Vanadium—an element of atypical biological significance. Toxicol. Lett. 2004, 150, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Kalk, M. Absorption of Vanadium by Tunicates. Nature 1963, 198, 1010–1011. [Google Scholar] [CrossRef]
- Ishii, T.; Nakai, I.; Numako, C.; Okoshi, K.; Otake, T. Discovery of a new vanadium accumulator, the fan worm Pseudopotamilla occelata. Naturwissenschaften 1993, 80, 268–270. [Google Scholar] [CrossRef]
- Kneifel, H.; Bayer, E. Stereochemistry and total synthesis of amavadin, the naturally occurring vanadium compound of Amanita muscaria. J. Am. Chem. Soc. 1986, 108, 3075–3077. [Google Scholar] [CrossRef]
- Nowakowski, W. Vanadium bioaccumulation in Pisum sativum seedlings. Biol. Plantarum 1993, 35, 461–465. [Google Scholar] [CrossRef]
- Mackey, E.A.; Becker, P.R.; Demiralp, R.; Greenberg, R.R.; Koster, B.J.; Wise, S.A. Bioaccumulation of vanadium and other trace metals in livers of Alaskan cetaceans and pinnipeds. Arch. Environ. Con. Tox. 1996, 30, 503–512. [Google Scholar] [CrossRef]
- Sadiq, M.; Alam, I.A.; Al-Mohanna, H. Bioaccumulation of nickel and vanadium by clams (Meretrix meretrix) living in different salinities along the Saudi coast of the Arabian Gulf. Environ. Pollut. 1992, 76, 225–231. [Google Scholar] [CrossRef]
- Crans, D.C.; Chatterjee, P.B. Vanadium Biochemistry. In Comprehensive Inorganic Chemistry II From Elements to Applications, 2nd ed.; Reedijk, J., Poeppelmeier, K.R., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 3, pp. 323–342. ISBN 9780080977744. [Google Scholar]
- Hirata, J.; Michibata, H. Valency of vanadium in the vanadocytes of Ascidia gemmata separated by density-gradient centrifugation. J. Exp. Zool. 1991, 257, 160–165. [Google Scholar] [CrossRef]
- Kim, D.; Li, Y.; Horenstein, B.A.; Nskanishi, K. Synthesis of tunichromes mm-l and mm-2, blood pigments of the iron. Assimilating tunicate, Molgula manhattensis. Tetrahedron Lett. 1990, 31, 7119–7122. [Google Scholar] [CrossRef]
- Bayer, E.; Schiefer, G.; Waidelich, D.; Scippa, S.; de Vicentiis, M. Structure of the Tunichrome of Tunicates and its Role in Concentrating Vanadium. Angew. Chem. Int. Ed. Engl. 1992, 31, 52–54. [Google Scholar] [CrossRef]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The Chemistry and Biochemistry of Vanadium and the Biological Activities Exerted by Vanadium Compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D. The role of vanadium in biology. Metallomics 2015, 7, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Antipov, A.N.; Lyalikova, N.N.; L’vov, N.P. Vanadium-Binding Protein Excreted by Vanadate-Reducing Bacteria. IUBMB Life 2000, 49, 137–141. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301–302, 24–48. [Google Scholar] [CrossRef]
- Reul, B.A.; Amin, S.S.; Buchet, J.-P.; Ongemba, L.N.; Crans, D.C.; Brichard, S.M. Effects of vanadium complexes with organic ligands on glucose metabolism: A comparison study in diabetic rats. Br. J. Pharmacol. 1999, 126, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Gambino, D. Potentiality of vanadium compounds as anti-parasitic agents. Coord. Chem. Rev. 2011, 255, 2193–2203. [Google Scholar] [CrossRef]
- Evangelou, A.; Karkabounas, S.; Kalpouzos, G.; Malamas, M.; Liasko, R.; Stefanou, D.; Vlahosd, A.T.; Kabanos, T.A. Comparison of the therapeutic effects of two vanadium complexes administered at low doses on benzo[a]pyrene-induced malignant tumors in rats. Cancer Lett. 1997, 119, 221–225. [Google Scholar] [CrossRef]
- Yousef Ebrahimipour, S.; Sheikhshoaie, I.; Simpson, J.; Ebrahimnejad, H.; Dusek, M.; Kharazmi, N.; Eigner, V. Antimicrobial activity of aroylhydrazone-based oxido vanadium(v) complexes: In vitro and in silico studies. New J. Chem. 2016, 40, 2401–2412. [Google Scholar] [CrossRef]
- Rehder, D. The future of/for vanadium. Dalton Trans. 2013, 42, 11749. [Google Scholar] [CrossRef] [PubMed]
- Bernroitner, M.; Zamocky, M.; Furtmüller, P.G.; Peschek, G.A.; Obinger, C. Occurrence, phylogeny, structure, and function of catalases and peroxidases in cyanobacteria. J. Exp. Bot. 2009, 60, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Rohde, M.; Grunau, K.; Djurdjevic, I.; Trncik, C.; Schneider, F.; Gies-Elterlein, J.; Einsle, O. Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; ISBN 9781119951438. [Google Scholar]
- Rebelein, J.G.; Hu, Y.; Ribbe, M.W. Differential Reduction of CO2 by Molybdenum and Vanadium Nitrogenases. Angew. Chem. Int. Ed. 2014, 53, 11543–11546. [Google Scholar] [CrossRef]
- Lin, C.Y.; Manley, S.L. Bromoform production from seawater treated with bromoperoxidase. Limnol. Oceanogr. 2012, 57, 1857–1866. [Google Scholar] [CrossRef]
- Jensen, B.R. The Synthesis of 1-Phenyl-3-methyl-4-acyl-pyrazolones-5. Acta Chem. Scand. 1959, 13, 1660–1670. [Google Scholar] [CrossRef]
- Clejan, L.A.; Cederbaum, A.I. Oxidation of Pyrazole by Reconstituted Systems Containing Cytochrome P-450 IIE1. Biochim. Biophys. Acta 1990, 1034, 233–237. [Google Scholar] [CrossRef]
- Brown, E.G.; Flayeh, K.A.M.; Gallon, J.R. The biosynthetic origin of the pyrazole moiety of β-pyrazol-1-yl-l-alanine. Phytochemistry 1982, 21, 863–867. [Google Scholar] [CrossRef]
- Brown, E.G.; Diffin, F.M. Biosynthesis and metabolism of pyrazole by Cucumis sativus: Enzymic cyclization and dehydrogenation of 1,3-diaminopropane. Phytochemistry 1990, 29, 469–478. [Google Scholar] [CrossRef]
- Park, M.H.; Wolff, E.C. Cell-free synthesis of deoxyhypusine. Separation of protein substrate and enzyme and identification of 1,3-diaminopropane as a product of spermidine cleavage. J. Biol. Chem. 1988, 263, 15264–15269. [Google Scholar]
- Baeza, I.; Ibáñez, M.; Wong, C.; Chávez, P.; Gariglio, P.; Oró, J. Possible prebiotic significance of polyamines in the condensation, protection, encapsulation, and biological properties of DNA. Origins Life Evol. B. 1992, 21, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Bachrach, U. The early history of polyamine research. Plant. Physiol. Bioch. 2010, 48, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Chun-Hui, H.; Freiser, H. The extraction of lanthanides with halogen substituted 4-acyl-pyrazolones. Solvent Extr. Ion. Exc. 1986, 4, 41–59. [Google Scholar] [CrossRef]
- Umetani, S.; Kawase, Y.; Le, Q.T.H.; Matsui, M. Acylpyrazolone derivatives of high selectivity for lanthanide metal ions: Effect of the distance between the two donating oxygens. J. Chem. Soc. Dalton Trans. 2000, 16, 2787–2791. [Google Scholar] [CrossRef]
- Holzer, W.; Mereiter, K.; Plagens, B. 4-Acyl-5-methyl-2-phenylpyrazolones: NMR and X-Ray Structure Investigations. Heterocycles 1999, 50, 799. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, R.; Pettinari, C. Recent advances in acylpyrazolone metal complexes and their potential applications. Coord. Chem. Rev. 2015, 303, 1–31. [Google Scholar] [CrossRef]
- Pettinari, R.; Marchetti, F.; Pettinari, C.; Petrini, A.; Skelton, B.W.; White, A.H.; Bonfili, L.; Cuccioloni, M.; Eleuteri, A.M. Dinuclear (η6-arene) ruthenium(II) acylpyrazolone complexes: Synthesis, characterization and cytotoxicity. J. Organomet. Chem. 2015, 791, 1–5. [Google Scholar] [CrossRef]
- De Pascali, S.A.; Migoni, D.; Monari, M.; Pettinari, C.; Marchetti, F.; Muscella, A.; Fanizzi, F.P. Synthesis, Crystal Structure, and Biological Study of PtII Complexes with 4-Acyl-5-pyrazolones. Eur. J. Inorg. Chem. 2014, 7, 1249–1259. [Google Scholar] [CrossRef]
- Thaker, B.T.; Surati, K.R.; Modi, C.K. Synthesis, spectral, thermal, and antibacterial investigation of mixed ligand complexes of oxovanadium(IV). Russ. J. Coord. Chem. 2008, 34, 25–33. [Google Scholar] [CrossRef]
- El-Deen, I.M.; Shoair, A.F.; El-Bindary, M.A. Synthesis, characterization and biological properties of oxovanadium(IV) complexes. J. Mol. Struct. 2018, 1180, 420–437. [Google Scholar] [CrossRef]
- Hassan, H.M.A.; Betiha, M.A.; El-Sharkawy, E.A.; Elshaarawy, R.F.M.; El-Assy, N.B.; Essawy, A.A.; Tolba, A.M.; Rabie, A.M. Highly selective epoxidation of olefins using vanadium (IV) schiff base-amine-tagged graphene oxide composite. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124520. [Google Scholar] [CrossRef]
- Coletti, A.; Sabuzi, F.; Floris, B.; Galloni, P.; Conte, V. Efficient and sustainable V-catalyzed oxidative desulfurization of fuels assisted by ionic liquids. J. Fuel Chem. Technol. 2018, 46, 1121–1129. [Google Scholar] [CrossRef]
- Meisch, H.-U.; Hoffmann, H.; Reinle, W. Vanadium Catalysis in the Nonenzymatic Transamination of δ-Aminolevulinic Acid. Z. Naturforsch. C 1978, 33, 623–628. [Google Scholar] [CrossRef]
- Velusamy, S.; Punniyamurthy, T. Novel Vanadium-Catalyzed Oxidation of Alcohols to Aldehydes and Ketones under Atmospheric Oxygen. Org. Lett. 2004, 6, 217–219. [Google Scholar] [CrossRef]
- Hara, T.; Kanai, S.; Mori, K.; Mizugaki, T.; Ebitani, K.; Jitsukawa, K.; Kaneda, K. Highly Efficient C−C Bond-Forming Reactions in Aqueous Media Catalyzed by Monomeric Vanadate Species in an Apatite Framework. J. Org. Chem. 2006, 71, 7455–7462. [Google Scholar] [CrossRef]
- Nizova, G.V.; Shul’pin, G.B.; Nizova, G.V.; Süss-Fink, G.; Stanislas, S. Carboxylation of methane with CO or CO2 in aqueous solution catalysed by vanadium complexes. Chem. Commun. 1998, 17, 1885–1886. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, C.; Di Nicola, C.; Pettinari, R.; Crispini, A.; Crucianelli, M.; Di Giuseppe, A. Synthesis and characterization of novel oxovanadium(IV) complexes with 4-acyl-5-pyrazolone donor ligands: Evaluation of their catalytic activity for the oxidation of styrene derivatives. Appl. Cat. A 2010, 378, 211–220. [Google Scholar] [CrossRef]
- Di Giuseppe, A.; Di Nicola, C.; Pettinari, R.; Ferino, I.; Meloni, D.; Passacantando, M.; Crucianelli, M. Selective catalytic oxidation of olefins by novel oxovanadium(iv) complexes having different donor ligands covalently anchored on SBA-15: A comparative study. Catal. Sci. Technol. 2013, 3, 1972–1984. [Google Scholar] [CrossRef]
- Aschi, M.; Crucianelli, M.; Di Giuseppe, A.; Di Nicola, C.; Marchetti, F. Insights on the mechanistic features of catalytic oxidations of simple and conjugated olefins promoted by VO(acac)2/H2O2 system, in acetonitrile: A computational study. Catal. Today 2012, 192, 56–62. [Google Scholar] [CrossRef]
- Cecchini, M.M.; De Angelis, F.; Iacobucci, C.; Reale, S.; Crucianelli, M. Mild catalytic oxidations of unsaturated fatty acid methyl esters (FAMEs) by oxovanadium complexes. Appl. Cat. A 2016, 517, 120–128. [Google Scholar] [CrossRef]
- Campitelli, P.; Aschi, M.; Di Nicola, C.; Pettinari, R.; Marchetti, F.; Crucianelli, M. Ionic Liquids vs conventional solvents: A comparative study in the selective catalytic oxidations promoted by oxovanadium (IV) complexes. Appl. Cat. A 2020, 599, 117622. [Google Scholar] [CrossRef]
- Carrascoza Mayén, J.F.; Rydzewski, J.; Szostak, N.; Blazewicz, J.; Nowak, W. Prebiotic Soup Components Trapped in Montmorillonite Nanoclay Form New Molecules: Car-Parrinello Ab Initio Simulations. Life 2019, 9, 46. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campitelli, P.; Crucianelli, M. On the Capability of Oxidovanadium(IV) Derivatives to Act as All-Around Catalytic Promoters Since the Prebiotic World. Molecules 2020, 25, 3073. https://doi.org/10.3390/molecules25133073
Campitelli P, Crucianelli M. On the Capability of Oxidovanadium(IV) Derivatives to Act as All-Around Catalytic Promoters Since the Prebiotic World. Molecules. 2020; 25(13):3073. https://doi.org/10.3390/molecules25133073
Chicago/Turabian StyleCampitelli, Patrizio, and Marcello Crucianelli. 2020. "On the Capability of Oxidovanadium(IV) Derivatives to Act as All-Around Catalytic Promoters Since the Prebiotic World" Molecules 25, no. 13: 3073. https://doi.org/10.3390/molecules25133073
APA StyleCampitelli, P., & Crucianelli, M. (2020). On the Capability of Oxidovanadium(IV) Derivatives to Act as All-Around Catalytic Promoters Since the Prebiotic World. Molecules, 25(13), 3073. https://doi.org/10.3390/molecules25133073