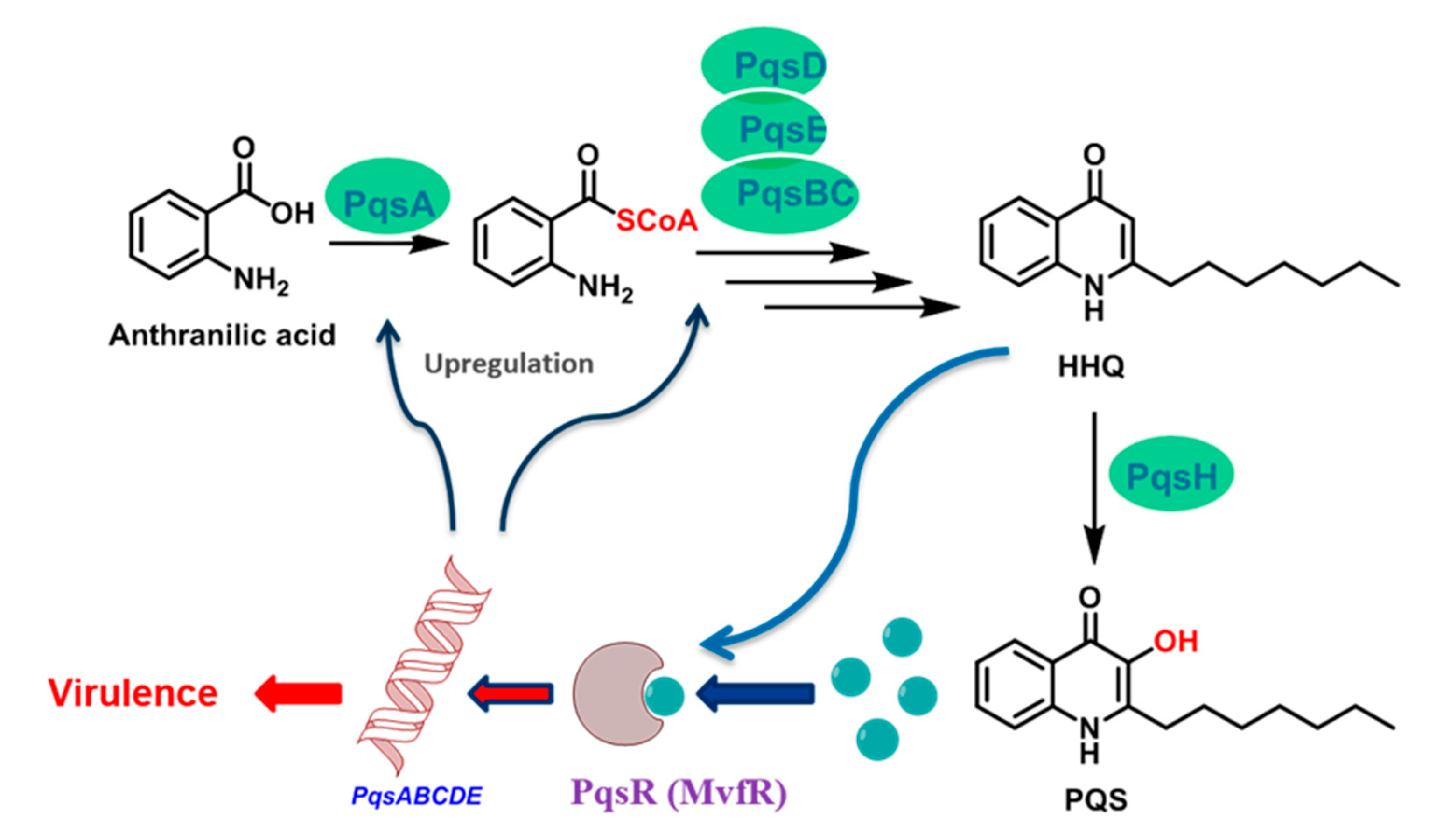
4.1. Synthesis
All the reagents and solvents were purchased from commercial sources (Combi-Blocks, Oakwood Chemicals, Sigma-Aldrich, Sydney, Australia) and used without further purification. Reactions were performed using oven-dried glassware under an atmosphere of nitrogen (if required). Room temperature refers to the ambient temperature (25 °C). Yields refer to compounds isolated after flash column chromatography unless otherwise stated. Progress of the reactions were monitored by thin layer chromatography (TLC) precoated with Merck silica gel 60 F254 plates and visualization using UV light (254 nm). Flash column chromatography was carried out using Grace Davison LC60A 40–63 µm silica gel as the stationary phase and solvent gradient (methanol in ethyl acetate) used as the mobile phase. High-resolution mass spectrometry (HRMS) was performed at Bioanalytical Mass Spectrometry Facility, UNSW by electrospray (ESI) ionization using a Thermo LTQ Orbitrap XL instrument (Thermo Scientific, Waltham, MA, USA). 1H- and 13C-NMR was recorded in deuterated solvent (DMSO-d6) using Bruker Avance 300, 400 or 600 MHz instruments (Bruker Pty Ltd., Preston, NSW, Australia) at 24 °C. Chemical shifts (δ) are reported as relative to the corresponding solvent peak, with tetramethylsilane as the internal standard and quoted in parts per million (ppm). Multiplicities in the NMR spectra are described as: s—singlet; d—doublet; t—triplet; q—quartet; m—multiplet; b—broad; coupling constants (J) are reported in hertz (Hz).
General procedure (A) for the ring opening of isatoic anhydrides (Synthesis of compounds 3a–3e): To a solution of isatoic anhydride (3.0 mmol) in THF (20 mL), propargyl amine (4.5 mmol) was added at 0 °C, and reaction stirred at rt for 2 h. After completion of the reaction as indicated by TLC, the solvent was evaporated and residue was dissolved in minimum DCM, then, excess hexane was added, and precipitate was collected and dried, which gives desired ring opened terminal alkyne products from 89–99% yields.
2-Amino-5-methoxy-N-(prop-2-yn-1-yl)benzamide (3b): Following the general procedure A, the title product was obtained as brown solid (607mg, 99%); 1H-NMR (400 MHz, DMSO-d6): δ 8.65 (t, J = 5.6 Hz, 1H, NH), 7.06 (d, J = 2.9 Hz, 1H, Ar-H), 6.85 (dd, J = 8.9, 2.9 Hz, 1H, Ar-H), 6.66 (d, J = 8.9 Hz, 1H, Ar-H), 6.03 (s, 2H, NH2), 4.00 (dd, J = 5.6, 2.5 Hz, 2H, CH2), 3.68 (s, 3H, CH3), 3.08 (t, J = 2.5 Hz, 1H, CH); 13C-NMR (101 MHz, DMSO-d6): δ 168.32, 149.31, 144.17, 120.20, 117.92, 113.80, 111.61, 81.62, 72.60, 55.58, 28.12; HRMS (ESI) m/z calcd. for C11H12N2O2 [M + H]+ 205.0972, found 205.0971.
2-Amino-5-fluoro-N-(prop-2-yn-1-yl)benzamide (3c): Following the general procedure A, the title product was obtained as off-white solid (515 mg, 89%); 1H-NMR (400 MHz, DMSO-d6): δ 8.70 (t, J = 5.4, 1H, NH), 7.34 (dd, J = 10.3, 3.0 Hz, 1H, Ar-H), 7.06 (td, J = 9.0, 8.1, 3.0 Hz, 1H, Ar-H), 6.72 (dd, J = 9.1, 5.0 Hz, 1H, Ar-H), 6.35 (s, 2H, NH2), 3.99 (dd, J = 5.5, 2.5 Hz, 2H, CH2), 3.09 (t, J = 2.5, 1H, CH); 13C-NMR (101 MHz, DMSO–d6): δ 167.60, 167.57, 153.75, 151.46, 146.66, 119.67, 119.44, 117.78, 117.71, 113.58, 113.36, 113.30, 81.37, 72.71, 28.20; HRMS (ESI) m/z calcd. For C10H9FN2O [M + H]+ 193.0772, found 193.0771.
2-Amino-5-chloro-N-(prop-2-yn-1-yl)benzamide (3d): Following the general procedure A, the title product was obtained as white solid (591 mg, 94%); 1H-NMR (400 MHz, DMSO-d6): δ 8.77 (t, J = 5.3, 1H, NH), 7.55 (d, J = 2.5 Hz, 1H, Ar-H), 7.17 (dd, J = 8.8, 2.5 Hz, 1H, Ar-H), 6.73 (d, J = 8.8 Hz, 1H, Ar-H), 6.59 (s, 2H, NH2), 3.98 (dd, J = 5.5, 2.5 Hz, 2H, CH2), 3.09 (t, J = 2.5 1H, CH); 13C-NMR (101 MHz, DMSO-d6): δ 167.41, 148.78, 131.76, 127.39, 118.17, 117.75, 114.46, 81.32, 72.72, 28.21; HRMS (ESI) m/z calcd. for C9H10ClN2O [M + H]+ 209.0476, found 209.0476.
General procedure (B) for the azide–alkyne cycloaddition reaction: To a mixture of nucleoside azide (0.2 mmol) and corresponding alkyne (10a–e) (0.2 mmol) in (1:1) tert-butanol: water (4 mL), sodium ascorbate (0.04 mmol) and CuSO4.5H2O (0.02 mmol) were added. The reaction mixture was stirred at rt for 16 h. After completion of the reaction as indicated by TLC, solvent was evaporated in vacuo. The crude was purified by silica gel column chromatography (silica gel was pre-neutralized with 1% triethylamine in ethyl acetate) using 0–20% methanol in ethyl acetate gradient system. The polarity was gradually increased after every 100 mL of eluent. Desired compounds were eluted with 15–20% methanol in ethyl acetate system as off-white to light brown solid between 52–74% yields.
2-Amino-N-((1-(((2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)benzamide (7a): Following the general procedure B, the title product was obtained as off-white solid (54 mg, 61%); 1H-NMR (400 MHz, DMSO-d6): δ 11.24 (bs, 1H, NH), 8.72 (t, J = 5.7 Hz, 1H, NH), 7.93 (s, 1H, Ar-H), 7.49 (dd, J = 8.0, 1.5 Hz, 1H, Ar-H), 7.37 (d, J = 1.4 Hz, 2H, NH2 ), 7.12 (td, J = 8.5, 7.1, 1.5 Hz, 1H, Ar-H), 6.68 (dd, J = 8.3, 1.2 Hz, 1H, Ar-H), 6.48 (td, J = 8.1, 7.1, 1.2 Hz, 1H, Ar-H), 6.42 (s, 2H, NH2), 6.17 (t, J = 7.7, 6.3 Hz, 1H, CH), 5.48 (d, J = 4.3 Hz, 1H, OH), 4.67–4.51 (m, 2H, CH2), 4.44 (d, J = 5.7 Hz, 2H, CH2), 4.30–4.23 (m, 1H, CH), 4.09–4.05 (m, 1H, CH), 2.20–2.04 (m, 2H, CH2), 1.78 (d, J = 1.2 Hz, 3H, CH3); 13C-NMR (101 MHz, DMSO-d6): δ 168.80, 163.64, 150.41, 149.77, 145.45, 136.01, 131.79, 128.11, 123.59, 116.35, 114.50, 114.15, 109.88, 84.07, 84.02, 70.79, 51.13, 37.86, 34.46, 12.04; HRMS (ESI) m/z calcd. for C20H23N7O5 [M + H]+ 442.1833, found 442.1832.
2-Amino-N-((1-(((2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-5-methoxybenzamide (7b): Following the general procedure B, the title product was obtained as light brown solid (56 mg, 59%) 1H-NMR (300 MHz, DMSO-d6): δ 11.31 (bs, 1H, NH), 8.79 (t, J = 5.7 Hz, 1H, NH), 7.95 (s, 1H, Ar-H), 7.38 (d, J = 1.5 Hz, 1H, Ar-H), 7.10 (d, J = 2.9 Hz, 1H, Ar-H), 6.85 (dd, J = 8.8, 2.9 Hz, 1H, Ar-H), 6.67 (d, J = 8.9 Hz, 1H, Ar-H), 6.17 (t, J = 7.0 Hz, 3H, CH, NH2), 5.49 (d, J = 4.3 Hz, 1H, OH), 4.65 (dd, J = 15.7, 6.0 Hz, 2H, CH2), 4.46 (d, J = 5.6 Hz, 2H, CH2), 4.31–4.22 (m, 1H, CH), 4.13–4.05 (m, 1H, CH), 3.68 (s, 3H, OCH3), 2.23–2.04 (m, 2H, CH2), 1.79 (s, 3H, CH3); 13C-NMR (76 MHz, DMSO-d6): δ 168.47, 163.63, 150.40, 149.43, 145.35, 136.01, 123.64, 119.82, 117.92, 114.58, 111.85, 109.87, 84.06, 84.02, 70.79, 55.57, 51.14, 37.87, 34.47, 12.02; HRMS (ESI) m/z calcd. for C20H25N7O6 [M + Na]+ 494.1759, found 494.1755.
2-Amino-5-fluoro-N-((1-(((2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)benzamide (7c): Following the general procedure B, the title product was obtained as off-white solid (60 mg, 65%) 1H-NMR (400 MHz, DMSO-d6): δ 11.29 (bs, 1H, NH), 8.80 (t, J = 5.6 Hz, 1H, NH), 7.96 (s, 1H, Ar-H), 7.40–7.30 (m, 2H, Ar-H), 7.04 (td, J = 8.5, 2.9 Hz, 1H, Ar-H), 6.70 (dd, J = 9.0, 5.0 Hz, 1H, Ar-H), 6.31 (s, 2H, NH2), 6.17 (t, J = 7.0 Hz, 1H, CH), 5.48 (d, J = 4.2 Hz, 1H, OH), 4.72–4.55 (m, 2H, CH2), 4.45 (d, J = 5.6 Hz, 2H, CH2), 4.30–4.22 (m, 1H, CH), 4.11–4.04 (m, 1H, CH), 2.19–1.99 (m, 2H, CH2), 1.78 (s, 3H, CH3); 13C-NMR (101 MHz, DMSO-d6): δ 166.72, 163.64, 150.41, 146.20, 136.00, 123.85, 119.58, 119.12, 117.56, 113.66, 113.06, 109.88, 84.04, 70.80, 51.16, 37.88, 34.52, 12.02; HRMS (ESI) m/z calcd. for C20H22FN7O5 [M + Na]+ 482.1559, found 482.1556.
2-Amino-5-chloro-N-((1-(((2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)benzamide (7d): Following the general procedure B, the title product was obtained as off-white solid (70 mg, 74%) 1H-NMR (400 MHz, DMSO-d6): δ 11.30 (bs, 1H, NH), 8.87 (t, J = 5.7 Hz, 1H, NH), 7.97 (s, 1H, Ar-H), 7.57 (d, J = 2.4 Hz, 1H, Ar-H), 7.36 (s, 1H, Ar-H), 7.16 (dd, J = 8.8, 2.4 Hz, 1H, Ar-H), 6.71 (d, J = 8.8 Hz, 1H, Ar-H), 6.57 (s, 2H, NH2), 6.17 (t, J = 7.0 Hz, 1H, CH), 5.50 (d, J = 4.3 Hz, 1H, OH), 4.74–4.53 (m, 2H, CH2), 4.45 (d, J = 5.5 Hz, 2H, CH2), 4.30–4.25 (m, 1H, CH), 4.12–4.04 (m, 1H, CH), 2.22–2.02 (m, 2H, CH2), 1.78 (s, 3H, CH3); 13C-NMR (101 MHz, DMSO-d6): δ 167.61, 163.65, 150.42, 148.68, 136.01, 131.56, 127.43, 123.66, 118.06, 117.73, 114.96, 109.90, 84.05, 70.81, 51.17, 37.90, 34.52, 31.31, 12.04; HRMS (ESI) m/z calcd. for C20H22ClN7O5 [M + H]+ 476.1444, found 476.1443.
2-Amino-5-bromo-N-((1-(((2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)benzamide (7e): Following the general procedure B, the title product was obtained as light orange solid (68 mg, 65%) 1H-NMR (400 MHz, DMSO-d6): δ 11.29 (bs, 1H, NH), 8.87 (t, J = 5.5 Hz, 1H, NH), 7.96 (s, 1H, Ar-H), 7.67 (s, 1H, Ar-H), 7.36 (s, 1H, Ar-H), 7.26 (d, J = 8.8 Hz, 1H, Ar-H), 6.67 (d, J = 8.8 Hz, 1H, Ar-H), 6.58 (s, 2H, NH2), 6.17 (t, J = 7.0 Hz, 1H, CH), 5.48 (d, J = 4.2 Hz, 1H, OH), 4.87–4.52 (m, 2H, CH2), 4.44 (d, J = 5.5 Hz, 2H, CH2), 4.32–4.22 (m, 1H, CH), 4.15–4.04 (m, 1H, CH), 2.32–2.01 (m, 2H, CH2), 1.78 (s, 3H, CH3); 13C-NMR (101 MHz, DMSO-d6): δ 167.52, 163.65, 150.42, 149.00, 136.01, 134.25, 130.24, 128.36, 126.47, 123.73, 118.47, 115.58, 109.89, 104.90, 84.04, 70.80, 51.16, 37.90, 34.52, 12.05; HRMS (ESI) m/z calcd. for C20H22BrN7O5 [M + H]+ 520.0938, found 520.0939.
2-Amino-N-((1-(((2R,3S,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-3-hydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)benzamide (9a): Following the general procedure B, the title product was obtained as off-white solid (60 mg, 70%); 1H-NMR (400 MHz, DMSO-d6): δ 11.32 (bs, J = 2.2 Hz, 1H, NH), 8.72 (t, J = 5.7 Hz, 1H, NH), 7.92 (s, 1H, Ar-H), 7.56 (d, J = 8.1 Hz, 1H, Ar-H), 7.49 (dd, J = 8.0, 1.5 Hz, 1H, Ar-H), 7.13 (td, J = 8.4, 7.0, 1.5 Hz, 1H, Ar-H), 6.69 (dd, J = 8.3, 1.2 Hz, 1H, Ar-H), 6.56–6.44 (m, 1H, Ar-H), 6.41 (s, 2H, NH2), 6.15 (t, J = 6.9 Hz, 1H, CH), 5.62 (dd, J = 8.1, 2.2 Hz, 1H, Ar-H), 5.51 (s, 1H, OH), 4.67 (dd, J = 14.2, 4.5 Hz, 1H, CH2), 4.57 (dd, J = 14.3, 7.7 Hz, 1H, CH2), 4.45 (d, J = 5.7 Hz, 2H, CH2), 4.25–4.22 (m, 1H, CH), 4.10–4.06 (m, 1H, CH), 2.22–2.04 (m, 2H, CH2);13C-NMR (101 MHz, DMSO-d6): δ 168.85, 163.02, 150.41, 149.74, 145.46, 140.72, 131.82, 128.15, 123.59, 116.40, 114.59, 114.26, 102.11, 84.42, 84.28, 70.78, 51.20, 38.08, 34.49; HRMS (ESI) m/z calcd. for C19H21N7O5 [M + H]+ 428.1677, found 428.1673.
2-Amino-N-((1-(((2R,3S,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-3-hydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-5-methoxybenzamide (9b): Following the general procedure B, the title product was obtained as off-white solid (55 mg, 60%) 1H-NMR (400 MHz, DMSO-d6): δ 11.34 (bs, J = 2.2 Hz, 1H, NH), 8.79 (t, J = 5.7 Hz, 1H, NH), 7.94 (s, 1H, Ar-H), 7.57 (d, J = 8.1 Hz, 1H, Ar-H), 7.08 (d, J = 2.9 Hz, 1H, Ar-H), 6.83 (dd, J = 8.9, 2.8 Hz, 1H, Ar-H), 6.65 (d, J = 8.9 Hz, 1H, Ar-H), 6.15 (t, J = 6.9 Hz, 1H, CH), 6.03 (s, 2H, NH2), 5.62 (dd, J = 8.1, 2.0 Hz, 1H, Ar-H), 5.51 (d, J = 4.3 Hz, 1H, OH), 4.72–4.64 (m, 1H, CH2), 4.58 (dd, J = 14.2, 7.7 Hz, 1H, CH2), 4.45 (d, J = 5.7 Hz, 2H, CH2), 4.25–4.23 (m, 1H, CH), 4.10–4.05 (m, 1H, CH), 3.67 (s, 3H, OCH3) 2.21–2.10 (m, 2H, CH2); 13C-NMR (101 MHz, DMSO-d6): δ 168.56, 163.01, 150.41, 149.33, 145.39, 144.00, 140.74, 123.64, 119.90, 117.84, 114.33, 111.82, 102.10, 84.40, 84.29, 70.78, 55.58, 51.22, 38.08, 34.49; HRMS (ESI) m/z calcd. for C20H24N7O6 [M + H]+ 458.1783, found 458.1781.
2-Amino-N-((1-(((2R,3S,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-3-hydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-5-fluorobenzamide (9c): Following the general procedure B, the title product was obtained as off-white solid (64 mg, 72%); 1H-NMR (400 MHz, DMSO-d6): δ 11.32 (bs, 1H, NH), 8.80 (t, J = 5.7, 1H, NH), 7.95 (s, 1H, Ar-H), 7.55 (d, J = 8.1 Hz, 1H, Ar-H), 7.36 (dd, J = 10.3, 3.1 Hz, 1H, Ar-H), 7.03 (dt, J = 8.5, 4.3 Hz, 1H, Ar-H), 6.70 (dd, J = 9.1, 5.0 Hz, 1H, Ar-H), 6.31 (s, 2H, NH2), 6.14 (t, J = 6.9 Hz, 1H, CH), 5.62 (d, J = 8.1 Hz, 1H, Ar-H), 5.51 (s, 1H, OH), 4.67 (dd, J = 14.2, 4.5 Hz, 1H, CH2), 4.57 (dd, J = 14.3, 7.7 Hz, 1H, CH2), 4.67–4.44 (d, J = 5.7 Hz, 2H, CH2), 4.28–4.19 (m, 1H, CH), 4.12–4.02 (m, 1H, CH), 2.25–2.04 (m, 2H, CH2); 13C-NMR (101 MHz, DMSO-d6): δ 167.81, 163.02, 153.79, 151.53, 150.41, 146.52, 145.18, 140.74, 123.64, 119.43, 119.20, 117.68, 117.62, 113.96, 113.71, 113.48, 102.10, 84.44, 84.27, 70.79, 51.23, 38.10, 34.55; HRMS (ESI) m/z calcd. for C19H20FN7O5 [M + Na]+ 468.1402, found 468.1401.
2-Amino-5-bromo-N-((1-(((2R,3S,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-3-hydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)benzamide (9e): Following the general procedure B, the title product was obtained as off-white solid (53 mg, 52%); 1H-NMR (400 MHz, DMSO-d6): δ 11.32 (bs, 1H, NH), 8.87 (t, J = 5.7, 1H, NH), 7.95 (s, 1H, Ar-H), 7.67 (d, J = 2.4 Hz, 1H, Ar-H), 7.56 (d, J = 8.1 Hz, 1H, Ar-H), 7.26 (dd, J = 8.8, 2.3 Hz, 1H, Ar-H), 6.67 (d, J = 8.9 Hz, 1H, Ar-H), 6.58 (s, 2H, NH2), 6.14 (t, J = 6.9 Hz, 1H, CH), 5.62 (d, J = 8.0 Hz, 1H, Ar-H), 5.50 (d, J = 4.3 Hz, 1H, OH), 4.61 (d, J = 4.5 Hz, 1H, CH2), 4.49 (d, J = 7.6 Hz, 1H, CH2), 4.43 (d, J = 5.6 Hz, 2H, CH2), 4.26–4.21 (m, 1H, CH), 4.14–4.02 (m, 1H, CH), 2.23–2.04 (m, 2H, CH2); 13C-NMR (101 MHz, DMSO-d6): δ 168.00, 163.45, 150.85, 149.44, 145.57, 141.18, 134.74, 134.71, 130.71, 124.05, 118.94, 116.10, 105.38, 102.55, 84.87, 84.72, 71.24, 38.54, 34.96; HRMS (ESI) m/z calcd. for C19H20BrN7O5 [M + Na]+ 528.0602, found 528.0600.
2-Amino-N-((1-(((2R,3S,5R)-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-3-hydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)benzamide (12a): Following the general procedure B, the title product was obtained as off-white solid (65 mg, 61%); 1H-NMR (400 MHz, DMSO-d6): δ 11.24 (bs, 1H, NH), 8.73 (t, 1H, NH), 8.12 (d, J = 7.5 Hz, 1H, Ar-H), 8.05–7.98 (m, 3H, Ar-H), 7.62 (t, J = 7.4 Hz, 1H, Ar-H), 7.56–7.46 (m, 3H, Ar-H), 7.37 (s, 1H, Ar-H), 7.13–7.07 (m, 1H, Ar-H), 6.67 (dd, J = 8.2, 1.2 Hz, 1H, Ar-H), 6.48 (t, J = 1.0 Hz, 1H, Ar-H), 6.41 (s, 2H, NH2), 6.16 (t, J = 6.5 Hz, 1H, CH), 5.54 (d, J = 4.2 Hz, 1H, OH), 4.76–4.61 (m, 2H, CH2), 4.46 (d, J = 5.7 Hz, 2H, CH2), 4.30–4.22 (m, 1H, CH), 4.22–4.14 (m, 1H, CH), 2.37–2.09 (m, 2H, CH2); 13C-NMR (151 MHz, DMSO-d6): δ 168.82, 149.76, 145.52, 132.76, 131.77, 128.46, 128.13, 123.66, 116.35, 114.52, 114.19, 87.18, 84.94, 70.77, 51.22, 34.47; HRMS (ESI) m/z calcd. for C26H26N8O5 [M + H]+ 531.2099, found 531.2098.
2-Amino-N-((1-(((2R,3S,5R)-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-3-hydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-5-methoxybenzamide (12b): Following the general procedure B, the title product was obtained as light brown solid (70 mg, 62%); 1H-NMR (400 MHz, DMSO-d6): δ 11.25 (bs, 1H, NH), 8.79 (t, J = 5.7 Hz, 1H, NH), 8.13 (d, J = 7.6 Hz, 1H, Ar-H), 8.06–7.96 (m, 3H, Ar-H), 7.68–7.59 (m, 1H, Ar-H), 7.53 (t, J = 8.3, 7.0 Hz, 2H, Ar-H), 7.38 (s, 1H, Ar-H), 7.09 (d, J = 2.9 Hz, 1H, Ar-H), 6.82 (dd, J = 8.9, 2.8 Hz, 1H, Ar-H), 6.65 (d, J = 8.9 Hz, 1H, Ar-H), 6.16 (t, J = 6.5 Hz, 1H, CH), 6.00 (s, 2H, NH2), 5.56 (d, J = 4.3 Hz, 1H, OH), 4.78–4.62 (m, 2H, CH2), 4.47 (d, J = 5.7 Hz, 2H, CH2), 4.34–4.23 (m, 1H, CH), 4.24–4.12 (m, 1H, CH), 3.66 (s, 3H, CH3) 2.38–2.09 (m, 2H, CH2); 13C-NMR (151 MHz, DMSO-d6): δ 168.56, 149.31, 145.46, 144.04, 132.78, 128.50, 128.47, 123.71, 119.89, 117.81, 114.32, 111.81, 86.53, 84.95, 70.79, 55.56, 51.26, 34.49; HRMS (ESI) m/z calcd. for C27H29N8O6 [M + H]+ 561.2205, found 561.2204.
2-Amino-N-((1-(((2R,3S,5R)-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-3-hydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-5-fluorobenzamide (12c): Following the general procedure B, the title product was obtained as light yellowish solid (80 mg, 73%); 1H-NMR (400 MHz, DMSO-d6): δ 11.24 (bs, 1H, NH), 8.81 (t, J = 5.7 Hz, 1H, NH), 8.12 (d, J = 7.5 Hz, 1H, Ar-H), 8.05–7.98 (m, 3H, Ar-H), 7.67–7.59 (m, 1H, Ar-H), 7.52 (t, J = 7.7 Hz, 2H, Ar-H), 7.36 (dd, J = 10.3, 3.0 Hz, 2H, Ar-H), 7.06–6.92 (m, 1H, Ar-H), 6.69 (dd, J = 9.0, 5.0 Hz, 1H, Ar-H), 6.31 (s, 2H, NH2), 6.16 (t, J = 6.5 Hz, 1H, CH), 5.54 (d, J = 4.3 Hz, 1H, OH), 4.69 (dd, J = 8.9, 6.1 Hz, 2H, CH2), 4.46 (d, J = 5.6 Hz, 2H, CH2), 4.30–4.23 (m, 1H, CH), 4.26–4.15 (m, 1H, CH), 2.38–2.10 (m, 1H, CH2); 13C-NMR (101 MHz, DMSO-d6): δ 167.85, 153.71, 151.86, 148.73, 146.50, 145.22, 132.74, 128.43, 123.68, 119.48, 119.14, 117.61, 113.67, 113.45, 86.76, 84.92, 70.77, 50.80, 34.52; HRMS (ESI) m/z calcd. for C25H26FN8O5 [M + Na]+ 571.1824, found 571.1823.
2-Amino-N-((1-(((2R,3S,5R)-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-3-hydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-5-chlorobenzamide (12d): Following the general procedure B, the title product was obtained as off-white solid (70 mg, 62%); 1H-NMR (400 MHz, DMSO-d6): δ 11.22 (bs, 1H, NH), 8.90 (t, 1H, NH), 8.12 (d, J = 7.3 Hz, 1H, Ar-H), 8.06–7.94 (m, 4H, Ar-H), 7.62 (t, 1H, Ar-H), 7.57 (d, J = 2.5 Hz, 1H, Ar-H), 7.52 (t, J = 7.7 Hz, 2H, Ar-H), 7.13 (dd, J = 8.8, 2.5 Hz, 1H, Ar-H), 6.71 (d, J = 8.8 Hz, 1H, Ar-H), 6.56 (s, 2H, NH2,), 6.15 (t, 1H, CH), 5.63 (d, J = 4.3 Hz, 1H, OH), 4.78–4.62 (m, 2H, CH2), 4.45 (d, J = 5.6 Hz, 2H, CH2), 4.30–4.22 (m, 1H, CH), 4.26–4.15 (m, 1H, CH), 2.41–2.10 (m, 1H, CH2); 13C-NMR (151 MHz, DMSO-d6): δ 167.63, 148.69, 145.20, 132.78, 131.53, 128.50, 128.47, 127.47, 123.72, 118.06, 117.72, 114.97, 96.44, 84.93, 70.73, 51.26, 34.52; HRMS (ESI) m/z calcd. for C26H26ClN8O5 [M + H]+ 565.1709, found 565.1709.
2-Amino-N-((1-(((2R,3S,5R)-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-3-hydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-5-bromobenzamide (12e): Following the general procedure B, the title product was obtained as off-white solid (65 mg, 53%); 1H-NMR (600 MHz, DMSO-d6): δ 11.26 (bs, 1H, NH), 8.89 (t, J = 5.9 Hz, 1H, NH), 8.12 (d, J = 7.6 Hz, 1H, Ar-H), 8.06–7.98 (m, 3H, Ar-H), 7.68 (d, J = 2.4 Hz, 1H, Ar-H), 7.63 (t, J = 7.3 Hz, 1H, Ar-H), 7.52 (t, J = 7.7 Hz, 2H, Ar-H), 7.40–7.34(m, 1H, Ar-H) 7.24 (dd, J = 8.8, 2.4 Hz, 1H, Ar-H), 6.67 (d, J = 8.8 Hz, 1H, Ar-H), 6.59 (s, 2H, NH2), 6.17 (t, J = 6.5 Hz, 1H, CH), 5.56 (d, J = 4.3 Hz, 1H, OH), 4.81–4.64 (m, 2H CH2), 4.45 (d, J = 5.8 Hz, 2H, CH2), 4.29–4.24 (m, 1H, CH), 4.24–4.17 (m, 1H, CH), 2.32–2.02 (m, 2H, CH2); 13C-NMR (151 MHz, DMSO-d6): δ 167.98, 149.46, 145.65, 134.69, 130.72, 128.95, 128.90, 124.14, 118.92, 116.06, 105.36, 85.39, 79.66, 79.44, 79.22, 71.24, 51.72, 34.97; HRMS (ESI) m/z calcd. for C26H25BrN8O5 [M + Na]+ 631.1023, found 631.1022.
General procedure (C) for the deprotection of benzoyl group: To a solution of N-benzoyl triazole (0.1 mmol) in methanol (6 mL), Aq. NH3 (28%) (6 mL) was added. The resulting mixture was stirred at rt for 16 h. After completion of the reaction, solvent was evaporated in vacuo, crude was purified by dissolving in methanol (1 mL), then, excess diethyl ether was added, and solid precipitate was collected and dried, which gives desired compound as off-white to light brown solid between (81–92% yields).
2-Amino-N-((1-(((2R,3S,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3-hydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)benzamide (13a): Following the general procedure C, the title product was obtained as off-white solid (38 mg, 88%); 1H-NMR (600 MHz, DMSO-d6): δ 8.73 (t, J = 5.8, 1H, NH), 7.94 (s, 1H, Ar-H), 7.52 (d, J = 7.5 Hz, 1H, Ar-H), 7.50 (dd, J = 8.0, 1.5 Hz, 1H, Ar-H), 7.14 (s, 2H, NH2), 7.13 (td, J = 8.4, 7.0, 1.5 Hz, 1H, Ar-H), 6.69 (dd, J = 8.2, 1.2 Hz, 1H, Ar-H), 6.50 (td, J = 8.1, 7.0, 1.2 Hz, 1H, Ar-H), 6.42 (s, 2H, NH2), 6.18 (t, J = 7.5, 6.2 Hz, 1H, CH), 5.75 (d, J = 7.4, 1H, Ar-H), 5.45 (d, J = 4.3 Hz, 1H, OH), 4.66 (dd, J = 14.3, 4.4 Hz, 1H, CH2), 4.58 (dd, J = 14.2, 7.7 Hz, 1H, CH2), 4.45 (d, J = 5.7 Hz, 2H, CH2), 4.22–4.21 (m, 3.6 Hz, 1H, CH), 4.09–4.06 (m, 4.2 Hz, 1H, CH), 2.14–2.08 (m, 1H, CH2), 2.05–1.98 (m, 1H, CH2); 13C-NMR (101 MHz, DMSO-d6): δ 168.84, 165.55, 154.99, 149.76, 145.42, 141.12, 131.83, 128.17, 123.60, 116.39, 114.60, 114.25, 94.41, 85.17, 84.17, 70.98, 51.38, 39.55, 34.48. HRMS (ESI) m/z calcd. for C19H22N8O4 [M + H]+ 449.1656, found 449.1655.
2-Amino-N-((1-(((2R,3S,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3-hydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-5-methoxybenzamide (13b): Following the general procedure C, the title product was obtained as light brown solid (37 mg, 81%); 1H-NMR (400 MHz, DMSO-d6): δ 8.77 (t, J = 5.7 Hz, 1H, NH), 7.95 (s, 1H, Ar-H), 7.53 (d, J = 7.4 Hz, 1H, Ar-H), 7.28–7.12 (m, 2H, NH2), 7.09 (d, J = 2.9 Hz, 1H, Ar-H), 6.84 (dd, J = 8.9, 2.9 Hz, 1H, Ar-H), 6.66 (d, J = 8.9 Hz, 1H), 6.18 (t, J = 6.8 Hz, 1H, CH), 6.01 (s, 2H, NH2), 5.75 (d, J = 7.4 Hz, 1H, Ar-H), 5.47 (d, J = 4.2 Hz, 1H, OH), 4.62 (dd, J = 21.4, 6.1 Hz, 2H, CH2), 4.46 (d, J = 5.6 Hz, 2H, CH2), 4.23–4.22 (m, 1H, CH), 4.12–4.04 (m, 1H, CH), 3.67 (s, 3H, OCH3), 2.23–1.96 (m, 2H, CH2); 13C-NMR (101 MHz, DMSO-d6): δ 169.00, 165.99, 155.42, 149.78, 145.78, 144.47, 141.57, 124.08, 120.35, 118.27, 114.83, 112.30, 94.83, 85.62, 84.61, 71.43, 56.04, 51.84, 34.94; HRMS (ESI) m/z calcd. for C20H24N8O5 [M + Na]+ 479.1762, found 479.1760.
2-Amino-N-((1-(((2R,3S,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3-hydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-5-fluorobenzamide (13c): Following the general procedure C, the title product was obtained as off-white solid (41 mg, 92%); 1H-NMR (400 MHz, DMSO-d6): δ 8.82 (t, J = 5.6 Hz, 1H, NH), 7.97 (s, 1H, Ar-H), 7.52 (d, J = 7.5 Hz, 1H, Ar-H), 7.37 (dd, J = 10.3, 3.0 Hz, 1H, Ar-H), 7.17 (d, J = 13.1 Hz, 2H, NH2), 7.09–7.00 (m, 1H, Ar-H), 6.70 (dd, J = 9.0, 5.0 Hz, 1H, Ar-H), 6.33 (s, 2H, NH2), 6.17 (t, 1H, J = 6.8 Hz, CH), 5.73 (d, J = 7.4 Hz, 1H, Ar-H), 5.45 (d, J = 4.3 Hz, 1H, OH), 4.75–4.53 (m, 2H, CH2), 4.44 (d, J = 5.7 Hz, 2H, CH2) 4.24–4.16 (m, 1H, CH), 4.11–4.03 (m, 1H, CH), 2.16–1.95 (m, 2H, CH2); 13C-NMR (101 MHz, DMSO-d6): δ 167.78, 165.54, 154.98, 153.80, 151.48, 146.51, 145.13, 141.13, 123.62, 119.42, 119.19, 117.66, 117.58, 113.96, 113.90, 113.72, 113.50, 94.39, 85.19, 84.15, 70.98, 51.40, 34.52; HRMS (ESI) m/z calcd. for C19H22FN8O4 [M + Na]+ 567.1562, found 567.1564.
2-Amino-N-((1-(((2R,3S,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3-hydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-5-chlorobenzamide (13d): Following the general procedure C, the title product was obtained as off-white solid (38 mg, 82%); 1H-NMR (600 MHz, DMSO-d6): δ 8.88 (t, J = 5.7 Hz, 1H, NH), 7.96 (s, 1H, Ar-H), 7.57 (d, J = 2.5 Hz, 1H, Ar-H), 7.52 (d, J = 7.4 Hz, 1H, Ar-H), 7.26–6.99 (m, 3H, NH2, Ar-H), 6.72 (d, J = 8.8 Hz, 1H, Ar-H), 6.57 (s, 2H, NH2), 6.17 (t, J = 7.5, 6.2 Hz, 1H, CH), 5.74 (d, J = 7.4 Hz, 1H, Ar-H), 5.46 (d, J = 4.0 Hz, 1H, OH), 4.66 (dd, J = 14.2, 4.4 Hz, 1H, CH2), 4.57 (dd, J = 14.2, 7.7 Hz, 1H, CH2), 4.44 (d, J = 5.6 Hz, 2H, CH2), 4.25–4.19 (m, 1H, CH), 4.08–4.05 (m, 1H, CH), 2.12–2.07 (m, 1H, CH2), 2.03–1.99 (m, 1H, CH2); 13C-NMR (151 MHz, DMSO-d6): δ 168.06, 165.98, 155.41, 149.12, 145.53, 141.57, 132.02, 127.92, 124.06, 118.51, 118.19, 115.45, 108.11, 94.83, 85.61, 84.60, 71.42, 51.85, 34.96; HRMS (ESI) m/z calcd. for C19H21ClN8O4 [M + H]+ 483.1267, found 483.1264.
2-Amino-N-((1-(((2R,3S,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3-hydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-5-bromobenzamide (13e): Following the general procedure C, the title product was obtained as off-white solid (43 mg, 85%); 1H-NMR (400 MHz, DMSO-d6): δ 8.87 (t, J = 5.5 Hz, 1H, NH), 7.96 (s, 1H, Ar-H), 7.68 (d, J = 2.4 Hz, 1H, Ar-H), 7.52 (d, J = 7.4 Hz, 1H, Ar-H), 7.26 (dd, J = 8.9, 2.3 Hz, 1H, Ar-H), 7.17 (s, 2H, NH2), 6.67 (d, J = 8.8 Hz, 1H, Ar-H), 6.58 (s, 2H, NH2), 6.17 (t, J = 6.8 Hz, 1H, CH), 5.76 (s, 1H, Ar-H), 5.45 (d, J = 4.3 Hz, 1H, OH), 4.65– 4.58 (m, 2H, CH2), 4.43 (d, J = 5.5 Hz, 2H, CH2), 4.24–4.16 (m, 1H, CH), 4.11–4.03 (m, 1H, CH), 2.10–1.95 (m, 2H, CH2); 13C-NMR (151 MHz, DMSO-d6): δ 167.52, 165.54, 148.99, 145.07, 141.12, 134.26, 130.27, 123.61, 118.47, 115.62, 104.92, 85.18, 84.15, 70.97, 69.79, 51.39, 34.50; HRMS (ESI) m/z calcd. for C19H21BrN8O4 [M + H]+ 505.0942, found 505.0942.