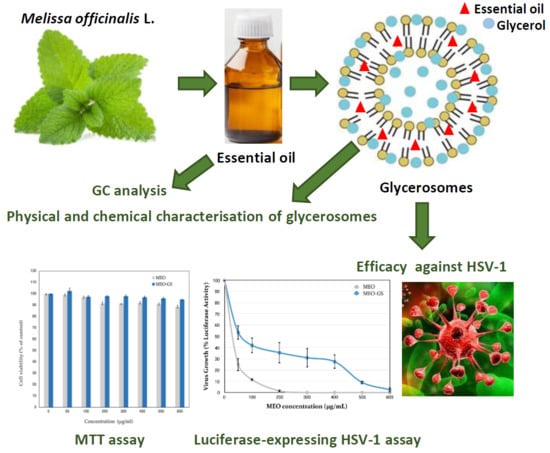
Glycerosome of Melissa officinalis L. Essential Oil for Effective Anti-HSV Type 1
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Analysis of MEO by Gas Chromatography–Mass Spectrometry (GC–MS)
2.2. Vesicle Preparation and Physical Characterization
2.3. Encapsulation Efficiency (EE) and Recovery (R) of MEO-GS
2.4. Deformability
2.5. In Vitro Release
2.6. Stability Studies
2.7. Antiviral Assays Using Luciferase-Expressing HSV-1
2.8. Cytotoxicity Assays
3. Materials and Methods
3.1. Chemicals
3.2. Chemical Analysis of MEO by Gas Chromatography–Mass Spectrometry (GC–MS)
3.3. HPLC-DAD Analysis
3.4. Preparation of Vesicles
3.5. Physical Characterization of MEO-GS
3.6. Deformability
3.7. Chemical Characterization of MEO-GS
3.8. In Vitro Release
3.9. Stability Studies
3.10. Cells, Viruses and Growth Conditions
3.11. Antiviral Assays
3.12. Luciferase Assays
3.13. MTT Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DAD | Diode Array Detector |
| DLS | Dynamic Light Scattering |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| ELS | Electrophoretic Light Scattering |
| EE | Encapsulation Efficiency |
| FCS | Fetal Bovine Calf Serum |
| GC–MS | Gas Chromatography–Mass Spectrometry |
| HSV | Herpes Simplex Virus |
| HPLC | High Performance Liquid Chromatograph |
| MEO | Melissa officinalis Essential Oil |
| MEO-GS | Melissa officinalis Essential Oil loaded in Glycerosomes |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyletrazolium bromide |
| NLC | Nanostructured Lipid Carrier |
| P90G | Phosphatidylcholine |
| PTA | Phosphotungstic acid |
| PdI | Polydispersity Index |
| R | Recovery |
| SLN | Solid Lipid Nanoparticles |
| TEM | Transmission Electron Microscopy |
| DMSO | Dimethyl sulfoxide |
References
- Whitley, R.; Baines, J. Clinical management of herpes simplex virus infections: Past, present, and future. F1000 Res. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isacchi, B.; Fabbri, V.; Galeotti, N.; Bergonzi, M.C.; Karioti, A.; Ghelardini, C.; Vannucchi, M.G.; Bilia, A.R. Salvianolic acid B and its liposomal formulations: Anti-hyperalgesic activity in the treatment of neuropathic pain. Eur. J. Pharm. Sci. 2011, 44, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Guccione, C.; Oufir, M.; Piazzini, V.; Eigenmann, D.E.; Jähne, E.A.; Zabela, V.; Faleschini, M.T.; Bergonzi, M.C.; Smiesko, M.; Hamburger, M.; et al. Andrographolide-loaded nanoparticles for brain delivery: Formulation, characterisation and in vitro permeability using hCMEC/D3 cell line. Eur. J. Pharm. Biopharm. 2017, 120, 146. [Google Scholar] [CrossRef]
- Isacchi, B.; Bergonzi, M.C.; Grazioso, M.; Righeschi, C.; Pietretti, A.; Severini, C.; Bilia, A.R. Artemisinin and artemisinin plus curcumin liposomal formulations: Enhanced antimalarial efficacy against Plasmodium berghei-infected mice. Eur. J. Pharm. Biopharm. 2012, 80, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, P. Essential Oils for the Treatment of Herpes Simplex Virus Infections. Chemotherapy 2019, 64, 1–7. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative Study on the Antiviral Activity of Selected Monoterpenes Derived from Essential Oils. Phytother. Res. 2010, 24, 673–679. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for antiviral activities of isolated compounds from essential oils. Evid Based Complement Altern. Med. 2011, 253643. [Google Scholar] [CrossRef] [Green Version]
- European Scientific Cooperative on Phytotherapy (ESCOP). Melissae folium, Melissa officinalis L. leaf. In ESCOP Monograph; Thieme Publisher: New York, NY, USA, 2013. [Google Scholar]
- Astani, A.; Heidary Navid, M.; Schnitzler, P. Attachment and Penetration of Acyclovir-resistant Herpes Simplex Virus are inhibited by Melissa officinalis Extract. Phytother. Res. 2014, 28, 1547–1552. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Melissa officinalis extract inhibits attachment of herpes simplex virus in vitro. Chemotherapy 2012, 58, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Mazzanti, G.; Battinelli, L.; Pompeo, C.; Serrilli, A.M.; Rossi, R.; Sauzullo, I.; Vullo, V. Inhibitory activity of Melissa officinalis L. extract on Herpes simplex virus type 2 replication. Nat. Prod. Res. 2008, 22, 1433–1440. [Google Scholar] [CrossRef]
- Nolkemper, S.; Reichling, J.; Stintzing, F.C.; Carle, R.; Schnitzler, P. Antiviral effect of aqueous extracts from species of the Lamiaceae family against Herpes simplex virus type 1 and type 2 in vitro. Planta Med. 2006, 72, 1378–1382. [Google Scholar] [CrossRef] [Green Version]
- Schnitzler, P.; Schuhmacher, A.; Astani, A.; Reichling, J. Melissa officinalis oil affects infectivity of enveloped herpesviruses. Phytomedicine 2008, 15, 734–740. [Google Scholar] [CrossRef]
- Allahverdiyev, A.; Duran, N.; Ozguven, M.; Koltas, S. Antiviral activity of the volatile oils of Melissa officinalis L. against Herpes simplex virus type-2. Phytomedicine 2004, 11, 657–661. [Google Scholar] [CrossRef]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid Based Complement Altern. Med. 2014, 651593. [Google Scholar] [CrossRef] [Green Version]
- Manca, M.L.; Zaru, M.; Manconi, M.; Lai, F.; Valenti, D.; Sinico, C.; Fadda, A.M. Glycerosomes: A new tool for effective dermal and transdermal drug delivery. Int. J. Pharm. 2013, 455, 66–74. [Google Scholar] [CrossRef]
- de Matos, S.P.; Teixeira, H.F.; de Lima, Á.A.; Veiga-Junior, V.F.; Koester, L.S. Essential oils and isolated terpenes in nanosystems designed for topical administration: A review. Biomolecules 2019, 9, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.; Jain, P.; Umamaheshwari, R.B.; Jain, N.K. Transfersomes—a novel vesicular carrier for enhanced transdermal delivery: Development, characterization, and performance evaluation. Drug Dev. Ind. Pharm. 2003, 29, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Masada, Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry; John Wiley & Sons: New York, NY, USA, 1976. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured publishing corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- de Almeida Borges, V.R.; Ribeiro, A.F.; de Souza Anselmo, C.; Cabral, L.M.; de Sousa, V.P. Development of a high performance liquid chromatography method for quantification of isomers β-caryophyllene and α-humulene in copaiba oleoresin using the Box-Behnken design. J. Chromatogr. B 2013, 940, 35–41. [Google Scholar] [CrossRef]
- Gaonkar, R.; Yallappa, S.; Dhananjaya, B.L.; Hegde, G. Development and validation of reverse phase high performance liquid chromatography for citral analysis from essential oils. J. Chromatogr. B 2016, 1036, 50–56. [Google Scholar] [CrossRef]
- van Hoogevest, P. Review–An update on the use of oral phospholipid excipients. Eur. J. Pharm. Sci. 2017, 108, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Li, Z.; Li, N.; Feng, N. Essential oil-mediated glycerosomes increase transdermal paeoniflorin delivery: Optimization, characterization, and evaluation in vitro and in vivo. Int. J. Nanomed. 2017, 12, 3521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Vanti, G.; Bani, D.; Salvatici, M.C.; Bergonzi, M.C.; Bilia, A.R. Development and percutaneous permeation study of escinosomes, escin-based nanovesicles loaded with berberine chloride. Pharmaceutics 2019, 15, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilia, A.R.; Nardiello, P.; Piazzini, V.; Leri, M.; Bergonzi, M.C.; Bucciantini, M.; Casamenti, F. Successful Brain Delivery of Andrographolide Loaded in Human Albumin Nanoparticles to TgCRND8 Mice, an Alzheimer’s disease Mouse Model. Front Pharmacol. 2019, 10, 910. [Google Scholar] [CrossRef]
- Moreno-Bautista, G.; Tam, K.C. Evaluation of dialysis membrane process for quantifying the in vitro drug-release from colloidal drug carriers. Colloids Surf. A Physicochem. Eng. Asp. 2011, 389, 299–303. [Google Scholar] [CrossRef]
- Risaliti, L.; Kehagia, A.; Daoultzi, E.; Lazari, D.; Bergonzi, M.C.; Vergkizi-Nikolakaki, S.; Hadjipavlou-Litina, D.; Bilia, A.R. Liposomes loaded with Salvia triloba and Rosmarinus officinalis essential oils: In vitro assessment of antioxidant, antiinflammatory and antibacterial activities. J. Drug Deliv. Sci. Technol. 2019, 51, 493–498. [Google Scholar] [CrossRef]
- Asprea, M.; Tatini, F.; Piazzini, V.; Rossi, F.; Bergonzi, M.C.; Bilia, A.R. Stable, monodisperse, and highly cell-permeating nanocochleates from natural soy lecithin liposomes. Pharmaceutics 2019, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Matta, M.K.; Panagiotidis, C.A. High-mobility group protein A1 binds herpes simplex virus gene regulatory sequences and affects their expression. Arch. Virol. 2008, 153, 1251–1262. [Google Scholar] [CrossRef]
- Nishioka, Y.; Silverstein, S. Degradation of cellular mRNA during infection by herpes simplex virus. Proc. Natl. Acad. Sci. USA 1977, 74, 2370–2374. [Google Scholar] [CrossRef] [Green Version]
- Kyratsous, C.A.; Walters, M.S.; Panagiotidis, C.A.; Silverstein, S.J. Complementation of a herpes simplex virus ICP0 null mutant by varicella-zoster virus ORF61p. J. Virol. 2009, 83, 10637–10643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panagiotidis, C.A.; Silverstein, S.J. The host-cell architectural protein HMG I (Y) modulates binding of herpes simplex virus type 1 ICP4 to its cognate promoter. Virology 1999, 256, 64–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armaka, M.; Papanikolaou, E.; Sivropoulou, A.; Arsenakis, M. Antiviral properties of isoborneol, a potent inhibitor of herpes simplex virus type 1. Antivir. Res. 1999, 43, 79–92. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds and vesicles are available from the authors. |







| Constituents | % |
|---|---|
| 1-Octen-3-ol | 0.30 |
| Methyl heptenone | 1.88 |
| Limonene | 0.04 |
| cis-Ocimene | 0.05 |
| trans-Ocimene | 0.37 |
| Linalool | 0.51 |
| cis-Rose oxide | 0.11 |
| exo-Isocitral | 0.49 |
| α-trans-Necrodol | 0.56 |
| Citronellal | 4.31 |
| (Ε)-Isocitral | 1.75 |
| 4-trans-Caranone | 2.62 |
| Citronellol | 0.19 |
| Nerol | 0.20 |
| Neral | 27.31 |
| Geraniol | 0.18 |
| Methyl citronellate | 0.28 |
| Geranial | 36.73 |
| Methyl geranate | 0.34 |
| α-Copaene | 0.15 |
| β-Bourbonene | 0.14 |
| β-Cubebene | 0.06 |
| β-Elemene | 0.14 |
| β-Caryophyllene | 14.85 |
| α-Humulene | 0.76 |
| Germacrene D | 1.55 |
| α-Muurolene | 0.05 |
| γ-Cadinene | 0.06 |
| δ-Cadinene | 0.14 |
| Caryophyllene oxide | 1.09 |
| Total identified constituents | 97.21 |
| Sample | Size (nm) | PdI | ζ-potential (mV) | R (%) | EE (%) | ||
|---|---|---|---|---|---|---|---|
| Citral | β-Car | Citral | β-Car | ||||
| MEO-GS * | 83.09 ± 5.04 | 0.20 ± 0.05 | −27.85 ± 4.03 | 73.80 ± 3.11 | 79.01 ± 8.71 | 51.27 ± 2.76 | 66.04 ± 8.76 |
| Sample | Size before Extrusion (nm) | Size after Extrusion (nm) | PdI before Extrusion | PdI after Extrusion | Deformability |
|---|---|---|---|---|---|
| MEO-GS * | 83.92 ± 3.53 | 82.61 ± 2.56 | 0.25 ± 0.02 | 0.23 ± 0.01 | 1.02 ± 0.01 |
| GS ** | 80.11 ± 6.92 | 79.68 ± 4.70 | 0.39 ± 0.04 | 0.36 ± 0.03 | 1.00 ± 0.03 |
| P90G:Chol Ratio (mg/mL) | MEO Conc (mg/mL) | Hydration Time (min) | Hydration Volume (mL) | Ultrasonication Bath |
|---|---|---|---|---|
| 33:1 | 10 | 30 | 10 | no |
| 60:1 | 10 | 30 | 10 | yes |
| 60:1 | 10 | 30 | 10 | no |
| 60:1 | 10 | 30 + 30 | 5 + 5 | yes |
| 60:1 | 10 | 30 + 30 | 5 + 5 | no |
| 60:1 | 10 | 60 | 10 | no |
| 60:1 | 10 | 60 + 60 | 5 + 5 | no |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanti, G.; Ntallis, S.G.; Panagiotidis, C.A.; Dourdouni, V.; Patsoura, C.; Bergonzi, M.C.; Lazari, D.; Bilia, A.R. Glycerosome of Melissa officinalis L. Essential Oil for Effective Anti-HSV Type 1. Molecules 2020, 25, 3111. https://doi.org/10.3390/molecules25143111
Vanti G, Ntallis SG, Panagiotidis CA, Dourdouni V, Patsoura C, Bergonzi MC, Lazari D, Bilia AR. Glycerosome of Melissa officinalis L. Essential Oil for Effective Anti-HSV Type 1. Molecules. 2020; 25(14):3111. https://doi.org/10.3390/molecules25143111
Chicago/Turabian StyleVanti, Giulia, Sotirios G. Ntallis, Christos A. Panagiotidis, Virginia Dourdouni, Christina Patsoura, Maria Camilla Bergonzi, Diamanto Lazari, and Anna Rita Bilia. 2020. "Glycerosome of Melissa officinalis L. Essential Oil for Effective Anti-HSV Type 1" Molecules 25, no. 14: 3111. https://doi.org/10.3390/molecules25143111
APA StyleVanti, G., Ntallis, S. G., Panagiotidis, C. A., Dourdouni, V., Patsoura, C., Bergonzi, M. C., Lazari, D., & Bilia, A. R. (2020). Glycerosome of Melissa officinalis L. Essential Oil for Effective Anti-HSV Type 1. Molecules, 25(14), 3111. https://doi.org/10.3390/molecules25143111