5-Aryl-2-(3,5-dialkyl-4-hydroxyphenyl)-4,4-dimethyl-4H-imidazole 3-Oxides and Their Redox Species: How Antioxidant Activity of 1-Hydroxy-2,5-dihydro-1H-imidazoles Correlates with the Stability of Hybrid Phenoxyl–Nitroxides
Abstract
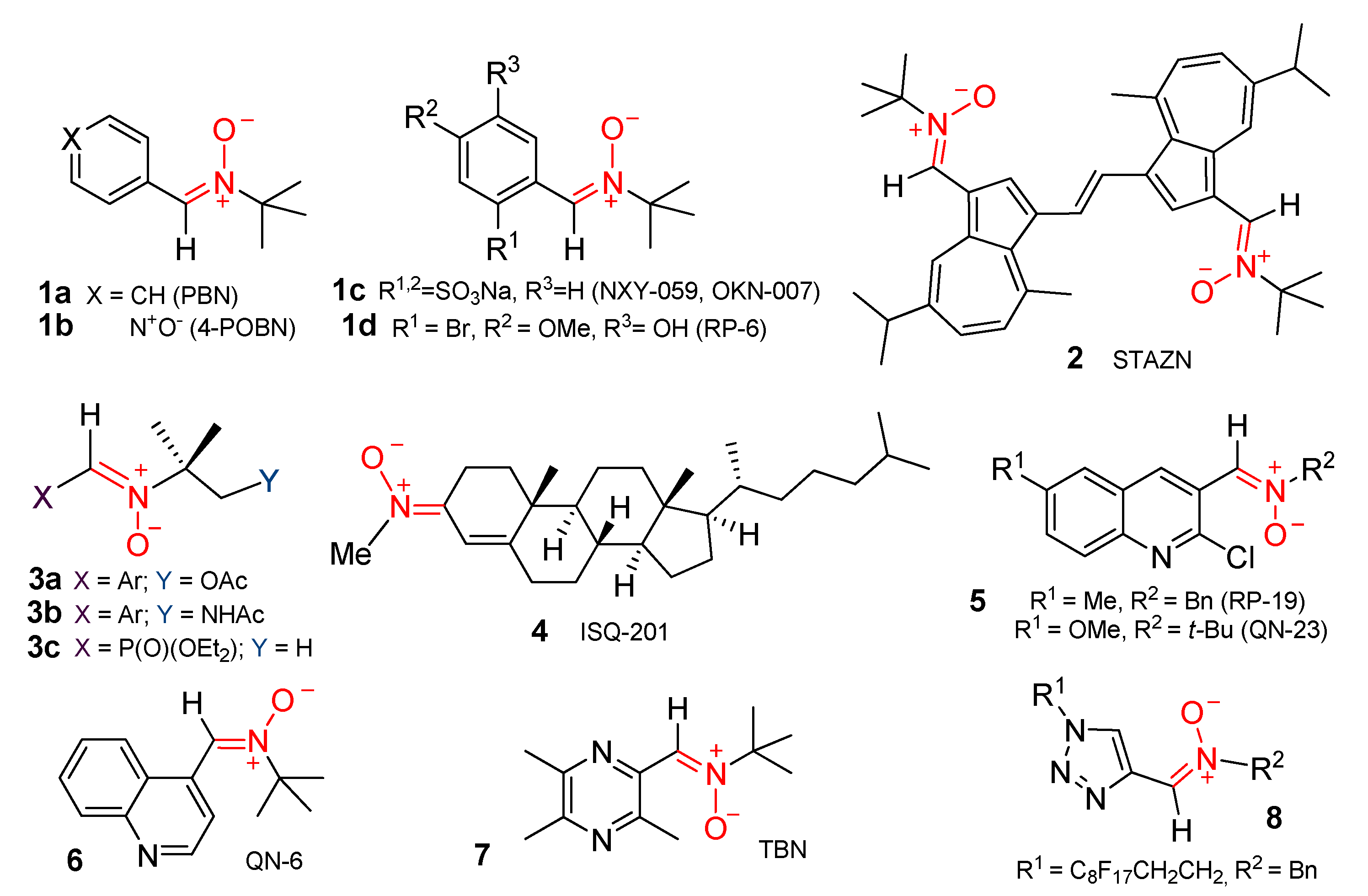
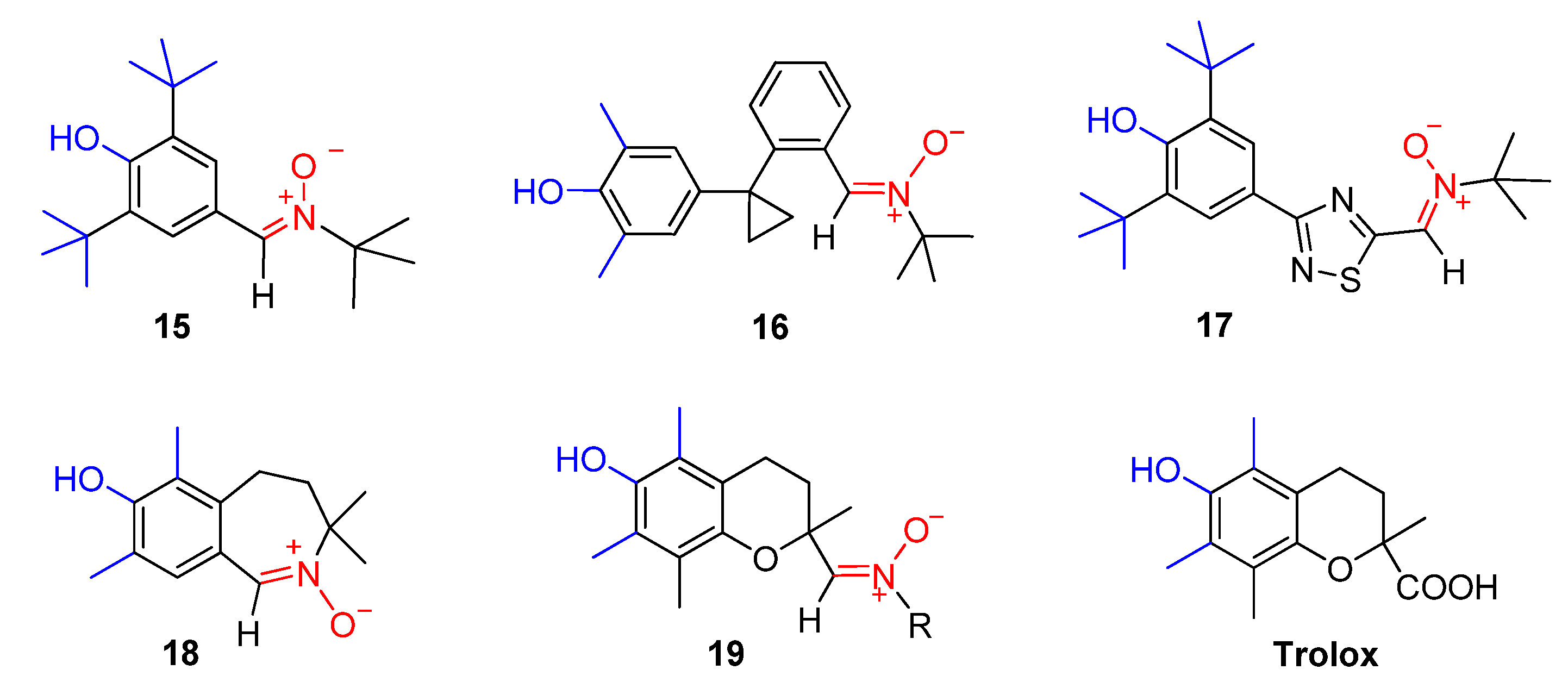
1. Introduction
- (i)
- the synthesis of 2-(3,5-dialkyl-4-hydroxyphenyl)-4,4-dimethyl-substituted imidazole derivatives 20 and 21
- (ii)
- assessment of their antiradical activity toward the model reaction of cumene oxidation, and
- (iii)
- 4H-imidazole 3-oxide 21–based preparation of new persistent HPNs 22 and their characterization by EPR in solution.
2. Results
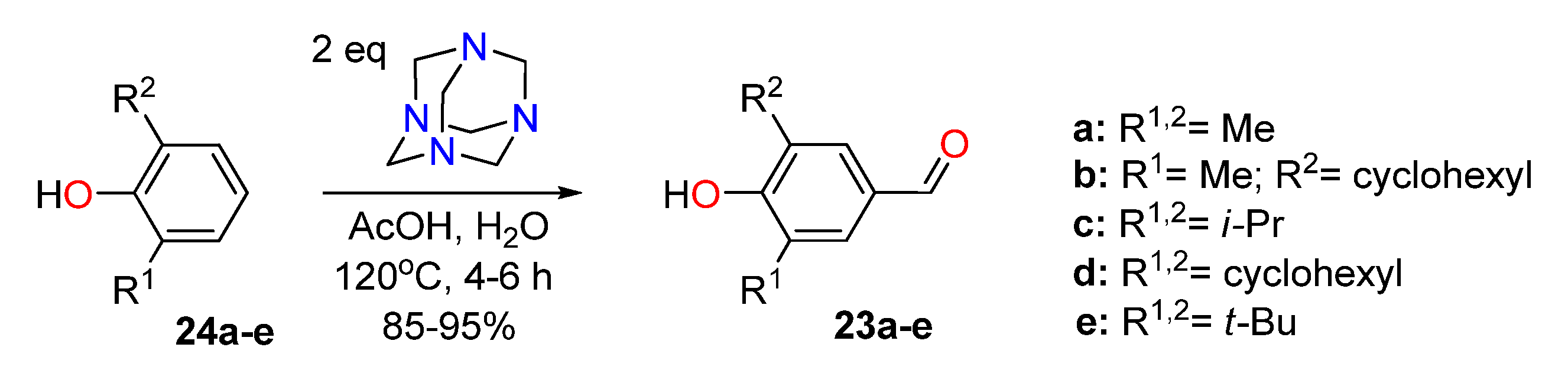
2.1. Preparation of Key Compounds
2.1.1. Synthesis of 4-formylphenols 23 and Their Condensation with p-X-Ar-substituted 2-hydroxylamino Ketones 25
2.1.2. Oxidation of 2,5-dihydroimidazoles 20 to 4H-imidazole 3-Oxides 21
2.2. Antiradical Activity of the 1-Hydroxy-2,5-Dihydroimidazole 20 and 2,5-Diaryl-4H-Imidazole 3-Oxide 21 Derivatives
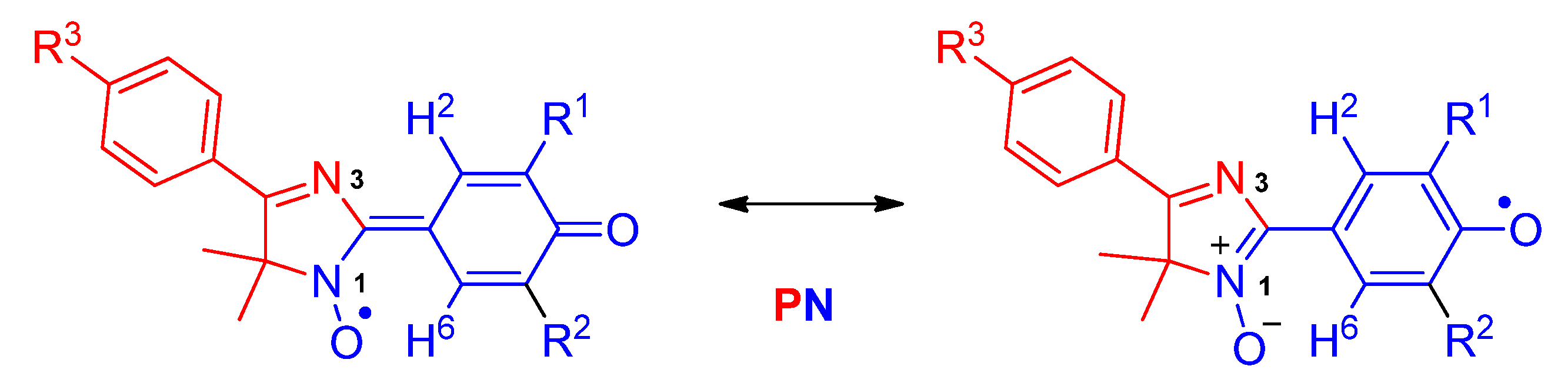
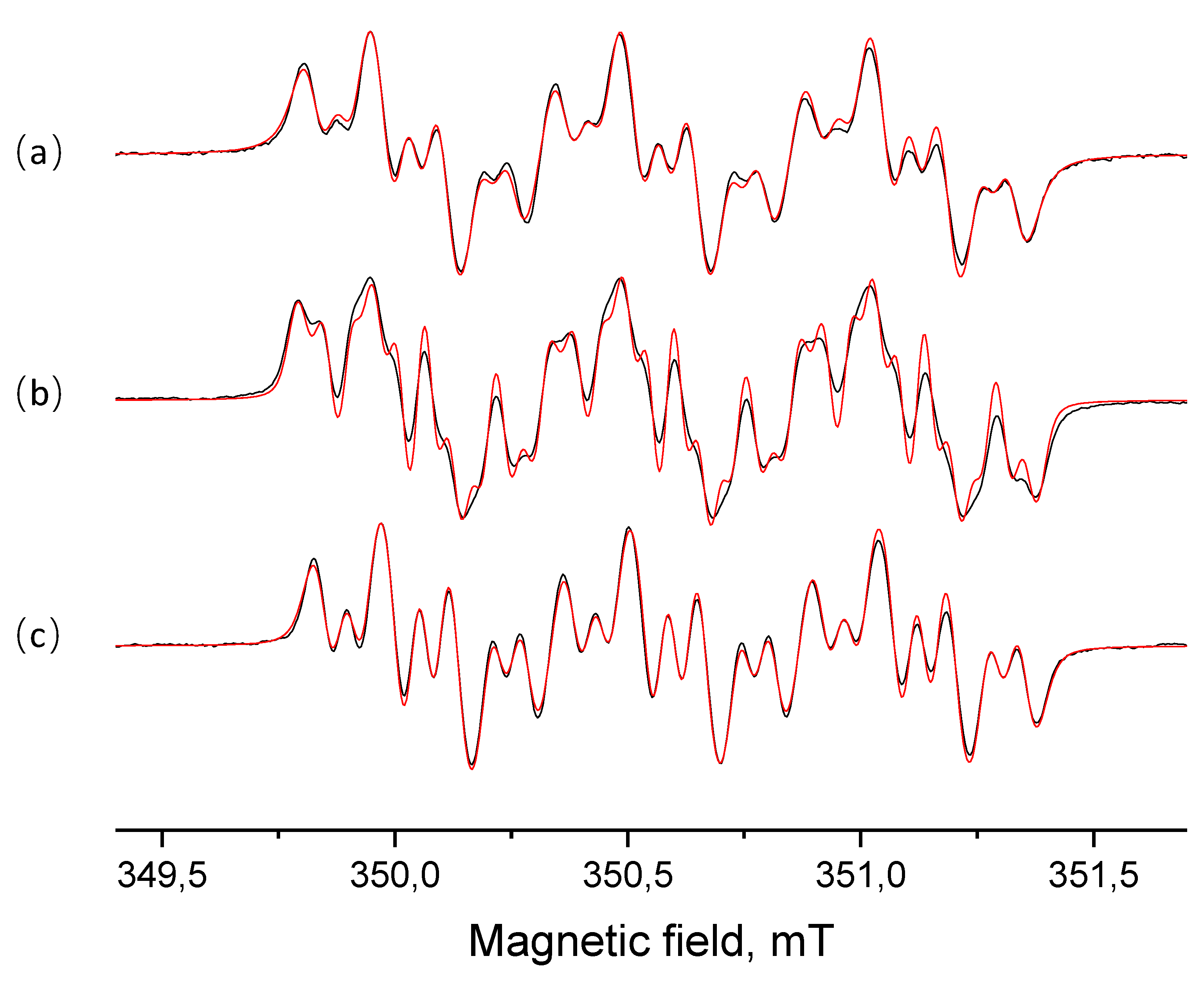
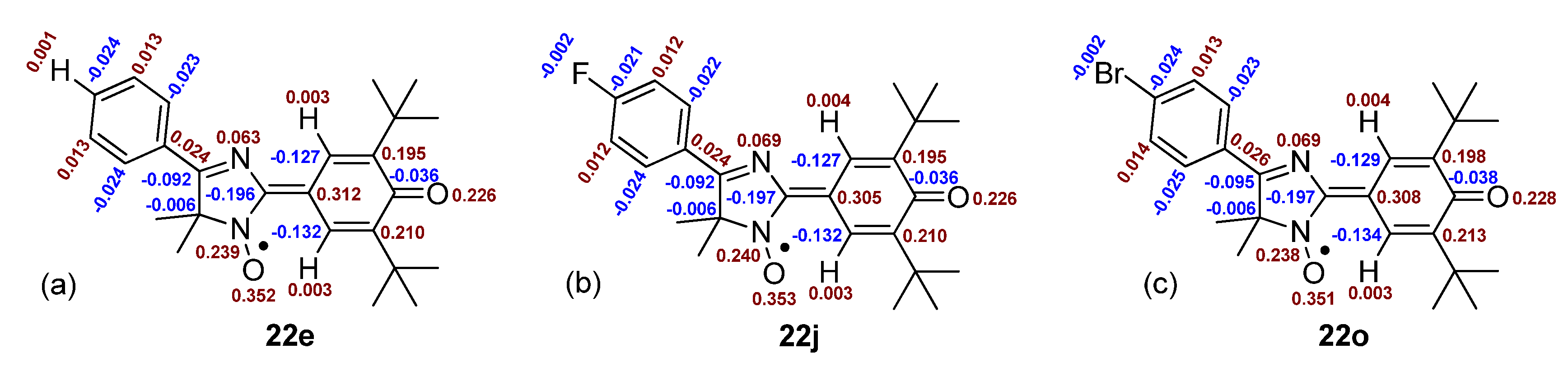
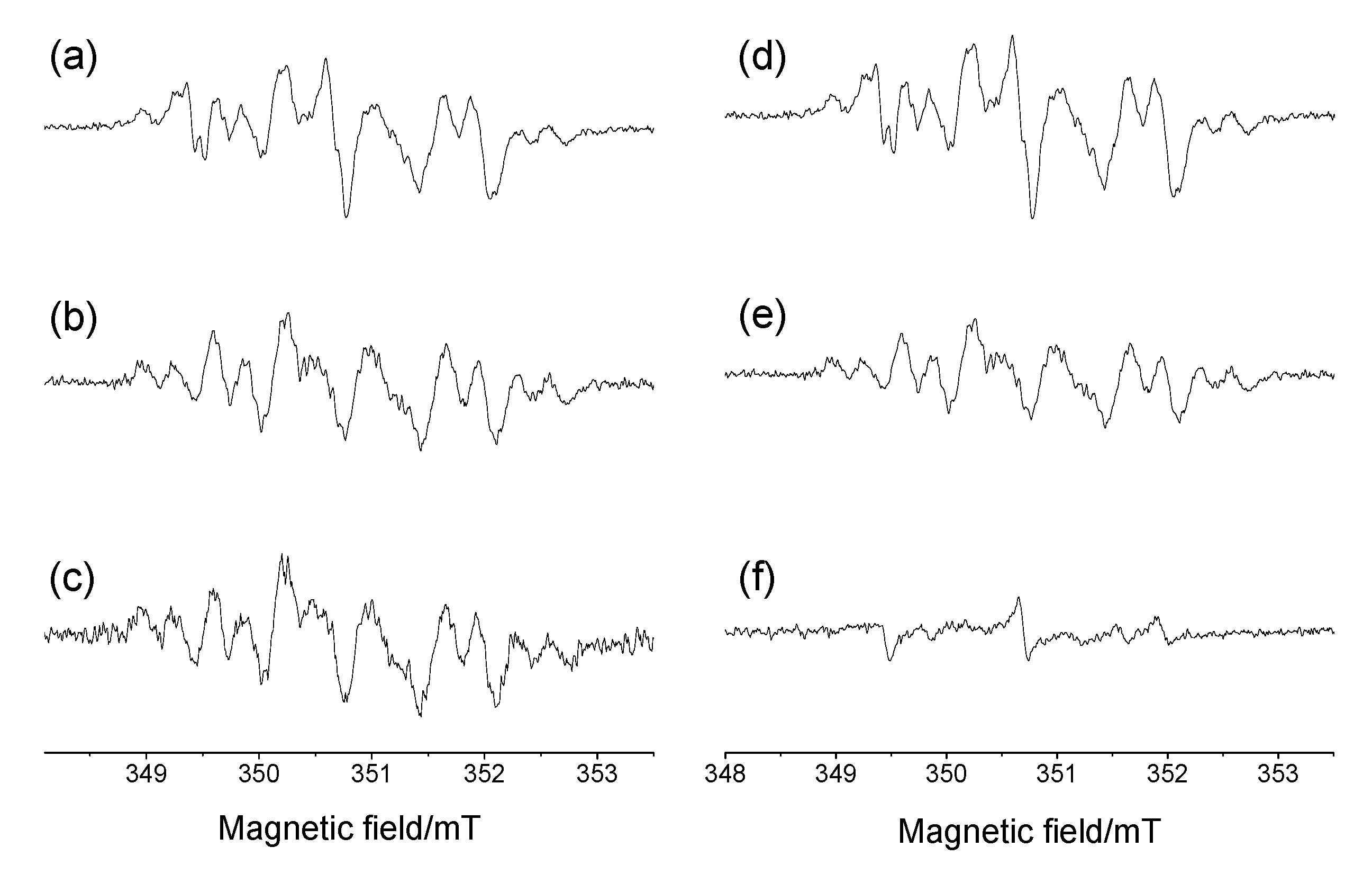
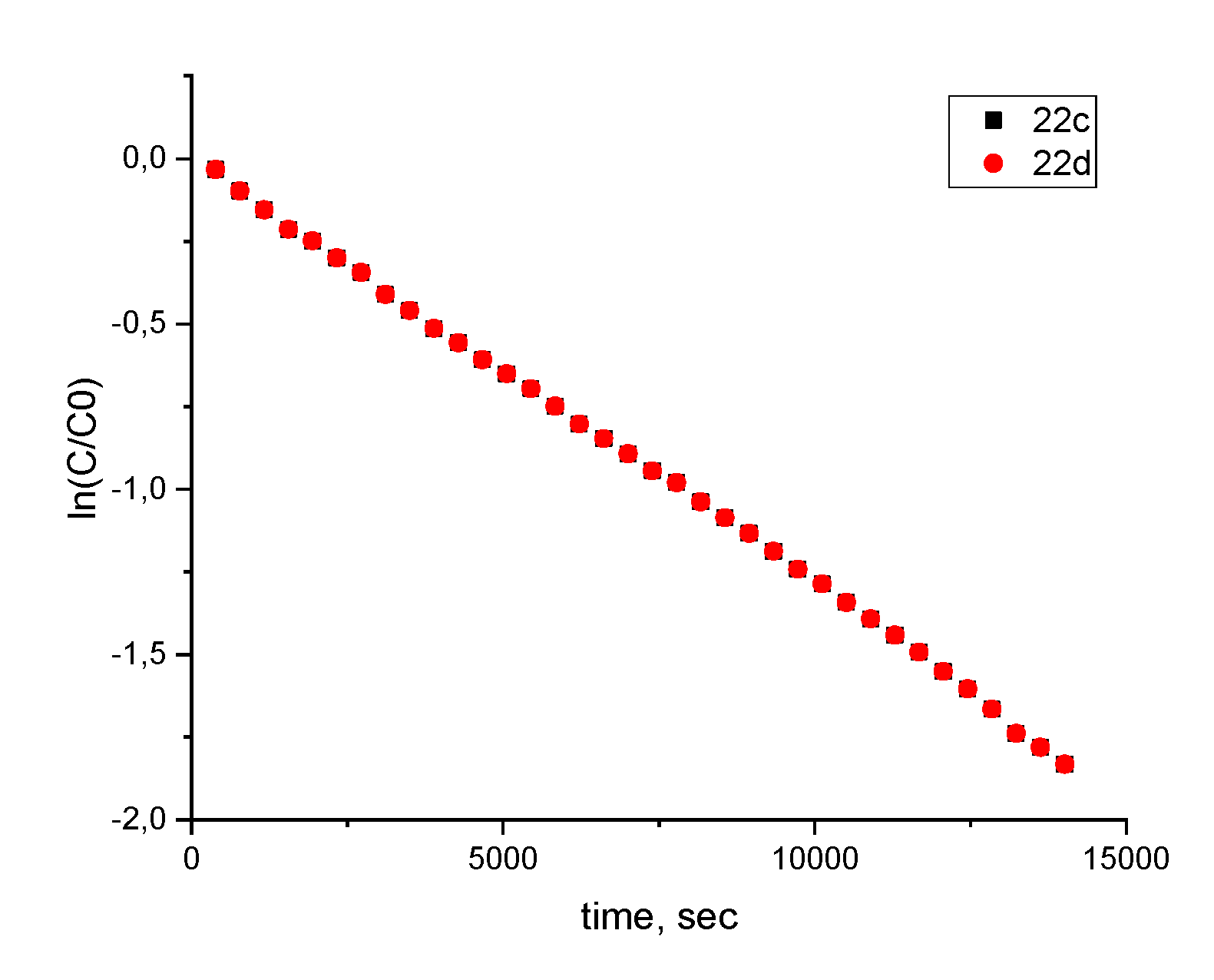
2.3. Generation and EPR Study of New Hybrid Phenoxyl–Nitroxides 22
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.2.1. General Procedure for Formylation of 2,6-dialkylphenols 24a–e Using the Synthesis of 4-hydroxy-3-cyclohexyl-5-methylbenzaldehyde (23b) as an Example.
3.2.2. General Synthetic Procedure for 2-(3,5-dialkyl-4-hydroxyphenyl)-4-aryl-1-hydroxy-4,4-dimethyl-2,5-dihydro-1H-imidazoles 20a–s.
3.2.3. General Synthetic Procedure for 5-aryl-2-(3,5-dialkyl-4-hydroxyphenyl)-4,4-dimethyl-4H-imidazole 3-oxides 21a–s
3.2.4. Preparation of Hybrid Phenoxyl–Nitroxides 22a–o
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grigor’ev, I.A. Nitrones: Novel strategies in synthesis. In Nitrile Oxides, Nitrones and Nitronates in Organic Synthesis: Novel Strategies in Synthesis, 2nd ed.; Feuer, H., Ed.; John Wiley@Sons Inc.: Hoboken, NJ, USA, 2007; pp. 129–434. [Google Scholar] [CrossRef]
- Murahashi, S.-I.; Imada, Y. Synthesis and transformations of nitrones for organic synthesis. Chem. Rev. 2019, 119, 4684–4716. [Google Scholar] [CrossRef]
- Towner, R.A.; Floyd, R.A. Nitrones as potent anticancer therapeutics. In Redox-Active Therapeutics, Oxidative Stress in Applied Basic Research and Clinical Practice; Batinić-Haberle, I., Rebouças, J.S., Spasojević, I., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 245–264. [Google Scholar] [CrossRef]
- Rosselin, M.; Poeggeler, B.; Durand, G. Nitrone derivatives as therapeutics: From chemical modification to specific-targeting. Curr. Topics Med. Chem. 2017, 17, 2006–2022. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Benfeito, S.; Fernandes, C.; Cagide, F.; Silva, T.; Borges, F. NO and HNO donors, nitrones, and nitroxides: Past, present, and future. Med. Res. Rev. 2018, 38, 1159–1187. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Peso, A.; Chioua, M.; Frezza, V.; Martínez-Alonso, E.; Marco-Contelles, J.; Alcázar, A. Nitrones, old fellows for new therapies in ischemic stroke. In Neuroprotective Therapy for Stroke and Ischemic Disease; Lapchak, P.A., Zhang, J.H., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 251–283. [Google Scholar] [CrossRef]
- Phillis, J.W.; Clough-Helfman, C. Protection from cerebral ischemic injury in gerbils with the spin trap agent N-tert-butyl-α-phenylnitrone (PBN). Neurosci. Lett. 1990, 116, 315–319. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Kadiiska, M.B.; Mason, R.P. An in vivo ESR spin-trapping study: Free radical generation in rats from formate intoxication-role of the Fenton reaction. Proc. Natl. Acad. Sci. USA 2001, 98, 13549–13553. [Google Scholar] [CrossRef]
- Deletraz, A.; Zéamari, K.; Hua, K.; Combes, M.; Villamena, F.A.; Tuccio, B.; Callizot, N.; Durand, G. Substituted α-Phenyl and α-Naphthlyl-N-tert-butyl Nitrones: Synthesis, Spin-Trapping and Neuroprotection Evaluation. J. Org. Chem. 2020, 85, 6073–6085. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, S.; Tsuchidate, R.; Smith, M.-L.; Maples, K.R.; Siesjo, B.K. Neuroprotective effects of a novel nitrone, NXY-059, after transient focal cerebral ischemia in the rat. J. Cereb. Blood Flow Metab. 1999, 19, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Battiste, J.D.; Ikeguchi, A.; Woo, S.; Sharan, S.; Zhao, Y.D.; Cohoon, A.; Sung, S.; Wright, D.; Teague, A.M.; Jensen, R.L.; et al. Phase Ib clinical trial of OKN-007 in recurrent malignant glioma. J. Clin. Oncol. 2020, 38, 2538. [Google Scholar] [CrossRef]
- Becker, D.A.; Ley, J.J.; Echegoyen, L.; Alvarado, R. Stilbazulenyl Nitrone (STAZN): A Nitronyl-substituted hydrocarbon with the potency of classical phenolic chain-breaking antioxidants. J. Amer.Chem. Soc. 2002, 124, 4678–4684. [Google Scholar] [CrossRef]
- Lapchak, P.A.; Schubert, D.R.; Maher, P.A. De-Risking of Stilbazulenyl Nitrone (STAZN), a lipophilic nitrone to treat stroke using a unique panel of in vitro assays. Transl. Stroke Res. 2011, 2, 209–217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arce, C.; Diaz-Castroverde, S.; Canales, M.J.; Marco-Contelles, J.; Samadi, A.; Oset-Gasque, M.J.; González, M.P. Drugs for stroke: Action of nitrone (Z)-N-(2-bromo-5-hydroxy-4-methoxybenzylidene)-2-methylpropan-2-amine oxide on rat cortical neurons in culture subjected to oxygeneglucose-deprivation. Eur. J. Med. Chem. 2012, 55, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, M.I.; Martínez-Alonso, E.; Chioua, M.; Escobar-Peso, A.; Gonzalo-Gobernado, R.; Montaner, J.; Marco-Contelles, J.; Alcázar, A. Quinolinyl Nitrone RP19 induces neuroprotection after transient brain ischemia. ACS Chem. Neurosci. 2017, 8, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Chioua, M.; Martínez-Alonso, E.; Gonzalo-Gobernado, R.; Ayuso, M.I.; Escobar-Peso, A.; Infantes, L.; Hadjipavlou-Litina, D.J.; Montoya, J.J.; Montaner, J.; Alcazar, A.; et al. New quinolylnitrones for stroke therapy: Antioxidant and neuroprotective (Z)-N-tert-Butyl-1-(2-chloro-6-methoxyquinolin-3- yl)methanimine Oxide as a new lead-compound for ischemic stroke treatment. J. Med. Chem. 2019, 62, 2184–2201. [Google Scholar] [CrossRef] [PubMed]
- Chioua, M.; Salgado-Ramos, M.; Diez-Iriepa, D.; Escobar-Peso, A.; Iriepa, I.; Hadjipavlou-Litina, D.; Martínez-Alonso, E.; Alcázar, A.; Marco-Contelles, J. Novel quinolylnitrones combining neuroprotective and antioxidant properties. ACS Chem. Neurosci. 2019, 10, 2703–2706. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, D.G.; Mediavilla, L.; Cuarental, L.; Głowacka, I.E.; Marco-Contelles, J.; Hadjipavlou-Litina, D.; López-Muñoz, F.; Oset-Gasque, M.J. Synthesis and neuroprotective properties of N-Substituted C-Dialkoxyphosphorylated nitrones. ACS Omega 2019, 4, 8581–8587. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, M.I.; Chioua, M.; Martínez-Alonso, E.; Soriano, E.; Montaner, J.; Masjuán, J.; Hadjipavlou-Litina, D.J.; Marco-Contelles, J.; Alcázar, A. CholesteroNitrones for stroke. J. Med. Chem. 2015, 58, 6704–6709. [Google Scholar] [CrossRef]
- Martínez-Alonso, E.; Escobar-Peso, A.; Ayuso, M.I.; Gonzalo-Gobernado, R.; Chioua, M.; Montoya, J.J.; Montaner, J.; Fernández, I.; Marco-Contelles, J.; Alcazar, A. Characterization of a CholesteroNitrone (ISQ-201), a novel drug candidate for the treatment of ischemic stroke. Antioxidants 2020, 9, 291. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, P.; Zhang, G.; Wang, L.; Zhong, H.; Zhai, Z.; Wang, L.; Wang, Y. Therapeutic effects of tetramethylpyrazine nitrone in rat ischemic stroke models. J. Neurosci. Res. 2012, 90, 1662–1669. [Google Scholar] [CrossRef]
- Rao, P.S.; Kurumurthy, C.; Veeraswamy, B.; Kumar, G.S.; Poornachandra, Y.; Kumar, C.G.; Vasamsetti, S.B.; Kotamraju, S.; Narsaiah, B. Synthesis of novel 1,2,3-triazole substituted-N-alkyl/aryl nitrones derivatives, their anti-inflammatory and anticancer activity. Eur. J. Med. Chem. 2014, 80, 184–191. [Google Scholar] [CrossRef]
- Finkelstein, E.; Rosen, G.M.; Rauckman, E.J. Spin trapping. Kinetics of the reaction of superoxide and hydroxyl radicals with nitrones. J. Amer. Chem. Soc. 1980, 102, 4994–4999. [Google Scholar] [CrossRef]
- Olive, G.; Mercier, A.; Le Moigne, F.; Rockenbauer, A.; Tordo, P. 2-Ethoxycarbonyl-2-methyl-3,4-dihydro-2H-pyrrole-1-oxide: Evaluation of the spin trapping properties. Free Rad. Bio. Med. 2000, 28, 403–408. [Google Scholar] [CrossRef]
- Zhao, H.; Joseph, J.; Zhang, H.; Karoui, H.; Kalyanaraman, B. Synthesis and biochemical applications of a solid cyclic nitrone spin trap: A relatively superior trap for detecting superoxide anions and glutathiyl radicals. Free Rad. Bio. Med. 2001, 31, 599–606. [Google Scholar] [CrossRef]
- Frejaville, C.; Karoui, H.; Tuccio, B.; Le Moigne, F.; Culcasi, M.; Pietri, S.; Lauricella, R.; Tordo, P. 5-(Diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide: A New Efficient Phosphorylated Nitrone for the in vitro and in vivo Spin Trapping of Oxygen-Centered Radicals. J. Med. Chem. 1995, 38, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.; Kirilyuk, I.; Grigor’ev, I. Spin trapping of O-, C-, and S-centered radicals and peroxynitrite by 2H-imidazole-1-oxides. Biochem. Biophys. Res. Commun. 1996, 218, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Krainev, A.G.; Williams, T.D.; Bigelow, D.J. Oxygen-centered spin adducts of 5,5-dimethyl-1-pyrroline N-oxide (DMPO) and 2H-imidazole 1-oxides. J. Magn. Res. (B) 1996, 111, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, K.; Conradi, M.; Chavant, P.-Y.; Blandin, V.; Barner-Kowollik, C.; Junkers, T. Enhanced Spin-capturing polymerization and radical coupling mediated by cyclic nitrones. Austr. J. Chem. 2012, 65, 1110–1116. [Google Scholar] [CrossRef]
- Hatano, B.; Miyoshi, K.; Sato, H.; Ito, T.; Ogata, T.; Kijima, T. Synthesis and spin trapping properties of 1,1-dimethyl-3-(trifluoromethyl)-1H-isoindole N-oxide. Tetrahedron Lett. 2010, 51, 5399–5401. [Google Scholar] [CrossRef]
- Bernotas, R.C.; Thomas, C.E.; Carr, A.A.; Nieduzak, T.R.; Adams, G.; Ohlweiler, D.F.; Hay, D.A. Synthesis and radical scavenging activity of 3,3-Dialkyl-3,4-Dihydro-isoquinoline 2-Oxides. Bioorg. Med. Chem. Lett. 1996, 6, 1105–1110. [Google Scholar] [CrossRef]
- Ramana, C.V.; Patel, P.; Vanka, K.; Miao, B.; Degterev, A. A Combined experimental and density functional theory study on the Pd-Mediated Cycloisomerization of o-Alkynylnitrobenzenes—Synthesis of Isatogens and their evaluation as modulators of ROS-Mediated cell death. Eur. J. Org. Chem. 2010, 5955–5966. [Google Scholar] [CrossRef]
- Nepveu, F.; Kim, S.; Boyer, J.; Chatriant, O.; Ibrahim, H.; Reybier, K.; Monje, M.-C.; Chevalley, S.; Perio, P.; Lajoie, B.H.; et al. Synthesis and antiplasmodial activity of new indolone N-Oxide derivatives. J. Med. Chem. 2010, 53, 699–714. [Google Scholar] [CrossRef]
- Ten, Y.A.; Salnikov, O.G.; Amitina, S.A.; Stass, D.V.; Rybalova, T.V.; Kazantsev, M.S.; Bogomyakov, A.S.; Mostovich, E.A.; Mazhukin, D.G. The Suzuki–Miyaura reaction as a tool for modification of phenoxyl-nitroxyl radicals of the 4H-imidazole N-oxide series. RSC Adv. 2018, 8, 26099–26107. [Google Scholar] [CrossRef]
- Zaytseva, E.; Shiomi, D.; Ten, Y.; Gatilov, Y.V.; Lomanovich, A.; Stass, D.; Bogomyakov, A.; Yu, A.; Sugisaki, K.; Sato, K.; et al. Magnetic Properties of π-Conjugated hybrid Phenoxyl−Nitroxide radicals with extended π-Spin delocalization. J. Phys. Chem. A 2020, 124, 2416–2426. [Google Scholar] [CrossRef]
- Pacifici, J.G.; Browning, H.L., Jr. α-(3,5-Di-tert-butyl-4-hydroxyphenyl)-N-tert-butylnitrone. A Novel Probe for Radical Detection and Identification. J. Amer. Chem. Soc. 1970, 92, 5231–5233. [Google Scholar] [CrossRef]
- Pryor, W.A.; Terauchi, K.; Davis, W.H., Jr. Electron spin resonance (ESR) study of cigarette smoke by use of spin trapping techniques. Environ. Health Perspect. 1976, 16, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, T.; Noda, Y.; Yamauchi, S.; Yamauchi, J. Multi-Frequency ESR Study of the Polycrystalline Phenoxyl Radical of α-(3,5-Di-tert-butyl-4-hydroxyphenyl)-N-tert-butylnitrone in the Diamagnetic Matrix. J. Phys. Chem. A 2006, 110, 1196–1200. [Google Scholar] [CrossRef]
- Caldwell, S.T.; Quin, C.; Edge, R.; Hartley, R.C. A Dual sensor spin trap for use with EPR spectroscopy. Org. Lett. 2007, 9, 3499–3502. [Google Scholar] [CrossRef] [PubMed]
- Porcal, W.; Hernández, P.; González, M.; Ferreira, A.; Olea-Azar, C.; Cerecetto, H.; Castro, A. Heteroarylnitrones as drugs for neurodegenerative diseases: Synthesis, neuroprotective properties, and free radical scavenger properties. J. Med. Chem. 2008, 51, 6150–6159. [Google Scholar] [CrossRef]
- Chavarria, C.; Perez, D.I.; Perez, C.; Garcia, J.A.M.; Alonso-Gil, S.; Perez-Castillo, A.; Gil, C.; Souza, J.M.; Porcal, W. Microwave-assisted synthesis of hydroxyphenyl nitrones with protective action against oxidative stress. Eur. J. Med. Chem. 2012, 58, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Chioua, M.; Sucunza, D.; Soriano, E.; Hadjipavlou-Litina, D.; Alcazar, A.; Ayuso, I.; Oset-Gasque, M.J.; Gonzalez, M.P.; Monjas, L.; Rodriguez-Franco, M.I.; et al. α-Aryl-N-alkyl nitrones, as potential agents for stroke treatment: Synthesis, theoretical calculations, antioxidant, anti-inflammatory, neuroprotective, and brain–blood barrier permeability properties. Med. Chem. 2012, 55, 153–168. [Google Scholar] [CrossRef]
- Cancela, S.; Canclini, L.; Mourglia-Ettlin, G.; Hernández, P.; Merlino, A. Neuroprotective effects of novel nitrones: In vitro and in silico studies. Eur. J. Pharm. 2020, 871, 172926. [Google Scholar] [CrossRef]
- Fevig, T.L.; Bowen, S.M.; Janowick, D.A.; Jones, B.K.; Munson, H.R.; Ohlweiler, D.F.; Thomas, C.E. Design, synthesis, and in vitro evaluation of cyclic nitrones as free radical traps for the treatment of stroke. J. Med. Chem. 1996, 39, 4988–4996. [Google Scholar] [CrossRef] [PubMed]
- Balogh, G.T.; Vukics, K.; Könczöl, A.; Kis-Varga, A.; Gere, A.; Fischer, J. Nitrone derivatives of trolox as neuroprotective agents. Bioorg. Med. Chem. Lett. 2005, 15, 3012–3015. [Google Scholar] [CrossRef]
- Decken, A.; Mailman, A.; Passmore, J.; Rautiainen, J.M.; Scherer, W.; Scheidt, E.-W. A prototype hybrid 7π quinone-fused 1,3,2-dithiazolyl radical. Dalton Trans. 2011, 40, 868–879. [Google Scholar] [CrossRef]
- Winter, S.M.; Balo, A.R.; Roberts, R.J.; Lekin, K.; Assoud, A.; Dube, P.A.; Oakley, R.T. Hybrid dithiazolothiadiazinyl radicals; versatile building blocks for magnetic and conductive materials. Chem. Commun. 2013, 49, 1603–1605. [Google Scholar] [CrossRef]
- Vasko, P.; Hurmalainen, J.; Mansikkamäki, A.; Peuronen, A.; Mailman, A.; Tuononen, H.M. Synthesis of new hybrid 1,4-thiazinyl-1,2,3-dithiazolyl radicals via Smiles rearrangement. Dalton Trans. 2017, 46, 16004–16008. [Google Scholar] [CrossRef] [PubMed]
- Buehler, E.; Brown, G.B. General Synthesis of N-Hydroxyamino Acids. J. Org. Chem. 1967, 32, 265–268. [Google Scholar] [CrossRef]
- Roginsky, V.A. Phenolic antioxidants. Reactivity and Efficiency; Nauka: Moscow, USSR, 1988; pp. 1–247. [Google Scholar]
- Oleynik, A.S.; Trubnikova, Y.N.; Kandalintseva, N.V.; Grigor’ev, I.A. Study of antioxidant properties of nitrones of 3-imidazoline 3-oxide, dihydropyrazine 1,4-dioxide, and 2H-imidazole 1-oxide series in reactions with peroxide radicals. Chem. Sustain. Develop. 2007, 15, 555–559. [Google Scholar]
- Buchachenko, A.L.; Vasserman, A.M. Stable radicals, Electronic Structure, Reactivity and Application; Khimiya: Moscow, USSR, 1973; pp. 1–408. [Google Scholar]
- Prosenko, A.E. Multifunctional Sulfur-, Nitrogen-, Phosphorus-Containing Antioxidants Based on Alkylated Phenols: Synthesis, Properties, Application Prospects. Ph.D. Thesis, Novosibirsk State Pedagogical University, Novosibirsk, Russia, 2010. [Google Scholar]
- Grigor’ev, I.A.; Kirilyuk, I.A.; Volodarskii, L.B. NMR Spectra of Cyclic Nitrones. 4. Synthesis and 13C NMR Spectra of 4H-Imidazole N-Oxides and N,N’-Dioxides. Chem. Heterocycl. Compd. 1988, 24, 1355–1362. [Google Scholar] [CrossRef]
- Tsepalov, V.F. A Method of Quantitative Analysis of Antioxidants by means of a Model Reaction of Initiated Oxidation. In Investigation of Synthetic and Natural Antioxidants In Vitro and In Vivo; Burlakova, E.B., Kruglyakova, K.E., Shishkina, L.N., Eds.; Nauka: Moscow, Russia, 1992; pp. 16–26. [Google Scholar]
- Dhimitruka, I.; Velayutham, M.; Bobko, A.A.; Khramtsov, V.V.; Villamena, F.A.; Hadad, C.M.; Zweier, J.L. Large-scale synthesis of a persistent trityl radical for use in biomedical EPR applications and imaging. Bioorg. Med. Chem. Lett. 2007, 17, 6801–6805. [Google Scholar] [CrossRef]
- Kozlikovskii, Y.B.; Koshchii, V.A.; Butov, S.A.; Sokolova, A.V. Ortho alkylation of o-, m-, and p-cresols with cyclohexene in the presence of aluminum cresolates. Zh. Org. Khim. 1987, 23, 1918–1924. [Google Scholar]
- Volodarsky, L.B. Imidazoline Nitroxides: Synthesis and Properties; CRC Press: Boca Raton, FL, USA, 1988; pp. 1–176. [Google Scholar]
- Volodarskii, L.B.; Lapik, A.S.; Russkikh, V.V.; Kobrin, V.S.; Lavretskaya, E.F.; Volkova, L.I.; Sarkisyan, D.A.; Borisov, M.M. Derivatives of (hydroxylamino) ketone with neurotropic activity. Chem. Abstr. 1979, 91, 68767. [Google Scholar]
- Jähnert, T.; Hager, M.D.; Schubert, U.S. Application of phenolic radicals for antioxidants, as active materials in batteries, magnetic materials and ligands for metal-complexes. J. Mater. Chem. A 2014, 2, 15234–15251. [Google Scholar] [CrossRef]
- Suwa, K.; Suga, T.; Oyaizu, K.; Segawa, H.; Nishide, H. Phenolic antioxidant-incorporated durable perovskite layers and their application for a solar cell. MRS Commun. 2020. [Google Scholar] [CrossRef]
- Dunn, P.; Johnson, S.I.; Kaminsky, W.; Bullock, R.M. Diversion of Catalytic C-N Bond Formation to Catalytic Oxidation of NH3 through Modification of the Hydrogen Atom Abstractor. J. Am. Chem. Soc. 2020, 142, 3361–3365. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of compounds 20a–s, 21a–s, and 22e,j,o are available from the authors (D.G.M., Y.A.T., or S.A.A.). |




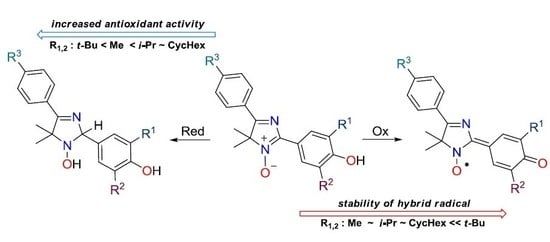
| Compound (s) | R3 | R1 | R2 | Yield of 20, % | Yield of 21, % |
|---|---|---|---|---|---|
| Number (s) | |||||
| 20–22a | H | Me | Me | 87 | 87 |
| 20–22b | H | Me | Cy * | 87 | 81 |
| 20–22c | H | i-Pr | i-Pr | 75 | 87 |
| 20–22d | H | Cy | Cy | 91 | 93 |
| 20–22e | H | t-Bu | t-Bu | 78 | 97 |
| 20–22f | F | Me | Me | 87 | 86 |
| 20–22g | F | Me | Cy | 91 | 86 |
| 20–22h | F | i-Pr | i-Pr | 85 | 96 |
| 20–22i | F | Cy | Cy | 82 | 96 |
| 20–22j | F | t-Bu | t-Bu | 76 | 90 |
| 20–22k | Br | Me | Me | 98 | 91 |
| 20–22l | Br | Me | Cy | 93 | 94 |
| 20–22m | Br | i-Pr | i-Pr | 78 | 97 |
| 20–22n | Br | Cy | Cy | 89 | 100 |
| 20–22o | Br | t-Bu | t-Bu | 88 | 93 |
| 20–21p | OH | Me | Me | 68 | 83 |
| 20–21q | OH | i-Pr | i-Pr | 70 | 90 |
| 20–21r | OH | Cy | Cy | 73 | 85 |
| 20–21s | OH | t-Bu | t-Bu | 76 | 82 |
| Compound | R3 | R1 | R2 | f | k7 × 10−4, M−1c−1 |
|---|---|---|---|---|---|
| 20a | H | Me | Me | 3.9 ± 0.3 | 5.1 ± 0.8 |
| 20b | H | Me | Cy | 4.5 ± 0.5 | 5.0 ± 1.2 |
| 20c | H | i-Pr | i-Pr | 4.0 ± 0.1 | 4.5 ± 0.1 |
| 20d | H | Cy | Cy | 4.4 ± 0.1 | 5.0 ± 0.8 |
| 20e | H | t-Bu | t-Bu | 3.2 ± 0.3 | 4.0 ± 0.3 |
| 20f | F | Me | Me | 4.1 ± 0.1 | 4.9 ± 0.4 |
| 20g | F | Me | Cy | 4.7 ± 0.2 | 5.6 ± 0.6 |
| 20h | F | i-Pr | i-Pr | 4.5 ± 0.1 | 4.5 ± 0.3 |
| 20j | F | t-Bu | t-Bu | 2.8 ± 0.3 | 4.7 ± 0.8 |
| 20k | Br | Me | Me | 4.44 ± 0.02 | 6.4 ± 0.1 |
| 20l | Br | Me | Cy | 4.83 ± 0.02 | 4.1 ± 0.5 |
| 20m | Br | i-Pr | i-Pr | 4.5 ± 0.1 | 3.7 ± 0.1 |
| 20n | Br | Cy | Cy | 4.7 ± 0.2 | 5.1 ± 0.9 |
| 20o | Br | t-Bu | t-Bu | 3.2 ± 0.2 | 4.0 ± 0.2 |
| 21a | H | Me | Me | 2.1 ± 0.1 | 6.0 ± 0.6 |
| 21b | H | Me | Cy | 2.05 ± 0.02 | 6.0 ± 0.6 |
| 21c | H | i-Pr | i-Pr | 2.1 ± 0.1 | 5.1 ± 0.5 |
| 21e | H | t-Bu | t-Bu | 1.9 ± 0.2 | 4.1 ± 0.7 |
| 21f | F | Me | Me | 2.11 ± 0.04 | 4.9±0.2 |
| 21g | F | Me | Cy | 2.1 ± 0.1 | 6.6 ± 0.9 |
| 21h | F | i-Pr | i-Pr | 2.1 ± 0.1 | 5.5 ± 0.7 |
| 21j | F | t-Bu | t-Bu | 1.9 ± 0.2 | 3.9 ± 0.3 |
| 21k | Br | Me | Me | 2.4 ± 0.1 | 5.5 ± 0.4 |
| 21l | Br | Me | CyH | 2.3 ± 0.1 | 5.2 ± 0.4 |
| 21m | Br | i-Pr | i-Pr | 2.34 ± 0.04 | 4.3 ± 0.4 |
| 21n | Br | Cy | Cy | 2.5 ± 0.1 | 5.9 ± 0.9 |
| 21o | Br | t-Bu | t-Bu | 2.0 ± 0.2 | 3.8 ± 0.1 |
| HPNs | giso | AN1, mT | AN3, mT | AH2, mT | AH6, mT | AH(Me), mT | AH(Cy), mT | AH(i-Pr), mT | AF, mT | [R2h]/[Ro] |
|---|---|---|---|---|---|---|---|---|---|---|
| 22a | 2.0049 | 0.343 | 0.045 | 0.216 | 0.100 | 0.767 | - | - | - | 0.85 |
| 0.612 | ||||||||||
| 22b | 2.0059 | 0.437 | 0.042 | 0.150 | 0.132 | 0.534 | 0.256 | - | - | 0.46 |
| 22c | 2.0063 | 0.539 | 0.066 | 0.265 | 0.265 | - | - | 0.150 | - | 0.77 |
| 0.150 | ||||||||||
| 22d | 2.0063 | 0.536 | 0.062 | 0.247 | 0.247 | - | 0.163 | - | - | 0.75 |
| 0.163 | ||||||||||
| 22e | 2.0059 | 0.550 | 0.062 | 0.163 | 0.155 | - | - | - | - | 1 |
| 22f | 2.0049 | 0.377 | 0.051 | 0.312 | 0.100 | 0.775 | - | - | - | 0.83 |
| 0.620 | ||||||||||
| 22g | 2.0059 | 0.439 | 0.042 | 0.160 | 0.130 | 0.529 | 0.258 | 0.51 | ||
| 22h | 2.0063 | 0.541 | 0.064 | 0.267 | 0.267 | - | - | 0.155 | - | 0.81 |
| 0.155 | ||||||||||
| 22i | 2.0063 | 0.539 | 0.060 | 0.249 | 0.249 | - | 0.161 | - | - | 0.79 |
| 0.161 | ||||||||||
| 22j | 2.0059 | 0.552 | 0.061 | 0.166 | 0.152 | - | - | - | 0.043 | 1 |
| 22k | 2.0049 | 0.358 | 0.049 | 0.225 | 0.066 | 0.747 | - | - | - | 0.81 |
| 0.656 | ||||||||||
| 22l | 2.0059 | 0.439 | 0.042 | 0.155 | 0.133 | 0.529 | 0.260 | - | - | 0.43 |
| 22m | 2.0064 | 0.538 | 0.065 | 0.263 | 0.263 | - | - | 0.156 | - | 0.67 |
| 0.156 | ||||||||||
| 22n | 2.0063 | 0.541 | 0.071 | 0.258 | 0.258 | - | 0.162 | - | - | 0.68 |
| 0.162 | ||||||||||
| 22o | 2.0059 | 0.548 | 0.063 | 0.163 | 0.158 | - | - | - | - | 1 |
| HPN | AN1, mT | AN3, mT | AH2, mT | AH6, mT | AH(Me), mT | AH(Cy), mT | AH(i-Pr), mT | AF, mT |
|---|---|---|---|---|---|---|---|---|
| 22a | 0.526 | 0.110 | 0.249 | 0.232 | 0.425 | - | - | - |
| 0.462 | ||||||||
| 22b | 0.523 | 0.110 | 0.231 | 0.247 | 0.459 | 0.243 | - | - |
| 22c | 0.519 | 0.110 | 0.233 | 0.250 | - | - | 0.125 | - |
| 0.134 | ||||||||
| 22d | 0.522 | 0.110 | 0.231 | 0.247 | - | 0.179 | - | - |
| 0.194 | ||||||||
| 22e | 0.525 | 0.109 | 0.228 | 0.246 | - | - | - | - |
| 22j | 0.526 | 0.110 | 0.228 | 0.246 | - | - | - | −0.122 |
| 22o | 0.522 | 0.113 | 0.232 | 0.249 | - | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amitina, S.A.; Zaytseva, E.V.; Dmitrieva, N.A.; Lomanovich, A.V.; Kandalintseva, N.V.; Ten, Y.A.; Artamonov, I.A.; Markov, A.F.; Mazhukin, D.G. 5-Aryl-2-(3,5-dialkyl-4-hydroxyphenyl)-4,4-dimethyl-4H-imidazole 3-Oxides and Their Redox Species: How Antioxidant Activity of 1-Hydroxy-2,5-dihydro-1H-imidazoles Correlates with the Stability of Hybrid Phenoxyl–Nitroxides. Molecules 2020, 25, 3118. https://doi.org/10.3390/molecules25143118
Amitina SA, Zaytseva EV, Dmitrieva NA, Lomanovich AV, Kandalintseva NV, Ten YA, Artamonov IA, Markov AF, Mazhukin DG. 5-Aryl-2-(3,5-dialkyl-4-hydroxyphenyl)-4,4-dimethyl-4H-imidazole 3-Oxides and Their Redox Species: How Antioxidant Activity of 1-Hydroxy-2,5-dihydro-1H-imidazoles Correlates with the Stability of Hybrid Phenoxyl–Nitroxides. Molecules. 2020; 25(14):3118. https://doi.org/10.3390/molecules25143118
Chicago/Turabian StyleAmitina, Svetlana A., Elena V. Zaytseva, Natalya A. Dmitrieva, Alyona V. Lomanovich, Natalya V. Kandalintseva, Yury A. Ten, Ilya A. Artamonov, Alexander F. Markov, and Dmitrii G. Mazhukin. 2020. "5-Aryl-2-(3,5-dialkyl-4-hydroxyphenyl)-4,4-dimethyl-4H-imidazole 3-Oxides and Their Redox Species: How Antioxidant Activity of 1-Hydroxy-2,5-dihydro-1H-imidazoles Correlates with the Stability of Hybrid Phenoxyl–Nitroxides" Molecules 25, no. 14: 3118. https://doi.org/10.3390/molecules25143118
APA StyleAmitina, S. A., Zaytseva, E. V., Dmitrieva, N. A., Lomanovich, A. V., Kandalintseva, N. V., Ten, Y. A., Artamonov, I. A., Markov, A. F., & Mazhukin, D. G. (2020). 5-Aryl-2-(3,5-dialkyl-4-hydroxyphenyl)-4,4-dimethyl-4H-imidazole 3-Oxides and Their Redox Species: How Antioxidant Activity of 1-Hydroxy-2,5-dihydro-1H-imidazoles Correlates with the Stability of Hybrid Phenoxyl–Nitroxides. Molecules, 25(14), 3118. https://doi.org/10.3390/molecules25143118