Synthesis of Medium-Sized Heterocycles by Transition-Metal-Catalyzed Intramolecular Cyclization
Abstract
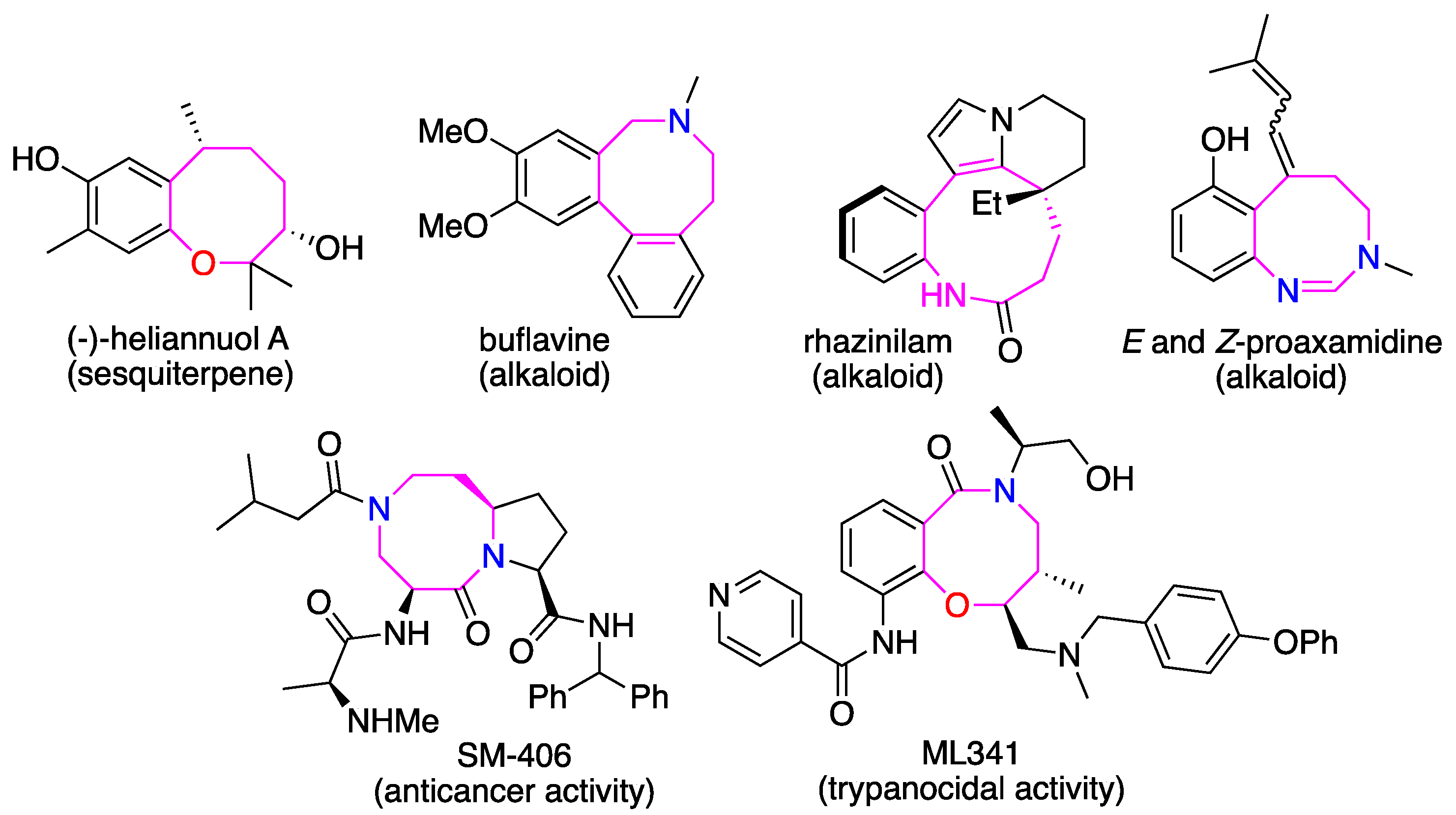
:1. Introduction
2. Methods Using Palladium-Catalyzed Reactions
2.1. Carbon–Carbon Bond Formation
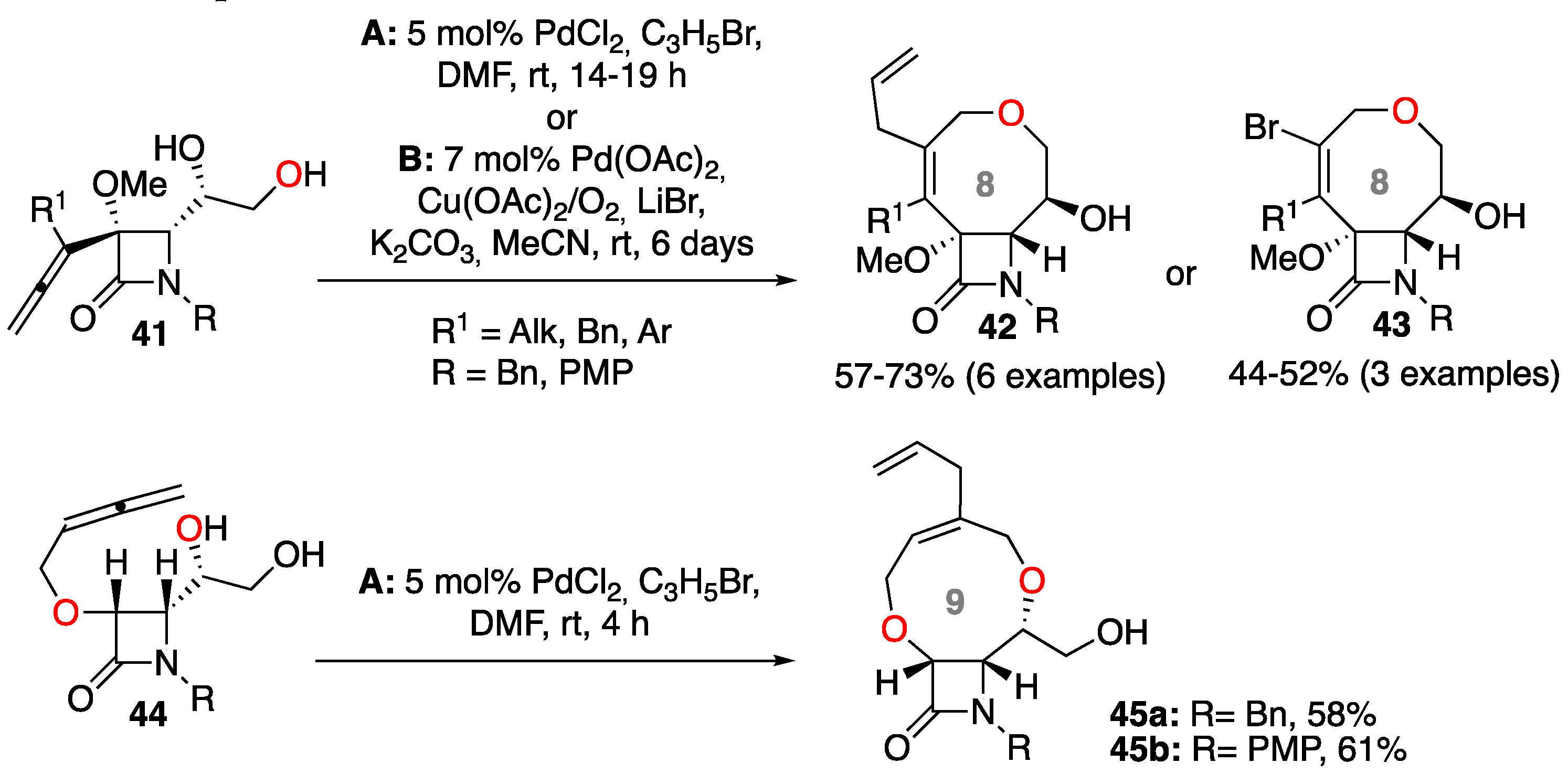
2.1.1. Intramolecular Heck Reaction
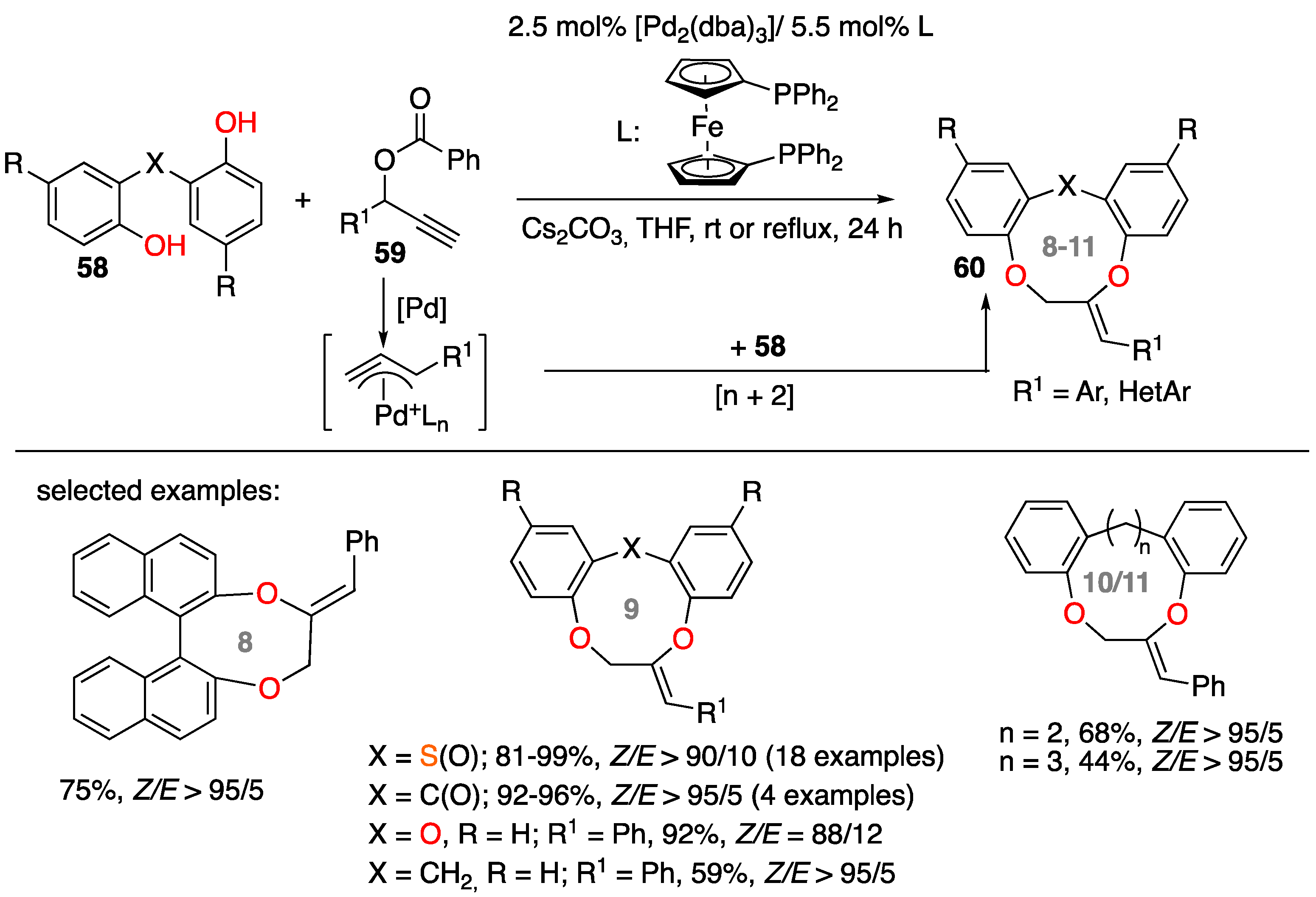
2.1.2. Intramolecular Pd-Catalyzed Cyclization of Alkynes
2.2. Carbon–Heteroatom Bond Formation
2.2.1. C–N Bond Formation
2.2.2. C–O Bond Formation
2.2.3. C–S Bond Formation
2.3. Cyclization of Bromoallenes
2.4. Formal Cycloaddition
3. Methods Using Copper-Catalyzed Reactions
3.1. Carbon–Carbon Bond Formation
3.2. Carbon-Heteroatom Bond Formation
3.2.1. C–N Bond Formation
N-arylation
N-Vinylation
Hydroamination
3.2.2. C–O Bond Formation
4. Methods Using Gold- or Silver-Catalyzed Reactions
4.1. Carbon–Carbon Bond Formation
4.2. Carbon–Heteroatom Bond Formation
C–O Bond Formation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakamura, I.; Yamamoto, Y. Transition-Metal-Catalyzed Reactions in Heterocyclic Synthesis. Chem. Rev. 2004, 104, 2127–2198. [Google Scholar] [CrossRef] [PubMed]
- Gulevich, A.V.; Dudnik, A.S.; Chernyak, N.; Gevorgyan, V. Transition Metal-Mediated Synthesis of Monocyclic Aromatic Heterocycles. Chem. Rev. 2013, 113, 3084–3213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newkome, G.R. Eight-Membered and Larger Rings. In Progress in Heterocyclic Chemistry; Suschitzky, H., Scriven, E.F.V., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 3, pp. 319–330. [Google Scholar]
- Quirke, J.M.E. Eight-Membered and Larger Rings Systems. In Heterocyclic Chemistry; Suschitzky, H., Ed.; The Royal Society of Chemistry’s Books; Royal Society of Chemistry: London, UK, 1986; Volume 5, pp. 455–481. [Google Scholar]
- Illuminati, G.; Mandolini, L. Ring Closure Reactions of Bifunctional Chain Molecules. Acc. Chem. Res. 1981, 14, 95–102. [Google Scholar] [CrossRef]
- Galli, C.; Mandolini, L. The Role of Ring Strain on the Ease of Ring Closure of Bifunctional Chain Molecules. Eur. J. Org. Chem. 2000, 2000, 3117–3125. [Google Scholar] [CrossRef]
- Maier, M.E. Synthesis of Medium-Sized Rings by the Ring-Closing Metathesis Reaction. Angew. Chem. Int. Ed. 2000, 39, 2073–2077. [Google Scholar] [CrossRef]
- Michaut, A.; Rodriguez, J. Selective Construction of Carbocyclic Eight-Membered Rings by Ring-Closing Metathesis of Acyclic Precursors. Angew. Chem. Int. Ed. 2006, 45, 5740–5750. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.K.; Karmakar, S.; Biswas, T.; Majumdar, K.C.; Rahaman, H.; Roy, B. Formation of medium-ring heterocycles by diene and enyne metathesis. Tetrahedron 2007, 63, 3919–3952. [Google Scholar] [CrossRef]
- Yet, L. Free Radicals in the Synthesis of Medium-Sized Rings. Tetrahedron 1999, 55, 9349–9403. [Google Scholar] [CrossRef]
- Molander, G.A. Diverse Methods for Medium Ring Synthesis. Acc. Chem. Res. 1998, 31, 603–609. [Google Scholar] [CrossRef]
- Majhi, T.P.; Achari, B.; Chattopadhyay, P. Advances in the Synthesis and Biological Perspectives of Benzannulated Medium Ring Heterocycles. Heterocycles 2007, 71, 1011–1052. [Google Scholar] [CrossRef]
- Donald, J.R.; Unsworth, W.P. Ring-Expansion Reactions in the Synthesis of Macrocycles and Medium-Sized Rings. Chem. Eur. J. 2017, 23, 8780–8799. [Google Scholar] [CrossRef] [PubMed]
- Yet, L. Metal-Mediated Synthesis of Medium-Sized Rings. Chem. Rev. 2000, 100, 2963–3007. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N. Metal catalysts: Applications in higher-membered N-heterocycles synthesis. J. Iran. Chem. Soc. 2015, 12, 9–45. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Chattopadhyay, B. New Synthetic Strategies for Medium-Sized and Macrocyclic Compounds by Palladium-Catalyzed Cyclization. Curr. Org. Chem. 2009, 13, 731–757. [Google Scholar] [CrossRef]
- Majumdar, K.C. Regioselective formation of medium-ring heterocycles of biological relevance by intramolecular cyclization. RSC Adv. 2011, 1, 1152–1170. [Google Scholar] [CrossRef]
- Sharma, A.; Appukkuttan, P.; Van der Eycken, E. Microwave-assisted synthesis of medium-sized heterocycles. Chem. Commun. 2012, 48, 1623–1637. [Google Scholar] [CrossRef]
- Ma, S.; Negishi, E. Palladium-Catalyzed Cyclization of Haloallenes. A New General Route to Common, Medium, and Large Ring Compounds via Cyclic Carbopalladation. J. Am. Chem. Soc. 1995, 117, 6345–6357. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Ansary, I.; Sinha, B.; Chattopadhyay, B. Palladium(0)-Catalyzed Intramolecular Heck Reaction: A Resourceful Route for the Synthesis of Naphthoxepine and Naphthoxocine Derivatives. Synthesis 2009, 2009, 3593–3602. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Chattopadhyay, B.; Sinha, B. Novel Synthesis of Oxathiocine Derivatives by Wittig Olefination and Intramolecular Heck Reaction via an 8-endo-trig Cyclization. Synthesis 2008, 2008, 3857–3863. [Google Scholar] [CrossRef]
- Nandi, S.; Singha, R.; Ray, J.K. Palladium catalyzed intramolecular cascade type cyclizations: Interesting Approach towards naphthoquinone derivatives having an O-containing heterocyclic skeleton. Tetrahedron 2015, 71, 669–675. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Chattopadhyay, B. Novel Synthesis of Nine-Membered Oxa-Heterocycles by Pd(0)-Catalyzed Intramolecular Heck Reaction via Unusual 9-endo-trig-Mode Cyclization. Synlett 2008, 2008, 979–982. [Google Scholar] [CrossRef]
- Arnold, L.A.; Luo, W.; Guy, R.K. Synthesis of Medium Ring Heterocycles Using an Intramolecular Heck Reaction. Org. Lett. 2004, 6, 3005–3007. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Yousuf, S.K.; Sharma, D.K.; Mallikharjuna Rao, L.M.; Singh, B.; Mukherjee, D. Design and synthesis of carbohydrate based medium sized sulfur containing benzannulated macrocycles: Applications of Sonogashira and Heck coupling. Tetrahedron 2013, 69, 5517–5524. [Google Scholar] [CrossRef]
- Jeffery, T. Palladium-catalysed vinylation of organic halides under solid–liquid phase transfer conditions. J. Chem. Soc. Chem. Commun. 1984, 19, 1287–1289. [Google Scholar] [CrossRef]
- Gibson (Thomas), S.E.; Guillo, N.; Middleton, R.J.; Thuilliez, A.; Tozer, M.J. Synthesis of conformationally constrained phenylalanine analogues via 7-, 8- and 9-endo Heck cyclisations. J. Chem. Soc. Perkin Trans. 1 1997, 1, 447–455. [Google Scholar] [CrossRef]
- Sunderhaus, J.D.; Dockendorff, C.; Martin, S.F. Applications of Multicomponent Reactions for the Synthesis of Diverse Heterocyclic Scaffolds. Org. Lett. 2007, 9, 4223–4226. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, K.C.; Mondal, S.; Ghosh, D. Concise Synthesis of Pyrimido-azocine Derivatives via Aza-Claisen Rearrangement and Intramolecular Heck Reaction. Synthesis 2010, 2010, 1315–1320. [Google Scholar] [CrossRef]
- Bowie, A.L.; Chambers, C.H.; Trauner, D. Concise synthesis of (±)-rhazinilam through direct coupling. Org. Lett. 2005, 7, 5207–5209. [Google Scholar] [CrossRef]
- Huang, L.; Shi, M. A cascade reaction of pyrrole-2-carbaldehyde substituted Morita–Baylis–Hillman adducts in the presence of tetrabutylammonium hydroxide or acetate to construct aza-heterocycles. Chem. Commun. 2012, 48, 4501–4503. [Google Scholar] [CrossRef]
- Yamamoto, Y. Synthesis of heterocycles via transition-metal- catalyzed hydroarylation of alkynes. Chem. Soc. Rev. 2014, 43, 1575–1600. [Google Scholar] [CrossRef]
- Düfert, A.; Werz, D.B. Carbopalladation Cascades Using Carbon–Carbon Triple Bonds: Recent Advances to Access Complex Scaffolds. Chem. Eur. J. 2016, 22, 16718–16732. [Google Scholar] [CrossRef]
- Blouin, S.; Blond, G.; Donnard, M.; Gulea, M.; Suffert, J. Cyclocarbopalladation as a Key Step in Cascade Reactions: Recent Developments. Synthesis 2017, 49, 1767–1784. [Google Scholar]
- Donets, P.A.; Van der Eycken, E.V. Efficient Synthesis of the 3-Benzazepine Framework via Intramolecular Heck Reductive Cyclization. Org. Lett. 2007, 9, 3017–3020. [Google Scholar] [CrossRef] [PubMed]
- Peshkov, V.A.; Van Hove, S.; Donets, P.A.; Pereshivko, O.P.; Van Hecke, K.; Van Meervelt, L.; Van der Eycken, E. Synthesis of the Azocino[cd]indole Framework through Pd-Catalyzed Intramolecular Acetylene Hydroarylation. Eur. J. Org. Chem. 2011, 2011, 1837–1840. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Ghosh, T.; Chakravorty, S. Palladium-mediated reductive Mizoroki–Heck cyclization strategy for the regioselective formation of dibenzoazocinone framework. Tet. Lett. 2010, 51, 3372–3375. [Google Scholar] [CrossRef]
- Greenaway, R.L.; Campbell, C.D.; Chapman, H.A.; Anderson, E.A. Reductive Cyclization of Bromoenynamides with Alcohols as Hydride Source: Synthesis and Reactions of 2-Amidodienes. Adv. Synth. Catal. 2012, 354, 3187–3194. [Google Scholar] [CrossRef]
- Castanheiro, T.; Donnard, M.; Gulea, M.; Suffert, J. Cyclocarbopalladation/Cross-Coupling Cascade Reactions in Sulfide Series: Access to Sulfur Heterocycles. Org. Lett. 2014, 16, 3060–3063. [Google Scholar] [CrossRef]
- Castanheiro, T.; Schoenfelder, A.; Suffert, J.; Donnard, M.; Gulea, M. Comparative study on the reactivity of propargyl and alkynylsulfides in palladium-catalyzed domino reactions. Comptes Rendus Chim. 2017, 20, 624–633. [Google Scholar] [CrossRef]
- Castanheiro, T.; Schoenfelder, A.; Donnard, M.; Chataigner, I.; Gulea, M. Synthesis of Sulfur-Containing Exo-Bicyclic Dienes and Their Diels—Alder Reactions to Access Thiacycle-Fused Polycyclic Systems. J. Org. Chem. 2018, 83, 4505–4515. [Google Scholar] [CrossRef]
- Basilio Lopes, A.; Wagner, P.; Gulea, M. Synthesis of Benzimidazole-Fused Medium-Sized N,S-Heterocycles via Palladium-Catalyzed Cyclizations. Eur. J. Org. Chem. 2019, 2019, 1361–1370. [Google Scholar] [CrossRef]
- Basilio Lopes, A.; Choury, M.; Wagner, P.; Gulea, M. Tandem Double-Cross-Coupling/Hydrothiolation Reaction of 2-Sulfenyl Benzimidazoles with Boronic Acids. Org. Lett. 2019, 21, 5943–5947. [Google Scholar] [CrossRef] [PubMed]
- Grigg, R.; Savic, V.; Sridharan, V.; Terrier, C. Palladium catalysed queuing processes. Part 4: Termolecular cyclisation–anion capture cascades employing allene as a relay switch and secondary amines as nucleophiles. Tetrahedron 2002, 58, 8613–8620. [Google Scholar] [CrossRef]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef] [PubMed]
- Bariwal, J.; Van der Eycken, E. C–N bond forming cross-coupling reactions: An overview. Chem. Soc. Rev. 2013, 42, 9283–9303. [Google Scholar] [CrossRef] [PubMed]
- Cuny, G.; Bois-Choussy, M.; Zhu, J. One-Pot Synthesis of Polyheterocycles by a Palladium-Catalyzed Intramolecular N-Arylation/ C-H Activation/Aryl–Aryl Bond-Forming Domino Process. Angew. Chem. Int. Ed. 2003, 42, 4774–4777. [Google Scholar] [CrossRef] [PubMed]
- Neogi, A.; Majhi, T.P.; Mukhopadhyay, R.; Chattopadhyay, P. Palladium-Mediated Intramolecular Aryl Amination on Furanose Derivatives: An Expedient Approach to the Synthesis of Chiral Benzoxazocine Derivatives and Tricyclic Nucleosides. J. Org. Chem. 2006, 71, 3291–3294. [Google Scholar] [CrossRef] [PubMed]
- Mari, M.; Bartoccini, F.; Piersanti, G. Synthesis of (−)-Epi-Indolactam V by an Intramolecular Buchwald–Hartwig C–N Coupling Cyclization Reaction. J. Org. Chem. 2013, 78, 7727–7734. [Google Scholar] [CrossRef]
- Balàzs, A.; Hetényi, A.; Szakonyi, Z.; Sillanpää, R.; Fülöp, F. Solvent-Enhanced Diastereo- and Regioselectivity in the PdII-Catalyzed Synthesis of Six- and Eight-Membered Heterocycles via cis-Aminopalladation. Chem. Eur. J. 2009, 15, 7376–7381. [Google Scholar] [CrossRef]
- Ohno, H.; Hamaguchi, H.; Ohata, M.; Kosaka, S.; Tanaka, T. Palladium(0)-Catalyzed Synthesis of Medium-Sized Heterocycles by Using Bromoallenes as an Allyl Dication Equivalent. J. Am. Chem. Soc. 2004, 126, 8744–8754. [Google Scholar] [CrossRef]
- Larock, R.C.; Tu, C.; Pace, P. Synthesis of Medium-Ring Nitrogen Heterocycles via Palladium-Catalyzed Heteroannulation of 1,2-Dienes. J. Org. Chem. 1998, 63, 6859–6866. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-M.; Alper, H. Intramolecular Carbonylation Reactions with Recyclable Palladium-Complexed Dendrimers on Silica: Synthesis of Oxygen, Nitrogen, or Sulfur-Containing Medium Ring Fused Heterocycles. J. Am. Chem. Soc. 2005, 127, 14776–14784. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, B.; Almendros, P.; Carrascosa, R.; Casarrubios, L.; Soriano, E. A Versatile Synthesis of -Lactam-Fused Oxacycles through the Palladium-Catalyzed Chemo-, Regio-, and Diastereoselective Cyclization of Allenic Diols. Chem. Eur. J. 2015, 21, 2200–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bürger, M.; Loch, M.N.; Jones, P.G.; Werz, D.B. From 1,2-difunctionalisation to cyanide-transfer cascades–Pd-catalysed cyanosulfenylation of internal (oligo)alkynes. Chem. Sci. 2020, 11, 1912–1917. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.-C.; Rong, Z.-Q.; Wang, Y.-N.; Tan, Z.Y.; Wang, M.; Zhao, Y. Construction of Nine-Membered Heterocycles through Palladium- Catalyzed Formal [5+4] Cycloaddition. Angew. Chem. Int. Ed. 2017, 56, 2927–2931. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.-Q.; Yang, L.-C.; Liu, S.; Yu, Z.; Wang, Y.-N.; Tan, Z.Y.; Huang, R.-Z.; Lan, Y.; Zhao, Y. Nine-Membered Benzofuran-Fused Heterocycles: Enantioselective Synthesis by Pd-Catalysis and Rearrangement via Transannular Bond Formation. J. Am. Chem. Soc. 2017, 139, 15304–15307. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, Y.; Wang, D.; Zhang, Z.; Wang, C.; Zhou, L.; Zhang, C.; Song, B.; Guo, H. Formal [5 + 3] Cycloaddition of Zwitterionic Allylpalladium Intermediates with Azomethine Imines for Construction of N,O- Containing Eight-Membered Heterocycles. Adv. Synth. Catal. 2018, 360, 652–658. [Google Scholar] [CrossRef]
- Liu, Z.-T.; Hu, X.-P. Palladium-Catalyzed Propargylic [n + 2] Cycloaddition: An Efficient Strategy for Construction of Benzo-Fused Medium-Sized Heterocycles. Adv. Synth. Catal. 2019, 361, 836–841. [Google Scholar] [CrossRef]
- Sambiagio, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper catalysed Ullmann type chemistry: From mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550. [Google Scholar] [CrossRef]
- Evano, G. Blanchard, N. Copper-Mediated Cross-Coupling Reactions; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Bhunia, S.; Pawar, G.G.; Vijay Kumar, S.V.; Jiang, Y.; Ma, D. Selected Copper-Based Reactions for C-N, C-O, C-S, and C-C Bond Formation. Angew. Chem. Int. Ed. 2017, 56, 16136–16179. [Google Scholar] [CrossRef]
- Surry, D.S.; Buchwald, S.L. Diamine ligands in copper-catalyzed reactions. Chem. Sci. 2010, 1, 13–31. [Google Scholar] [CrossRef] [Green Version]
- Spring, D.R.; Krishnan, S.; Schreiber, S.L. Towards Diversity-Oriented, Stereoselective Syntheses of Biaryl- or Bis(aryl)metal-Containing Medium Rings. J. Am. Chem. Soc. 2000, 122, 5656–5657. [Google Scholar] [CrossRef]
- Vo, C.-V.T.; Luescher, M.U.; Bode, J.W. SnAP reagents for the one-step synthesis of medium-ring saturated N-heterocycles from aldehydes. Nat. Chem. 2014, 6, 310–314. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.-D.; Zhu, X.-Q.; Bu, H.-Z.; Deng, G.; Chen, Y.-B.; Liu, R.-S.; Ye, L.-W. Copper-Catalyzed Cascade Cyclization of Indolyl Homopropargyl Amides: Stereospecific Construction of Bridged Aza-[n.2.1] Skeletons. Angew. Chem. Int. Ed. 2019, 58, 9632–9639. [Google Scholar] [CrossRef]
- Rolfe, A.; Lushington, G.H.; Hanson, P.R. Reagent based DOS: A “Click, Click, Cyclize” strategy to probe chemical space. Org. Biomol. Chem. 2010, 8, 2198–2203. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Lin, C.; Fu, H.; Jiang, Y.; Zhao, Y. Copper-Catalyzed Synthesis of Medium- and Large-Sized Nitrogen Heterocycles via N-Arylation of Phosphoramidates and Carbamates. Org. Lett. 2005, 7, 4781–4784. [Google Scholar] [CrossRef]
- Srinivasulu, V.; Janda, K.D.; Abu-Yousef, I.A.; O’Connor, M.J.; Al-Tel, T.H. A modular CuI-L-proline catalyzed one-pot route for the rapid access of constrained and privileged hetero-atom-linked medium-sized ring systems. Tetrahedron 2017, 73, 2139–2150. [Google Scholar] [CrossRef]
- Lu, H.; Yuan, X.; Zhu, S.; Sun, C.; Li, C. Copper-Catalyzed Intramolecular N-Vinylation of Sulfonamides: General and Efficient Synthesis of Heterocyclic Enamines and Macrolactams. J. Org. Chem. 2008, 73, 8665–8668. [Google Scholar] [CrossRef]
- Dai, X.-J.; Engl, O.D.; León, T.; Buchwald, S.L. Catalytic Asymmetric Synthesis of α-Arylpyrrolidines and Benzo-fused Nitrogen Heterocycles. Angew. Chem. Int. Ed. 2019, 58, 3407–3411. [Google Scholar] [CrossRef] [PubMed]
- Kenwright, J.L.; Galloway, W.R.J.D.; Blackwell, D.T.; Isidro-Llobet, A.; Hodgkinson, J.; Wortmann, L.; Bowden, S.D.; Welch, M.; Spring, D.R. Novel and Efficient Copper-Catalysed Synthesis of Nitrogen-Linked Medium-Ring Biaryls. Chem. Eur. J. 2011, 17, 2981–2986. [Google Scholar] [CrossRef]
- Mestichelli, P.; Scott, M.J.; Galloway, W.R.J.D.; Selwyn, J.; Parker, J.S.; Spring, D.R. Concise Copper-Catalyzed Synthesis of Tricyclic Biaryl Ether-Linked Aza-Heterocyclic Ring Systems. Org. Lett. 2013, 15, 5448–5451. [Google Scholar] [CrossRef]
- Bandini, M. (Ed.) Topics in Heterocyclic Chemistry. In Au-Catalyzed Synthesis and Functionalization of Heterocycles; Springer International Publishing: Cham, Switzerland, 2016; Volume 46. [Google Scholar] [CrossRef]
- Ferrer, C.; Echavarren, A.M. Gold-Catalyzed Intramolecular Reaction of Indoles with Alkynes: Facile Formation of Eight-Membered Rings and an Unexpected Allenylation. Angew. Chem. Int. Ed. 2006, 45, 1105–1109. [Google Scholar] [CrossRef]
- Ferrer, C.; Amijs, C.H.M.; Echavarren, A.M. Intra- and Intermolecular Reactions of Indoles with Alkynes Catalyzed by Gold. Chem. Eur. J. 2007, 13, 1358–1373. [Google Scholar] [CrossRef]
- Ferrer, C.; Escribano-Cuesta, A.; Echavarren, A.M. Synthesis of the Tetracyclic Core Skeleton of the Lundurines by a Gold-Catalyzed Cyclization. Tetrahedron 2009, 65, 9015–9020. [Google Scholar] [CrossRef]
- Peshkov, V.A.; Pereshivko, O.P.; Van der Eycken, E.V. Synthesis of Azocino[5,4- b ]Indoles via Gold-Catalyzed Intramolecular Alkyne Hydroarylation. Adv. Synth. Catal. 2012, 354, 2841–2848. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Inuki, S.; Tokimizu, Y.; Oishi, S.; Ohno, H. Gold(I)-Catalyzed Cascade Cyclization of Anilines with Diynes: Controllable Formation of Eight-Membered Ring-Fused Indoles and Propellane-Type Indolines. J. Org. Chem. 2020, 85, 2543–2559. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, Q.; Wei, Y.; Shi, M. Synthesis of Indolizine Derivatives Containing Eight-Membered Rings via a Gold-Catalyzed Two-Fold Hydroarylation of Diynes. Chem. Commun. 2018, 54, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-W.; Tang, X.-Y.; Shi, M. A Gold-Catalyzed 1,2-Acyloxy Migration/Intramolecular Cyclopropanation/Ring Enlargement Cascade: Syntheses of Medium-Sized Heterocycles. Chem. Commun. 2015, 51, 13937–13940. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-Y.; Wei, Y.; Marek, I.; Tang, X.-Y.; Shi, M. Gold(I)-Catalyzed Cycloisomerization of Vinylidenecyclopropane-Enes via Carbene or Non-Carbene Processes. Chem. Sci. 2015, 6, 5519–5525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittstein, K.; Kumar, K.; Waldmann, H. Gold(I)-Catalyzed Synthesis of Benzoxocines by an 8-Endo-Dig Cyclization. Angew. Chem. Int. Ed. 2011, 50, 9076–9080. [Google Scholar] [CrossRef]
- Zhao, C.; Xie, X.; Duan, S.; Li, H.; Fang, R.; She, X. Gold-Catalyzed 1,2-Acyloxy Migration/Intramolecular [3+2] 1,3-Dipolar Cycloaddtion Cascade Reaction: An Efficient Strategy for Syntheses of Medium-Sized-Ring Ethers and Amines. Angew. Chem. Int. Ed. 2014, 53, 10789–10793. [Google Scholar] [CrossRef] [PubMed]
- Nolla-Saltiel, R.; Robles-Marín, E.; Porcel, S. Silver(I) and Gold(I)-Promoted Synthesis of Alkylidene Lactones and 2H-Chromenes from Salicylic and Anthranilic Acid Derivatives. Tetrahedron Lett. 2014, 55, 4484–4488. [Google Scholar] [CrossRef]
- Scully, S.S.; Zheng, S.-L.; Wagner, B.K.; Schreiber, S.L. Synthesis of Oxazocenones via Gold(I)-Catalyzed 8- Endo -Dig Hydroalkoxylation of Alkynamides. Org. Lett. 2015, 17, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Chou, D.H.-C.; Duvall, J.R.; Gerard, B.; Liu, H.; Pandya, B.A.; Suh, B.-C.; Forbeck, E.M.; Faloon, P.; Wagner, B.K.; Marcaurelle, L.A. Synthesis of a Novel Suppressor of β-Cell Apoptosis via Diversity-Oriented Synthesis. ACS Med. Chem. Lett. 2011, 2, 698–702. [Google Scholar]
- Scully, S.S.; Tang, A.J.; Lundh, M.; Mosher, C.M.; Perkins, K.M.; Wagner, B.K.J. Small-Molecule Inhibitors of Cytokine-Mediated STAT1 Signal Transduction in β-Cells with Improved Aqueous Solubility. Med. Chem. 2013, 56, 4125–4129. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Guo, X.-Y.; Gu, Z.-Y.; Wei, L.-S.; Liu, L.-L.; Mo, D.-L.; Pan, C.; Su, G.-F. Silver(I)-Catalyzed Selective Hydroalkoxylation of C2-Alkynyl Quinazolinones to Synthesize Quinazolinone-Fused Eight-Membered N.,O-Heterocycles. Org. Chem. Front. 2020. [Google Scholar] [CrossRef]
- Tokimizu, Y.; Oishi, S.; Fujii, N.; Ohno, H. Gold-Catalyzed Cascade Cyclization of 2-Alkynyl- N -Propargylanilines by Rearrangement of a Propargyl Group. Angew. Chem. Int. Ed. 2015, 54, 7862–7866. [Google Scholar] [CrossRef] [Green Version]









































| Entry | Product a | Reaction Conditions | Yields (%) (Examples) b | Ref. |
|---|---|---|---|---|
| 1 |  | cat. PdCl2(PPh3)2, K2CO3, EtOH, DMF, 100 °C, 2 h | 52 | [19] |
| 2 |  | cat. Pd(OAc)2, AcOK, TBAB, DMF, 100 °C, 4 h | 60–86 (10) | [20] |
| 3 |  | cat. Pd(OAc)2, K2CO3, MeCN, 80 °C, 1 h | 61–70 (4) | [21] |
| 4 |  | cat. Pd(OAc)2/PPh3, Cs2CO3, TBAC, DMF, 90 °C, 2 h | 80–84% | [22] |
| 5 |  | cat. Pd(OAc)2, AcOK, TBAB, DMF, 100 °C, 4 h | 79–91 (4) | [23] |
| 6 |  | cat. Pd(OAc)2, TEAC, Cy2NMe, DMA, 100 °C, 12 h | 69 | [24] |
| 7 |  | cat. Pd(OAc)2, Cs2CO3, TBAB, DMF, 100 °C, 12 h | 59 | [25] |
| 8 |  | cat. Pd2(dba)3.CHCl3, PPh3, NEt3, DMF, 130 °C | 72 | [26] |
| 9 |  | cat. Pd(OAc)2, NaHCO3, TBAC, DMF, 110 °C, 16 h | 73–86 (2) | [27] |
| 10 |  | cat. Pd(OAc)2, TEAC, Cy2NMe, DMA, 95 °C, 6 h | 72 | [24] |
| 11 |  | cat. Pd(OAc)2, PPh3, NEt3, MeCN, MW (125 °C), 6 h | 85 | [28] |
| 12 |  | cat. Pd(OAc)2, AcOK, TBAB, DMF | 80–85 (5) | [29] |
| 13 |  | cat. Pd(OAc)2/DavePhos, K2CO3, MeCN, 80 °C, 1 h | 47 | [30] |
| 14 |  | cat. Pd(OAc)2/JohnPhos, TBAA, DMF, 100 °C | 60 | [31] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choury, M.; Basilio Lopes, A.; Blond, G.; Gulea, M. Synthesis of Medium-Sized Heterocycles by Transition-Metal-Catalyzed Intramolecular Cyclization. Molecules 2020, 25, 3147. https://doi.org/10.3390/molecules25143147
Choury M, Basilio Lopes A, Blond G, Gulea M. Synthesis of Medium-Sized Heterocycles by Transition-Metal-Catalyzed Intramolecular Cyclization. Molecules. 2020; 25(14):3147. https://doi.org/10.3390/molecules25143147
Chicago/Turabian StyleChoury, Mickael, Alexandra Basilio Lopes, Gaëlle Blond, and Mihaela Gulea. 2020. "Synthesis of Medium-Sized Heterocycles by Transition-Metal-Catalyzed Intramolecular Cyclization" Molecules 25, no. 14: 3147. https://doi.org/10.3390/molecules25143147
APA StyleChoury, M., Basilio Lopes, A., Blond, G., & Gulea, M. (2020). Synthesis of Medium-Sized Heterocycles by Transition-Metal-Catalyzed Intramolecular Cyclization. Molecules, 25(14), 3147. https://doi.org/10.3390/molecules25143147






