Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review
Abstract
1. Introduction
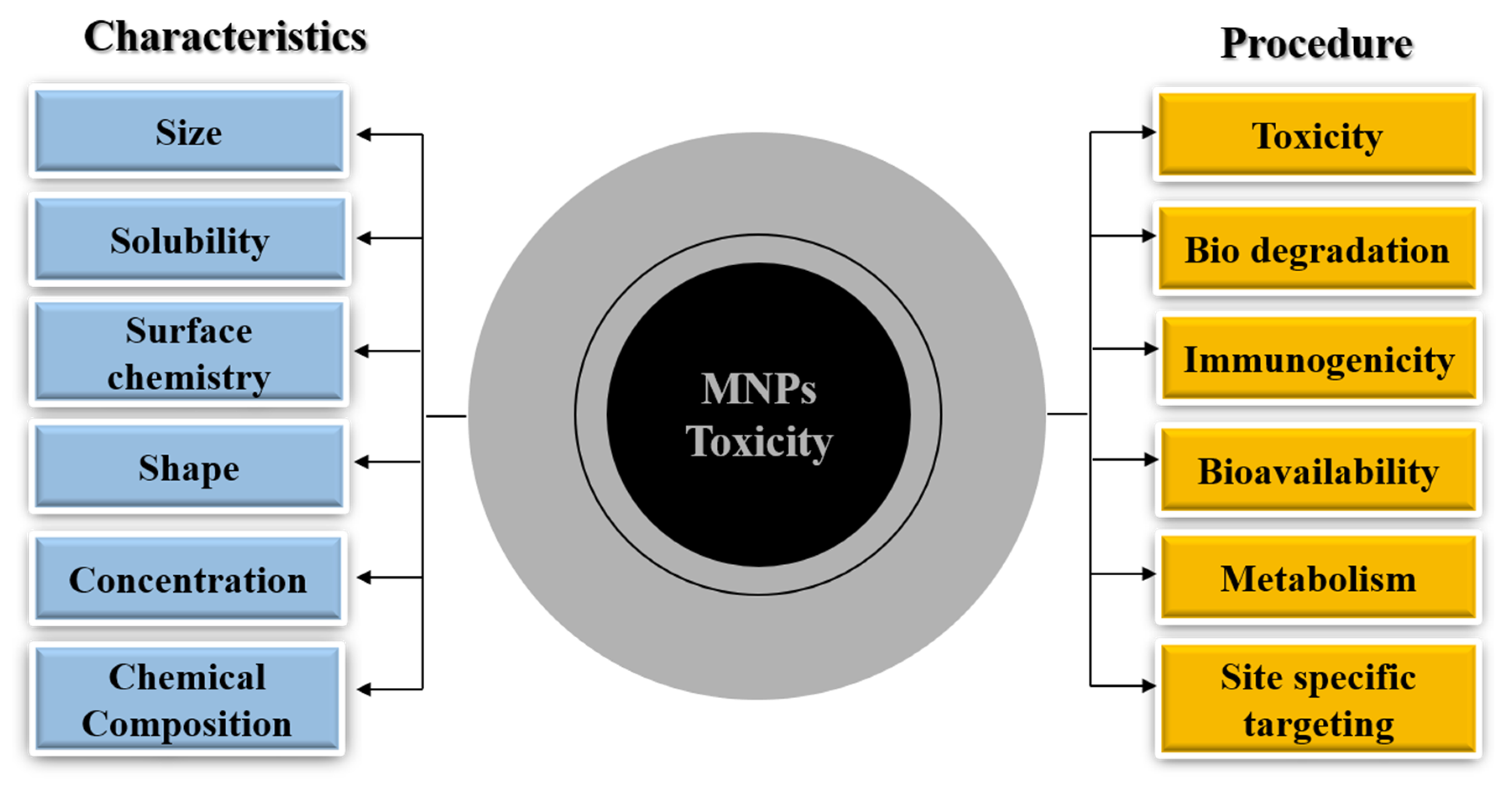
2. Formulation of Magnetic Nanoparticles
Desirable Properties of Magnetic Nanoparticles
3. MNP Metabolism and Toxicity
3.1. MNP Biodistribution and Metabolism
3.2. MNP Toxicity
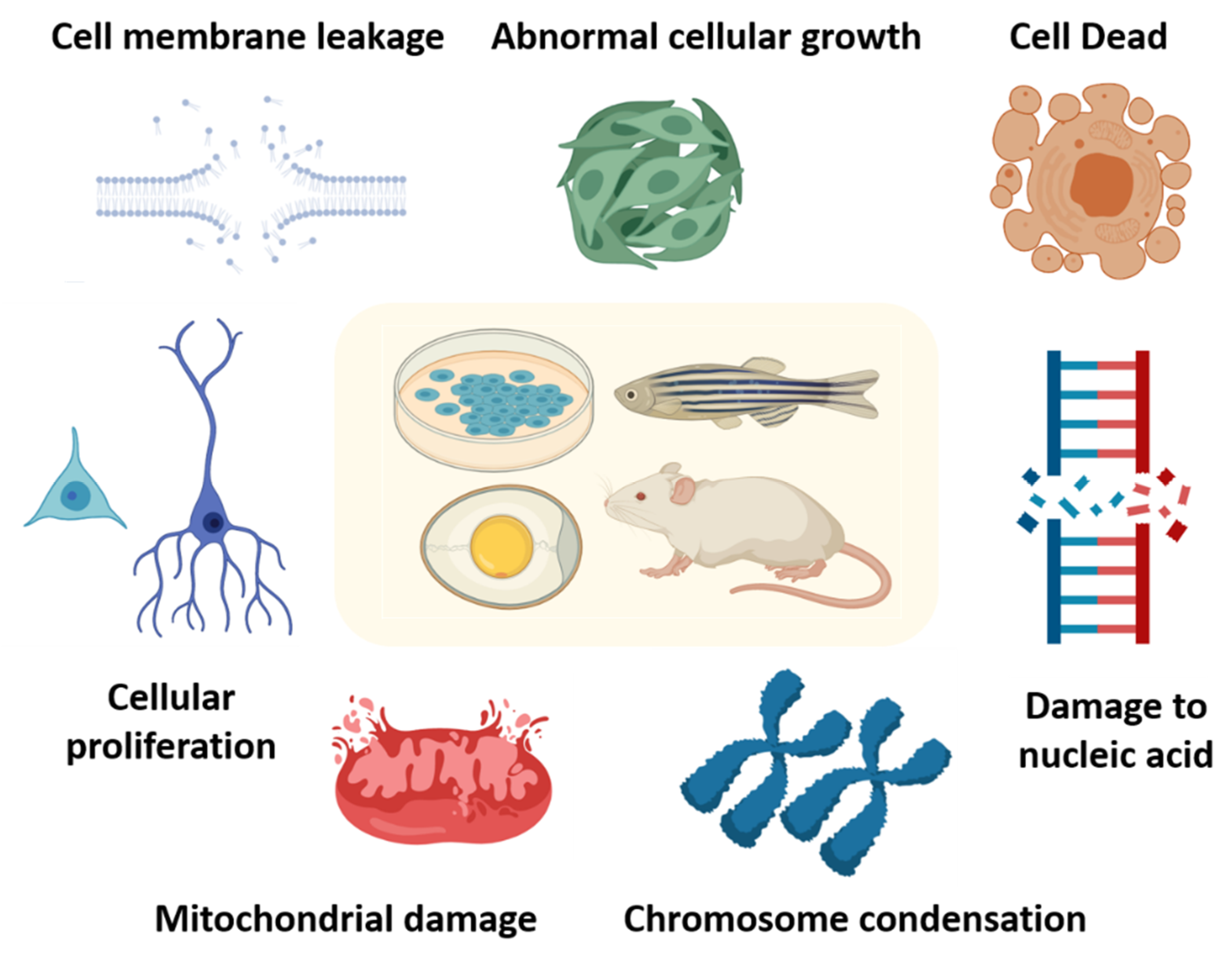
4. MNP Toxicity In Vitro and In Vivo
4.1. MNP Toxicity In Vitro
4.2. MNPs Toxicity In Vivo
4.3. MNP Toxicity in Invertebrates
4.4. MNP Toxicity in Vertebrates
5. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Feynman, R.P. There’s plenty of room at the bottom. Calif. Inst. Technol. Eng. Sci. Mag. 1960, 23, 22–36. [Google Scholar]
- Park, S.; Lim, J.; Kim, J.; Yun, H.; Kim, C. Toxicity estimation of magnetic fluids in a biological test. J. Magn. Magn. Mater. 2006, 304, e406–e408. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Harisinghani, M.G.; Barentsz, J.; Hahn, P.F.; Deserno, W.M.; Tabatabaei, S.; van de Kaa, C.H.; de la Rosette, J.; Weissleder, R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N. Engl. J. Med. 2003, 348, 2491–2499. [Google Scholar] [CrossRef]
- Agency, E.M. Withdrawal Assessment Report for Sinerem; European Medicines Agency: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Wang, Y.-X.J. Current status of superparamagnetic iron oxide contrast agents for liver magnetic resonance imaging. World J. Gastroenterol. 2015, 21, 13400. [Google Scholar] [CrossRef] [PubMed]
- Varallyay, C.G.; Toth, G.B.; Fu, R.; Netto, J.P.; Firkins, J.; Ambady, P.; Neuwelt, E.A. What does the boxed warning tell us? Safe practice of using ferumoxytol as an mri contrast agent. Am. J. Neuroradiol. 2017, 38, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Chertow, G.M.; Rosner, M. Ferumoxytol for the treatment of iron deficiency anemia. Expert Rev. Hematol. 2018, 11, 829–834. [Google Scholar] [CrossRef]
- Coricovac, D.-E.; Moacă, E.-A.; Pinzaru, I.; Cîtu, C.; Soica, C.; Mihali, C.-V.; Păcurariu, C.; Tutelyan, V.A.; Tsatsakis, A.; Dehelean, C.-A. Biocompatible colloidal suspensions based on magnetic iron oxide nanoparticles: Synthesis, characterization and toxicological profile. Front. Pharmacol. 2017, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Dias, J.T.; Grazú, V.; Moros, M.; Baptista, P.V.; de la Fuente, J.M. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front. Chem. 2014, 2, 48. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.I.; Laia, C.A.; Carvalho, A.; Pereira, L.C.; Coutinho, J.T.; Ferreira, I.M.; Novo, C.M.; Borges, J.P. Iron oxide nanoparticles stabilized with a bilayer of oleic acid for magnetic hyperthermia and mri applications. Appl. Surf. Sci. 2016, 383, 240–247. [Google Scholar] [CrossRef]
- Medeiros, S.F.; Filizzola, J.O.; Fonseca, V.F.; Oliveira, P.F.; Silva, T.M.; Elaissari, A.; Santos, A.M. Synthesis and characterization of stable aqueous dispersion of functionalized double-coated iron oxide nanoparticles. Mater. Lett. 2015, 160, 522–525. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Fernandez-Bertolez, N.; Kiliç, G.; Costa, C.; Costa, S.; Fraga, S.; Bessa, M.J.; Pasaro, E.; Teixeira, J.P.; Laffon, B. Are iron oxide nanoparticles safe? Current knowledge and future perspectives. J. Trace Elem. Med. Biol. 2016, 38, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Webster, T.J. Magnetic nanoparticles: Biomedical applications and challenges. J. Mater. Chem. 2010, 20, 8760–8767. [Google Scholar] [CrossRef]
- Shete, P.; Patil, R.; Tiwale, B.; Pawar, S. Water dispersible oleic acid-coated fe3o4 nanoparticles for biomedical applications. J. Magn. Magn. Mater. 2015, 377, 406–410. [Google Scholar] [CrossRef]
- Tran, T.T.-D.; Van Vo, T.; Tran, P.H.-L. Design of iron oxide nanoparticles decorated oleic acid and bovine serum albumin for drug delivery. Chem. Eng. Res. Des. 2015, 94, 112–118. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, S.; Hou, P.; Yang, Y.; Weng, J.; Li, X.; Li, M. Synthesis and characterization of biocompatible fe3o4 nanoparticles. J. Biomed. Mater. Res. Part A 2007, 80, 333–341. [Google Scholar] [CrossRef]
- Sun, C.; Sze, R.; Zhang, M. Folic acid-peg conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by mri. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater Aust. Soc. Biomater. Korean Soc. Biomater. 2006, 78, 550–557. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed]
- Arbab, A.S.; Bashaw, L.A.; Miller, B.R.; Jordan, E.K.; Lewis, B.K.; Kalish, H.; Frank, J.A. Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular mr imaging. Radiology 2003, 229, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Bulte, J.W.; Kraitchman, D.L. Iron oxide mr contrast agents for molecular and cellular imaging. NMR Biomed. Internatl. J. Devot. Dev. Appl. Magn. Res. Vivo 2004, 17, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Pardoe, H.; Chua-Anusorn, W.; Pierre, T.G.S.; Dobson, J. Structural and magnetic properties of nanoscale iron oxide particles synthesized in the presence of dextran or polyvinyl alcohol. J. Magn. Magn. Mater. 2001, 225, 41–46. [Google Scholar] [CrossRef]
- Petri-Fink, A.; Steitz, B.; Finka, A.; Salaklang, J.; Hofmann, H. Effect of cell media on polymer coated superparamagnetic iron oxide nanoparticles (spions): Colloidal stability, cytotoxicity, and cellular uptake studies. Eur. J. Pharm. Biopharm. 2008, 68, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Józefczak, A.; Hornowski, T.; Skumiel, A.; Závišová, V.; Koneracká, M.; Tomašovičová, N.; Timko, M.; Kopčanský, P.; Kelani, H. Effect of the molecular weight of poly (ethylene glycol) on the properties of biocompatible magnetic fluids. Internatl. J. Thermophys. 2012, 33, 640–652. [Google Scholar] [CrossRef]
- Soleymani, M.; Edrissi, M. Synthesis of bilayer surfactant-coated magnetic nanoparticles for application in magnetic fluid hyperthermia. J. Dispers. Sci. Technol. 2016, 37, 693–698. [Google Scholar] [CrossRef]
- Mamalis, A. Recent advances in nanotechnology. J. Mater. Process. Technol. 2007, 181, 52–58. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar]
- Srivastava, V.; Gusain, D.; Sharma, Y.C. Critical review on the toxicity of some widely used engineered nanoparticles. Ind. Eng. Chem. Res. 2015, 54, 6209–6233. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Srivastava, V.; Singh, V.; Kaul, S.; Weng, C. Nano-adsorbents for the removal of metallic pollutants from water and wastewater. Environ. Technol. 2009, 30, 583–609. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Gao, L.; Guo, J. A new sol-gel route using inorganic salt for synthesizing al2o3 nanopowders. Nanostruct. Mater. 1998, 10, 543–550. [Google Scholar] [CrossRef]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Vallabani, N.S.; Singh, S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech 2018, 8, 279. [Google Scholar] [CrossRef]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- McBain, S.C.; Yiu, H.H.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169. [Google Scholar]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef]
- Carpenter, E.E. Iron nanoparticles as potential magnetic carriers. J. Magn. Magn. Mater. 2001, 225, 17–20. [Google Scholar] [CrossRef]
- Koneracka, M.; Kopčanský, P.; Timko, M.; Ramchand, C.; De Sequeira, A.; Trevan, M. Direct binding procedure of proteins and enzymes to fine magnetic particles. J. Mol. Catal. B Enzym. 2002, 18, 13–18. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Fatima, H.; Lee, D.-W.; Yun, H.J.; Kim, K.-S. Shape-controlled synthesis of magnetic fe 3 o 4 nanoparticles with different iron precursors and capping agents. RSC Adv. 2018, 8, 22917–22923. [Google Scholar] [CrossRef]
- Kalantari, K.; Ahmad, M.B.; Shameli, K.; Hussein, M.Z.B.; Khandanlou, R.; Khanehzaei, H. Size-controlled synthesis of fe3o4 magnetic nanoparticles in the layers of montmorillonite. J. Nanomater. 2014, 2014, 181. [Google Scholar] [CrossRef]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.-s.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size-and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284. [Google Scholar] [CrossRef]
- Li, W.; Lee, S.S.; Wu, J.; Hinton, C.H.; Fortner, J.D. Shape and size controlled synthesis of uniform iron oxide nanocrystals through new non-hydrolytic routes. Nanotechnology 2016, 27, 324002. [Google Scholar] [CrossRef] [PubMed]
- Agostini, P.; Meffre, A.; Lacroix, L.-M.; Ugnati, D.; Ondarçuhu, T.; Respaud, M.; Lassagne, B. Electrospray deposition of isolated chemically synthesized magnetic nanoparticles. J. Nanopart. Res. 2016, 18, 11. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Bomatí-Miguel, O.; Mazeina, L.; Navrotsky, A.; Veintemillas-Verdaguer, S. Calorimetric study of maghemite nanoparticles synthesized by laser-induced pyrolysis. Chem. Mater. 2008, 20, 591–598. [Google Scholar] [CrossRef]
- Majidi, S.; Zeinali Sehrig, F.; Farkhani, S.M.; Soleymani Goloujeh, M.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.; Vali, H.; Phelps, T.; Moon, J.-W. Extracellular synthesis of magnetite and metal-substituted magnetite nanoparticles. J. Nanosci. Nanotechnol. 2006, 6, 3517–3520. [Google Scholar] [CrossRef] [PubMed]
- Bharde, A.A.; Parikh, R.Y.; Baidakova, M.; Jouen, S.; Hannoyer, B.; Enoki, T.; Prasad, B.; Shouche, Y.S.; Ogale, S.; Sastry, M. Bacteria-mediated precursor-dependent biosynthesis of superparamagnetic iron oxide and iron sulfide nanoparticles. Langmuir 2008, 24, 5787–5794. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Navas, P.M.; Garcia-Martin, M.L. Application of inorganic nanoparticles for diagnosis based on mri. In Frontiers of Nanoscience; Elsevier: Barcelona, Spain, 2012; Volume 4, pp. 233–245. [Google Scholar]
- Hofmann-Amtenbrink, M.; Hofmann, H.; Montet, X. Superparamagnetic nanoparticles–a tool for early diagnostics. Swiss Med. Wkly. 2010, 140, w13081. [Google Scholar] [CrossRef]
- Kim, J.-E.; Shin, J.-Y.; Cho, M.-H. Magnetic nanoparticles: An update of application for drug delivery and possible toxic effects. Arch. Toxicol. 2012, 86, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.H.; Salabas, E.E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Edit. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Guo, L.; Sun, H. Manufacture of biomaterials. Ref. Modul. Biomed. Sci. Encycl. Biomed. Eng. 2019, 9, 26252–26262. [Google Scholar]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Ann. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- Gupta, A.K.; Naregalkar, R.R.; Vaidya, V.D.; Gupta, M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine 2007, 2, 23–39. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, H. Synthesis and surface modification of magnetic particles for application in biotechnology and biomedicine. China Part. 2007, 5, 1–10. [Google Scholar] [CrossRef]
- Shapiro, E.M. Biodegradable, polymer encapsulated, metal oxide particles for mri-based cell tracking. Magn. Res. Med. 2015, 73, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Senthilnathan, B.; Ameerkhan, H.; Aswini, S.; Abirami, M.; Bharath, T.; Ajithkumar, T.; Maheswaran, A. Review on various approaches on preparation, characterisation and applications of polymeric nanoparticles. World J. Pharm. Res. 2015, 4, 645–663. [Google Scholar]
- Srivastava, A.; Yadav, T.; Sharma, S.; Nayak, A.; Kumari, A.A.; Mishra, N. Polymers in drug delivery. J. Biosci. Med. 2015, 4, 69–84. [Google Scholar] [CrossRef]
- Shubayev, V.I.; Pisanic, T.R., II; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Coll. Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wischke, C.; Schwendeman, S.P. Principles of encapsulating hydrophobic drugs in pla/plga microparticles. Int. J. Pharm. 2008, 364, 298–327. [Google Scholar] [CrossRef]
- Martinho, N.; Damgé, C.; Reis, C.P. Recent advances in drug delivery systems. J. Biomater. Nanobiotechnol. 2011, 2, 510. [Google Scholar] [CrossRef]
- Aguilar-Arteaga, K.; Rodriguez, J.; Barrado, E. Magnetic solids in analytical chemistry: A review. Anal. Chim. Acta 2010, 674, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Carregal-Romero, S.; Caballero-Díaz, E.; Beqa, L.; Abdelmonem, A.M.; Ochs, M.; Hühn, D.; Suau, B.S.; Valcarcel, M.; Parak, W.J. Multiplexed sensing and imaging with colloidal nano-and microparticles. Annu. Rev. Anal. Chem. 2013, 6, 53–81. [Google Scholar] [CrossRef]
- Cole, A.J.; Yang, V.C.; David, A.E. Cancer theranostics: The rise of targeted magnetic nanoparticles. Trends Biotechnol. 2011, 29, 323–332. [Google Scholar] [CrossRef]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Zhang, Y.; Kohler, N.; Zhang, M. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials 2002, 23, 1553–1561. [Google Scholar] [CrossRef]
- Leung, K.C.F.; Xuan, S. Noble metal-iron oxide hybrid nanomaterials: Emerging applications. Chem. Rec. 2016, 16, 458–472. [Google Scholar] [CrossRef]
- Chak, C.-P.; Xuan, S.; Mendes, P.M.; Yu, J.C.; Cheng, C.H.; Leung, K.C.-F. Discrete functional gold nanoparticles: Hydrogen bond-assisted synthesis, magnetic purification, supramolecular dimer and trimer formation. ACS Nano 2009, 3, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-S.; Xu, H.; Xu, H.-J.; Yu, G.-J.; Gong, X.-L.; Fang, Q.-L.; Leung, K.C.-F.; Xuan, S.-H.; Xiong, Q.-R. A facile ultrasonication assisted method for fe 3 o 4@ sio 2-ag nanospheres with excellent antibacterial activity. Dalton Trans. 2015, 44, 9140–9148. [Google Scholar] [CrossRef] [PubMed]
- Duguet, E.; Vasseur, S.; Mornet, S.; Devoisselle, J.-M. Magnetic nanoparticles and their applications in medicine. Nanomedicine 2006, 1, 157–168. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Donaldson, K.; Stone, V.; Tran, C.; Kreyling, W.; Borm, P.J. Nanotoxicology. Occup. Environ. Med. 2004, 61, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Unfried, K.; Albrecht, C.; Klotz, L.-O.; Von Mikecz, A.; Grether-Beck, S.; Schins, R.P. Cellular responses to nanoparticles: Target structures and mechanisms. Nanotoxicology 2007, 1, 52–71. [Google Scholar] [CrossRef]
- Ran, Q.; Xiang, Y.; Liu, Y.; Xiang, L.; Li, F.; Deng, X.; Xiao, Y.; Chen, L.; Chen, L.; Li, Z. Eryptosis indices as a novel predictive parameter for biocompatibility of fe 3 o 4 magnetic nanoparticles on erythrocytes. Sci. Rep. 2015, 5, 16209. [Google Scholar] [CrossRef]
- Malhotra, N.; Chen, J.-R.; Sarasamma, S.; Audira, G.; Siregar, P.; Liang, S.-T.; Lai, Y.-H.; Lin, G.-M.; Ger, T.-R.; Hsiao, C.-D. Ecotoxicity assessment of fe3o4 magnetic nanoparticle exposure in adult zebrafish at an environmental pertinent concentration by behavioral and biochemical testing. Nanomaterials 2019, 9, 873. [Google Scholar] [CrossRef]
- Shen, S.; Wang, S.; Zheng, R.; Zhu, X.; Jiang, X.; Fu, D.; Yang, W. Magnetic nanoparticle clusters for photothermal therapy with near-infrared irradiation. Biomaterials 2015, 39, 67–74. [Google Scholar] [CrossRef]
- Correia Carreira, S.; Walker, L.; Paul, K.; Saunders, M. The toxicity, transport and uptake of nanoparticles in the in vitro bewo b30 placental cell barrier model used within nanotest. Nanotoxicology 2015, 9, 66–78. [Google Scholar] [CrossRef]
- Shukla, S.; Jadaun, A.; Arora, V.; Sinha, R.K.; Biyani, N.; Jain, V. In vitro toxicity assessment of chitosan oligosaccharide coated iron oxide nanoparticles. Toxicol. Rep. 2015, 2, 27–39. [Google Scholar] [CrossRef]
- Gholami, A.; Rasoul-amini, S.; Ebrahiminezhad, A.; Seradj, S.H.; Ghasemi, Y. Lipoamino acid coated superparamagnetic iron oxide nanoparticles concentration and time dependently enhanced growth of human hepatocarcinoma cell line (hep-g2). J. Nanomater. 2015, 16, 150. [Google Scholar] [CrossRef]
- Di Bona, K.; Xu, Y.; Gray, M.; Fair, D.; Hayles, H.; Milad, L.; Montes, A.; Sherwood, J.; Bao, Y.; Rasco, J. Short-and long-term effects of prenatal exposure to iron oxide nanoparticles: Influence of surface charge and dose on developmental and reproductive toxicity. Int. Ernational J. Mol. Sci. 2015, 16, 30251–30268. [Google Scholar] [CrossRef]
- Ahmad, F.; Liu, X.; Zhou, Y.; Yao, H. An in vivo evaluation of acute toxicity of cobalt ferrite (CoFe2O4) nanoparticles in larval-embryo Zebrafish (Danio rerio). Aquat. Toxicol. 2015, 166, 21–28. [Google Scholar] [CrossRef]
- Calero, M.; Chiappi, M.; Lazaro-Carrillo, A.; Rodríguez, M.J.; Chichón, F.J.; Crosbie-Staunton, K.; Prina-Mello, A.; Volkov, Y.; Villanueva, A.; Carrascosa, J.L. Characterization of interaction of magnetic nanoparticles with breast cancer cells. J. Nanobiotechnol. 2015, 13, 16. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Fathy, M.M.; Khalil, W.M. Doxorubicin loaded magnetic gold nanoparticles for in vivo targeted drug delivery. Int. J. Pharm. 2015, 490, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Tse, B.W.-C.; Cowin, G.J.; Soekmadji, C.; Jovanovic, L.; Vasireddy, R.S.; Ling, M.-T.; Khatri, A.; Liu, T.; Thierry, B.; Russell, P.J. PSMA-targeting iron oxide magnetic nanoparticles enhance MRI of preclinical prostate cancer. Nanomedicine 2015, 10, 375–386. [Google Scholar] [CrossRef]
- Marcus, M.; Karni, M.; Baranes, K.; Levy, I.; Alon, N.; Margel, S.; Shefi, O. Iron oxide nanoparticles for neuronal cell applications: Uptake study and magnetic manipulations. J. Nanobiotechnol. 2016, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Buteică, S.; Mihaiescu, D.; Rogoveanu, I.; Mărgăritescu, D.; Mîndrilă, I. Chick chorioallantoic membrane model as a preclinical tool for nanoparticles biology study. Rom. Biotechnol. Lett. 2016, 21, 11684–11690. [Google Scholar]
- Sanz, B.; Calatayud, M.P.; Torres, T.E.; Fanarraga, M.L.; Ibarra, M.R.; Goya, G.F. Magnetic hyperthermia enhances cell toxicity with respect to exogenous heating. Biomaterials 2017, 114, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Salehiabar, M.; Attari, E.; Davaran, S.; Danafar, H.; Manjili, H.K. Green and one-pot surface coating of iron oxide magnetic nanoparticles with natural amino acids and biocompatibility investigation. Appl. Organomet. Chem. 2018, 32, e4069. [Google Scholar] [CrossRef]
- Trabulo, S.; Aires, A.; Aicher, A.; Heeschen, C.; Cortajarena, A.L. Multifunctionalized iron oxide nanoparticles for selective targeting of pancreatic cancer cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Saatchi, K.; Tod, S.E.; Leung, D.; Nicholson, K.E.; Andreu, I.; Buchwalder, C.; Schmitt, V.; Häfeli, U.O.; Gray, S.L. Characterization of alendronic-and undecylenic acid coated magnetic nanoparticles for the targeted delivery of rosiglitazone to subcutaneous adipose tissue. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Rashidi, N.; Danafar, H.; Manjili, H.K. Anticancer activity of tamoxifen loaded tyrosine decorated biocompatible fe 3 o 4 magnetic nanoparticles against breast cancer cell lines. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1178–1186. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef]
- Nosrati, H.; Sefidi, N.; Sharafi, A.; Danafar, H.; Manjili, H.K. Bovine serum albumin (bsa) coated iron oxide magnetic nanoparticles as biocompatible carriers for curcumin-anticancer drug. Bioorg. Chem. 2018, 76, 501–509. [Google Scholar] [CrossRef]
- Shevtsov, M.; Nikolaev, B.; Marchenko, Y.; Yakovleva, L.; Skvortsov, N.; Mazur, A.; Tolstoy, P.; Ryzhov, V.; Multhoff, G. Targeting experimental orthotopic glioblastoma with chitosan-based superparamagnetic iron oxide nanoparticles (cs-dx-spions). Int. J. Nanomed. 2018, 13, 1471. [Google Scholar] [CrossRef]
- Alam, S.; Ahmad, R.; Pranaw, K.; Mishra, P.; Khare, S.K. Asparaginase conjugated magnetic nanoparticles used for reducing acrylamide formation in food model system. Bioresour. Technol. 2018, 269, 121–126. [Google Scholar] [CrossRef]
- Caro, C.; Egea-Benavente, D.; Polvillo, R.; Royo, J.L.; Leal, M.P.; García-Martín, M.L. Comprehensive toxicity assessment of pegylated magnetic nanoparticles for in vivo applications. Coll. Surf. B Biointerfaces 2019, 177, 253–259. [Google Scholar] [CrossRef]
- Ma, W.; Gehret, P.M.; Hoff, R.E.; Kelly, L.P.; Suh, W.H. The investigation into the toxic potential of iron oxide nanoparticles utilizing rat pheochromocytoma and human neural stem cells. Nanomaterials 2019, 9, 453. [Google Scholar] [CrossRef]
- Malhotra, N.; Audira, G.; Chen, J.-R.; Siregar, P.; Hsu, H.-S.; Lee, J.-S.; Ger, T.-R.; Hsiao, C.-D. Surface modification of magnetic nanoparticles by carbon-coating can increase its biosafety: Evidences from biochemical and neurobehavioral tests in zebrafish. Molecules 2020, 25, 2256. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-T.; Hwang, S.-K.; Jin, H.; Kim, D.-S.; Minai-Tehrani, A.; Yoon, H.-J.; Choi, M.; Yoon, T.-J.; Han, D.-Y.; Kang, Y.-W. Body distribution of inhaled fluorescent magnetic nanoparticles in the mice. J. Occup. Health 2008, 50, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.K.; Reddy, M.K.; Morales, M.A.; Leslie-Pelecky, D.L.; Labhasetwar, V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol. Pharm. 2008, 5, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.J.; David, A.E.; Wang, J.; Galbán, C.J.; Yang, V.C. Magnetic brain tumor targeting and biodistribution of long-circulating peg-modified, cross-linked starch-coated iron oxide nanoparticles. Biomaterials 2011, 32, 6291–6301. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Yang, X.; Tang, Z.; Hu, Y.; Chen, B.; Tang, J. In vivo assessment of hepatotoxicity, nephrotoxicity and biodistribution using 3-aminopropyltriethoxysilane-coated magnetic nanoparticles (apts-mnps) in icr mice. Chin. Sci. Bull. 2014, 59, 1800–1808. [Google Scholar] [CrossRef]
- Markides, H.; Rotherham, M.; El Haj, A. Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J. Nanomater. 2012, 2012, 13. [Google Scholar] [CrossRef]
- Kumamoto, Y.; Camporez, J.P.G.; Jurczak, M.J.; Shanabrough, M.; Horvath, T.; Shulman, G.I.; Iwasaki, A. Cd301b+ mononuclear phagocytes maintain positive energy balance through secretion of resistin-like molecule alpha. Immunity 2016, 45, 583–596. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Bhatti, S.K.; Bajwa, A.; DiNicolantonio, J.J.; Lavie, C.J. Alcohol and cardiovascular health: The dose makes the pois or the remedy. Mayo Clin. Proc. 2014, 89, 382–393. [Google Scholar] [CrossRef]
- Huang, D.-M.; Chung, T.-H.; Hung, Y.; Lu, F.; Wu, S.-H.; Mou, C.-Y.; Yao, M.; Chen, Y.-C. Internalization of mesoporous silica nanoparticles induces transient but not sufficient osteogenic signals in human mesenchymal stem cells. Toxicol. Appl. Pharm. 2008, 231, 208–215. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Hofmann, H.; Rothen-Rutishauser, B.; Petri-Fink, A. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem. Rev. 2011, 112, 2323–2338. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Hsiao, J.-K.; Tai, M.-F.; Chen, S.-T.; Cheng, H.-Y.; Wang, J.-L.; Liu, H.-M. Direct labeling of hmsc with spio: The long-term influence on toxicity, chondrogenic differentiation capacity, and intracellular distribution. Mol. Imaging Biol. 2011, 13, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.; De Cuyper, M. How to assess cytotoxicity of (iron oxide-based) nanoparticles. A technical note using cationic magnetoliposomes. Contrast Media Mol. Imaging 2011, 6, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Schrand, A.M.; Dai, L.; Schlager, J.J.; Hussain, S.M. Toxicity testing of nanomaterials. In New Technologies for Toxicity Testing; Springer: Toledo, OH, USA, 2012; pp. 58–75. [Google Scholar]
- Villanueva, A.; Canete, M.; Roca, A.G.; Calero, M.; Veintemillas-Verdaguer, S.; Serna, C.J.; del Puerto Morales, M.; Miranda, R. The influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cells. Nanotechnology 2009, 20, 115103. [Google Scholar] [CrossRef]
- Soenen, S.J.; Illyes, E.; Vercauteren, D.; Braeckmans, K.; Majer, Z.; De Smedt, S.C.; De Cuyper, M. The role of nanoparticle concentration-dependent induction of cellular stress in the internalization of non-toxic cationic magnetoliposomes. Biomaterials 2009, 30, 6803–6813. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Samim, M.; Abdin, M.; Ahmed, F.J.; Maitra, A.; Prashant, C.; Dinda, A.K. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomed. 2010, 5, 983. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Burtea, C.; Thirifays, C.; Häfeli, U.O.; Mahmoudi, M. Crucial ignored parameters on nanotoxicology: The importance of toxicity assay modifications and “cell vision”. PLoS ONE 2012, 7, e29997. [Google Scholar] [CrossRef]
- Jiang, W.; Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145. [Google Scholar] [CrossRef]
- Kunzmann, A.; Andersson, B.; Vogt, C.; Feliu, N.; Ye, F.; Gabrielsson, S.; Toprak, M.S.; Buerki-Thurnherr, T.; Laurent, S.; Vahter, M. Efficient internalization of silica-coated iron oxide nanoparticles of different sizes by primary human macrophages and dendritic cells. Toxicol. Appl. Pharm. 2011, 253, 81–93. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Simchi, A.; Milani, A.; Stroeve, P. Cell toxicity of superparamagnetic iron oxide nanoparticles. J. Coll. Interface Sci. 2009, 336, 510–518. [Google Scholar] [CrossRef]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.C.; Chan, W.C. Nanotoxicity: The growing need for in vivo study. Curr. Opin. Biotechnol. 2007, 18, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Riviere, N.; Inman, A.; Zhang, L. Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicol. Appl. Pharm. 2009, 234, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. Nih image to imagej: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Kedziorek, D.A.; Muja, N.; Walczak, P.; Ruiz-Cabello, J.; Gilad, A.A.; Jie, C.C.; Bulte, J.W. Gene expression profiling reveals early cellular responses to intracellular magnetic labeling with superparamagnetic iron oxide nanoparticles. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2010, 63, 1031–1043. [Google Scholar] [CrossRef]
- Buyukhatipoglu, K.; Clyne, A.M. Superparamagnetic iron oxide nanoparticles change endothelial cell morphology and mechanics via reactive oxygen species formation. J. Biomed. Mater. Res. Part A 2011, 96, 186–195. [Google Scholar] [CrossRef]
- Hsu, J.-L.; Huang, S.-Y.; Chow, N.-H.; Chen, S.-H. Stable-isotope dimethyl labeling for quantitative proteomics. Anal. Chem. 2003, 75, 6843–6852. [Google Scholar] [CrossRef]
- Lin, Y.-R.; Kuo, C.-J.; Lin, H.Y.-H.; Wu, C.-J.; Liang, S.-S. A proteomics analysis to evaluate cytotoxicity in nrk-52e cells caused by unmodified nano-fe3o4. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Wilhelm, C.; Fortin, J.-P.; Gazeau, F. Tumour cell toxicity of intracellular hyperthermia mediated by magnetic nanoparticles. J. Nanosci. Nanotechnol. 2007, 7, 2933–2937. [Google Scholar] [CrossRef]
- Labusca, L.; Herea, D.-D.; Danceanu, C.-M.; Minuti, A.E.; Stavila, C.; Grigoras, M.; Gherca, D.; Stoian, G.; Ababei, G.; Chiriac, H. The effect of magnetic field exposure on differentiation of magnetite nanoparticle-loaded adipose-derived stem cells. Mater. Sci. Eng. C 2020, 109, 110652. [Google Scholar] [CrossRef]
- Häfeli, U.O.; Riffle, J.S.; Harris-Shekhawat, L.; Carmichael-Baranauskas, A.; Mark, F.; Dailey, J.P.; Bardenstein, D. Cell uptake and in vitro toxicity of magnetic nanoparticles suitable for drug delivery. Mol. Pharm. 2009, 6, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Cengelli, F.; Maysinger, D.; Tschudi-Monnet, F.; Montet, X.; Corot, C.; Petri-Fink, A.; Hofmann, H.; Juillerat-Jeanneret, L. Interaction of functionalized superparamagnetic iron oxide nanoparticles with brain structures. J. Pharm. Exp. Ther. 2006, 318, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Skepper, J.N.; Posfai, M.; Trivedi, R.; Howarth, S.; Corot, C.; Lancelot, E.; Thompson, P.W.; Brown, A.P.; Gillard, J.H. Effect of ultrasmall superparamagnetic iron oxide nanoparticles (ferumoxtran-10) on human monocyte-macrophages in vitro. Biomaterials 2007, 28, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Mejías, R.; Gutiérrez, L.; Salas, G.; Pérez-Yagüe, S.; Zotes, T.M.; Lázaro, F.J.; Morales, M.P.; Barber, D.F. Long term biotransformation and toxicity of dimercaptosuccinic acid-coated magnetic nanoparticles support their use in biomedical applications. J. Control. Release 2013, 171, 225–233. [Google Scholar] [CrossRef]
- Shen, C.-C.; Wang, C.-C.; Liao, M.-H.; Jan, T.-R. A single exposure to iron oxide nanoparticles attenuates antigen-specific antibody production and t-cell reactivity in ovalbumin-sensitized balb/c mice. Int. J. Nanomed. 2011, 6, 1229. [Google Scholar]
- Schlachter, E.K.; Widmer, H.R.; Bregy, A.; Lönnfors-Weitzel, T.; Vajtai, I.; Corazza, N.; Bernau, V.J.; Weitzel, T.; Mordasini, P.; Slotboom, J. Metabolic pathway and distribution of superparamagnetic iron oxide nanoparticles: In vivo study. Int. J. Nanomed. 2011, 6, 1793. [Google Scholar]
- Malindretos, P.; Sarafidis, P.A.; Rudenco, I.; Raptis, V.; Makedou, K.; Makedou, A.; Grekas, D.M. Slow intravenous iron administration does not aggravate oxidative stress and inflammatory biomarkers during hemodialysis: A comparative study between iron sucrose and iron dextran. Am. J. Nephrol. 2007, 27, 572–579. [Google Scholar] [CrossRef]
- Hu, J.; Wang, D.; Wang, J.; Wang, J. Bioaccumulation of fe2o3 (magnetic) nanoparticles in ceriodaphnia dubia. Environ. Pollut. 2012, 162, 216–222. [Google Scholar] [CrossRef]
- Taze, C.; Panetas, I.; Kalogiannis, S.; Feidantsis, K.; Gallios, G.P.; Kastrinaki, G.; Konstandopoulos, A.G.; Václavíková, M.; Ivanicova, L.; Kaloyianni, M. Toxicity assessment and comparison between two types of iron oxide nanoparticles in mytilus galloprovincialis. Aquat. Toxicol. 2016, 172, 9–20. [Google Scholar] [CrossRef]
- Zhang, W.; Rittmann, B.; Chen, Y. Size effects on adsorption of hematite nanoparticles on E. coli cells. Environ. Sci. Technol. 2011, 45, 2172–2178. [Google Scholar] [CrossRef]
- Cao, J.; Feng, Y.; Lin, X.; Wang, J.; Xie, X. Iron oxide magnetic nanoparticles deteriorate the mutual interaction between arbuscular mycorrhizal fungi and plant. J. Soils Sediments 2017, 17, 841–851. [Google Scholar] [CrossRef]
- Baumann, J.; Köser, J.; Arndt, D.; Filser, J. The coating makes the difference: Acute effects of iron oxide nanoparticles on daphnia magna. Sci. Total Environ. 2014, 484, 176–184. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Dringen, R.; Petters, C.; Rastedt, W.; Köser, J.; Filser, J.; Stolte, S. Toxicity of dimercaptosuccinate-coated and un-functionalized magnetic iron oxide nanoparticles towards aquatic organisms. Environ. Sci. Nano 2016, 3, 754–767. [Google Scholar] [CrossRef]
- Pappus, S.A.; Mishra, M. A drosophila model to decipher the toxicity of nanoparticles taken through oral routes. In Cellular and Molecular Toxicology of Nanoparticles; Springer: Odisha, India, 2018; pp. 311–322. [Google Scholar]
- Sayadi, M.H.; Mansouri, B.; Shahri, E.; Tyler, C.R.; Shekari, H.; Kharkan, J. Exposure effects of iron oxide nanoparticles and iron salts in blackfish (capoeta fusca): Acute toxicity, bioaccumulation, depuration, and tissue histopathology. Chemosphere 2020, 247, 125900. [Google Scholar] [CrossRef]
- Hafiz, S.M.; Kulkarni, S.S.; Thakur, M.K. In-vivo toxicity assessment of biologically synthesized iron oxide nanoparticles in zebrafish (danio rerio). Biosci. Biotechnol. Res. Asia 2018, 15, 419–425. [Google Scholar] [CrossRef]
- Patel, S.; Jana, S.; Chetty, R.; Thakore, S.; Singh, M.; Devkar, R. Toxicity evaluation of magnetic iron oxide nanoparticles reveals neuronal loss in chicken embryo. Drug Chem. Toxicol. 2019, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Marin-Barba, M.; Gavilán, H.; Gutierrez, L.; Lozano-Velasco, E.; Rodríguez-Ramiro, I.; Wheeler, G.; Morris, C.J.; Morales, M.; Ruiz, A. Unravelling the mechanisms that determine the uptake and metabolism of magnetic single and multicore nanoparticles in a xenopus laevis model. Nanoscale 2018, 10, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Rivero, M.; Marín-Barba, M.; Gutiérrez, L.; Lozano-Velasco, E.; Wheeler, G.; Sánchez-Marcos, J.; Muñoz-Bonilla, A.; Morris, C.; Ruiz, A. Toxicity and biodegradation of zinc ferrite nanoparticles in xenopus laevis. J. Nanopart. Res. 2019, 21, 181. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Aly, E.M.; Mohamed, F.F.; Noor, N.A.; Elsayed, A.A. Neurotoxicity of green-synthesized magnetic iron oxide nanoparticles in different brain areas of wistar rats. Neurotoxicology 2019, 77, 80–93. [Google Scholar] [CrossRef]
- Volkovova, K.; Handy, R.D.; Staruchova, M.; Tulinska, J.; Kebis, A.; Pribojova, J.; Ulicna, O.; Kucharská, J.; Dusinska, M. Health effects of selected nanoparticles in vivo: Liver function and hepatotoxicity following intravenous injection of titanium dioxide and na-oleate-coated iron oxide nanoparticles in rodents. Nanotoxicology 2015, 9, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Awaad, A.; Seleem, A. Histochemical changes in neonatal liver caused by vaginal instillation of magnetic nanoparticles in pregnant mice. Biotech. Histochem. 2016, 91, 48–62. [Google Scholar] [CrossRef] [PubMed]
| Type of MNPs | Size and Shape of Tested MNPs | Model Organism (In Vitro or in Vivo Test) | Method of Toxicity Analysis | Treatment Condition (Time and Dose) | Results | Ref. |
|---|---|---|---|---|---|---|
| Uncoated Magnetic Nanoparticles (MNPs) | ||||||
| Bare Fe3O4-MNPs | 72.6 ± 0.6 nm spheroid | THP-1 cells and female CD(R) IGS rats | Biochemical marker in rat blood after treatment | In vitro: 100, 800 and 1600 μg/mL 24 h In vivo: 12 mg/kg/intravenous injection 6 days | Fe3O4-MNPs cytotoxicity in erythrocytes in vitro and in vivo | [79] |
| 15 nm | Adult zebrafish | Behavioral and biochemical assessment in adult zebrafish | 14 days waterborne incubation at 1 and 10 ppm | Uncoated MNPs exhibited behavior and biochemical safety at 1ppm but display neurobehavioral toxicity at 10 ppm | [80] | |
| 15 nm and 225 nm spherical | A549 cells and Male Balb/c mice | Cell viability assay | In vitro:10–80 μg/mL In vivo: Subcutaneous injection of 2 × 106 cells suspended in 100 µL PBS | Magnetic nanomaterials did not indicate inherent toxicity | [81] | |
| Surface coated/modified MNPs | ||||||
| (OC-Fe3O4) NPs (Fl-SiO2) | 8 nm, 25 nm and 50 nm | BeWo b30 placental barrier model | Lactase dehydrogenase (LDH) in cell culture | 4, 24 or 48 h 75, 15, 3, 0.6 and 0.12 µg/cm2 | Iron oxide MNPS triggers cytotoxicity at lower doses and shorter exposure compared with silica NPs | [82] |
| CSO-INPs | 6 ± 1.2 nm 8 ± 2.7 nm | HeLa, A549 and HeK293 cells | MTT assay | 24, 48 and 72 h 0.5, 2, 4 μg/µL | INPs triggers toxic effects in Hek293, A549 and Hela cells in comparison to CSO-INPs | [83] |
| L14@Fe3O4 L4@Fe3O4 Gly@Fe3O4 | 11 ± 3 nm 7 ± 2 nm 9 ± 2 nm spherical | HeP G2 cells | MTT assay | 24 and 48 h 1–500 μg/mL | Cytotoxicity of naked SPION increased in relation to increasing concentration | [84] |
| Fe2O3-NPs PEI-NPs PAA-NPs | 28–30 nm | Male and female Crl:CD1(ICR) (CD-1) mice | Dams: gestation period of toxicity Cesarean: Histopathology analysis | Gestation day 8, 9, or 10 low dose:10 mg/kg high dose:100 mg/kg | A low dose of NPs, regardless of charge, did not induce toxicity; high exposure led to charge-dependent fetal loss | [85] |
| (HLC) Fe3O4 NPs | 8.4 nm spherical | NIH3T3 cells | FluoStar Optima microplate reader | 24 h 25 to 250 μg/mL | Reduced toxicity towards normal cells, enhancing the potential of magnetic hyperthermia in cancer treatment | [86] |
| DMSA-SPION | 15 nm | MCF-7 cells | MTT assay Trypan blue exclusion test | 1 h–72 h 0.4 mg/mL | MCF-7 accumulated NPs without effect on cell morphology, ROS generation and cell viability | [87] |
| Dox-gold coated MNPs MGNPs-DOX-M-group | MNP: 10 nm MGNPs: 22 nm spherical | Ehrlich ascites carcinoma cells injected intraperitoneally into female Balb/c mice | Histological examination Tumor size (AST, ALT, CK-MB, LDH) | 20 mice group 10 mg/kg/group external application of neodymium–iron–boron magnetic disc (1.14 T) at tumor site for 3 h | Best therapeutic anti-cancer activity and lowest systemic toxicity compared to free DOX | [88] |
| PLGA NPs sorafenib SPION SRF/FA-PEG-PLGA NP | 205 ± 3 nm spherical | BEL7402 cancer cells | MTT assay Apoptosis assay Anticancer efficacy | 72 h 10 and 40 mg iron/mL | Concentration dependent cytotoxicity in BEL7402 cancer cells | [89] |
| Starch- Fe3O4 MNPs Dextran-Fe3O4 MNPs | 100 nm | Rat PC 12 cells (ATCC) | Cell-viability assay | 1 h–72 h 0.01–0.5 mg/mL | Uncoated- Fe3O4 MNPs maximum interaction and entered inside cell with no cytotoxic effect | [90] |
| Fe3O4/salicylic acid NPs | MNPs 33–277.9 nm Embryos injected: 60.3 nm and 79.9 nm MNPs | chick embryo chorioallantoic membrane model (CAM) | Morphological analysis | 24 h Autopsied to harvest embryo viscera (heart, kidney, liver, and lung). 0.15 mL MNPs | 50–100 nm diameter range MNPs had no embolic risk, on a safety intravenous administration. Tissue MNPs deposits were biocompatible with embryos and chicken | [91] |
| PEI-MNP | Not available | Human neuroblastoma SH-SY5Y cells (ATCC CRL-2266) | Quantitative/qualitative flow cytometry of apoptosis and necrosis | External hyperthermia (EHT), Magnetic hyperthermia (MHT) | A maximum difference in cytotoxicity approximately 45% was observed at T0 = 46 °C. | [92] |
| AA coated IONPs | 3.98, 4.09, 3.41, 4.32, 2.35 nm globular | HFF2 cell lines | MTT assay | 72 h 0.049, 0.073, 0.110, 0.165, 0.248 and 0.373 mg/mL | IONPs were biocompatible and nontoxic with the cell line HFF2 | [93] |
| Multifunctional MNPs Anti-CD47 antibodyGemcitabine | 109 ± 1 nm | CD47-positive pancreatic cancer cells | Resazurin dye | 24 h Free Gem (0.1, 0.4 and 1 µM) MNP-Gem and MNP Gem-anti-CD47 (0.2 mg Fe/mL, 4.8 µM Gem, Ab 20 μg/mg Fe) | Cytotoxic activity of the multifunctional Nano formulation is not increased in the in vitro studies | [94] |
| Rosi-MNPs Al-MNPs Un-MNPs | 21 ± 4 nm | Magnet and Sham mice | MTT assay | 24 h 48 h 0.5, 5, 50, and 500 μg/mL | Al-MNPs only caused a significant reduction in cell viability at 500 μg/mL | [95] |
| MTX F-Lys-MTX NPs | 43.72 ± 4.73 nm | MCF-7 cell lines | MTT assay | 48 and 72 h 100 mL | MTX-conjugated NPs: reduction in cellular viability in human breast cancer (MCF-7) cells compared to free MTX over time | [96] |
| F@Tyr NPs F@Tyr@TMX NPs | 22.19 ± 3.58 nm | HEK-293 MCF-7 cells | Hemolysis test and MTT assays | 72 h F@Tyr NPs, Bare Fe3O4 0.025, 0.05, 0.1, 0.2, 0.4 and 0.8 mg/mL | Cytotoxicity study, F@Tyr@TMX NPs exhibited more cytotoxic effects than free TMX | [96] |
| IONPs-PEG IONPs-PEI SEI-10 SMG-10 SMG-30 | 10–30 nm | SKOV-3 RAW 264.7 Nude mice BALB/c mice | LDH assay, Hemolysis, ROS, MMP Cell cycle analysis, in vivo bio-distribution, toxicity | Hemolysis: 200 µL, 4 h. In vivo biodistribution: dose of 1.5 mg Fe/kg. In vivo toxicity: 1.5, 2.5, or 5 mg/kg | No obvious toxicity was found for PEGylated IONPs in BALB/c mice, whereas PEI-coated IONPs exhibited dose-dependent lethal toxicity | [97] |
| F@BSA@CURNPs | 56 ± 11.43 nm, spherical | HFF2 MCF-7 cells | Cell viability by MTT assay | 72 and 96 h Serial dilution 15–950 µM | F@BSA@CUR NPs had much higher cytotoxicity against MCF7 cells | [98] |
| CS-DX-SPIONs | 55 nm round shape | In vitro: Rat C6 glioma, human U87 glioma, and human cervix carcinoma HeLa cells and Male Wistar rats | Histology analysis | 24 h In vitro: 1, 10, 50, and 150 μg/mL 1, 3, 6, 12 intravenous injections of PBS via tail vein; DX-SPIONs (Fe concentration of 2.5 mg/kg); CS-DX-SPIONs (Fe at 2.5 mg/kg). | Increase in surface charge of the NPs due to the chitosan coating enhanced the intracellular uptake of particles and thus increased their cytotoxic activity. | [99] |
| Asparaginase enzyme-immobilized on APTES modified MNPs | 50–100 nm | In vitro: Reduction of acrylamide in food model system | Deactivation rate constant (Kd) of free and immobilized enzyme | Five cycles of pretreatment | It was found to be more than three-fold increase their thermal stability from free enzyme and retained 90% activity after fifth cycle | [100] |
| MnFe2O4 MnFe1 MnFe2 | 3–20 nm | Mouse microglial cell line N13 and Zebrafish embryos Male Balb/c mice | Teratogenicity assay | In vitro: 0.1 to 100 μg/mL In vivo: 0.01, 0.1, 1, 10, 100 μg/mL In vivo: Fe 1, Fe 2. PEGylated Cubic (20 nm) | No significant cytotoxicity, till 24 h; No mortality or malformations were observed in the embryos exposed to different doses of particles at 48 hpf. At 100 μg/mL high percentage of mortality 6 dpf | [101] |
| n-octyltriethoxysilane coated-MNPs | 17.9 ± 3.9 nm 18.7 ± 4.4 nm | PC12 and ReN cell VM | Cell Viability LIVE/DEAD Staining Prussian Blue and Nuclear Fast Red Staining | 24 h 4, 8, 16, and 32 µg | Coated MNPs decreased cytotoxic effects; Significant differences in toxicological profiles in two mammalian cell lines | [102] |
| Carbon-coated MNPs | 24 nm | Adult zebrafish | Multiple behavioral and biochemical tests | 1 and 10 ppm exposure for 14 days | Carbon-coated MNPs can significantly enhance its biosafety by reducing neurobehavioral toxicities compared to the bare MNPs | [103] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malhotra, N.; Lee, J.-S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.-R.; Hsiao, C.-D. Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules 2020, 25, 3159. https://doi.org/10.3390/molecules25143159
Malhotra N, Lee J-S, Liman RAD, Ruallo JMS, Villaflores OB, Ger T-R, Hsiao C-D. Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules. 2020; 25(14):3159. https://doi.org/10.3390/molecules25143159
Chicago/Turabian StyleMalhotra, Nemi, Jiann-Shing Lee, Rhenz Alfred D. Liman, Johnsy Margotte S. Ruallo, Oliver B. Villaflores, Tzong-Rong Ger, and Chung-Der Hsiao. 2020. "Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review" Molecules 25, no. 14: 3159. https://doi.org/10.3390/molecules25143159
APA StyleMalhotra, N., Lee, J.-S., Liman, R. A. D., Ruallo, J. M. S., Villaflores, O. B., Ger, T.-R., & Hsiao, C.-D. (2020). Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules, 25(14), 3159. https://doi.org/10.3390/molecules25143159








