Syntheses and Reactivity of New Zwitterionic Imidazolium Trihydridoborate and Triphenylborate Species
Abstract
:1. Introduction
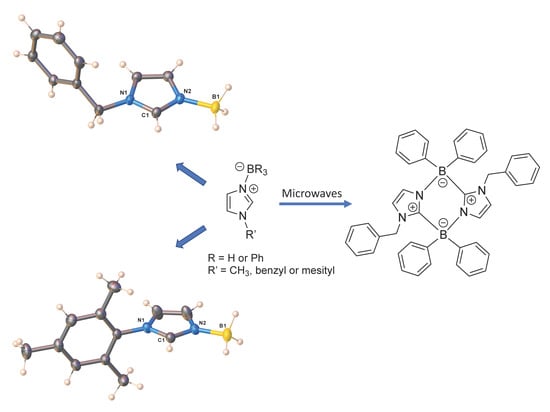
2. Result and Discussion
Synthesis and Characterization
3. Experimental Section
3.1. Materials and General Methods
3.1.1. Synthesis of (HImBn)BH3 (1)
3.1.2. Synthesis of (HImMes)BH3 (2)
3.1.3. Synthesis of (HImCH3)BPh3 (3)
3.1.4. Synthesis of (HImBn)BPh3 (4)
3.1.5. Synthesis of (ImBnBPh2)2 (5)
3.2. Crystallographic Data Collection and Refinement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arduengo, A.J.; Dias, H.V.R.; Harlow, R.L.; Kline, M. Electronic stabilization of nucleophilic carbenes. J. Am. Chem. Soc. 1992, 114, 5530–5534. [Google Scholar] [CrossRef]
- Igau, A.; Grutzmacher, H.; Baceiredo, A.; Bertrand, G. Analogous.alpha.,.alpha.’-bis-carbenoid, triply bonded species: Synthesis of a stable.lambda.3-phosphino carbene-.lambda.5-phosphaacetylene. J. Am. Chem. Soc. 1988, 110, 6463–6466. [Google Scholar] [CrossRef]
- Arduengo, A.J.; Harlow, R.L.; Kline, M. A stable crystalline carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Martin, C.D.; Soleilhavoup, M.; Bertrand, G. Carbene-Stabilized Main Group Radicals and Radical Ions. Chem. Sci. 2013, 4, 3020–3030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, F.E.; Jahnke, M.C. Heterocyclic Carbenes: Synthesis and Coordination Chemistry. Angew. Chem. Int. Ed. 2008, 47, 3122–3172. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.P. N-Heterocyclic Carbenes in Synthesis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Dröge, T.; Glorius, F. The Measure of All Rings-N-Heterocyclic Carbenes. Angew. Chem. Int. Ed. 2010, 49, 6940–6952. [Google Scholar] [CrossRef]
- Hahn, F.E. Heterocyclic Carbenes. Angew. Chem. Int. Ed. 2006, 45, 1348–1352. [Google Scholar] [CrossRef]
- Díez-González, S. N-Heterocyclic Carbenes: From Laboratory Curiosities to Efficient Synthetic Tools: Edition 2; Royal Society of Chemistry: Cambridge, UK, 2017. [Google Scholar]
- Öfele, K.; Herrmann, W.A.; Mihalios, D.; Elison, M.; Herdtweck, E.; Scherer, W.; Mink, J. Multiple bonds between Main-Group elements and transition metals. CXXVI. Heterocyclene-carbenes as phosphine-analog ligands in metal complexes. J. Organomet. Chem. 1993, 459, 177–184. [Google Scholar] [CrossRef]
- Kühl, O. Functionalised N-Heterocyclic Carbene Complexes; John Wiley & Sons Ltd.: Chichester, UK, 2010. [Google Scholar]
- Zeng, X.; Soleilhavoup, M.; Bertrand, G. Gold-Catalyzed Intermolecular Markovnikov Hydroamination of Allenes with Secondary Amines. Org. Lett. 2009, 11, 3166–3169. [Google Scholar] [CrossRef] [Green Version]
- Crabtree, R.H. NHC ligands versus cyclopentadienyls and phosphines as spectator ligands in organometallic catalysis. J. Organomet. Chem. 2005, 690, 5451–5457. [Google Scholar] [CrossRef]
- Nesterov, V.; Reiter, D.; Bag, P.; Frisch, P.; Holzner, R.; Porzelt, A.; Inoue, S. NHCs in Main Group Chemistry. Chem. Rev. 2018, 118, 9678–9842. [Google Scholar] [CrossRef] [PubMed]
- Arduengo, A.J.; Bertrand, G. Carbenes Introduction. Chem. Rev. 2009, 109, 3209–3210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doddi, A.; Peters, M.; Tamm, M. N-Heterocyclic Carbene Adducts of Main Group Elements and Their Use as Ligands in Transition Metal Chemistry. Chem. Rev. 2019, 119, 6994–7112. [Google Scholar] [CrossRef]
- Arnold, P.L.; Casely, I.J. F-Block N-Heterocyclic Carbene Complexes. Chem. Rev. 2009, 109, 3599–3611. [Google Scholar] [CrossRef]
- Cazin, C.S.J. N-Heterocyclic Carbenes in Transition Metal Catalysis and Organocatalysis; Springer Science & Business Media: Dordrecht, The Netherlands, 2011; Volume 32. [Google Scholar]
- Lin, J.C.Y.; Huang, R.T.W.; Lee, C.S.; Bhattacharyya, A.; Hwang, W.S.; Lin, I.J.B. Coinage Metal−N-Heterocyclic Carbene Complexes. Chem. Rev. 2009, 109, 3561–3598. [Google Scholar] [CrossRef]
- Dash, C.; Kroll, P.; Yousufuddin, M.; Dias, H.V.R. Isolable, gold carbonyl complexes supported by N-heterocyclic carbenes. Chem. Commun. 2011, 47, 4478. [Google Scholar] [CrossRef]
- Celik, M.A.; Dash, C.; Adiraju, V.A.; Das, A.; Yousufuddin, M.; Frenking, G.; Dias, H.V.R. End-On and Side-On π-Acid Ligand Adducts of Gold(I): Carbonyl, Cyanide, Isocyanide, and Cyclooctyne Gold(I) Complexes Supported by N-Heterocyclic Carbenes and Phosphines. Inorg. Chem. 2012, 52, 729–742. [Google Scholar] [CrossRef]
- Dash, C.; Das, A.; Yousufuddin, M.; Dias, H.V.R. Isolable, Copper(I) Dicarbonyl Complexes Supported by N-Heterocyclic Carbenes. Inorg. Chem. 2013, 52, 1584–1590. [Google Scholar] [CrossRef]
- Dash, C.; Yousufuddin, M.; Cundari, T.R.; Dias, H.V.R.; Dias, H.V.R. Gold-Mediated Expulsion of Dinitrogen from Organic Azides. J. Am. Chem. Soc. 2013, 135, 15479–15488. [Google Scholar] [CrossRef]
- Wang, G.; Ponduru, T.T.; Wang, Q.; Zhao, L.; Frenking, G.; Dias, H.V.R. Heterobimetallic Complexes Featuring Fe(CO)5 as a Ligand on Gold. Chem. - A Eur. J. 2017, 23, 17222–17226. [Google Scholar] [CrossRef] [PubMed]
- Dash, C.; Wang, G.; Muñoz-Castro, A.R.; Ponduru, T.T.; Zacharias, A.O.; Yousufuddin, M.; Dias, H.V.R. Organic Azide and Auxiliary-Ligand-Free Complexes of Coinage Metals Supported by N-Heterocyclic Carbenes. Inorg. Chem. 2019, 59, 2188–2199. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Narouz, M.R.; Lummis, P.A.; Singh, I.; Nazemi, A.; Li, C.H.; Crudden, C.M. N-Heterocyclic Carbenes in Materials Chemistry. Chem. Rev. 2019, 119, 4986–5056. [Google Scholar] [CrossRef] [PubMed]
- Rovis, T.; Nolan, S.P. Stable Carbenes: From ‘Laboratory Curiosities’ to Catalysis Mainstays. Synlett 2013, 24, 1188–1189. [Google Scholar] [CrossRef] [Green Version]
- Schaper, L.-A.; Hock, S.J.; Herrmann, W.A.; Kuehn, F.E. Synthesis and Application of Water-Soluble NHC Transition-Metal Complexes. Angew. Chem. int. Ed. 2013, 44, 270–289. [Google Scholar] [CrossRef]
- He, Y.; Lv, M.-F.; Cai, C. A simple procedure for polymer-supported N-heterocyclic carbene silver complex via click chemistry: An efficient and recyclable catalyst for the one-pot synthesis of propargylamines. Dalton Trans. 2012, 41, 12428–12433. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Song, Y.; Fang, L.; Zou, G. Well-defined N-heterocyclic carbene silver halides of 1-cyclohexyl-3-arylmethylimidazolylidenes: Synthesis, structure and catalysis in A3-reaction of aldehydes, amines and alkynes. Dalton Trans. 2011, 40, 2046. [Google Scholar] [CrossRef]
- Herrmann, W.A. N-heterocyclic carbenes: A new concept in organometallic catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Glorius, F.; Glorius, F. N-Heterocyclic Carbenes in Transition Metal Catalysis; Springer-Verlag Berlin Heidelberg: Heidelberg, Germany, 2007. [Google Scholar]
- Marion, N.; Nolan, S.P.; Díez-González, S. N-Heterocyclic Carbenes as Organocatalysts. Angew. Chem. Int. Ed. 2007, 46, 2988–3000. [Google Scholar] [CrossRef]
- Izquierdo, J.; Hutson, G.E.; Cohen, D.T.; Scheidt, K.A. A continuum of progress: Applications of N-hetereocyclic carbene catalysis in total synthesis. Angew. Chem. Int. Ed. 2012, 51, 11686–11698. [Google Scholar] [CrossRef] [Green Version]
- Bugaut, X.; Glorius, F. Organocatalytic umpolung: N-heterocyclic carbenes and beyond. Chem. Soc. Rev. 2012, 41, 3511. [Google Scholar] [CrossRef] [PubMed]
- Díez-González, S.; Marion, N.; Nolan, S.P. N-Heterocyclic Carbenes in Late Transition Metal Catalysis. Chem. Rev. 2009, 109, 3612–3676. [Google Scholar] [CrossRef] [PubMed]
- Kantchev, E.A.B.; O’Brien, C.J.; Organ, M.G. Palladium Complexes of N-Heterocyclic Carbenes as Catalysts for Cross-Coupling Reactions — A Synthetic Chemist′s Perspective. Angew. Chem. Int. Ed. 2007, 38, 2768–2813. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.J.; Lin, I.J.B. Facile Synthesis of Silver(I)−Carbene Complexes. Useful Carbene Transfer Agents. Organometallics 1998, 17, 972–975. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Aher, S.B.; Muskawar, P.N.; Thenmozhi, K.; Bhagat, P.R. Recent developments of metal N-heterocyclic carbenes as anticancer agents. Eur. J. Med. Chem. 2014, 81, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, C.; Bravin, A.; Cavaletti, G.; Pellei, M.; Santini, C. The combined therapeutical effect of metal-based drugs and radiation therapy: The present status of research. Curr. Med. Chem. 2014, 21, 2237–2265. [Google Scholar] [CrossRef]
- Budagumpi, S.; Haque, R.A.; Endud, S.; Rehman, G.U.; Salman, A.W. Biologically Relevant Silver(I)-N-Heterocyclic Carbene Complexes: Synthesis, Structure, Intramolecular Interactions, and Applications. Eur. J. Inorg. Chem. 2013, 2013, 4367–4388. [Google Scholar] [CrossRef]
- Liu, W.; Gust, R. Metal N-heterocyclic carbene complexes as potential antitumor metallodrugs. Chem. Soc. Rev. 2013, 42, 755–773. [Google Scholar] [CrossRef]
- Monteiro, D.C.F.; Phillips, R.M.; Crossley, B.D.; Fielden, J.; Willans, C.E. Enhanced cytotoxicity of silver complexes bearing bidentate N-heterocyclic carbeneligands. Dalton Trans. 2012, 41, 3720. [Google Scholar] [CrossRef] [Green Version]
- Hindi, K.M.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. The Medicinal Applications of Imidazolium Carbene−Metal Complexes. Chem. Rev. 2009, 109, 3859–3884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teyssot, M.-L.; Jarrousse, A.-S.; Manin, M.; Chevry, A.; Roche, S.; Norre, F.; Beaudoin, C.; Morel, L.; Boyer, D.; Mahiou, R.; et al. Metal-NHC complexes: A survey of anti-cancer properties. Dalton Trans. 2009, 35, 6894. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, C.G.; Dyson, P.J. Bioorganometallic chemistry—from teaching paradigms to medicinal applications. Chem. Soc. Rev. 2009, 38, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Porchia, M.; Pellei, M.; Marinelli, M.; Tisato, F.; Del Bello, F.; Santini, C. New insights in Au-NHCs complexes as anticancer agents. Eur. J. Med. Chem. 2018, 146, 709–746. [Google Scholar] [CrossRef] [PubMed]
- Arduengo, A.J.; Goerlich, J.R.; Marshall, W.J. A stable diaminocarbene. J. Am. Chem. Soc. 1995, 117, 11027–11028. [Google Scholar] [CrossRef]
- Alder, R.W.; Allen, P.R.; Murray, M.; Orpen, A.G. Bis(diisopropylamino)carbene. Angew. Chem. Int. Ed. 1996, 35, 1121–1123. [Google Scholar] [CrossRef]
- Santini, C.; Marinelli, M.; Pellei, M. Boron-Centered Scorpionate-Type NHC-Based Ligands and Their Metal Complexes. Eur. J. Inorg. Chem. 2016, 2016, 2312–2331. [Google Scholar] [CrossRef]
- Weiss, A.; Pritzkow, H.; Siebert, W. Synthesis, Structures and Reactivity ofN-Borane-Protected 1,1′-Bisimidazoles with Different Bridging Functions. Eur. J. Inorg. Chem. 2002, 2002, 1607–1614. [Google Scholar] [CrossRef]
- Kernbach, U.; Ramm, M.; Luger, P.; Fehlhammer, W.P. A Chelating Triscarbene Ligand and Its Hexacarbene Iron Complex. Angew. Chem. Int. Ed. 1996, 35, 310–312. [Google Scholar] [CrossRef]
- Lapointe, R.E.; Roof, G.R.; Abboud, K.A.; Klosin, J. New Family of Weakly Coordinating Anions. J. Am. Chem. Soc. 2000, 122, 9560–9561. [Google Scholar] [CrossRef]
- Asada, T.; Hoshimoto, Y.; Ogoshi, S. Rotation-Triggered Transmetalation on a Heterobimetallic Cu/Al N-Phosphine-Oxide-Substituted Imidazolylidene Complex. J. Am. Chem. Soc. 2020, 142, 9772–9784. [Google Scholar] [CrossRef]
- Vagedes, D.; Kehr, G.; König, D.; Wedeking, K.; Fröhlich, R.; Erker, G.; Mück-Lichtenfeld, C.; Grimme, S. Formation of Isomeric BAr3 Adducts of 2-Lithio-N-methylimidazole. Eur. J. Inorg. Chem. 2002, 2002, 2015–2021. [Google Scholar] [CrossRef]
- Wacker, A.; Pritzkow, H.; Siebert, W. Nucleophilic Substitution Reactions with the 3-Borane-1,4,5-trimethylimidazol-2-ylidene Anion. – Unexpected Formation of an Imidazabole Isomer. Eur. J. Inorg. Chem. 1999, 5, 789–793. [Google Scholar] [CrossRef]
- Wacker, A.; Pritzkow, H.; Siebert, W. Borane-substituted imidazol-2-ylidenes. Syntheses, structures, and reactivity. Eur. J. Inorg. Chem. 1998, 6, 843–849. [Google Scholar] [CrossRef]
- Padilla-Martínez, I.I.; Martínez-Martínez, F.J.; López-Sandoval, A.; Girón-Castillo, K.I.; Brito, M.A.; Contreras, R. New imidazabole derivatives: Dimers of carbene-borane adducts. Eur. J. Inorg. Chem. 1998, 10, 1547–1553. [Google Scholar]
- Okada, K.; Suzuki, R.; Oda, M. Novel boron–nitrogen containing compounds from the reaction of organolithiums with complexes between dimesitylfluoroborane and six- or five-membered aza aromatic compounds. J. Chem. Soc., Chem. Commun. 1995, 20, 2069–2070. [Google Scholar] [CrossRef]
- Padilla-Martínez, I.I.; Ariza-Castolo, A.; Contreras, R. NMR Study of isolobal N-CH3+, N-BH3 and N-BF3 imidazole derivatives. Magn. Reson. Chem. 1993, 31, 189–193. [Google Scholar] [CrossRef]
- Nasr, A.; Winkler, A.; Tamm, M. Anionic N-heterocyclic carbenes: Synthesis, coordination chemistry and applications in homogeneous catalysis. Coord. Chem. Rev. 2016, 316, 68–124. [Google Scholar] [CrossRef]
- Wacker, A.; Yan, C.G.; Kaltenpoth, G.; Ginsberg, A.; Arif, A.M.; Ernst, R.; Pritzkow, H.; Siebert, W. Metal complexes of anionic 3-borane-1-alkylimidazol-2-ylidene derivatives. J. Organomet. Chem. 2002, 641, 195–202. [Google Scholar] [CrossRef]
- Liu, W.-C.; Liu, Y.-H.; Lin, T.-S.; Peng, S.-M.; Chiu, C.-W. 1,2-Migration of N-Diarylboryl Imidazol-2-ylidene through Intermolecular Radical Process. Inorg. Chem. 2017, 56, 10543–10548. [Google Scholar] [CrossRef]
- Padilla-Martínez, I.I.; Rosalez-Hoz, M.D.J.; Contreras, R.; Kerschl, S.; Wrackmeyer, B. From Azole—Borane Adducts to Azaboles—Molecular Structure of an Imidazabole. Eur. J. Inorg. Chem. 1994, 127, 343–346. [Google Scholar] [CrossRef]
- Vagedes, D.; Erker, G.; Kehr, G.; Bergander, K.; Kataeva, O.; Fröhlich, R.; Grimme, S.; Mück-Lichtenfeld, C. Tris(pentafluorophenyl)borane adducts of substituted imidazoles: Conformational features and chemical behavior upon deprotonation. Dalton Trans. 2003, 7, 1337–1344. [Google Scholar] [CrossRef]
- Arrowsmith, M.; Heath, A.; Hill, M.S.; Hitchcock, P.B.; Kociok-Köhn, G. Tris(imidazolin-2-ylidene-1-yl)borate Complexes of the Heavier Alkaline Earths: Synthesis and Structural Studies. Organometallics 2009, 28, 4550–4559. [Google Scholar] [CrossRef]
- Crudden, C.M.; Allen, D.P. Stability and reactivity of N-heterocyclic carbene complexes. Co-ord. Chem. Rev. 2004, 248, 2247–2273. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, W.; Liu, T.; Wang, K.; Qi, X.; Zhang, J.; Zhang, Q. TowardsN-Alkylimidazole Borane-based Hypergolic Fuels. Chem. – Asian J. 2016, 11, 3528–3533. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gioia Lobbia, G.; Papini, G. Synthesis and properties of poly(pyrazolyl)borate and related boron-centered scorpionate ligands. Part A: Pyrazole-based systems. Mini-Rev. Org. Chem. 2010, 7, 84–124. [Google Scholar] [CrossRef]
- Pellei, M.; Lobbia, G.G.; Papini, G.; Santini, C. Synthesis and Properties of Poly(pyrazolyl)borate and Related Boron-Centered Scorpionate Ligands. Part B: Imidazole-, Triazole- and Other Heterocycle-Based Systems. Mini-Reviews Org. Chem. 2010, 7, 173–203. [Google Scholar] [CrossRef]
- Fränkel, R.; Birg, C.; Kernbach, U.; Habereder, T.; Noth, H.; Fehlhammer, W.P. A Homoleptic Carbene–Lithium Complex. Angew. Chem., Int. Ed. 2001, 40, 1907–1910. [Google Scholar] [CrossRef]
- Frankel, R.; Kernbach, U.; Bakola-Christianopoulou, M.; Plaia, U.; Suter, M.; Ponikwar, W.; Noth, H.; Moinet, C.; Fehlhammer, W.P. Homoleptic carbene complexes. Part VIII. Hexacarbene complexes. J. Organomet. Chem. 2001, 530–545. [Google Scholar] [CrossRef]
- Fränkel, R.; Kniczek, J.; Ponikwar, W.; Noth, H.; Polborn, K.; Fehlhammer, W.P. Homoleptic carbene complexes: Part IX. Bis(imidazolin-2-ylidene-1-yl)borate complexes of palladium(II), platinum(II) and gold(I). Inorg. Chim. Acta 2001, 312, 23–39. [Google Scholar]
- Nieto, I.; Bontchev, R.P.; Smith, J.M. Synthesis of a Bulky Bis(carbene)borate Ligand – Contrasting Structures of Homoleptic Nickel(II) Bis(pyrazolyl)borate and Bis(carbene)borate Complexes. Eur. J. Inorg. Chem. 2008, 2008, 2476–2480. [Google Scholar] [CrossRef]
- Biffis, A.; Lobbia, G.G.; Papini, G.; Pellei, M.; Santini, C.; Scattolin, E.; Tubaro, C. Novel scorpionate-type triscarbene ligands and their silver and gold complexes. J. Organomet. Chem. 2008, 693, 3760–3766. [Google Scholar] [CrossRef]
- Papini, G.; Bandoli, G.; Dolmella, A.; Lobbia, G.G.; Pellei, M.; Santini, C. New homoleptic carbene transfer ligands and related coinage metal complexes. Inorg. Chem. Commun. 2008, 11, 1103–1106. [Google Scholar] [CrossRef]
- Papini, G.; Pellei, M.; Lobbia, G.G.; Burini, A.; Santini, C. Sulfonate- or carboxylate-functionalized N-heterocyclic bis-carbene ligands and related water soluble silver complexes. Dalton Trans. 2009, 6985. [Google Scholar] [CrossRef]
- Giorgetti, M.; Aquilanti, G.; Pellei, M.; Gandin, V. The coordination core of Ag( i ) N-heterocyclic carbene (NHC) complexes with anticancer properties as revealed by synchrotron radiation X-ray absorption spectroscopy. J. Anal. At. Spectrom. 2014, 29, 491–497. [Google Scholar] [CrossRef]
- Pellei, M.; Gandin, V.; Marinelli, M.; Marzano, C.; Yousufuddin, M.; Dias, H.V.R.; Santini, C. Synthesis and Biological Activity of Ester- and Amide-Functionalized Imidazolium Salts and Related Water-Soluble Coinage Metal N-Heterocyclic Carbene Complexes. Inorg. Chem. 2012, 51, 9873–9882. [Google Scholar] [CrossRef]
- Gandin, V.; Pellei, M.; Marinelli, M.; Marzano, C.; Dolmella, A.; Giorgetti, M.; Santini, C. Synthesis and in vitro antitumor activity of water soluble sulfonate- and ester-functionalized silver(I) N-heterocyclic carbene complexes. J. Inorg. Biochem. 2013, 129, 135–144. [Google Scholar] [CrossRef]
- Maria, L.; Paulo, A.; Santos, I.C.; Santos, I.; Kurz, P.; Spingler, B.; Alberto, R. Very Small and Soft Scorpionates: Water Stable Technetium Tricarbonyl Complexes Combining a Bis-agostic (k3-H, H, S) Binding Motif with Pendant and Integrated Bioactive Molecules. J. Am. Chem. Soc. 2006, 128, 14590–14598. [Google Scholar] [CrossRef]
- Lu, D.; Tang, H. Theoretical survey of the ligand tunability of poly(azolyl)borates. Phys. Chem. Chem. Phys. 2015, 17, 17027–17033. [Google Scholar] [CrossRef]
- Pellei, M.; Papini, G.; Lobbia, G.G.; Ricci, S.; Yousufuddin, M.; Dias, H.V.R.; Santini, C.; Dias, H.V.R. Scorpionates bearing nitro substituents: Mono-, bis- and tris-(3-nitro-pyrazol-1-yl)borate ligands and their copper(i) complexes. Dalton Trans. 2010, 39, 8937. [Google Scholar] [CrossRef]
- Dias, H.V.R.; Alidori, S.; Lobbia, G.G.; Papini, G.; Pellei, M.; Santini, C.; Dias, H.V.R. Small Scorpionate Ligands: Silver(I)-Organophosphane Complexes of 5-CF3-Substituted Scorpionate Ligand Combining a B−H··Ag Coordination Motif. Inorg. Chem. 2007, 46, 9708–9714. [Google Scholar] [CrossRef] [PubMed]
- Meisters, M.; VandeBerg, J.T.; Cassaretto, F.P.; Posvic, H.; Moore, C.E. Studies in the tetraarylborates: Part V. The influence of substituents on the stability of tetraarylborates. Anal. Chim. Acta 1970, 49, 481–485. [Google Scholar] [CrossRef]
- Bakshi, P.K.; Linden, A.; Vincent, B.R.; Roe, S.P.; Adhikesavalu, D.; Cameron, T.S.; Knop, O. Crystal chemistry of tetraradial species. Part 4. Hydrogen bonding to aromatic π systems: Crystal structures of fifteen tetraphenylborates with organic ammonium cations. Can. J. Chem. 1994, 72, 1273–1293. [Google Scholar] [CrossRef] [Green Version]
- Belanger, S.; Beauchamp, A.L. (1-Methylimidazole- N 3 )triphenylboron. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1998, 54, IUC9800057. [Google Scholar] [CrossRef]
- Bélanger-Chabot, G.; Kaplan, S.M.; Deokar, P.; Szimhardt, N.; Haiges, R.; Christe, K.O. Synthesis and Characterization of Nitro-, Trinitromethyl-, and Fluorodinitromethyl-Substituted Triazolyl- and Tetrazolyl-trihydridoborate Anions. Chem. - A Eur. J. 2017, 23, 13087–13099. [Google Scholar] [CrossRef]
- Ridlen, S.G.; Kulkarni, N.; Dias, H.V.R. Monoanionic, Bis(pyrazolyl)methylborate [(Ph3B)CH(3,5-(CH3)2Pz)2)]−as a Supporting Ligand for Copper(I)-ethylene, cis-2-Butene, and Carbonyl Complexes. Inorg. Chem. 2017, 56, 7237–7246. [Google Scholar] [CrossRef] [PubMed]
- Good, C.D.; Ritter, D.M. Alkenylboranes. II. Improved Preparative Methods and New Observations on Methylvinylboranes. J. Am. Chem. Soc. 1962, 84, 1162–1166. [Google Scholar] [CrossRef]
- Winkelmann, O.H.; Navarro, O. Microwave-Assisted Synthesis of N-Heterocyclic Carbene- Palladium(II) Complexes. Adv. Synth. Catal. 2010, 352, 212–214. [Google Scholar] [CrossRef]
- Curran, D.P.; Solovyev, A.; Brahmi, M.M.; Fensterbank, L.; Malacria, M.; Lacôte, E. Synthesis and Reactions of N-Heterocyclic Carbene Boranes. Angew. Chem. Int. Ed. 2011, 50, 10294–10317. [Google Scholar] [CrossRef]
- Wrackmeyer, B. Nuclear Magnetic Resonance Spectroscopy of Boron Compounds Containing Two-, Three- and Four-Coordinate Boron. Annual Reports on NMR Spectroscopy 1988, 20, 61–203. [Google Scholar] [CrossRef]
- Kronig, S.; Theuergarten, E.; Daniliuc, C.; Jones, P.G.; Tamm, M. Anionic N-Heterocyclic Carbenes That Contain a Weakly Coordinating Borate Moiety. Angew. Chem. Int. Ed. 2012, 51, 3240–3244. [Google Scholar] [CrossRef] [PubMed]
- Kolychev, E.L.; Kronig, S.; Brandhorst, K.; Freytag, M.; Jones, P.G.; Tamm, M. Iridium(I) Complexes with Anionic N-Heterocyclic Carbene Ligands as Catalysts for the Hydrogenation of Alkenes in Nonpolar Media. J. Am. Chem. Soc. 2013, 135, 12448–12459. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Zhao, J.; Zhao, Y.; Li, L.; Zhang, H. A Modified Procedure for the Synthesis of 1-Arylimidazoles. Synthesis 2003, 17, 2661–2666. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history ofSHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2007, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.; Bourhis, L.J.; Gildea, R.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellei, M.; Vallesi, R.; Bagnarelli, L.; Dias, H.V.R.; Santini, C. Syntheses and Reactivity of New Zwitterionic Imidazolium Trihydridoborate and Triphenylborate Species. Molecules 2020, 25, 3184. https://doi.org/10.3390/molecules25143184
Pellei M, Vallesi R, Bagnarelli L, Dias HVR, Santini C. Syntheses and Reactivity of New Zwitterionic Imidazolium Trihydridoborate and Triphenylborate Species. Molecules. 2020; 25(14):3184. https://doi.org/10.3390/molecules25143184
Chicago/Turabian StylePellei, Maura, Riccardo Vallesi, Luca Bagnarelli, H. V. Rasika Dias, and Carlo Santini. 2020. "Syntheses and Reactivity of New Zwitterionic Imidazolium Trihydridoborate and Triphenylborate Species" Molecules 25, no. 14: 3184. https://doi.org/10.3390/molecules25143184
APA StylePellei, M., Vallesi, R., Bagnarelli, L., Dias, H. V. R., & Santini, C. (2020). Syntheses and Reactivity of New Zwitterionic Imidazolium Trihydridoborate and Triphenylborate Species. Molecules, 25(14), 3184. https://doi.org/10.3390/molecules25143184