Caenorhabditis elegans as a Model Organism to Evaluate the Antioxidant Effects of Phytochemicals
Abstract
:1. Introduction
2. Polyphenols and Oxidative Stress
3. Caenorhabditis elegans and Oxidative Stress
4. Methodological Approaches for Antioxidants Evaluation in C. elegans
4.1. Determination of Markers of Oxidative Damage
4.1.1. Intracellular ROS Levels
4.1.2. Glutathione Levels
4.1.3. Evaluation of Biological Molecules Damage
Protein Oxidation
Lipid Peroxidation
DNA Damage
4.2. Activity of Antioxidant Enzymes
4.3. Exploring Genes and Signaling Pathways Involved in Antioxidant Response
4.3.1. Mutant Worms
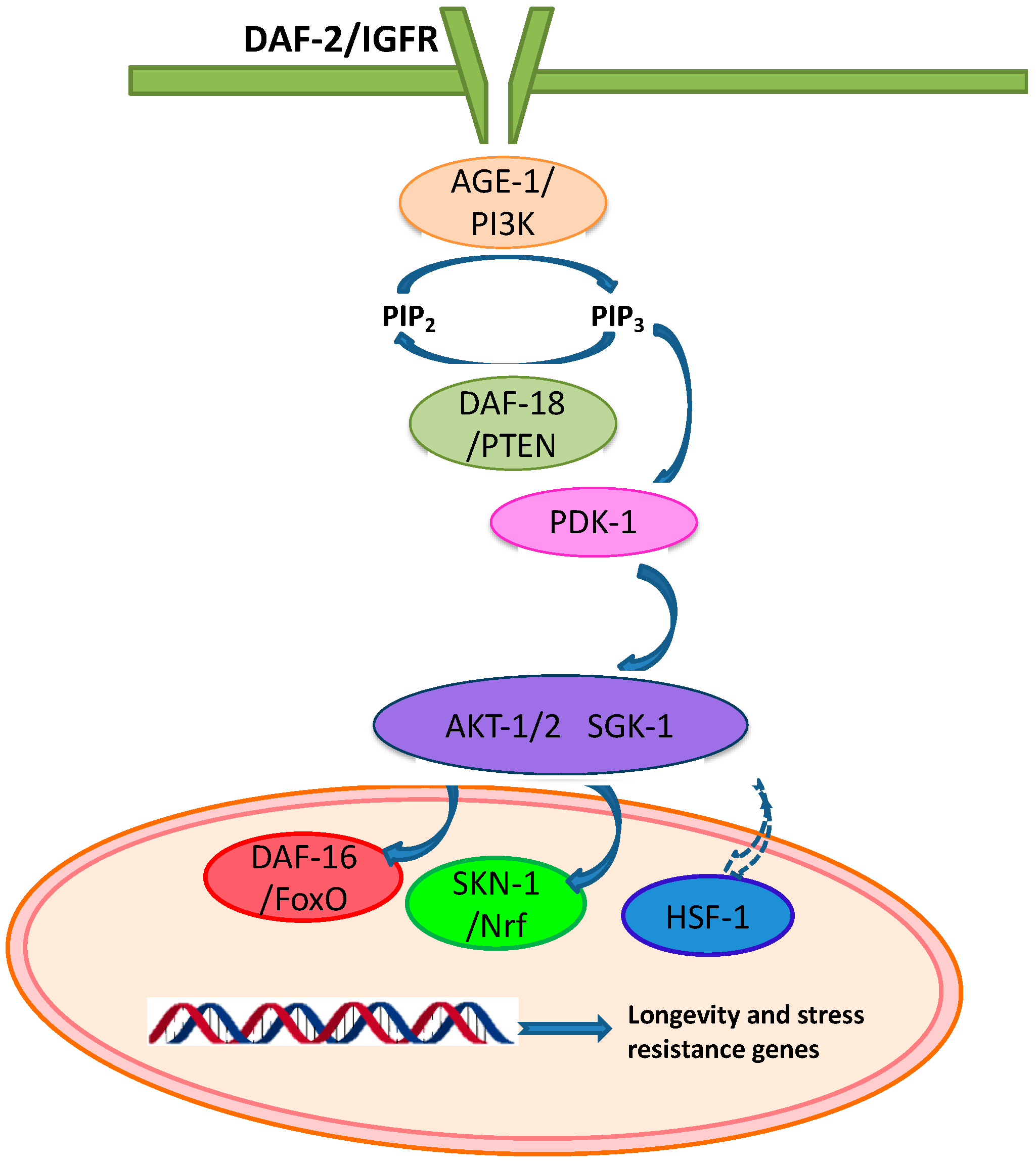
Insulin/IGF-1 Signaling (IIS) Pathway
Nrf2/SKN-1 Signaling Pathway
Heat Shock Protein Response
4.3.2. Transgenic Worms Containing Reporter Gene Fusions
4.3.3. RT-qPCR
5. Final Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H. Oxidative stress: Introductory remarks In Oxidative Stress; Sies, H., Ed.; Academic Press: London, UK, 1985; pp. 1–8. [Google Scholar]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals and antioxidants-quo vadis? Trends Pharmacol. Sci. 2011, 32, 125–130. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Paramás, A.M.; Oludemi, T.; Ayuda-Durán, B.; González-Manzano, S. Plant phenolics as functional food ingredients. Adv. Food Nutr. Res. 2019, 90, 183–257. [Google Scholar]
- Benthsáth, A.; Rusznyak, S.T.; Szent-Györgyi, A. Vitamin P. Nature 1937, 139, 326–327. [Google Scholar] [CrossRef]
- Vickery, H.B.; Nelson, E.M.; Almquist, H.J.; Elvehjem, C.A. Term “Vitamin P” recommended to be discontinued. Science 1950, 112, 628. [Google Scholar]
- Del Rio, D.; Rodríguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 8, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Laranjinha, J. Translation of chemical properties of polyphenols into biological activity with impact on human health. In Recent Advances in Polyphenols Research; Santos-Buelga, C., Escribano, M.T., Lattanzio, V., Eds.; Wiley-Blackwell: Chichester, UK, 2010; Volume 2, pp. 269–282. [Google Scholar]
- Barrajón-Catalán, E.; Herranz-López, M.; Joven, J.; Segura-Carretero, A.; Alonso-Villaverde, C.; Menéndez, J.A.; Micol, V. Molecular promiscuity of plant polyphenols in the management of age-related diseases: Far beyond their antioxidant properties. Adv. Exp. Med. Biol. 2014, 824, 141–159. [Google Scholar] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollman, P.C.H. Unravelling of the health effects of polyphenols is a complex puzzle complicated by metabolism. Arch. Biochem. Biophys. 2014, 559, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Kroon, P.A.; Clifford, M.N.; Crozier, A.; Day, A.J.; Donovan, J.L.; Manach, C.; Williamson, G. How should we assess the effects of exposure to dietary polyphenols in vitro? Am. J. Clin. Nutr. 2004, 80, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; González-Manzano, S.; González-Paramás, A.; Santos-Buelga, C. Antioxidant evaluation of O-methylated metabolites of catechin, epicatechin and quercetin. J. Pharm. Biomed. Anal. 2010, 51, 443–449. [Google Scholar] [CrossRef]
- Altintas, O.; Park, S.; Lee, S.J.V. The role of insulin/IGF-1 signaling in the longevity of model in vertebrates, C. elegans and D. Melanogaster. BMB Rep. 2016, 49, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Dueñas, M.; Surco-Laos, F.; Gonzalez-Manzano, S.; Gonzalez-Paramas, A.M.; Santos-Buelga, C. Antioxidant properties of major metabolites of quercetin. Eur. Food Res. Technol. 2011, 232, 103–111. [Google Scholar] [CrossRef]
- Barbieri, M.; Bonafe, M.; Franceschi, C.; Paolisso, G. Insulin/IGF-I-signaling pathway: An evolutionarily conserved mechanism of longevity from yeast to humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E1064–E1071. [Google Scholar] [CrossRef] [Green Version]
- Ayuda-Durán, B.; González-Manzano, S.; Miranda-Vizuete, A.; Dueñas, M.; Santos-Buelga, C.; González-Paramás, A.M. Epicatechin modulates stress-resistance in C. elegans via insulin/IGF-1 signaling pathway. PLoS ONE 2019, 14, e0199483. [Google Scholar]
- Ayuda-Durán, B.; González-Manzano, S.; Miranda-Vizuete, A.; Sánchez-Hernández, E.; Romero, R.R.; Dueñas, M.; Santos-Buelga, C.; González-Paramás, A.M. Exploring target genes involved in the effect of quercetin on the response to oxidative stress in Caenorhabditis elegans. Antioxidants 2019, 8, 585. [Google Scholar] [CrossRef] [Green Version]
- Asthana, J.; Yadav, D.; Pant, A.; Yadav, A.K.; Gupta, M.M.; Pandey, R. Acacetin 7-O-α-l-rhamnopyranosyl (1-2) β-D-xylopyranoside elicits life-span extension and stress resistance in Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1160–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietsch, K.; Saul, N.; Menzel, R.; Stürzenbaum, S.R.; Steinberg, C.E. Quercetin mediated lifespan extension in Caenorhabditis elegans is modulated by age-1, daf-2, sek-1 and unc-43. Biogerontology 2009, 10, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Büchter, C.; Ackermann, D.; Havermann, S.; Honnen, S.; Chovolou, Y.; Fritz, G.; Kampkötter, A.; Wätjen, W. Myricetin-mediated lifespan extension in Caenorhabditis elegans is modulated by DAF-16. Int. J. Mol. Sci. 2013, 14, 11895–11914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, S.; Hou, D.X. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol. Nutr. Food Res. 2016, 60, 1731–1755. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Lee-Hilz, Y.Y.; Boerboom, A.M.J.F.; Westphal, A.H.; van Berkel, W.J.H.; Aarts, J.M.M.J.G.; Rietjens, I.M.C.M. Pro-oxidant activity of flavonoids induces EpRE-mediated gene expression. Chem. Res. Toxicol. 2006, 19, 1499–1505. [Google Scholar] [CrossRef]
- Masella, R.; Di Benedetto, R.; Varì, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.C.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A.M. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Ferriola, P.C.; Cody, V.; Middleton, E. Protein kinase C inhibition by plant flavonoids: Kinetic mechanisms and structure–activity relationships. Biochem. Pharmacol. 1989, 38, 1617–1624. [Google Scholar] [CrossRef]
- Guarente, L.; Kenyon, C. Genetic pathways that regulate ageing in model organisms. Nature 2000, 408, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Kyriakakis, E.; Markaki, M.; Tavernarakis, N. Caenorhabditis elegans as a model for cancer research. Mol. Cell. Oncol. 2015, 2, e975027. [Google Scholar] [CrossRef] [Green Version]
- Tissenbaum, H.A. Using C. elegans for aging research. Invertebr. Reprod. Dev. 2015, 59, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tissenbaum, H.A. Genetics, life span, health span, and the aging process in Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 503–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaletta, T.; Hengartner, M.O. Finding function in novel targets. C. elegans as a model organism. Nat. Rev. Drug Discov. 2006, 5, 387–398. [Google Scholar] [CrossRef]
- The C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: A platform for investigating biology. Science 1998, 282, 2012–2018. [Google Scholar]
- Boulin, T.; Etchberger, J.F.; Hobert, O.; Hughes, H. Reporter gene fusions (April 5, 2006). In WormBook; The C. elegans Research Community, Ed.; WormBook: Pasadena, CA, USA, 2006; Available online: http://www.wormbook.org (accessed on 17 June 2020). [CrossRef] [Green Version]
- Kimura, K.D.; Tissenbaum, H.A.; Liu, Y.; Ruvkun, G. Daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 1997, 277, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Mohri-Shiomi, A.; Garsin, D.A. Insulin signaling and the heat shock response modulate protein homeostasis in the Caenorhabditis elegans intestine during infection. J. Biol. Chem. 2008, 283, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.T.; Hu, P.J. Insulin/Insulin-Like Growth Factor Signaling in C. elegans (26 December 2013). In WormBook; The C. elegans Research Community, Ed.; WormBook: Pasadena, CA, USA, 2013; Available online: http://www.wormbook.org (accessed on 3 April 2020). [CrossRef] [Green Version]
- Hsu, A.; Coleen, T.; Kenyon, C. Regulation of aging and age-related disease by DAF-16 and Heat-Shock Factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef] [Green Version]
- Tullet, J.M.; Hertweck, M.; An, J.H.; Baker, J.; Hwang, J.Y.; Liu, S.; Oliveira, R.P.; Baumeister, R.; Blackwell, T.K. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 2008, 132, 1025–1038. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, K.; Fukumoto, K.; Murakami, T.; Harada, S.; Hosono, R.; Wadhwa, R.; Mitsui, Y.; Ohkuma, S. Extended longevity of Caenorhabditis elegans by knocking in extra copies of hsp70F, a homolog of mot-2 (mortalin)/mthsp70/Grp75. FEBS Lett. 2002, 516, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Walker, G.A.; Lithgow, G.J. Reactive oxygen species and aging in Caenorhabditis elegans: Causal or casual relationship? Antiox. Redox Signal. 2003, 2, 131–139. [Google Scholar]
- Walker, G.A.; White, T.M.; McColl, G.; Jenkins, N.L.; Babich, S.; Candido, E.P.; Johnson, T.E.; Lithgow, G.J. Heat shock protein accumulation is upregulated in a long-lived mutant of Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B281–B287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, N.W.; Rea, S.L.; Moyle, S.; Kell, A.; Johnson, T.E. Proteasomal dysfunction activates the transcription factor SKN-1 and produces a selective oxidative-stress response in Caenorhabditis elegans. Biochem. J. 2008, 409, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef] [Green Version]
- Mertenskötter, A.; Keshet, A.; Gerke, P.; Paul, R.J. The p38 MAPK PMK-1 shows heat-induced nuclear translocation, supports chaperone expression, and affects the heat tolerance of Caenorhabditis elegans. Cell Stress Chaperones 2013, 18, 293–306. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Sudji, I.R.; Wang, E.; Joubert, E.; Van Wyk, B.E.; Wink, M. Ameliorative effect of aspalathin from rooibos (Aspalathus linearis) on acute oxidative stress in Caenorhabditis elegans. Phytomedicine 2013, 20, 380–386. [Google Scholar] [CrossRef]
- Grünz, G.; Haas, K.; Soukup, S.; Klingenspor, M.; Kulling, S.E.; Daniel, H.; Spanier, B. Structural features and bioavailability of four flavonoids and their implications for lifespan-extending and antioxidant actions in C. elegans. Mech. Ageing Dev. 2012, 133, 1–10. [Google Scholar]
- Kampkötter, A.; Timpel, C.; Zurawski, R.F.; Ruhl, S.; Chovolou, Y.; Proksch, P.; Wätjen, W. Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 149, 314–323. [Google Scholar] [CrossRef]
- Surco-Laos, F.; Cabello, J.; Gómez-Orte, E.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C.; Dueñas, M. Effects of O-methylated metabolites of quercetin on oxidative stress, thermotolerance, lifespan and bioavailability on Caenorhabditis elegans. Food Funct. 2011, 2, 445–456. [Google Scholar] [CrossRef]
- Surco-Laos, F.; Dueñas, M.; González-Manzano, S.; Juan Cabello, J.; Santos-Buelga, C.; González-Paramás, A.M. Influence of catechins and their methylated metabolites on lifespan and resistance to oxidative and thermal stress of Caenorhabditis elegans and epicatechin uptake. Food Res. Int. 2012, 46, 514–521. [Google Scholar] [CrossRef]
- Zhang, L.; Jie, G.; Zhang, J.; Zhao, B. Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Radic. Biol. Med. 2009, 46, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Bruskov, V.I.; Malakhova, L.V.; Masalimov, Z.K.; Chernikov, A.V. Heat-induced formation of reactive oxygen species and 8-oxoguanine, a biomarker of damage to DNA. Nucleic Acids Res. 2002, 30, 1354–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayuda-Durán, B.; González-Manzano, S.; Gil-Sánchez, I.; Moreno-Arribas, M.V.; Bartolomé, B.; Sanz-Buenhombre, M.; Guadarrama, A.; Santos-Buelga, C.; González-Paramás, A.M. Antioxidant characterization and biological effects of grape pomace extracts supplementation in Caenorhabditis elegans. Foods 2019, 8, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kampkötter, A.; Gombitang-Nkwonkam, C.; Zurawski, R.F.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and FoxO transcription factor DAF-16 in the model organism Caenorhabditis elegans. Arch. Toxicol. 2007, 81, 849–858. [Google Scholar] [CrossRef]
- Kampkötter, A.; Nkwonkam, C.G.; Zurawski, R.F.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Investigations of protective effects of the flavonoids quercetin and rutin on stress resistance in the model organism Caenorhabditis elegans. Toxicology 2007, 234, 113–123. [Google Scholar] [CrossRef]
- Kampkötter, A.; Pielarski, T.; Rohrig, R.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. The Ginkgo biloba Egb761 reduces stress sensitivity, ROS accumulation and expression of catalase and glutathione S-transferase 4 in Caenorhabditis elegans. Pharmacol. Res. 2007, 55, 139–147. [Google Scholar] [CrossRef]
- González-Manzano, S.; González-Paramás, A.M.; Delgado, L.; Patianna, S.; Surco-Laos, F.; Dueñas, M.; Santos-Buelga, C. Oxidative status of stressed Caenorhabditis elegans treated with epicatechin. J. Agric. Food Chem. 2012, 60, 8911–8916. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Kehrer, I.; Kozlov, A.V.; Haller, M.; Redl, H.; Hermann, M.; Grimm, M.; Troppmair, J. Mitochondrial ROS production under cellular stress: Comparison of different detection methods. Anal. Bioanal. Chem. 2011, 400, 2383–2390. [Google Scholar] [CrossRef]
- Labuschagne, C.F.; Brenkman, A.B. Current methods in quantifying ROS and oxidative damage in Caenorhabditis elegans and other model organism of aging. Ageing Res. Rev. 2013, 12, 918–930. [Google Scholar] [CrossRef]
- Zhao, H.; Joseph, J.; Fales, H.M.; Sokoloski, E.A.; Levine, R.L.; Vasquez-Vivar, J.; Kalyanaraman, B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl. Acad. Sci. USA 2005, 102, 5727–5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, A.; Fernandes, E.; Lima, J.L. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef]
- Esposti, M.D.; Hatzinisiriou, I.; McLennan, H.; Ralph, S. Bcl-2 and mitochondrial oxygen radicals. New approaches with reactive oxygen species-sensitive probes. J. Biol. Chem. 1999, 274, 29831–29837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J.; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikalov, S.I.; Harrison, D.G. Methods for detection of mitochondrial and cellular reactive oxygen species. Antiox. Redox Signal. 2014, 20, 372–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, L.G.; Chen, Y.J.; Tong, J.W.; Gong, Y.S.; Huang, J.A.; Liu, Z.H. Epigallocatechin-3-gallate promotes healthy lifespan through mitohormesis during early-to-mid adulthood in Caenorhabditis elegans. Redox Biol. 2018, 14, 305–315. [Google Scholar] [CrossRef]
- Urban, N.; Tsitsipatis, D.; Hausig, F.; Kreuzer, K.; Erler, K.; Stein, V.; Ristow, M.; Steinbrenner, H.; Klotz, L.O. Non-linear impact of glutathione depletion on C. elegans life span and stress resistance. Redox Biol. 2017, 11, 502–515. [Google Scholar] [CrossRef]
- Lüersen, K.; Stegehake, D.; Daniel, J.; Drescher, M.; Ajonina, I.; Ajonina, C.; Hertel, P.; Woltersdorf, C.; Liebau, E. The glutathione reductase GSR-1 determines stress tolerance and longevity in Caenorhabditis elegans. PLoS ONE 2013, 8, e60731. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, G.D.; Bridge, W.J. The glutathione system and the related thiol network in Caenorhabditis elegans. Redox Biol. 2019, 24, 101171. [Google Scholar] [CrossRef]
- Mari, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial glutathione, a key survival antioxidant. Antiox. Redox. Sign. 2009, 11, 2685–2700. [Google Scholar] [CrossRef] [Green Version]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Caito, S.W.; Aschner, M. Quantification of glutathione in Caenorhabditis elegans. Curr. Protoc. Toxicol. 2015, 64, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mergel, D.; Andermann, G.; Andermann, C. Simultaneous spectrophotometric determination of oxidized and reduced glutathione in human and rabbit red cells. Methods Find. Exp. Clin. Pharmacol. 1979, 1, 277–283. [Google Scholar] [PubMed]
- Rahman, I.; Kode, A.; Biswas, S. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Khayamian, T.; Hasanpour, F. Determination of glutathione in hemolysed erythrocyte by flow injection analysis with chemiluminescence detection. J. Pharm. Biomed. Anal. 2008, 48, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta. 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Van Raamsdonk, J.M.; Hekimi, S. Reactive oxygen species and aging in Caenorhabditis elegans: Causal or casual relationship? Antiox. Redox Signal. 2010, 13, 1911–1953. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Meth. Enzymol. 1990, 186, 464–478. [Google Scholar]
- Adachi, H.; Fujiwara, Y.; Ishii, N. Effects of oxygen on protein carbonyl and aging in Caenorhabditis elegans mutants with long (age-1) and short (mev-1) life spans. J. Gerontol. A Biol. Sci. Med. Sci. 1998, 53, B240–B244. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, K.; Adachi, H.; Fujiwara, Y.; Ishii, N. Protein carbonyl accumulation in aging dauer formation-defective (daf) mutants of Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 1999, 54, B47–B51. [Google Scholar] [CrossRef] [Green Version]
- Tambara, A.L.; de Los Santos-Moraes, L.; Dal Forno, A.H.; Boldori, J.R.; Gonçalves-Soares, A.T.; de Freitas-Rodrigues, C.; Mariutti, L.R.B.; Mercadante, A.Z.; de Ávila, D.S.; Denardin, C.C. Purple pitanga fruit (Eugenia uniflora L.) protects against oxidative stress and increase the lifespan in Caenorhabditis elegans via the DAF-16/FOXO pathway. Food Chem. Toxicol. 2018, 120, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Rea, S.L.; Ventura, N.; Johnson, T.E. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol. 2007, 5, e259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyr Dit Ruys, S.; Bonzom, J.M.; Frelon, S. Benchmarking of protein carbonylation analysis in Caenorhabditis elegans: Specific considerations and general advice. Free Radic. Biol. Med. 2016, 99, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.R.; De Waal, E.M.; Pierce, A.; Remmen, H.V.; Ward, W.F.; Richardson, A. Detection of protein carbonyls in aging liver tissue: A fluorescence-based proteomic approach. Mech. Ageing Devel. 2006, 127, 849–861. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Bhamidipaty, S.; Evans, M.K.; Rifkind, J.M. A fluorimetric semi-microplate format assay of protein carbonyls in blood plasma. Anal. Biochem. 2010, 400, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Leichert, L.I.; Gehrke, F.; Gudiseva, H.V.; Blackwell, T.; Ilbert, M.; Walker, A.K.; Strahler, J.R.; Andrews, P.C.; Jakob, U. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 8197–8202. [Google Scholar] [CrossRef] [Green Version]
- Knoefler, D.; Thamsen, M.; Koniczek, M.; Niemuth, N.J.; Diederich, A.K.; Jakob, U. Quantitative in vivo redox sensors uncover oxidative stress as an early event in life. Mol. Cell 2012, 47, 767–776. [Google Scholar] [CrossRef] [Green Version]
- Kumsta, C.; Thamsen, M.; Jakob, U. Effects of oxidative stress on behavior, physiology, and the redox thiol proteome of Caenorhabditis elegans. Antiox. Redox Signal. 2011, 14, 1023–1037. [Google Scholar] [CrossRef] [Green Version]
- Petersen, D.R.; Doorn, J.A. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic. Biol. Med. 2004, 37, 937–945. [Google Scholar] [CrossRef]
- Montine, K.S.; Kim, P.J.; Olson, S.J.; Markesbery, W.R.; Montine, T.J. 4-Hydroxy-2-nonenal pyrrole adducts in human neurodegenerative disease. J. Neuropathol. Exp. Neurol. 1997, 56, 866–871. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; An, J.; Bai, Y.; Li, H.; Chen, H.; Ou, D.; Liu, Y. Tris(1,3-dichloro-2-propyl) phosphate accelerated the aging process induced by the 4-hydroxynon-2-enal response to reactive oxidative species in Caenorhabditis elegans. Environ. Pollut. 2019, 246, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Ayyadevara, S.; Dandapat, A.; Singh, S.P.; Siegel, E.R.; Shmookler Reis, R.J.; Zimniak, L.; Zimniak, P. Life span and stress resistance of Caenorhabditis elegans are differentially affected by glutathione transferases metabolizing 4-hydroxynon-2-enal. Mech. Ageing Dev. 2007, 128, 196–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayyadevara, S.; Engle, M.R.; Singh, S.P.; Dandapat, A.; Lichti, C.F.; Benes, H.; Shmookler Reis, R.J.; Liebau, E.; Zimniak, P. Lifespan and stress resistance of Caenorhabditis elegans are increased by expression of glutathione transferases capable of metabolizing the lipid peroxidation product 4-hydroxynonenal. Aging Cell 2005, 4, 257–271. [Google Scholar] [CrossRef]
- Singh, S.P.; Niemczyk, M.; Zimniak, L.; Zimniak, P. Fat accumulation in Caenorhabditis elegans triggered by the electrophilic lipid peroxidation product 4-hydroxynonenal (4-HNE). Aging (Albany NY) 2008, 1, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Blanco, A.; Rodríguez-Matellán, A.; González-Paramás, A.; González-Manzano, S.; Kim, S.K.; Mollinedo, F. Dietary and microbiome factors determine longevity in Caenorhabditis elegans. Aging (Albany NY) 2016, 8, 1513–1539. [Google Scholar]
- Porta, E.A. Pigments in aging: An overview. Ann. N. Y. Acad. Sci. 2002, 959, 57–65. [Google Scholar] [CrossRef]
- Clokey, G.V.; Jacobson, L.A. The autofluorescent “lipofuscin granules” in the intestinal cells of Caenorhabditis elegans are secondary lysosome. Mech. Ageing. Dev. 1986, 35, 79–94. [Google Scholar] [CrossRef]
- Ha, M.K.; Cho, J.S.; Baik, O.R.; Lee, K.H.; Koo, H.S.; Chung, K.Y. Caenorhabditis elegans as a screening tool for the endothelial cell-derived putative aging-related proteins detected by proteomic analysis. Proteomics 2006, 6, 3339–3351. [Google Scholar] [CrossRef]
- Liao, V.H.C.; Yu, C.W.; Chu, Y.J.; Li, W.H.; Hsieh, Y.C.; Wang, T.T. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech. Ageing Dev. 2011, 132, 480–487. [Google Scholar] [CrossRef]
- Labuschagne, C.F.; Stigter, E.C.; Hendriks, M.M.; Berger, R.; Rokach, J.; Korswagen, H.C.; Brenkman, A.B. Quantification of in vivo oxidative damage in Caenorhabditis elegans during aging by endogenous F3-isoprostane measurement. Aging Cell 2013, 12, 214–223. [Google Scholar] [CrossRef]
- Arczewska, K.D.; Baumeier, C.; Kassahun, H.; Sengupta, T.; Bjørås, M.; Kuśmierek, J.T.; Nilsen, H. Caenorhabditis elegans NDX-4 is a MutT-type enzyme that contributes to genomic stability. DNA Repair 2011, 10, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Wang, P.; Liu, Y.H.; Wu, J.Y.; Chen, J.; Peng, R.X. Fast evaluation of oxidative DNA damage by Liquid Chromatography-Electrospray Tandem Mass Spectrometry coupled with precision-cut rat liver slices. Biomed. Environ. Sci. 2007, 20, 386–391. [Google Scholar] [PubMed]
- Delgado, L. Mecanismos de acción implicados en la bioactividad de flavonoides. Caenorhabditis elegans y líneas celulares como sistemas modelo. Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 27 November 2015. [Google Scholar]
- Hunter, S.E.; Jung, D.; Di Giulio, R.T.; Meyer, J.N. The QPCR assay for analysis of mitochondrial DNA damage, repair, and relative copy number. Methods 2010, 51, 444–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, J.N.; Boyd, W.A.; Azzam, G.A.; Haugen, A.C.; Freedman, J.H.; Van Houten, B. Decline of nucleotide excision repair capacity in aging Caenorhabditis elegans. Gen. Biol. 2007, 8, R70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grüber, J.; Ng, L.F.; Fong, S.; Wong, Y.T.; Koh, S.A.; Chen, C.B.; Shui, G.; Cheong, W.F.; Schaffer, S.; Wenk, M.R.; et al. Mitochondrial changes in ageing Caenorhabditis elegans –what do we learn from superoxide dismutase knockouts? PLoS ONE 2011, 6, e19444. [Google Scholar] [CrossRef] [Green Version]
- Corbisier, P.; Houbion, A.; Remacle, J. A new technique for highly sensitive detection of superoxide dismutase activity by chemiluminescence. Anal. Biochem. 1987, 164, 240–247. [Google Scholar] [CrossRef]
- Aebi, H.E. Catalase. In Methods of Enzymatic Analysis, 3rd ed.; Bergmeyer, J., Grossl, M., Eds.; VCH: Weinheim, Germany, 1987; Volume 3, pp. 273–286. [Google Scholar]
- Alia, M.; Ramos, S.; Mateos, R.; Bravo, L.; Goya, L. Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2). J. Biochem. Mol. Toxicol. 2005, 19, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Delgado, L.; González-Paramás, A.M.; González-Manzano, S.; Ayuda-Durán, B.; Santos-Buelga, C. Influence of flavonoids in ROS production and oxidative DNA damage in Caenorhabditis elegans submitted to thermal stress. Planta Med. 2014, 80, P2O5. [Google Scholar] [CrossRef]
- Houthoofd, K.; Braeckman, B.P.; Johnson, T.E.; Vanfleteren, J.R. Life extension via dietary restriction is independent of the Ins/IGF-1 signalling pathway in Caenorhabditis elegans. Exp. Gerontol. 2003, 38, 947–954. [Google Scholar] [CrossRef]
- Houthoofd, K.; Braeckman, B.P.; Lenaerts, I.; Brys, K.; De Vreese, A.; Van Eygen, S.; Vanfleteren, J.R. Ageing is reversed, and metabolism is reset to young levels in recovering dauer larvae of C. elegans. Exp. Gerontol. 2002, 37, 1015–1021. [Google Scholar] [CrossRef]
- Kennedy, M.C.; Emptage, M.H.; Dreyer, J.L.; Beinert, H. The role of iron in the activation-inactivation of aconitase. J. Biol. Chem. 1983, 258, 11098–11105. [Google Scholar] [PubMed]
- Yanase, S.; Onodera, A.; Tedesco, P.; Johnson, T.E.; Ishii, N. SOD-1 deletions in Caenorhabditis elegans alter the localization of intracellular reactive oxygen species and show molecular compensation. J. Gerontol. 2009, 64A, 530–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda-Vizuete, A.; Gonzalez, J.C.; Gahmon, G.; Burghoorn, J.; Navas, P.; Swoboda, P. Lifespan decrease in a Caenorhabditis elegans mutant lacking TRX-1, a thioredoxin expressed in ASJ sensory neurons. FEBS Lett. 2006, 580, 484–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, N.; Fujii, M.; Hartman, P.S.; Tsuda, M.; Yasuda, K.; Senoo-Matsuda, N.; Yanase, S.; Ayusawa, D.; Suzuki, K. A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature 1998, 394, 694–697. [Google Scholar] [CrossRef]
- Pietsch, K.; Saul, N.; Chakrabarti, S.; Stürzenbaum, S.R.; Menzel, R.; Steinberg, C.E. Hormetins, antioxidants and prooxidants: Defining quercetin-, caffeic acid- and rosmarinic acid-mediated life extension in C. elegans. Biogerontology 2011, 12, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Saul, N.; Pietsch, K.; Menzel, R.; Stürzenbaum, S.R.; Steinberg, C.E. Catechin induced longevity in C. elegans: From Key regulator genes to disposable soma. Mech. Ageing Dev. 2009, 130, 447–486. [Google Scholar] [CrossRef]
- Bartholome, A.; Kampkötter, A.; Tanner, S.; Sies, H.; Klotz, L.O. Epigallocatechin gallate-induced modulation of FoxO signaling in mammalian cells and C. elegans: FoxO stimulation is masked via PI3K/Akt activation by hydrogen peroxide formed in cell culture. Arch. Biochem. Biophys. 2010, 501, 58–64. [Google Scholar] [CrossRef]
- Oh, S.W.; Mukhopadhyay, A.; Svrzikapa, N.; Jiang, F.; Davis, R.J.; Tissenbaum, H.A. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl. Acad. Sci. USA 2005, 102, 4494–4499. [Google Scholar] [CrossRef] [Green Version]
- Troemel, E.R.; Chu, S.W.; Reinke, V.; Lee, S.S.; Ausubel, F.M.; Kim, D.H. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. Elegans. PLoS Genet. 2006, 2, e183. [Google Scholar] [CrossRef]
- Govindan, S.; Amirthalingam, M.; Duraisamy, K.; Govindhan, T.; Sundararaj, N.; Palanisamy, S. Phytochemicals-induced hormesis protects Caenorhabditis elegans against α-synuclein protein aggregation and stress through modulating HSF-1 and SKN-1/Nrf2 signaling pathways. Biomed. Pharmacother. 2018, 102, 812–822. [Google Scholar] [CrossRef]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–283. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Blackwell, T.K. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003, 17, 1882–1893. [Google Scholar] [CrossRef] [Green Version]
- Antebi, A. Genetics of Aging in Caenorhabditis elegans. PLoS Genet. 2007, 3, 1565–1571. [Google Scholar] [CrossRef]
- Kenyon, C. The plasticity of aging: Insights from long-lived mutants. Cell. 2005, 120, 449–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenyon, C.; Chang, J.; Gensch, E.; Rudner, A.; Tabtiang, R.A. C. elegans mutant that lives twice as long as wild type. Nature 1993, 366, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.B.; Johnson, T.E. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 1988, 118, 75–86. [Google Scholar]
- Cai, W.J.; Huang, J.H.; Zhang, S.Q.; Wu, B.; Kapahi, P.; Zhang, X.M.; Shen, Z.Y. Icariin and its derivative icariside II extend healthspan via insulin/IGF-1 pathway in C. elegans. PLoS ONE 2011, 6, e28835. [Google Scholar] [CrossRef] [Green Version]
- Martorell, P.; Vicent-Forment, P.; De Llanos, R.; Montón, F.; LLopis, S.; González, N.; Genovés, S.; Cienfuegos, E.; Monzó, H.; Ramón, D. Use of Saccharomyces cerevisiae and Caenorhabditis elegans as model organisms to study the effect of cocoa polyphenols in the resistance to oxidative stress. J. Agric. Food Chem. 2011, 59, 2077–2085. [Google Scholar] [CrossRef]
- Yu, C.W.; Wei, C.C.; Liao, V.H. Curcumin-mediated oxidative stress resistance in Caenorhabditis elegans is modulated by age-1, akt-1, pdk-1, osr-1, unc-43, sek-1, skn-1, sir-2.1, and mev-1. Free Rad. Res. 2014, 48, 371–379. [Google Scholar] [CrossRef]
- Li, Y.; Chu, Q.; Liu, Y.; Ye, X.; Jiang, Y.; Zheng, X. Radix Tetrastigma flavonoid ameliorates inflammation and prolongs the lifespan of Caenorhabditis elegans through JNK, p38 and Nrf2 pathways. Free Rad. Res. 2019, 53, 562–573. [Google Scholar] [CrossRef]
- Zheng, S.Q.; Huang, X.B.; Xing, T.K.; Ding, A.J.; Wu, G.S.; Luo, H.R. Chlorogenic acid extends the lifespan of Caenorhabditis elegans via Insulin/IGF-1 signaling pathway. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 464–472. [Google Scholar] [PubMed] [Green Version]
- Shen, P.; Yue, Y.; Zheng, J.; Park, Y. Caenorhabditis elegans: A convenient in vivo model for assessing the impact of food bioactive components on obesity, aging, and Alzheimer’s disease. Annu. Rev. Food Sci. Technol. 2018, 9, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Li, H.; Huang, C.; Zhao, T.; Zhang, Y.; Ba, X.; Li, Z.; Zhang, Y.; Huang, B.; Lu, J.; et al. Muscle-specific histone H3K36 dimethyltransferase SET-18 shortens lifespan of Caenorhabditis elegans by repressing daf-16a expression. Cell Rep. 2018, 22, 2716–2729. [Google Scholar] [CrossRef] [PubMed]
- Senchuk, M.M.; Dues, D.D.; Schaar, C.E.; Johnson, B.K.; Madaj, Z.B.; Bowman, M.J.; Winn, M.E.; Van Raamsdonk, J.V. Activation of DAF-16/FOXO by reactive oxygen species contributes to longevity in long-lived mitochondrial mutants in Caenorhabditis elegans. PLoS Genet. 2018, 14, e1007268. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Weldle, N.; Baier, S.; Büchter, C.; Wätjen, W. Hibiscus sabdariffa L. extract prolongs lifespan and protects against amyloid-β toxicity in Caenorhabditis elegans: Involvement of the FoxO and Nrf2 orthologues DAF-16 and SKN-1. Eur. J. Nutr. 2020, 59, 137–150. [Google Scholar] [CrossRef]
- Sobeh, M.; Mahmoud, M.F.; Abdelfattah, M.A.O.; Cheng, H.; El-Shazly, A.M.; Wink, M. A proanthocyanidin-rich extract from Cassia abbreviata exhibits antioxidant and hepatoprotective activities in vivo. J. Ethnopharmacol. 2018, 213, 38–47. [Google Scholar] [CrossRef]
- Paiva, F.A.; Bonomo, L.F.; Boasquivis, P.F.; de Paula, I.T.; Guerra, J.F.; Leal, W.M.; Silva, M.E.; Pedrosa, M.L.; Oliveira, R.P. Carqueja (Baccharis trimera) protects against oxidative stress and β-amyloid-induced toxicity in Caenorhabditis elegans. Oxid. Med. Cell Longev. 2015, 2015, 740162. [Google Scholar]
- Bonomo, L.F.; Silva, D.N.; Boasquivis, P.F.; Paiva, F.A.; Guerra, J.F.; Martins, T.A.; de Jesus-Torres, Á.G.; de Paula, I.T.; Caneschi, W.L.; Jacolot, P.; et al. Açaí (Euterpe oleracea Mart.) modulates oxidative stress resistance in Caenorhabditis elegans by direct and indirect mechanisms. PLoS ONE 2014, 9, e89933. [Google Scholar] [CrossRef] [Green Version]
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 2006, 5, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Tullet, J.M.A.; Green, J.W.; Au, C.; Benedetto, A.; Thompson, M.A.; Clark, E.; Gilliat, A.F.; Young, A.; Schmeisser, K.; Gems, D. The SKN-1/Nrf2 transcription factor can protect against oxidative stress and increase lifespan in C. elegans by distinct mechanisms. Aging Cell 2017, 16, 1191–1194. [Google Scholar] [CrossRef] [Green Version]
- Guha, S.; Cao, M.; Kane, R.M.; Savino, A.M.; Zou, S.; Dong, Y. The longevity effect of cranberry extract in Caenorhabditis elegans is modulated by daf-16 and osr-1. Age (Dordr.) 2012, 5, 1559–1574. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Chen, H.; Cheng, Y.; Nasir, M.A.; Kemper, N.; Bao, E. The interactive association between heat shock factor 1 and heat shock proteins in primary myocardial cells subjected to heat stress. Int. J. Mol. Med. 2016, 37, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Asthana, J.; Mishra, B.N.; Pandey, R. Acacetin promotes healthy aging by altering stress response in Caenorhabditis elegans. Free Rad. Res. 2016, 50, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Zhao, B.; Zhao-Wilson, X. Quinic acid could be a potential rejuvenating natural compound by improving survival of Caenorhabditis elegans under deleterious conditions. Rejuvenation Res. 2012, 15, 573–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangsinth, P.; Prasansuklab, A.; Duangjan, C.; Gu, X.; Meemon, K.; Wink, M.; Tencomnao, T. Leaf extract of Caesalpinia mimosoides enhances oxidative stress resistance and prolongs lifespan in Caenorhabditis elegans. BMC Complement. Altern. Med. 2019, 19, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havermann, S.; Humpf, H.-U.; Wätjen, W. Baicalein modulates stress-resistance and life span in C. Elegans via SKN-1 but not DAF-16. Fitoterapia 2016, 113, 123–127. [Google Scholar] [CrossRef]
- Duangjan, C.; Rangsintha, P.; Gub, X.; Zhangc, S.; Winkd, M.; Tencomnaoa, T. Glochidion zeylanicum leaf extracts exhibit lifespan extending and oxidative stress resistance properties in Caenorhabditis elegans via DAF-16/FoxO and SKN-1/Nrf-2 signaling pathways. Phytomedicine 2019, 64, 153061. [Google Scholar] [CrossRef]
- Abbas, S.; Wink, M. Epigallocatechin gallate from green tea (Camellia sinensis) increases lifespan and stress resistance in Caenorhabditis elegans. Planta Med. 2009, 75, 216–221. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Abbas, S.; Wink, M. Epigallocatechin gallate inhibits beta amyloid oligomerization in Caenorhabditis elegans and affects the daf-2/insulin-like signalling pathway. Phytomedicine 2010, 17, 902–909. [Google Scholar] [CrossRef]
- Calvo, D.R.; Martorell, P.; Genovés, S.; Gosálbez, L. Development of novel functional ingredients: Need for testing systems and solutions with Caenorhabditis elegans. Trends Food Sci. Technol. 2016, 54, 197–203. [Google Scholar] [CrossRef]

| Probe | Reaction | Specificity | Limitations |
|---|---|---|---|
| DCFH-DA | The colourless reduced form DFCH is oxidized to fluorescent DCF | Sensitive to H2O2, •HO and ROO• | No detection of •NO, HOCl or O2•− |
| MitoTracker® red CM-H(2)XRos | Oxidation of the reduced form to the red-fluorescent dye rosamine | Especially H2O2 | Poor detection of other ROS |
| MitoSOX™ | Dihydroethidium (DHE) is oxidized by O2•− to the fluorescent ethidium form | Mainly O2•− | Possible reaction with cell components like cytochrome C |
| Amplex red | Formation of fluorescent resorufin upon oxidation of 10-acetyl-3,7-dihydroxy- phenoxazine | H2O2 | Interference of reductants like glutathione or NADH. No detection of intracellular H2O2 |
| C. elegans | Cell Cultures | Yeasts | Drosophila melanogaster | Zebra Fish (Danio rerio) | Murine Models | |
|---|---|---|---|---|---|---|
| Handling and maintenance | Easy | Easy | Easy | Fair | Fair | Difficult |
| Consideration of bioavailability issues | Yes | No | No | Yes | Yes | Yes |
| Throughput | High | High | High | Moderate | Good | Low |
| Availability of disease models | Good | Good | Limited | Good | Limited | High |
| Human prediction capacity | Moderate | Poor | Poor | Poor | Moderate | Good |
| Ease for genetic manipulation | Good | Good | Good | Good | Limited | Poor |
| Ethical concerns | No | May exist | No | Yes | Yes | Yes |
| Drawbacks | Biologically far from mammals Primitive immune system | Not a physiological setting | Biologically far from mammals Low degree of homology with human genes | Difficult to scale and handling system (it flies) | Difficult testing of non-soluble molecules | Facilities and breeding requirements |
| Approach | Procedures | Observations |
|---|---|---|
| Phenotypical assessment | Evaluation of the survival or phenotypical modifications in worms treated with the compound after submission to an oxidative challenge (e.g., paraquat, H2O2, juglone, thermal stress) | Results highly by assay conditions (analyte concentration, treatment conditions, worm age or strength of the oxidative challenge) |
| Markers of oxidative damage | ||
| 1. ROS | Measurement after reaction colored or fluorescent probes: dichlorofluorescein, MitoTracker® red CM-H(2)XRos, MitoSOX™, Amplex red | Different probes have different specificity towards different probes |
| 2. Glutathione | Spectrophotometric or HPLC analysis after reaction with DTNB or OPA) | Determination of total glutathione (i.e., GSH + GSSG) requires previous GSSG reduction by glutathione reductase. |
| 3. Carbonylated proteins | Reaction with 2,4-dinitrophenyl hydrazine (DNPH) or fluorescein- 5-thiosemicarbazide (FTC). Spectrophotometrical HPLC, or immunoblotting (OxyBlot assay) measurement | Poor homogeneity Semiquantitative assessment (OxyBlot) |
| 4. Lipid oxidation products | LC-MS or ELISA analysis of lipid degradation products (MDA, HNE, isoprostanes). Assessment of lipofuscin accumulation by fluorescence microscopy | Different stages of the lipid oxidation are evaluated depending on the approach |
| 5. DNA damage | Measurement of 8-OHdG spectrophotometrically or by LC-MS/MS | Low sensitivity |
| Antioxidant enzymes | Measurement of the activity of different enzymes (e.g., SOD, CAT, GPXs, TRXs, GLRXs, PRDX, aconitase) typically in a microplate reader | Indirect measurement Different enzymes measure different processes Low sensitivity |
| Mutant worms | Assessment of the behavioral responses of worms with loss-of-function mutations in genes belonging to conserved stress or ageing pathways (e.g., insulin/IGF-1, SKN-1/Nrf2) treated with the compound. | Suited for evaluation of molecular mechanisms of action Highly variable results depending on the assay conditions. |
| Transgenic worms carrying fluorescent reporters | Microscopy observation of the fluorescence of different reporters: green fluorescent protein (GFP), βGAL (LacZ), Discosoma sp. red fluorescent protein (dsRED), yellow fluorescent protein (YFP) | Allow detection of subcellular location |
| RT-qPCR | Quantitative measurement of changes in expression of a gene | Information about the expression of a particular gene |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C. Caenorhabditis elegans as a Model Organism to Evaluate the Antioxidant Effects of Phytochemicals. Molecules 2020, 25, 3194. https://doi.org/10.3390/molecules25143194
Ayuda-Durán B, González-Manzano S, González-Paramás AM, Santos-Buelga C. Caenorhabditis elegans as a Model Organism to Evaluate the Antioxidant Effects of Phytochemicals. Molecules. 2020; 25(14):3194. https://doi.org/10.3390/molecules25143194
Chicago/Turabian StyleAyuda-Durán, Begoña, Susana González-Manzano, Ana M. González-Paramás, and Celestino Santos-Buelga. 2020. "Caenorhabditis elegans as a Model Organism to Evaluate the Antioxidant Effects of Phytochemicals" Molecules 25, no. 14: 3194. https://doi.org/10.3390/molecules25143194
APA StyleAyuda-Durán, B., González-Manzano, S., González-Paramás, A. M., & Santos-Buelga, C. (2020). Caenorhabditis elegans as a Model Organism to Evaluate the Antioxidant Effects of Phytochemicals. Molecules, 25(14), 3194. https://doi.org/10.3390/molecules25143194







