Antiproliferative Activity and Antioxidant Potential of Extracts of Garcinia gardneriana
Abstract
1. Introduction
2. Results
2.1. Identification of the Constituents by HPLC-DAD (Diode Array Detector)-MS
2.2. Centesimal Composition and Antioxidant Activity of Fruits and Leaves of Garcinia gardneriana
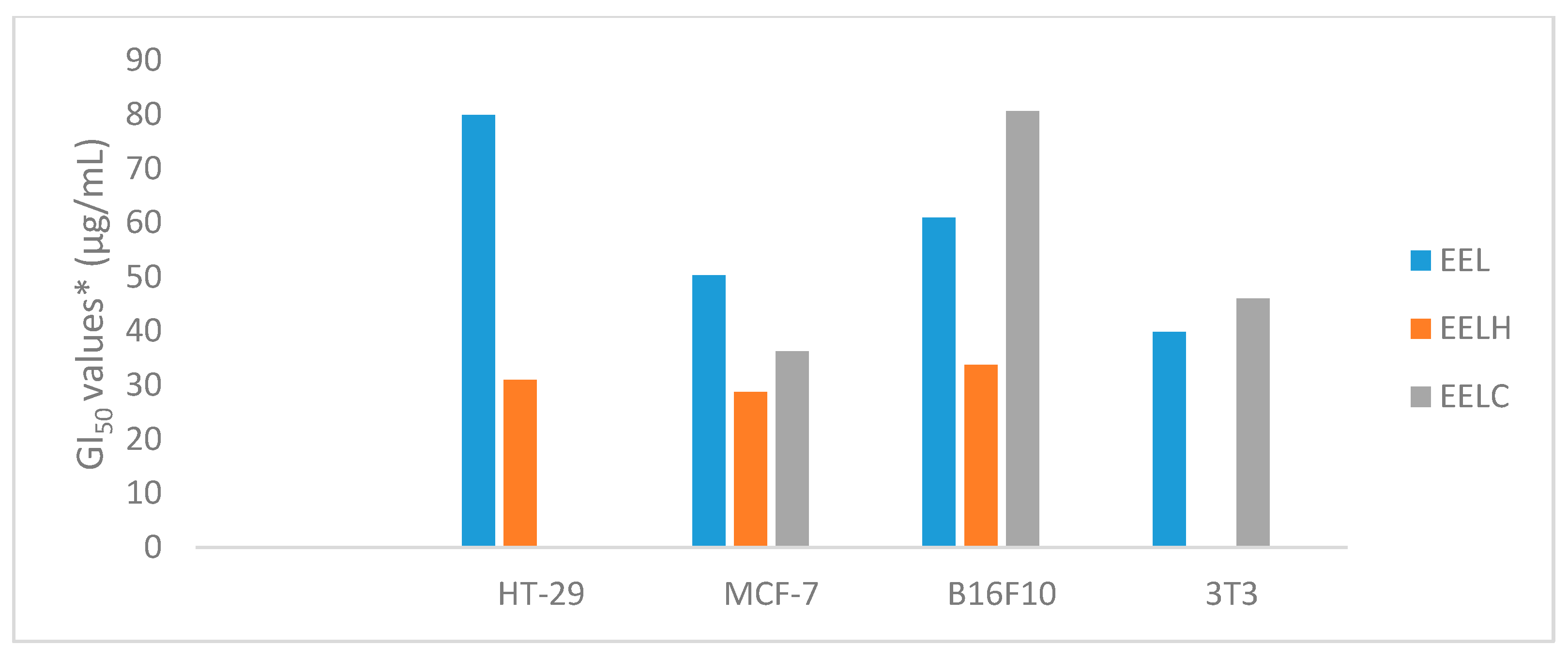
2.3. Evaluation of the Antiproliferative Activity of Extracts and Fractions of Garcinia gardneriana Fruits and Leaves in Cancer Cell Lines
3. Discussion
4. Materials and Methods
4.1. Plant Material and Samples’ Preparation
4.2. Chemical Profile of EEFH and EELH
4.3. Centesimal Composition of Fruits and Leaves of Garcinia gardneriana
4.4. In Vitro Cytotoxicity Assay
4.5. Antioxidant Property
4.5.1. Determination of Total Phenols
4.5.2. Evaluation of Antiradical Activity (DPPH)
4.5.3. Determination of Ascorbic Acid and Carotenoids
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation List:
| EEF | Fruit ethanolic extract |
| EEFA | Ethyl acetate fraction |
| EEFC | Chloroform fraction |
| EEFH | Hexane fraction |
| EEFW | Hydromethanolic fraction |
| EEL | Ethanol leaf extract |
| EELA | Ethyl acetate fraction |
| EELC | Chloroform fraction |
| EELH | Hexane fraction |
| EELW | Hydromethanolic fraction |
| MF | Molecular formula |
| MS/MS | Tandem mass spectrometry |
| Ppm | Parts per million |
| Rt | Retention time |
| [M-H] | Deprotonated ion |
| m/z | mass-to-charge ratio |
References
- Rufino, M.D.S.M.; Alves, R.E.; De Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Monache, F.; Mac-Quhae, M.; Monache, G.; Bettolo, G.; De Lima, R. Xanthones, xanthonolignoids and other constituents of the roots of vismia guaramirangae. Phytochemistry 1983, 22, 227–232. [Google Scholar] [CrossRef]
- Monache, G.D.; Monache, F.D.; Waterman, P.G.; Crichton, E.G.; De Limas, R.A. Minor xanthones from Rheedia gardneriana. Phytochemistry 1984, 23, 1757–1759. [Google Scholar] [CrossRef]
- Bennett, G.J.; Lee, H.H. Xantonas grom Guttiferae. Phytochemistry 1989, 4, 967–998. [Google Scholar] [CrossRef]
- Hay, A.-E.; Aumond, M.-C.; Mallet, S.; Dumontet, V.; Litaudon, M.; Rondeau, D.; Richomme, P. Antioxidant Xanthones fromGarcinia vieillardii. J. Nat. Prod. 2004, 67, 707–709. [Google Scholar] [CrossRef]
- Peres, V.; Nagem, T. Trioxygenated naturally occurring xanthones. Phytochemistry 1997, 44, 191–214. [Google Scholar] [CrossRef]
- Peres, V.; Nagem, T.J.; De Oliveira, F.F. Tetraoxygenated naturally occurring xanthones. Phytochemistry 2000, 55, 683–710. [Google Scholar] [CrossRef]
- Botta, B.; Mac-Quhae, M.; Delle-Monache, F.; Delle-Monache, G.; De Mello, J.F. Chemical investigation of the genus Rheedia. V. biflavonoids and xanthochymol. J. Nat. Prod. 1984, 47, 1053–1064. [Google Scholar] [CrossRef]
- Itoigawa, M.; Ito, C.; Tan, H.T.; Kuchide, M.; Tokuda, H.; Nishino, H.; Furukawa, H. Cancer chemopreventive agents, 4-phenylcoumarins from Calophyllum inophyllum. Cancer Lett. 2001, 169, 147–150. [Google Scholar] [CrossRef]
- Rao, A.; Sarma, M.; Venkataraman, K.; Yemul, S. A benzophenone and xanthone with unusual hydroxylation patterns from the heartwood of Garcinia pedunculata. Phytochemistry 1974, 13, 1241–1244. [Google Scholar] [CrossRef]
- Luzzi, R.; Guimaraes, C.; Verdi, L.; Simionatto, E.; Monache, F.D.; Yunes, R.; Floriani, A.; Cechinel-Filho, V. Isolation of biflavonoids with analgesic activity from Rheedia gardneriana leaves. Phytomedicine 1997, 4, 141–144. [Google Scholar] [CrossRef]
- Naves, V.M.L.; Santos, M.H.; Ribeiro, I.S.; Silva, C.A.; Silva, N.C.; Silva, M.A.; Silva, G.A.; Dias, A.L.T.; Ionta, M.; Dias, D.F. Antimicrobial and antioxidant activity of garciniabrasiliensis extracts. S. Afr. J. Bot. 2019, 124, 244–250. [Google Scholar] [CrossRef]
- Green, K.; Brand, M.D.; Murphy, M.P. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes 2004, 53, S110–S118. [Google Scholar] [CrossRef] [PubMed]
- Rolim, P.M.; Fidelis, G.P.; Padilha, C.E.A.; Santos, E.S.; Rocha, H.A.O.; Macedo, G.R. Phenolic profile and antioxidant activity from peels and seeds of melon (Cucumismelo L. var. reticulatus) and their antiproliferative effect in cancer cells. Braz. J. Med. Biol. Res. 2018, 51, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, L.A.B.M.; Ferreira, R.D.S.; Guimarães, R.D.C.A.; Castro, A.; Franco, O.L.; Matias, R.; Carvalho, C.M.E. The Complex Puzzle of Interactions Among Functional Food, Gut Microbiota, and Colorectal Cancer. Front. Oncol. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- De Souza, V.R.; Pereira, P.A.P.; Queiroz, F.; Borges, S.V.; Carneiro, J.D.D.S. Determination of bioactive compounds, antioxidant activity and chemical composition of Cerrado Brazilian fruits. Food Chem. 2012, 134, 381–386. [Google Scholar] [CrossRef]
- Neog, B.; Gogoi, N.; Gogoi, A.; Baruah, D.; Singh, K.D. Evaluation of antioxidant and hepatoprotective activity of fruit rind extract of Garcinia dulcis (Roxburgh) Kurz. Pharmacogn. Res. 2017, 9, 266–272. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Carrillo-Hormaza, L.; Ramírez, A.M.; Quintero-Ortiz, C.; Cossio, M.; Medina, S.; Ferreres, F.; Gil-Izquierdo, Á.; Osorio, E. Comprehensive characterization and antioxidant activities of the main biflavonoids of Garcinia madruno: A novel tropical species for developing functional products. J. Funct. Foods 2016, 27, 503–516. [Google Scholar] [CrossRef]
- Auranwiwat, C.; Laphookhieo, S.; Rattanajak, R.; Kamchonwongpaisan, S.; Pyne, S.G.; Ritthiwigrom, T. Antimalarial polyoxygenated and prenylated xanthones from the leaves and branches of Garcinia mckeaniana. Tetrahedron 2016, 72, 6837–6842. [Google Scholar] [CrossRef]
- Abderamane, B.; Tih, A.E.; Ghogomu, R.T.; Blond, A.; Bodo, B. New flavonoid C–O–C dimers and other chemical constituents from Garcinia brevipedicellata stem heartwood. Z. Nat. C 2016, 71, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Chen, B.; Zhang, Y.; Ou, H.; Li, Y.; Li, S.; Shi, P.; Lin, X. Analysis of the Total Biflavonoids Extract from Selaginella doederleinii by HPLC-QTOF-MS and Its In Vitro and In Vivo Anticancer Effects. Molecules 2017, 22, 325. [Google Scholar] [CrossRef] [PubMed]
- Stark, T.D.; Lösch, S.; Balemba, O.B.; Hofmann, T. Two new benzoyl glucuronosyl glycerols from the leaves of Garcinia buchananii Baker. Phytochem. Lett. 2017, 19, 187–190. [Google Scholar] [CrossRef]
- Pandey, R.; Chandra, P.; Kumar, S.; Srivastva, M.; Aravind, A.A.; Shameer, P.; Rameshkumar, K. Simultaneous determination of multi-class bioactive constituents for quality assessment of Garcinia species using UHPLC–QqQ LIT –MS/MS. Ind. Crop. Prod. 2015, 77, 861–872. [Google Scholar] [CrossRef]
- Na, Z.; Xu, Y. Chemical constituents from twigs of Garcinia xipshuanbannaensis. China J. Chin. Mater. Med. 2009, 34, 2338–2342. [Google Scholar]
- Du, X.-G.; Wang, W.; Zhang, Q.-Y.; Cheng, J.; Avula, B.; Khan, I.A.; Guo, D. Identification of xanthones fromSwertia puniceausing high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 2913–2923. [Google Scholar] [CrossRef]
- Mariano, L.N.B.; Da Silva-Santos, J.E.; De Souza, P.; Boeing, T.; Somensi, L.B.; Bonomini, T.J.; Monache, F.D.; Filho, V.C.; Andrade, S.; Niero, R. Gastroprotective xanthones isolated from Garcinia achachairu: Study on mucosal defensive factors and H+, K+-ATPase activity. Chem. Interact. 2016, 258, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Senthilkumar, H.A.; Wu, S.-B.; Liu, B.; Guo, Z.; Fata, J.E.; Kennelly, E.J.; Long, C. Comparative UPLC-QTOF-MS-based metabolomics and bioactivities analyses of Garcinia oblongifolia. J. Chromatogr. B 2016, 1011, 179–195. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; Lannang, A.M.; Fouotsa, H.; Mbazoa, C.D.; Nkengfack, A.E.; Sewald, N.; Jn, E. Anti-inflammatory activity of benzophenone and xanthone derivatives isolated from Garcinia (Clusiaceae) species. Phytochem. Lett. 2015, 14, 153–158. [Google Scholar] [CrossRef]
- Wu, C.; Xu, H.; Héritier, J.; Andlauer, W. Determination of catechins and flavonol glycosides in Chinese tea varieties. Food Chem. 2012, 132, 144–149. [Google Scholar] [CrossRef]
- Jaiswal, R.; Jayasinghe, L.; Kuhnert, N. Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC-MS. J. Mass Spectrom. 2012, 47, 502–515. [Google Scholar] [CrossRef]
- Suffness, M.; Pezzuto, J.M. Assays related to cancer drug discovery. In Methods in Plant Biochemistry: Assays for Bioactivity; Hostettmann, K., Ed.; Academic Press: London, UK, 1990; Volume 6, pp. 71–133. [Google Scholar]
- Yoshikawa, M.; Harada, E.; Miki, A.; Tsukamoto, K.; Liang, S.Q.; Yamahara, J.; Murakami, N. Antioxidant Constituents from the Fruit Hulls of Mangosteen (Garcinia mangostana L.) Originating in Vietnam. Yakugaku Zasshi 1994, 114, 129–133. [Google Scholar] [CrossRef]
- Ferreres, F.; Silva, B.M.; Andrade, P.B.; Seabra, R.; Ferreira, M.A. Approach to the study of C-glycosyl flavones by ion trap HPLC-PAD-ESI/MS/MS: Application to seeds of quince (Cydonia oblonga). Phytochem. Anal. 2003, 14, 352–359. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, P.; Chen, Y.; Fu, Y.; Shi, K.; Liu, L.; Liu, H.; Xiong, M.; Liu, Q.-H.; Yang, G.; et al. Depsidone and xanthones from Garcinia xanthochymus with hypoglycemic activity and the mechanism of promoting glucose uptake in L6 myotubes. Bioorg. Med. Chem. 2017, 25, 6605–6613. [Google Scholar] [CrossRef] [PubMed]
- Trisuwan, K.; Limtharakul, T. Benzophenone and xanthone derivatives from the inflorescences of Garcinia cowa. Arch. Pharmacal Res. 2012, 35, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Santa-Cecília, F.V.; Santos, G.B.; Fuzissaki, C.N.; Derogis, P.; Freitas, L.A.; Gontijo, V.S.; Stringheta, P.C.; Nagem, T.J.; Brigagão, M.R.; Dos Santos, M.H. 7-epiclusianone, the natural prenylated benzophenone, inhibits superoxide anions in the neutrophil respiratory burst. J. Med. Food 2012, 15, 200–205. [Google Scholar] [CrossRef]
- Derogis, P.; Martins, F.T.; De Souza, T.C.; Moreira, M.E.D.C.; Filho, J.D.S.; Doriguetto, A.C.; Souza, K.R.D.; Veloso, M.P.; Dos Santos, M.H. Complete assignment of the1H and13C NMR spectra of garciniaphenone and keto-enol equilibrium statements for prenylated benzophenones. Magn. Reson. Chem. 2008, 46, 278–282. [Google Scholar] [CrossRef]
- Mahamodo, S.; Rivière, C.; Christel, N.; Abedini, A.; Ranarivelo, H.; Duhal, N.; Roumy, V.; Hennebelle, T.; Sahpaz, S.; Lemoine, A.; et al. Antimicrobial prenylated benzoylphloroglucinol derivatives and xanthones from the leaves of Garcinia goudotiana. Phytochemistry 2014, 102, 162–168. [Google Scholar] [CrossRef]
- Verdi, L.G.; Pizzolatti, M.G.; Montanher, A.B.; Brighente, I.M.; Smânia Júnior, A.; Smânia-Ed Ede, F.; Simionatto, E.L.; Monache, F.D. Antibacterial and brine shrimp lethality tests of biflavonoids and derivatives of Rheedia gardneriana. Fitoterapia 2004, 75, 360–363. [Google Scholar] [CrossRef]
- Demarque, D.P.; Crotti, A.E.M.; Vessecchi, R.; Lopes, J.L.; Falcon, T. Fragmentation reactions using electrospray ionization mass spectrometry: An important tool for the structural elucidation and characterization of synthetic and natural products. Nat. Prod. Rep. 2016, 33, 432–455. [Google Scholar] [CrossRef]
- Virgolin, L.B.; Seixas, F.R.F.; Janzantti, N.S. Composition, content of bioactive compounds, and antioxidant activity of fruit pulps from the Brazilian Amazon biome. Pesqui. Agropecu. Brasileira 2017, 52, 933–941. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Phenolic Phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- Liu, R.H. Health-Promoting Components of Fruits and Vegetables in the Diet12. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, I.; Małecka, M.; Szlachta, M.; Gliszczyńska-Świgło, A. Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. J. Food Compos. Anal. 2007, 20, 313–322. [Google Scholar] [CrossRef]
- Gomes, F.S. Carotenoids: A Possible protection against cancer development. Rev. Nutr. 2007, 5, 537–548. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, M.; Yang, B.; Zhao, Q.; Jiang, Y. Phenolics from hull of Garcinia mangostana fruit and their antioxidant activities. Food Chem. 2007, 104, 176–181. [Google Scholar] [CrossRef]
- Jantan, I.; Saputri, F.C. Benzophenones and xanthones from Garcinia cantleyana var. cantleyana and their inhibitory activities on human low-density lipoprotein oxidation and platelet aggregation. Phytochemistry 2012, 80, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Okoko, T. In vitro antioxidant and free radical scavenging activities of Garcinia kola seeds. Food Chem. Toxicol. 2009, 47, 2620–2623. [Google Scholar] [CrossRef] [PubMed]
- Mackeen, M.; Ali, A.; Lajis, N.; Kawazu, K.; Hassan, Z.; Amran, M.; Habsah, M.; Mooi, L.; Mohamed, S. Antimicrobial, antioxidant, antitumour-promoting and cytotoxic activities of different plant part extracts of Garcinia atroviridis Griff. ex T. Anders. J. Ethnopharmacol. 2000, 72, 395–402. [Google Scholar] [CrossRef]
- Gao, X.-M.; Yu, T.; Cui, M.-Z.; Pu, J.-X.; Du, X.; Han, Q.-B.; Hu, Q.-F.; Liu, T.-C.; Luo, K.Q.; Xu, H. Identification and evaluation of apoptotic compounds from Garcinia oligantha. Bioorganic Med. Chem. Lett. 2012, 22, 2350–2353. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Park, R.; Kim, M.-S.; Kwon, D.-Y. Anti-adipogenic effects of Garcinia extract on the lipid droplet accumulation and the expression of transcription factor. BioFactors 2004, 22, 193–196. [Google Scholar] [CrossRef]
- Lin, K.-W.; Huang, A.-M.; Yang, S.-C.; Weng, J.-R.; Hour, T.-C.; Pu, Y.-S.; Lin, C.-N. Cytotoxic and antioxidant constituents from Garcinia subelliptica. Food Chem. 2012, 135, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Zeraik, M.L.; Serteyn, D.; Deby-Dupont, G.; Wauters, J.-N.; Tits, M.; Yariwake, J.; Angenot, L.; Franck, T. Evaluation of the antioxidant activity of passion fruit (Passiflora edulis and Passiflora alata) extracts on stimulated neutrophils and myeloperoxidase activity assays. Food Chem. 2011, 128, 259–265. [Google Scholar] [CrossRef]
- Uesato, S.; Kitagawa, Y.; Kamishimoto, M.; Kumagai, A.; Hori, H.; Nagasawa, H. Inhibition of green tea catechins against the growth of cancerous human colon and hepatic epithelial cells. Cancer Lett. 2001, 170, 41–44. [Google Scholar] [CrossRef]
- Nagao, T.; Hase, T.; Tokimitsu, I. A Green Tea Extract High in Catechins Reduces Body Fat and Cardiovascular Risks in Humans. Obesity 2007, 15, 1473–1483. [Google Scholar] [CrossRef]
- Moongkarndi, P.; Kosem, N.; Kaslungka, S.; Luanratana, O.; Pongpan, N.; Neungton, N. Antiproliferation, antioxidation and induction of apoptosis Bygarcinia mangostana (Mangosteen) on SKBR3 human breast cancer cell line. J. Ethnopharmacol. 2004, 90, 161–166. [Google Scholar] [CrossRef]
- Cano-Campos, M.; Díaz-Camacho, S.; Uribe-Beltrán, M.; López-Angulo, G.; Montes-Avila, J.; Paredes-López, O.; Delgado-Vargas, F. Bio-guided fractionation of the antimutagenic activity of methanolic extract from the fruit of Randia echinocarpa (Sessé et Mociño) against 1-nitropyrene. Food Res. Int. 2011. [Google Scholar] [CrossRef]
- Parveen, N.; Singh, M.P.; Khan, N.U.; Logani, M.K. Flavonoid constituents of Garcinia xanthochymus leaves. Phytother. Milano 1994, 65, 89–90. [Google Scholar]
- Jung, H.-A.; Su, B.-N.; Keller, W.J.; Mehta, R.G.; Kinghorn, A.D. Antioxidant Xanthones from the Pericarp of Garcinia mangostana(Mangosteen). J. Agric. Food Chem. 2006, 54, 2077–2082. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Al-Abd, A.M.; El-Halawany, A.M.; Abdallah, H.M.; Ibrahim, S.R.M. New xanthones and cytotoxic constituents from Garcinia mangostana fruit hulls against human hepatocellular, breast, and colorectal cancer cell lines. J. Ethnopharmacol. 2017, 198, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Castardo, J.C.; Prudente, A.; Ferreira, J.; Guimarães, C.L.; Monache, F.D.; Filho, V.C.; Otuki, M.F.; Cabrini, D.A. Anti-inflammatory effects of hydroalcoholic extract and two biflavonoids from Garcinia gardneriana leaves in mouse paw oedema. J. Ethnopharmacol. 2008, 118, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhong, F.; He, H.-W.; Hu, Y.; Zhu, D.; Yang, G. Structure elucidation and NMR spectral assignment of five new xanthones from the bark of Garcinia xanthochymus. Magn. Reson. Chem. 2008, 46, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.; Nagafuji, S.; Okabe, H.; Akahane, H.; Estrada-Muniz, E.; Huerta-Reyes, M.; Reyes-Chilpa, R. Trypanocidal constituents in plants 3 Leaves of Garcinia intermedia and heartwood of Calophyllum brasiliense. Biol. Pharm. Bull. 2004, 27, 141–143. [Google Scholar] [CrossRef]
- Kaikabo, A.A.; Samuel, B.B.; Eloff, J.N. Isolation and activity of two antibacterial bioflavonoids from leaf extracts of Garcinia livingstonei (Clusiaceae). Nat. Prod. Commun. 2009, 10, 1363–1366. [Google Scholar]
- Alves, T.M.; Alves, R.; Romanha, A.J.; Zani, C.L.; Santos, M.H.; Nagem, T.J. Biologicalactivitiesof 7-Epiclusianone. J. Nat. Prod. 1999, 62, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Branco-De-Almeida, L.; Murata, R.M.; Franco, E.M.; Dos Santos, M.H.; Alencar, S.M.; Koo, H.; Rosalen, P.L. Effects of 7-epiclusianone on Streptococcus mutans and caries development in rats. Planta Med. 2010, 77, 40–45. [Google Scholar] [CrossRef]
- Cruz, A.; Lemos, V.S.; Dos Santos, M.; Nagem, T.; Cortes, S. Vascular effects of 7-epiclusianone, a prenylated benzophenone from Rheedia gardneriana, on the rat aorta. Phytomedicine 2006, 13, 442–445. [Google Scholar] [CrossRef]
- Neves, J.; Coelho, L.; Cordeiro, R.; Veloso, M.E.; Silva, P.; Dos Santos, M.; Martins, M.A. Antianaphylactic Properties of 7-Epiclusianone, a Tetraprenylated Benzophenone Isolated from Garcinia brasiliensis. Planta Med. 2007, 73, 644–649. [Google Scholar] [CrossRef]
- Murata, R.M.; Branco-De-Almeida, L.; Yatsuda, R.; Dos Santos, M.H.; Nagem, T.J.; Rosalen, P.L.; Koo, H. Inhibitory effects of 7-epiclusianone on glucan synthesis, acidogenicity and biofilm formation by Streptococcus mutans. FEMS Microbiol. Lett. 2008, 282, 174–181. [Google Scholar] [CrossRef]
Sample Availability: The samples used are available from the authors. |
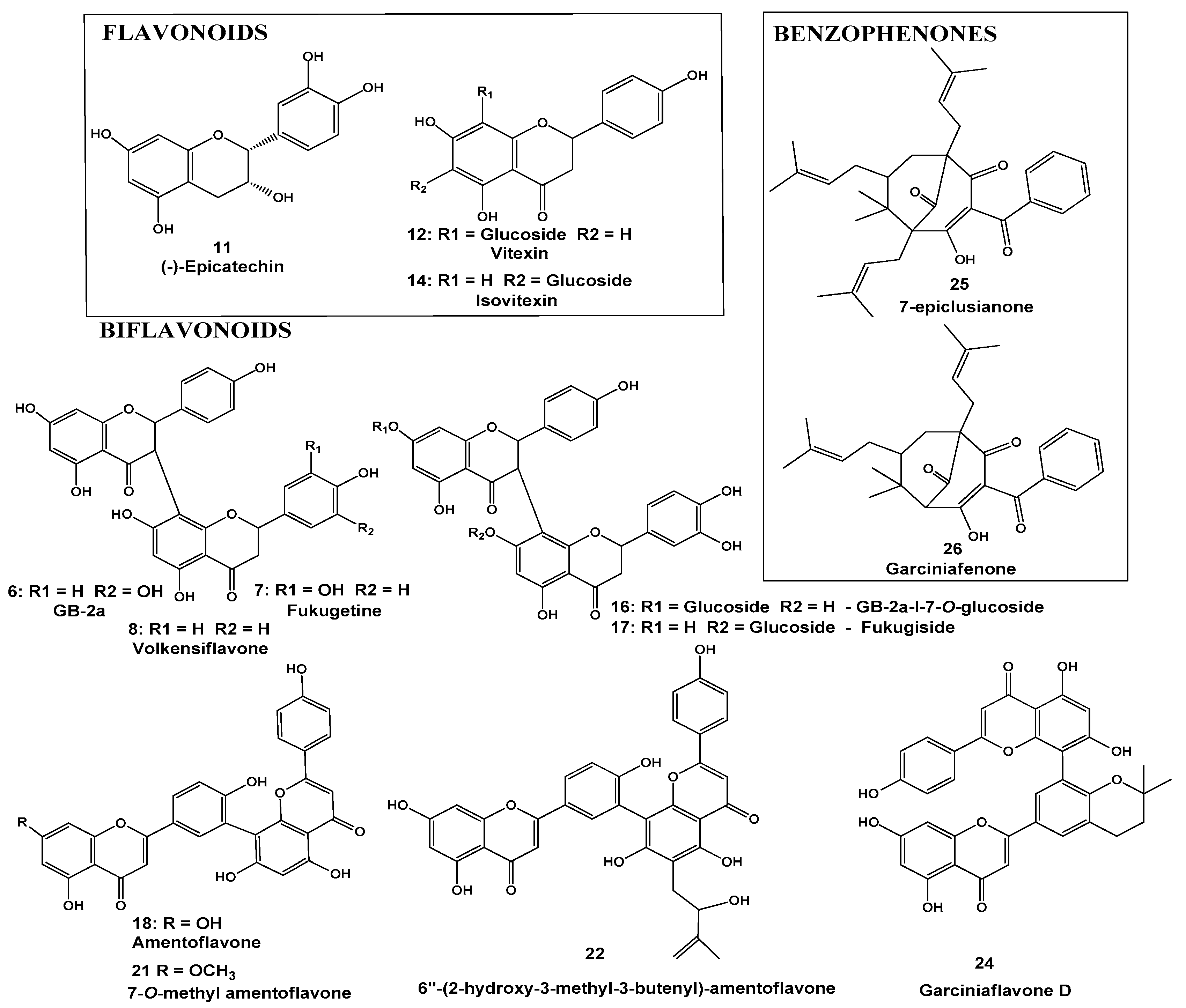
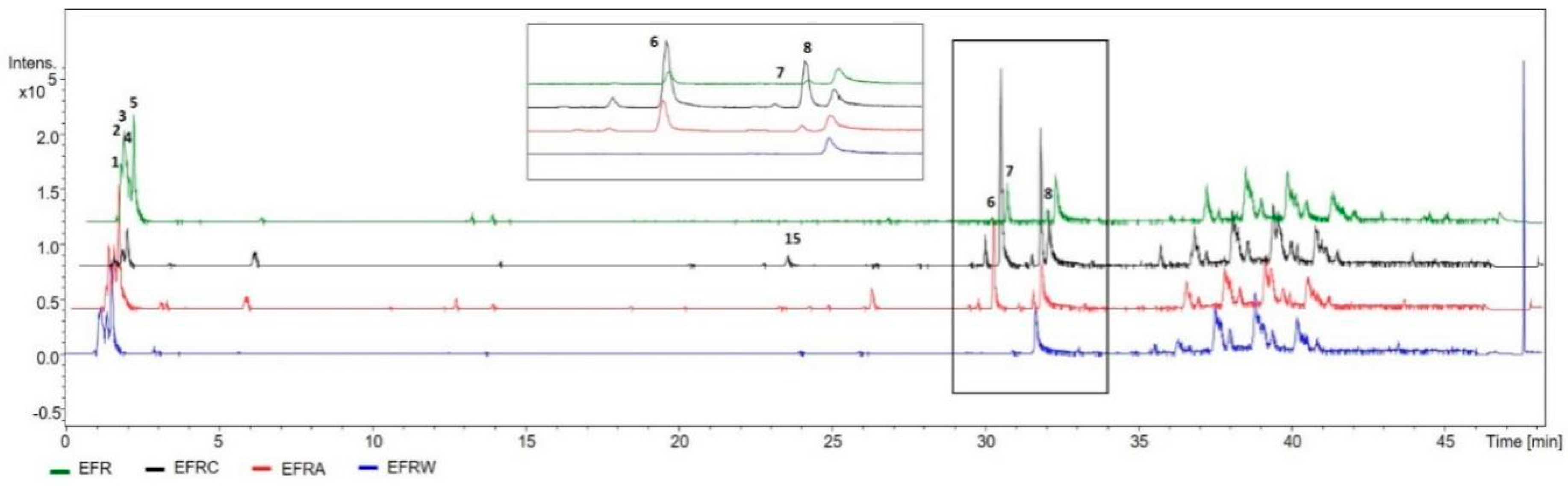

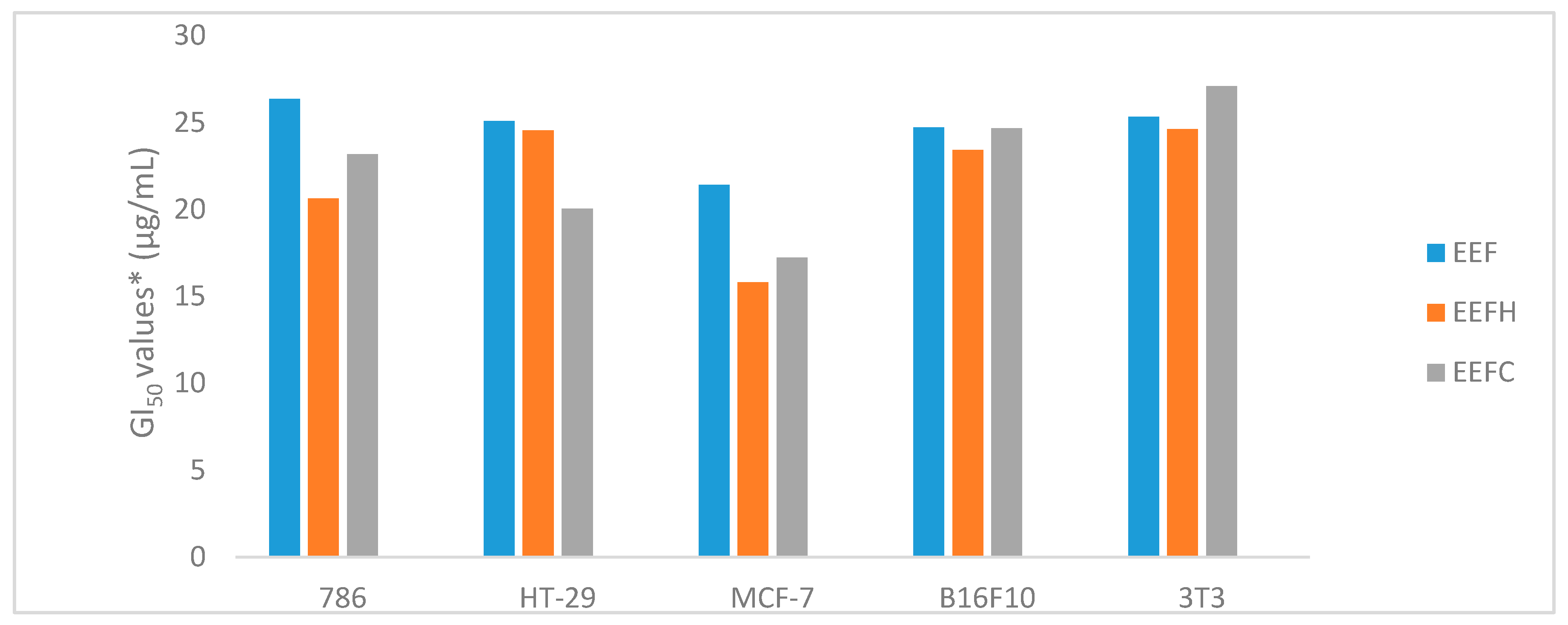
| Substance | Rt (min) | Compound | [M-H]− (MF) | Precursor ion (m/z) | Fragment Ions (m/z) | UV |
|---|---|---|---|---|---|---|
| 1 | 1.1 | Dimers of hexoses | C12H21O11 | 341.1097 | ||
| 2 | 1.1 | Monomers of hexoses | C6H11O6 | 179.0551 | ||
| 3 | 1.2 | Hydroxycitric acid lactone | C6H5O7 | 189.0034 | ||
| 4 | 1.3 | Glycosylated citric acid | C12H17O12 | 353.0725 | ||
| 5 | 1.4 | Citric acid | C6H7O7 | 191.0191 | ||
| 6 | 29.4 | GB-2a biflavonoid | C30H21O11 | 557.1105 | 280 | |
| 7 | 30.0 | Fukugetine | C30H19O11 | 555.0935 | 429, 403, 401, 295 | 280 |
| 8 | 31.3 | Volkensiflavone | C30H19O10 | 539.0982 | 413, 387 | 280 |
| Substance | Rt (min) | Compound | [M-H]− (MF) | Precursor Ion (m/z) | Fragment Ions (m/z) | UV |
|---|---|---|---|---|---|---|
| 1 | 1.2 | Dimers of hexoses | C12H21O11 | 341.1097 | ||
| 2 | 1.2 | Monomers of hexoses | C6H11O6 | 179.0551 | ||
| 3 | 1.2 | Hydroxycitric acid lactone | C6H5O7 | 189.0052 | ||
| 6 | 29.4 | GB-2a biflavonoid | C30H21O11 | 557.1105 | 431, 295 | 280 |
| 7 | 30.0 | Fukugetine | C30H19O11 | 555.0956 | 429, 403, 401, 295 | 280 |
| 8 | 31.3 | Volkensiflavone | C30H19O10 | 539.0982 | 413, 387 | 280 |
| 9 | 1.2 | Tetramer of hexoses | C24H43O22 | 683.2259 | 341, 179 | |
| 10 | 12.1 | Procyanidin B | C30H25O12 | 577.1361 | 407, 289 | 280 |
| 11 | 12.5 | epicatechin | C15H13O6 | 289.0724 | 280 | |
| 12 | 17.9 | Vitexin | C21H19O10 | 431.1000 | 311, 283 | 273, 330 |
| 13 | 18.2 | Vitexin-O-rhamnoside | C27H29O14 | 577.1564 | 413, 293 | 270, 340 |
| 14 | 18.6 | Isovitexin | C21H19O10 | 431.0996 | 273, 330 | |
| 15 | 23.0 | Tetrahydroxy-xanthone | C13H7O6 | 259.0251 | 215, 187 | 275, 315, 360 |
| 16 | 24.0 | GB-2a-I-7-O-glucoside | C36H31O16 | 719.1597 | 431, 313, 295 | 284 |
| 17 | 24.5 | Fukugiside | C36H29O16 | 717.1501 | 565, 493, 429, 403 | 276, 350 |
| 18 | 32.0 | Amentoflavone | C30H17O10 | 537.0834 | 443, 417, 399, 375, 331 | 269, 336 |
| 19 | 33.7 | Prenylated Xanthone | C18H15O6 | 32.0877 | 311, 295, 272 | 275, 316 |
| 20 | 34.0 | Prenylated Xanthone | C18H15O6 | 327.0888 | 283, 272, 258, 243 | 275, 320 |
| 21 | 34.4 | 7-O-methylamentoflavone | C31H19O10 | 551.0995 | 457, 431, 413, 389, 345 | 272, 330 |
| 22 | 34.5 | Amentoflavone | C35H25O11 | 621.1426 | 551, 441, 431, 389, 345 | 276, 328 |
| 23 | 35.4 | Prenylated Xanthone | C18H15O8 | 311.0920 | 295 283, 267, 255 | 277, 307 |
| 24 | 36.2 | Garciniaflavone D | C35H25O10 | 605.1458 | 511, 485, 467, 443, 399, 374, 309, 227 | 275, 333 |
| 25 | 42.8 | 7-epiclusianone | C33H41O4 | 501.3007 | 417, 347, 305, 175 | 276, 307 |
| 26 | 43.1 | Garciniaphenone | C28H33O4 | 433.2390 | 349, 295, 279, 241 | 278, 307 |
| Parameter | Fruit Mean ± MSE | Leaf Mean ± MSE |
|---|---|---|
| Moisture (%) | 82.17 ± 0.91 a | 30.51 ± 2.77 b |
| Ash (%) | 0.40 ± 0.015 a | 4.93 ± 0.06 b |
| Protein (%) | 1.35 ± 0.12 a | 7.4 ± 0.08 b |
| Lipids (%) | 5.41 ± 0.1a | 2.11 ± 0.09b |
| Carbohydrates (%) ** | 10.64 ± 0.83 a | 54.79 ± 2.89 b |
| Phenols (mg GAE 100 g−1) | 107.07 ± 9.65 a | 132.46 ± 2.32 a |
| Vit C (mg 100 g−1) | 25.23 ± 3.58 a | 30.26 ± 2.01 a |
| Carotenoids (mg β-carotene 100 g−1) | 27.05 ± 4.04 a | 76.25 ± 3.51 b |
| Extract and Fraction | Fruit (µg/mL) | Leaf (µg/mL) |
|---|---|---|
| Ethanol extract | 39.13 ± 0.09 a | 16.95 ± 0.80 b |
| Hexane fraction | 20.20 ± 1.21 a | 27.03 ± 1.54 b |
| Chloroform fraction | 103.37 ± 3.32 a | 14.27 ± 1.36 b |
| Ethyl acetate fraction | 73.40 ± 10.72 a | 16.68 ± 0.63 b |
| Hydromethanol fraction | 166.64 ± 2.70 a | 35.46 ± 1.70 b |
| Line | Sample *** | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EEF | EEFH | EEFC | EEFA | EEFHM | EEL | EELH | EELC | EELA | EELHM | Doxorubicin ** | |
| 786 | 26.36 | 20.63 | 23.17 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | 0.026 |
| HT-29 | 25.09 | 24.55 | 20.04 | >250 | >250 | 79.89 | 31.00 | >250 | >250 | >250 | 0.24 |
| MCF-7 | 21.42 | 15.81 | 17.22 | >250 | >250 | 50.29 | 28.7 | 36.29 | >250 | >250 | 0.025 |
| B16F10 | 24.71 | 23.43 | 24.67 | >250 | >250 | 60.92 | 33.79 | 80.59 | >250 | >250 | 0.026 |
| 3T3 | 25.32 | 24.63 | 27.10 | >250 | >250 | 39.87 | >250 | 46.03 | >250 | >250 | 0.36 |
| Classification | Compound | Activity | Reference |
|---|---|---|---|
| Xanthones | Tetrahydroxy-xanthone Prenylated xanthone | Anti-inflammatory, antiviral (herpes), antimicrobial, antifungal, cytotoxic, and antioxidant. | [34,38,44,45] |
| epicatechin | Antioxidant | [34,40,41] | |
| Biflavonoids | GB-2a biflavonoid | Anti-inflammatory, analgesic, antiviral, antioxidant activity | [11,46,47] |
| Procyanidin B2 | Anti-inflammatory | [11,46] | |
| Fukugetine | Anti-inflammatory, antibacterial | [11,32,46,48] | |
| Volkensiflavone | Analgesic, anti-tumor, antibacterial | [8,11,41] | |
| GB-2a-I-7-O-glucoside | Antibacterial | [32] | |
| Fukugisid | Analgesic | [11] | |
| 7-O-methylamentoflavone | [44] | ||
| Amentoflavone | Analgesic, antibacterial, antifungal, anti-inflammatory, contraceptive, antioxidant, antitumor, antiviral, and cytotoxicity | [45,49] | |
| Benzophenones | 7-epiclusianone | Antimicrobial, high concentrations (vasoconstrictor action)/low concentrations (vasodilator), anti-caries, anti-anaphylaxis anti-inflammatory, antiparasitic, trypanocide, antiproliferative, cytotoxic | [50,51] |
| Garciniaphenone | Antimicrobial, antiproliferative | [52] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demenciano, S.d.C.; Silva, M.C.B.L.e.; Alexandrino, C.A.F.; Kato Junior, W.H.; Figueiredo, P.d.O.; Garcez, W.S.; Campos, R.P.; Guimarães, R.d.C.A.; Sarmento, U.C.; Bogo, D. Antiproliferative Activity and Antioxidant Potential of Extracts of Garcinia gardneriana. Molecules 2020, 25, 3201. https://doi.org/10.3390/molecules25143201
Demenciano SdC, Silva MCBLe, Alexandrino CAF, Kato Junior WH, Figueiredo PdO, Garcez WS, Campos RP, Guimarães RdCA, Sarmento UC, Bogo D. Antiproliferative Activity and Antioxidant Potential of Extracts of Garcinia gardneriana. Molecules. 2020; 25(14):3201. https://doi.org/10.3390/molecules25143201
Chicago/Turabian StyleDemenciano, Simone da Cunha, Magalli Costa Barbosa Lima e Silva, Caroline Almeida Farias Alexandrino, Wilson Hino Kato Junior, Patrícia de Oliveira Figueiredo, Walmir Silva Garcez, Raquel Pires Campos, Rita de Cássia Avellaneda Guimarães, Ulana Chaves Sarmento, and Danielle Bogo. 2020. "Antiproliferative Activity and Antioxidant Potential of Extracts of Garcinia gardneriana" Molecules 25, no. 14: 3201. https://doi.org/10.3390/molecules25143201
APA StyleDemenciano, S. d. C., Silva, M. C. B. L. e., Alexandrino, C. A. F., Kato Junior, W. H., Figueiredo, P. d. O., Garcez, W. S., Campos, R. P., Guimarães, R. d. C. A., Sarmento, U. C., & Bogo, D. (2020). Antiproliferative Activity and Antioxidant Potential of Extracts of Garcinia gardneriana. Molecules, 25(14), 3201. https://doi.org/10.3390/molecules25143201





