Propofol Improved Glucose Tolerance Associated with Increased FGF-21 and GLP-1 Production in Male Sprague-Dawley Rats
Abstract
1. Introduction
2. Results
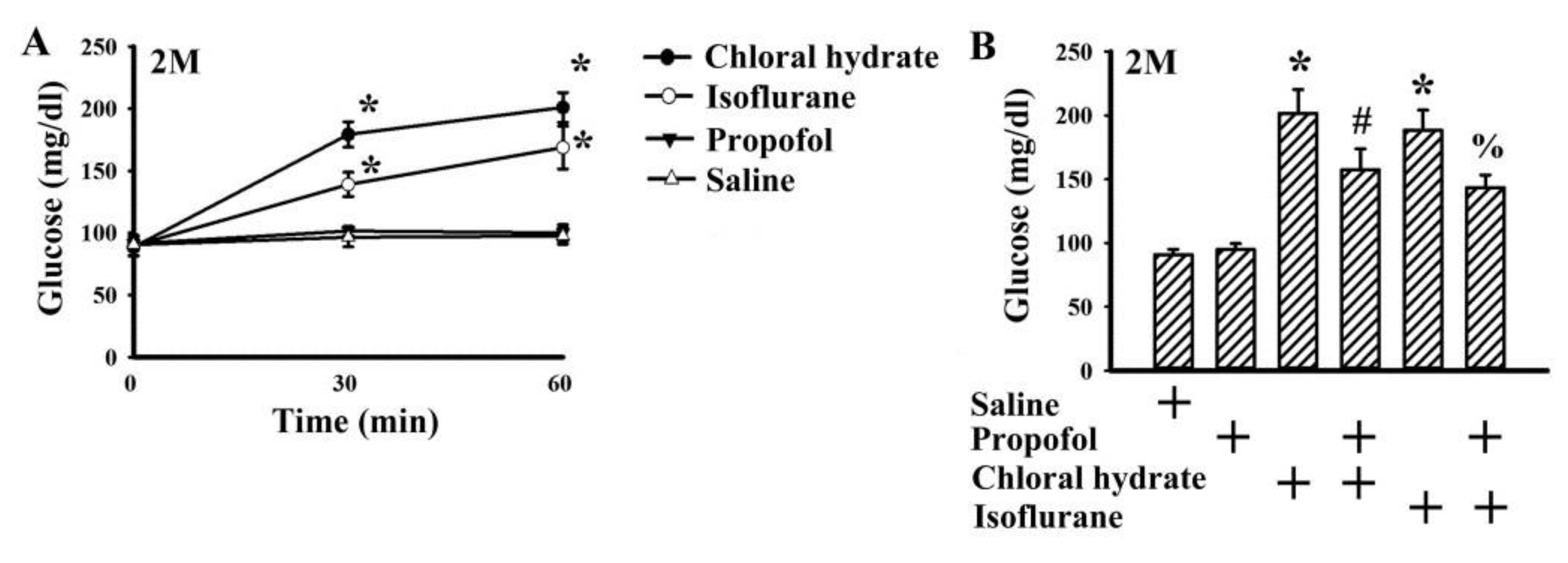
2.1. Propofol Had Minimal Effect on Basal Glucose Level
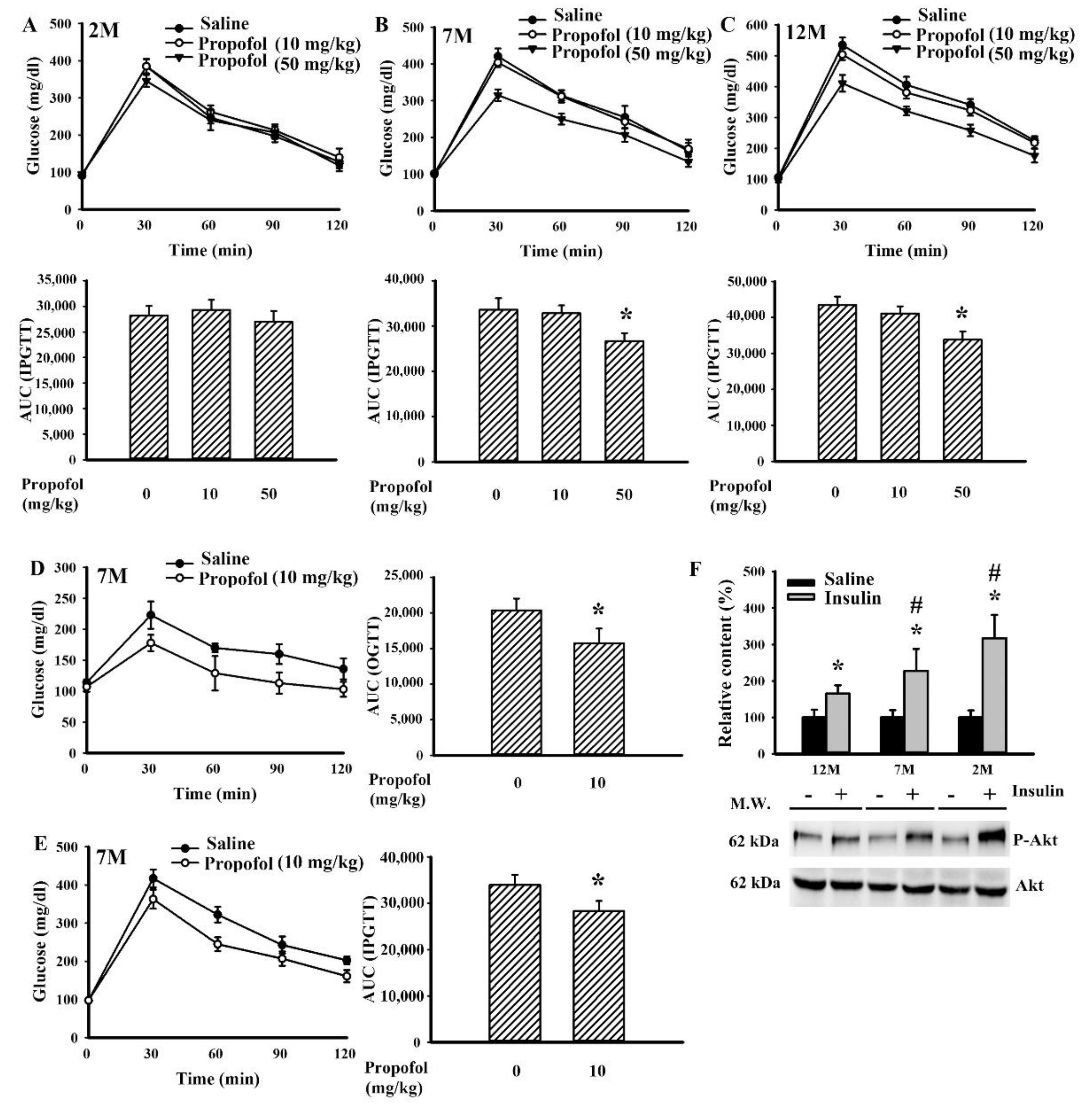
2.2. Propofol Improved Impaired Glucose Tolerance
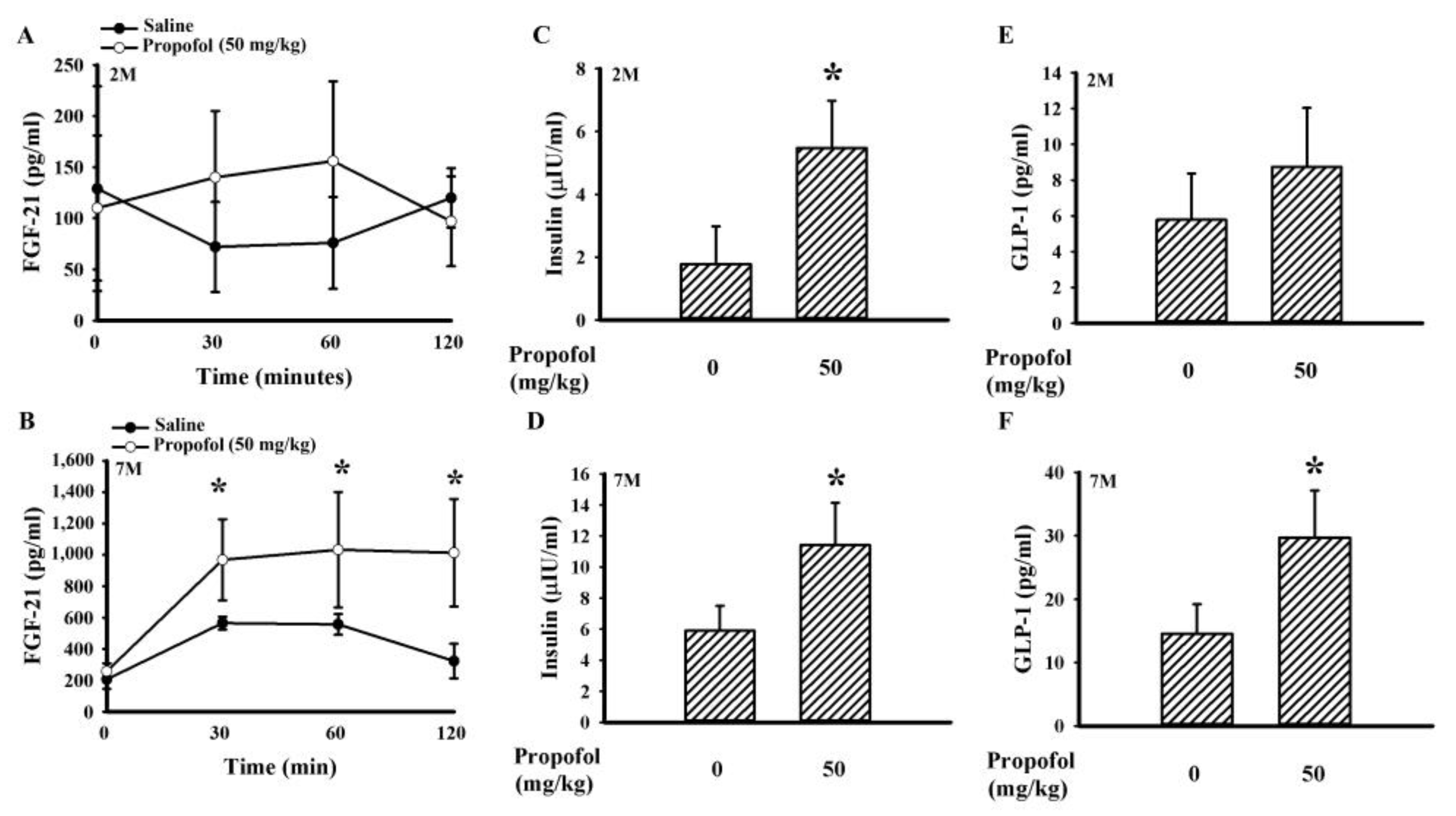
2.3. Propofol Increased the Circulating Level of Fibroblast Growth Factor-21 (FGF-21), Glucagon-like Peptide-1 (GLP-1), and Insulin
2.4. Propofol Upregulated FGF-21 Signaling in the Liver
3. Discussion
4. Materials and Methods
4.1. Animal Study
4.2. Blood Sample Reparation
4.3. Tissue Collection and Western Blot Analyses
4.4. RNA Preparation and Quantitative Real-Time Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Vanhorebeek, I.; Gunst, J.; Van den Berghe, G. Critical care management of stress-induced hyperglycemia. Curr. Diab. Rep. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.M.; Potter, D.J.; Kongable, G.L.; Cook, C.B. Update on inpatient glycemic control in hospitals in the United States. Endocr. Pract. 2011, 17, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Xia, T.; Xu, F.; Wu, H.; Ma, Z.; Zhao, X.; Gu, X. Isoflurane aggravates peripheral and central insulin resistance in high-fat diet/streptozocin-induced type 2 diabetic mice. Brain Res. 2020, 1727, 146511. [Google Scholar] [CrossRef] [PubMed]
- Høyer, K.F.; Nielsen, T.S.; Risis, S.; Treebak, J.T.; Jessen, N. Sevoflurane impairs insulin secretion and tissue-specific glucose uptake in vivo. Basic Clin. Pharmacol. Toxicol. 2018, 123, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Windeløv, J.A.; Pedersen, J.; Holst, J.J. Use of anesthesia dramatically alters the oral glucose tolerance and insulin secretion in C57Bl/6 mice. Physiol. Rep. 2016, 4, e12824. [Google Scholar] [CrossRef] [PubMed]
- Behdad, S.; Mortazavizadeh, A.; Ayatollahi, V.; Khadiv, Z.; Khalilzadeh, S. The effects of propofol and isoflurane on blood glucose during abdominal hysterectomy in diabetic patients. Diabetes Metab. J. 2014, 38, 311–316. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, L.; Yin, G.; Liu, Y.; Chen, L. Stress response to propofol versus isoflurane anesthesia in patients undergoing gastric surgery. J. Coll. Physicians Surg. Pak. 2019, 29, 201–204. [Google Scholar] [CrossRef]
- Li, X.; Kitamura, T.; Kawamura, G.; Mori, Y.; Sato, K.; Araki, Y.; Sato, R.; Yamada, Y. Comparison of mechanisms underlying changes in glucose utilization in fasted rats anesthetized with propofol or sevoflurane: Hyperinsulinemia is exaggerated by propofol with concomitant insulin resistance induced by an acute lipid load. Biosci. Trends. 2014, 8, 155–162. [Google Scholar] [CrossRef][Green Version]
- Kitamura, T.; Sato, K.; Kawamura, G.; Yamada, Y. The involvement of adenosine triphosphate-sensitive potassium channels in the different effects of sevoflurane and propofol on glucose metabolism in fed rats. Anesth. Analg. 2012, 114, 110–116. [Google Scholar] [CrossRef]
- Sato, K.; Kitamura, T.; Kawamura, G.; Mori, Y.; Sato, R.; Araki, Y.; Yamada, Y. Glucose use in fasted rats under sevoflurane anesthesia and propofol anesthesia. Anesth. Analg. 2013, 117, 627–633. [Google Scholar] [CrossRef]
- Yasuda, Y.; Fukushima, Y.; Kaneki, M.; Martyn, J.A. Anesthesia with propofol induces insulin resistance systemically in skeletal and cardiac muscles and liver of rats. Biochem. Biophys. Res. Commun. 2013, 431, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Chaki, T.; Hirata, N.; Yoshikawa, Y.; Tachibana, S.; Tokinaga, Y.; Yamakage, M.J. Lipid emulsion, but not propofol, induces skeletal muscle damage and lipid peroxidation. J. Anesth. 2019, 33, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Shin, J.S.; Yoon, I.H.; Min, B.H.; Jeong, W.Y.; Lee, G.E.; Kim, M.S.; Kim, J.E.; Jang, J.Y.; Park, C.G. The effect of propofol on intravenous glucose tolerance test in rhesus monkey. J. Med. Primatol. 2014, 43, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Ogawa, M.; Kawamura, G.; Sato, K.; Yamada, Y. The effects of sevoflurane and propofol on glucose metabolism under aerobic conditions in fed rats. Anesth. Analg. 2009, 109, 1479–1485. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, L.; Yang, B.; Zeng, J.; Zhang, Q.; Lei, H.; Xu, S. Protective effect of pretreatment with propofol against tumor necrosis factor-α-induced hepatic insulin resistance. Exp. Ther. Med. 2015, 10, 289–294. [Google Scholar] [CrossRef][Green Version]
- Rowlands, J.; Heng, J.; Newsholme, P.; Carlessi, R. Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front. Endocrinol. 2018, 9, 672. [Google Scholar] [CrossRef]
- Nonogaki, K.; Hazama, M.; Satoh, N. Liraglutide suppresses obesity and hyperglycemia associated with increases in hepatic fibroblast growth factor 21 production in KKAy mice. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, L.; Wang, C.; Liu, H.; Boden, G.; Yang, G.; Li, L. Liraglutide increases FGF-21 activity and insulin sensitivity in high fat diet and adiponectin knockdown induced insulin resistance. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Staiger, H.; Keuper, M.; Berti, L.; Hrabe de Angelis, M.; Häring, H.U. Fibroblast growth factor 21-metabolic role in mice and men. Endocr. Rev. 2017, 38, 468–488. [Google Scholar] [CrossRef]
- Liu, J.; Yang, K.; Yang, J.; Xiao, W.; Le, Y.; Yu, F.; Gu, L.; Lang, S.; Tian, Q.; Jin, T.; et al. Liver-derived fibroblast growth factor 21 mediates effects of glucagon-like peptide-1 in attenuating hepatic glucose output. EbioMedicine. 2019, 41, 73–84. [Google Scholar] [CrossRef]
- Gluvic, Z.; Zaric, B.; Resanovic, I.; Obradovic, M.M.; Mitrovic, A.A.; Radak, D.; Isenovic, E.R. Link between metabolic syndrome and insulin resistance. Curr. Vasc. Pharmacol. 2017, 15, 30–39. [Google Scholar] [CrossRef]
- Tresguerres, J.A.; Cuesta, S.; Kireev, R.A.; Garcia, C.; Acuña-Castroviejo, D.; Vara, E. Beneficial effect of melatonin treatment on age-related insulin resistance and on the development of type 2 diabetes. Horm. Mol. Biol. Clin. Investig. 2013, 16, 47–54. [Google Scholar] [CrossRef]
- Pham, H.; Marathe, C.S.; Phillips, L.K.; Trahair, L.G.; Hatzinikolas, S.; Huynh, L.; Wu, T.; Nauck, M.A.; Rayner, C.K.; Horowitz, M.; et al. Longitudinal changes in fasting and glucose-stimulated GLP-1 and GIP in healthy older subjects. J. Clin. Endocrinol. Metab. 2019, 104, 6201–6206. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Leung, P.S. Fibroblast growth factor 21: A regulator of metabolic disease and health span. Am. J Physiol. Endocrinol. Metab. 2017, 313, E292–E302. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.R.; Wang, L.; Jiang, W.; Qi, A.H.; Zhou, Q.H.; Zhang, X.L. Propofol prevents cerebral ischemia-triggered autophagy activation and cell death in the rat hippocampus through the NF-κB/p53 signaling pathway. Neuroscience. 2013, 246, 117–132. [Google Scholar] [CrossRef]
- Li, J.; Yu, W.; Li, X.T.; Qi, S.H.; Li, B. The effects of propofol on mitochondrial dysfunction following focal cerebral ischemia-reperfusion in rats. Neuropharmacology 2014, 77, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Tsuji, M.; Inagaki, M.; Tamura, Y.; Kato, M.; Niiya, A.; Usui, Y.; Oguchi, K. Neuroprotective effects of propofol on ER stress-mediated apoptosis in neuroblastoma SH-SY5Y cells. Eur. J. Pharmacol. 2014, 725, 47–54. [Google Scholar] [CrossRef]
- Zhou, R.; Yang, Z.; Tang, X.; Tan, Y.; Wu, X.; Liu, F. Propofol protects against focal cerebral ischemia via inhibition of microglia-mediated proinflammatory cytokines in a rat model of experimental stroke. PLoS ONE 2013, 12. [Google Scholar] [CrossRef]
- Tanaka, K.; Kawano, T.; Tsutsumi, Y.M.; Kinoshita, M.; Kakuta, N.; Hirose, K.; Kimura, M.; Oshita, S. Differential effects of propofol and isoflurane on glucose utilization and insulin secretion. Life. Sci. 2011, 88, 96–103. [Google Scholar] [CrossRef]
- Maruyama, R.; Shimizu, M.; Hashidume, T.; Inoue, J.; Itoh, N.; Sato, R. FGF21 alleviates hepatic endoplasmic reticulum stress under physiological conditions. J. Nutr. Sci. Vitaminol. 2018, 64, 200–208. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, S.; Ni, L.; Huang, L.; Wang, K.; Zhou, Y. Dexmedetomidine alleviates insulin resistance in hepatocytes by reducing endoplasmic reticulum stress. Endocrine 2020, 67, 87–94. [Google Scholar] [CrossRef]
- Nakajima, S.; Hira, T.; Hara, H. Postprandial glucagon-like peptide-1 secretion is increased during the progression of glucose intolerance and obesity in high-fat/high-sucrose diet-fed rats. Br. J. Nutr. 2015, 113, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Krasner, N.M.; Ido, Y.; Ruderman, N.B.; Cacicedo, J.M. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Wen, X.; Cho, H.; Koo, S.H. CREB/CRTC2 controls GLP-1-dependent regulation of glucose homeostasis. FASEB J. 2018, 32, 1566–1578. [Google Scholar] [CrossRef]
- Mauna, J.C.; Miyamae, T.; Pulli, B.; Thiels, E. Protein phosphatases 1 and 2A are both required for long-term depression and associated dephosphorylation of cAMP response element binding protein in hippocampal area CA1 in vivo. Hippocampus 2011, 21, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhao, Y.; Duan, W.; Liu, Y.; Chen, X.; Zhu, M. Propofol inhibits high glucose-induced PP2A expression. in human umbilical vein endothelial cells. Vascul. Pharmacol. 2017, 91, 18–25. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-C.; Hung, C.-J.; Wang, Y.-Y.; Lin, S.-Y.; Chen, W.-Y.; Kuan, Y.-H.; Liao, S.-L.; Yang, C.-P.; Chen, C.-J. Propofol Improved Glucose Tolerance Associated with Increased FGF-21 and GLP-1 Production in Male Sprague-Dawley Rats. Molecules 2020, 25, 3229. https://doi.org/10.3390/molecules25143229
Wu C-C, Hung C-J, Wang Y-Y, Lin S-Y, Chen W-Y, Kuan Y-H, Liao S-L, Yang C-P, Chen C-J. Propofol Improved Glucose Tolerance Associated with Increased FGF-21 and GLP-1 Production in Male Sprague-Dawley Rats. Molecules. 2020; 25(14):3229. https://doi.org/10.3390/molecules25143229
Chicago/Turabian StyleWu, Chih-Cheng, Chih-Jen Hung, Ya-Yu Wang, Shih-Yi Lin, Wen-Ying Chen, Yu-Hsiang Kuan, Su-Lan Liao, Ching-Ping Yang, and Chun-Jung Chen. 2020. "Propofol Improved Glucose Tolerance Associated with Increased FGF-21 and GLP-1 Production in Male Sprague-Dawley Rats" Molecules 25, no. 14: 3229. https://doi.org/10.3390/molecules25143229
APA StyleWu, C.-C., Hung, C.-J., Wang, Y.-Y., Lin, S.-Y., Chen, W.-Y., Kuan, Y.-H., Liao, S.-L., Yang, C.-P., & Chen, C.-J. (2020). Propofol Improved Glucose Tolerance Associated with Increased FGF-21 and GLP-1 Production in Male Sprague-Dawley Rats. Molecules, 25(14), 3229. https://doi.org/10.3390/molecules25143229





