Bio-Based Thermoplastic Starch Composites Reinforced by Dialdehyde Lignocellulose
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD Spectra
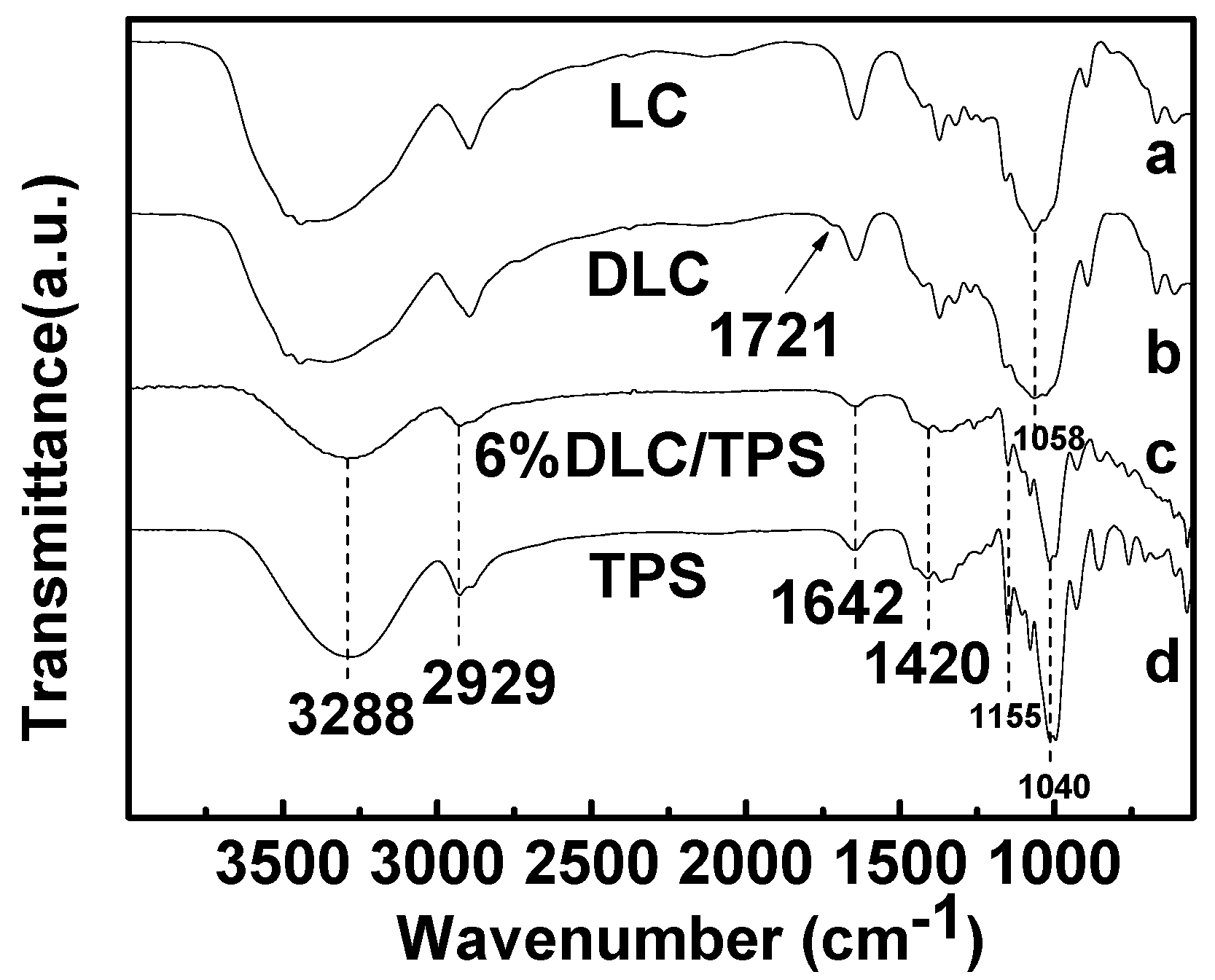
2.2. FTIR Spectra
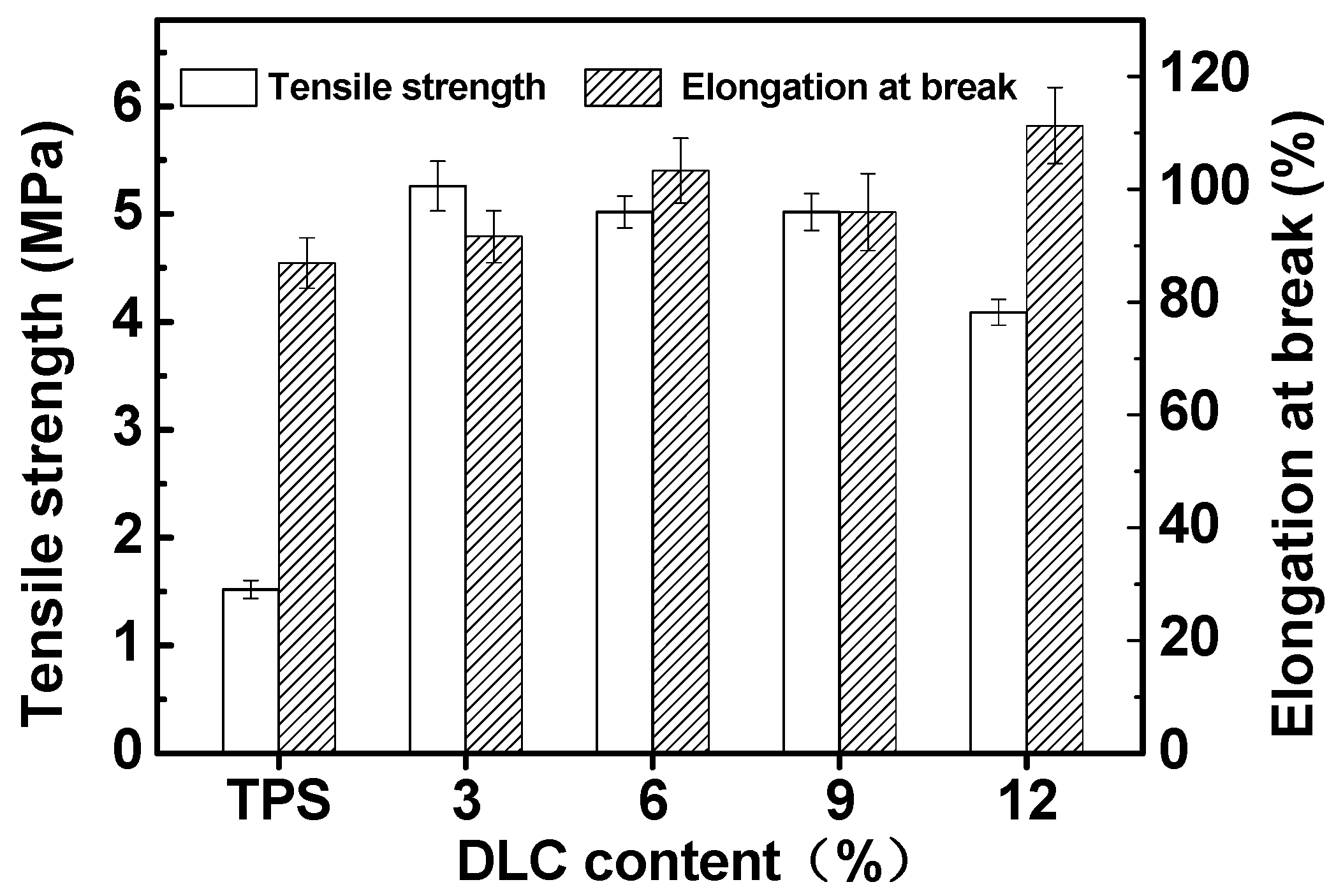
2.3. Mechanical Properties
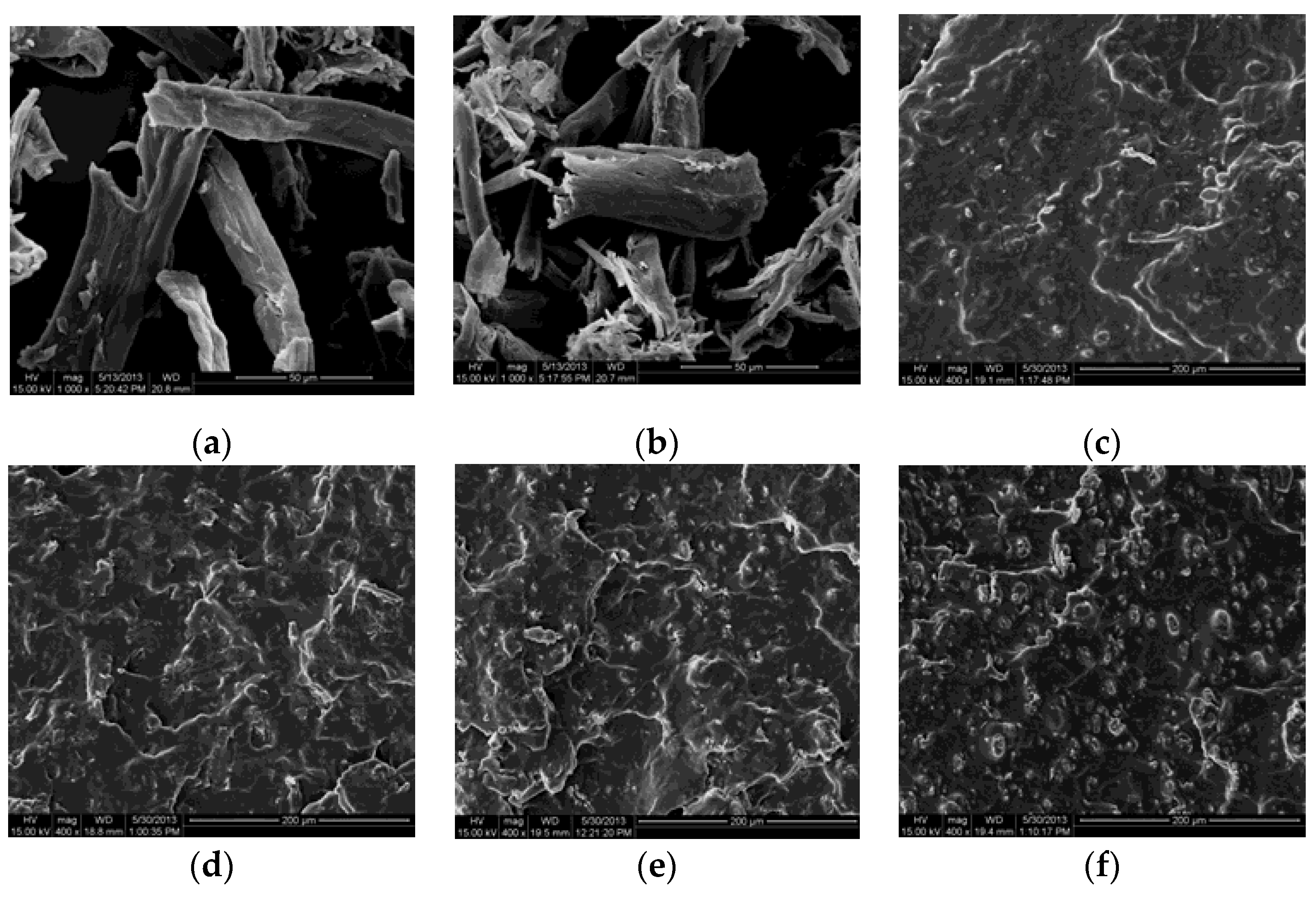
2.4. SEM
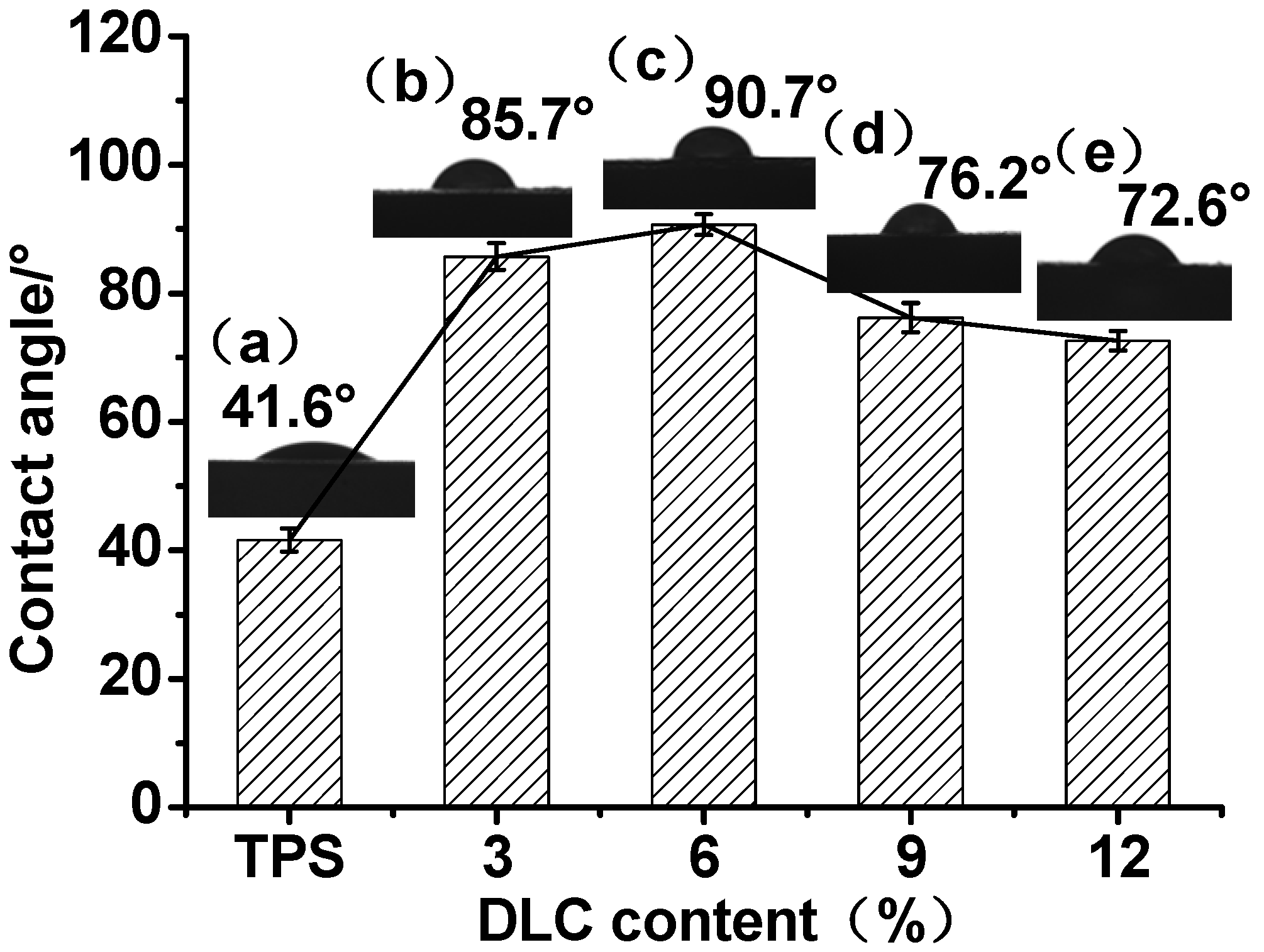
2.5. Contact Angle Measurement
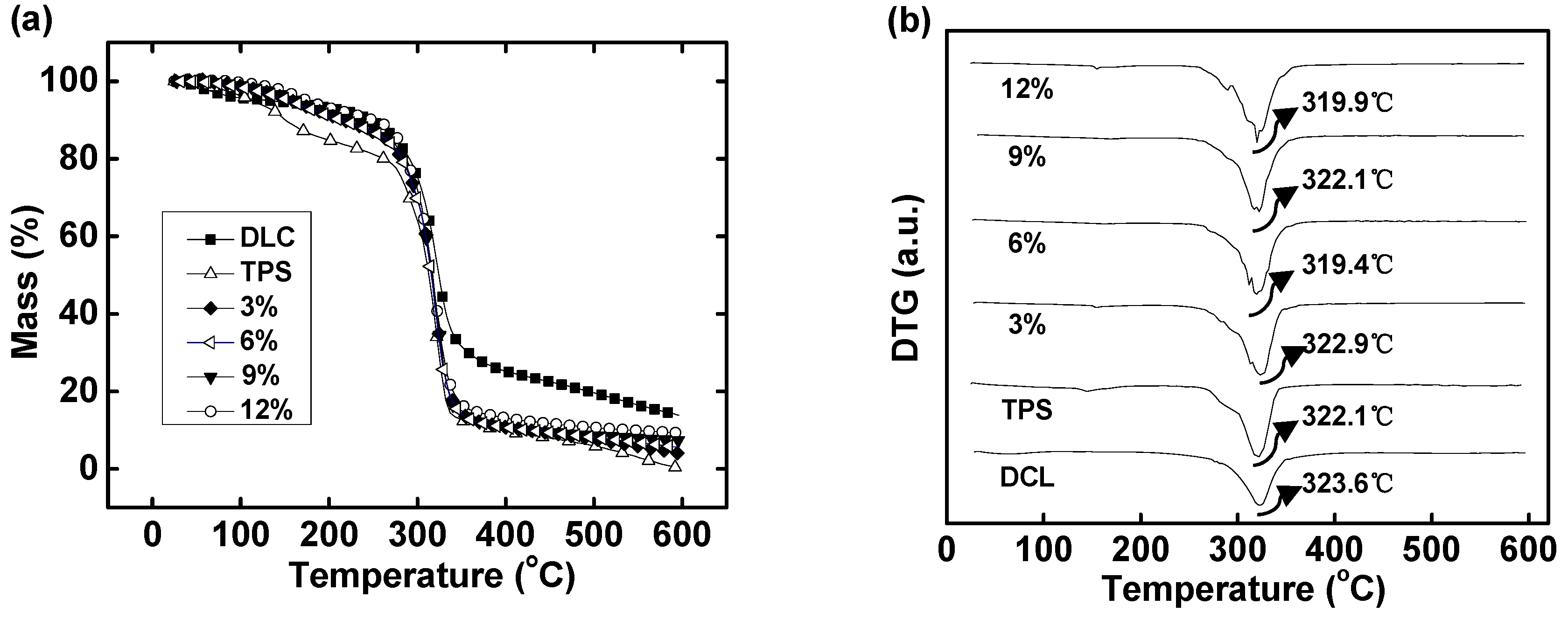
2.6. Thermal Stability
3. Materials and Methods
3.1. Materials
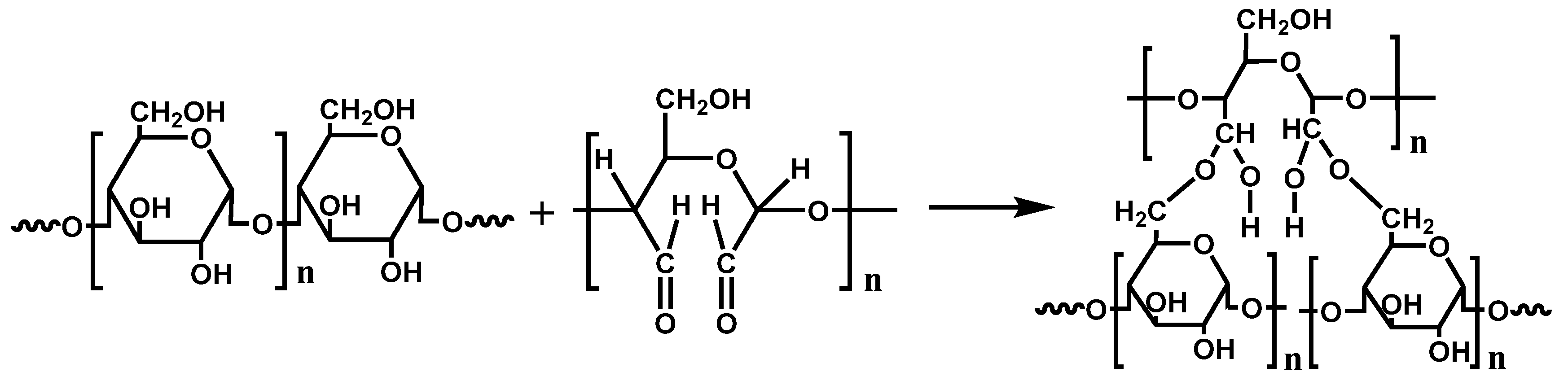
3.2. Preparation of DLC
3.3. Determination of Aldehyde Group Content
3.4. Preparation of DLC/TPS Composites
3.5. X-Ray Diffraction (XRD) Analysis
3.6. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.7. Mechanical Tests
3.8. Scanning Electron Microscopy (SEM) Analysis
3.9. Contact Angle Measurement
3.10. Thermal Stability Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Narancic, T.; O’Connor, K.E. Plastic waste as a global challenge: Are biodegradable plastics the answer to the plastic waste problem? Microbiology-Sgm. 2019, 165, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.F.; Yu, J.G. The plastcizers containing amide groups for thermoplastic starch. Carbohydr. Polym. 2004, 57, 197–203. [Google Scholar] [CrossRef]
- Prachayawarakorn, J.; Hommanee, L.; Phosee, D.; Chairapaksatien, P. Property improvement of thermoplastic mung bean starch using cotton fiber and low-density polyethylene. Starch/Stärke. 2010, 62, 435–443. [Google Scholar] [CrossRef]
- Prachayawarakorn, J.; Sangnitidej, P.; Boonpasith, P. Properties of thermoplastic rice starch composites reinforced by cotton fiber or low-density polyethylene. Carbohydr. Polym. 2010, 81, 425–433. [Google Scholar] [CrossRef]
- Romhany, G.; Karger-Kocsis, J.; Czigany, T. Tensile fracture and failure behavior of thermoplastic starch with unidirectional and cross-ply flax fiber reinforcements. Macromol. Mater. Eng. 2003, 288, 699–707. [Google Scholar] [CrossRef]
- Ramírez, M.G.L.; Satyanarayana, K.G.; Iwakiri, S.; Muniz, G.B.D.; Tanobe, V.; Flores-Sahagund, T.S. Study of the properties of biocomposites. Part I. Cassava starch-green coir fibers from Brazil. Carbohydr. Polym. 2011, 86, 1712–1722. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, E.D.; Pasquini, D.; Curvelo, A.A.S.C.; Corradini, E.; Belgacem, M.N.; Dufresne, A. Cassava bagasse cellulose nanofibrils reinforced thermoplastic cassava starch. Carbohydr. Polym. 2009, 78, 422–431. [Google Scholar] [CrossRef]
- Kaushik, A.; Singh, M.; Verma, G. Green nanocomposites based on thermoplastic starch and steam exploded cellulose nanofibrils from wheat straw. Carbohydr. Polym. 2010, 82, 337–345. [Google Scholar] [CrossRef]
- Park, H.; Li, X.; Jin, C.; Cho, W.; Ha, C. Preparation and properties of biodegradable thermoplastic starch/clay hybrids. Macromol. Mater. Eng. 2002, 287, 553–558. [Google Scholar] [CrossRef]
- Mbey, J.A.; Hoppe, S.; Thomas, F. Cassava starch-kaolinite composite film. Effect of clay content and clay modification on film properties. Carbohydr. Polym. 2012, 88, 213–222. [Google Scholar] [CrossRef]
- Schmitt, H.; Prashantha, K.; Soulestin, J.; Lacrampe, M.F.; Krawczak, P. Preparation and properties of novel melt-blended halloysite nanotubes/wheat starch nanocomposites. Carbohydr. Polym. 2012, 89, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.R.; Wu, D.L.; Anderson, D.P.; Ma, X.F. Nanocomposites based on plasticized starch and rectorite clay: Structure and properties. Carbohydr. Polym. 2012, 89, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.F.; Yu, J.G.; Kennedy, J.F. Studies on the properties of natural fibers-reinforced thermoplastic starch composites. Carbohydr. Polym. 2005, 62, 19–24. [Google Scholar] [CrossRef]
- Curvelo, A.A.S.; de Carvalho, A.J.F.; Agnelli, J.A.M. Thermoplastic starch-cellulosic fibers composites: Preliminary results. Carbohydr. Polym. 2001, 45, 183–188. [Google Scholar] [CrossRef]
- Ma, X.F.; Chang, P.R.; Yu, J.G. Properties of biodegradable thermoplastic pea starch/carboxymethyl cellulose and pea starch/microcrystalline cellulose composites. Carbohydr. Polym. 2008, 72, 369–375. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Fu, S.Y.; Zheng, L.M.; Zhan, H.Y. Effect of nanocellulose isolation techniques on the formation of reinforced poly(vinyl alcohol) nanocomposite films. Exp. Polym. Lett. 2012, 6, 794–804. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J. Excellent chemical and material cellulose from tunicates: Diversity in cellulose production yield and chemical and morphological structures from different tunicate species. Cellulose 2014, 21, 3427–3441. [Google Scholar] [CrossRef]
- Jedvert, K.; Heinze, T. Cellulose modification and shaping—A review. J. Polym. Eng. 2017, 37, 845–860. [Google Scholar] [CrossRef]
- Dincă, V.; Mocanu, A.; Isopencu, G.; Busuioc, C.; Brajnicov, S.; Vlad, A.; Icriverzi, M.; Roseanu, A.; Dinescu, M.; Stroescu, M.; et al. Biocompatible pure ZnO nanoparticles-3D bacterial cellulose biointerfaces with antibacterial properties. Arab. J. Chem. 2020, 13, 3521–3533. [Google Scholar] [CrossRef]
- Kim, U.J.; Kuga, S. Ion-exchange chromatography by dicarboxyl cellulose gel. J. Chromatogr. A. 2001, 919, 29–37. [Google Scholar] [CrossRef]
- Wu, M.; Kuga, S. Cationization of cellulose fabrics by polyallylamine binding. J. Appl. Polym. Sci. 2006, 100, 1668–1672. [Google Scholar] [CrossRef]
- Liu, X.L.; Wang, L.J.; Song, X.P.; Song, H.N.; Zhao, J.R.; Wang, S.F. A kinetic model for oxidative degradation of bagasse pulp fiber by sodium periodate. Carbohydr. Polym. 2012, 90, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.L.; Cao, Y.; Lu, F.; Li, F.; Cao, Y.; Wang, X.L.; Wang, Y.Z. Biodegradation behaviors of thermoplastic starch (TPS) and thermoplastic dialdehyde starch (TPDAS) under controlled composting conditions. Polym. Test. 2008, 27, 924–930. [Google Scholar] [CrossRef]
- Yu, J.G.; Ma, X.F.; Chang, P.R. The preparation and properties of dialdehyde starch and thermoplastic dialdehyde starch. Carbohydr. Polym. 2010, 79, 296–300. [Google Scholar] [CrossRef]
- Veelaert, S.; de Wit, D.; Gotlieb, K.F.; Verhé, R. Chemical and physical transitions of periodate oxidized potato starch in water. Carbohydr. Polym. 1997, 33, 153–162. [Google Scholar] [CrossRef]
- Shi, L.; Zhen, W.J.; Shan, Z.H. Preparation and characteristic of dialdehyde cellulose. Fine Chemicals (China) 2008, 25, 795–798. [Google Scholar] [CrossRef]
- Tao, F.R.; Wang, D.J.; Song, H.L.; Chou, L.J. Oxidation of microcrystalline cellulose to oxycellulose by sodium periodate. J. Mol. Catal. (China) 2011, 25, 119–123. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, P.; Li, Y.; He, X.; Dai, Y.; Tan, Z. Preparation and antibacterial activity of a cellulose-based Schiff base derived from dialdehyde cellulose and L-lysine. Ind. Crop Prod. 2020, 145, 112126. [Google Scholar] [CrossRef]
- Bakdev, R.; Sankar, U.K. Low density polyethylene/starch blend films for food packaging applications. Adv. Polym. Technol. 2004, 23, 32–45. [Google Scholar] [CrossRef]
- Kaewtatip, K.; Thongmee, J. Effect of kraft lignin and esterified lignin on the properties of thermoplastic starch. Mater Des. 2013, 49, 701–704. [Google Scholar] [CrossRef]
- Peng, X.L.; Kuo, M.C.; Huang, C.Y.; Wei, W.; Wang, D.W.; Sun, L.S.; Runt, J.; Yeh, J.T. Melt-processing, moisture-resistance and strength retention properties of supercritical CO2-processed thermoplastic starch resins. Express Polym. Lett. 2018, 12, 462–478. [Google Scholar] [CrossRef]
- Teixeira, E.D.; Lotti, C.; Correa, A.C.; Teodoro, K.B.R.; Marconcini, J.M.; Mattoso, L.H.C. Thermoplastic corn starch reinforced with cotton cellulose nanofibers. J. Appl. Polym. Sci. 2011, 120, 2428–2433. [Google Scholar] [CrossRef]
- Kvien, I.; Tanem, B.S.; Oksman, K. Characterization of cellulose whiskers and their nanocomposites by atomic force and electron microscopy. Biomacromolecules. 2006, 6, 3160–3165. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Dong, X.; Zhou, W.; Zha, D.; Xu, J.; Guo, B.; Li, P. A novel method to produce sustainable biocomposites based on thermoplastic corn-starch reinforced by polyvinyl alcohol fibers. RSC Adv. 2020, 10, 23632–23643. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, P.; Zhou, W.; Zhang, X.; Guo, B.; Li, P. Bio-Based Thermoplastic Starch Composites Reinforced by Dialdehyde Lignocellulose. Molecules 2020, 25, 3236. https://doi.org/10.3390/molecules25143236
Yin P, Zhou W, Zhang X, Guo B, Li P. Bio-Based Thermoplastic Starch Composites Reinforced by Dialdehyde Lignocellulose. Molecules. 2020; 25(14):3236. https://doi.org/10.3390/molecules25143236
Chicago/Turabian StyleYin, Peng, Wen Zhou, Xin Zhang, Bin Guo, and Panxin Li. 2020. "Bio-Based Thermoplastic Starch Composites Reinforced by Dialdehyde Lignocellulose" Molecules 25, no. 14: 3236. https://doi.org/10.3390/molecules25143236





