Synthetic Strategies, Reactivity and Applications of 1,5-Naphthyridines
Abstract
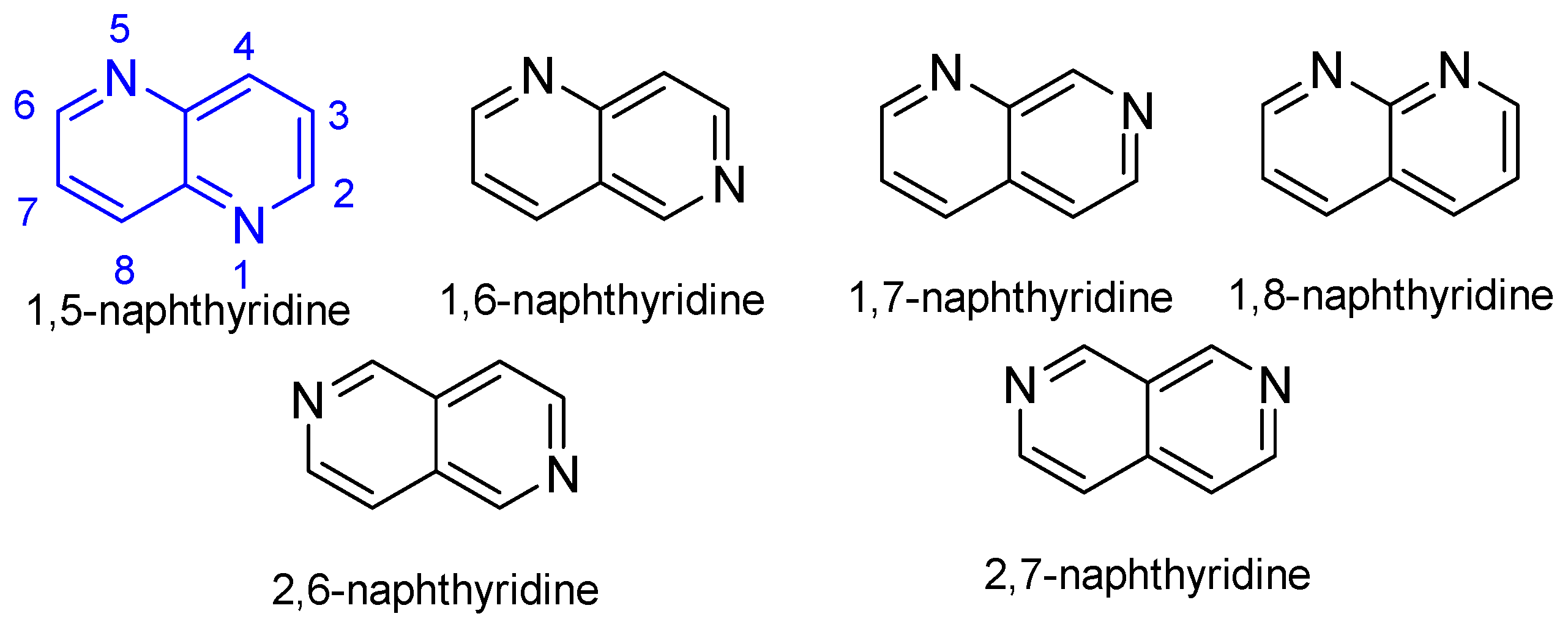
1. Introduction
2. Synthesis of 1,5-Naphthyridines
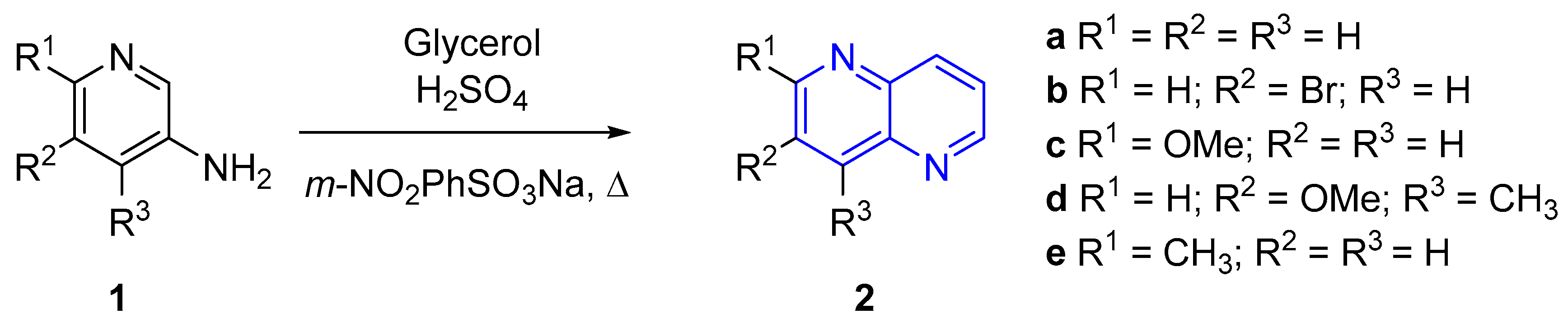
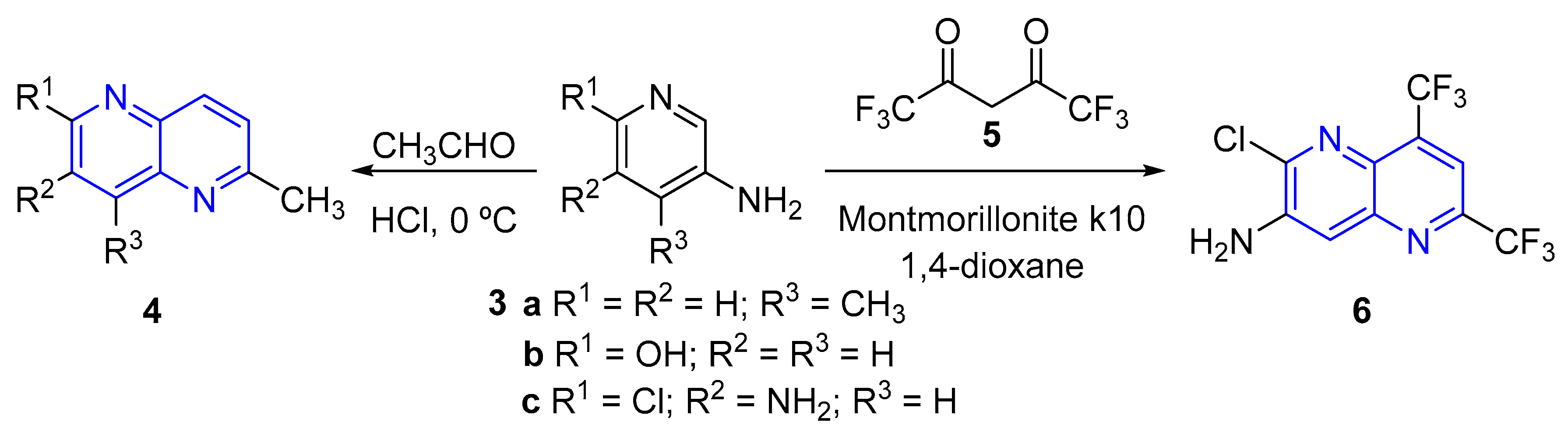
2.1. Synthesis of 1,5-Naphthyridines by Cyclization Reactions
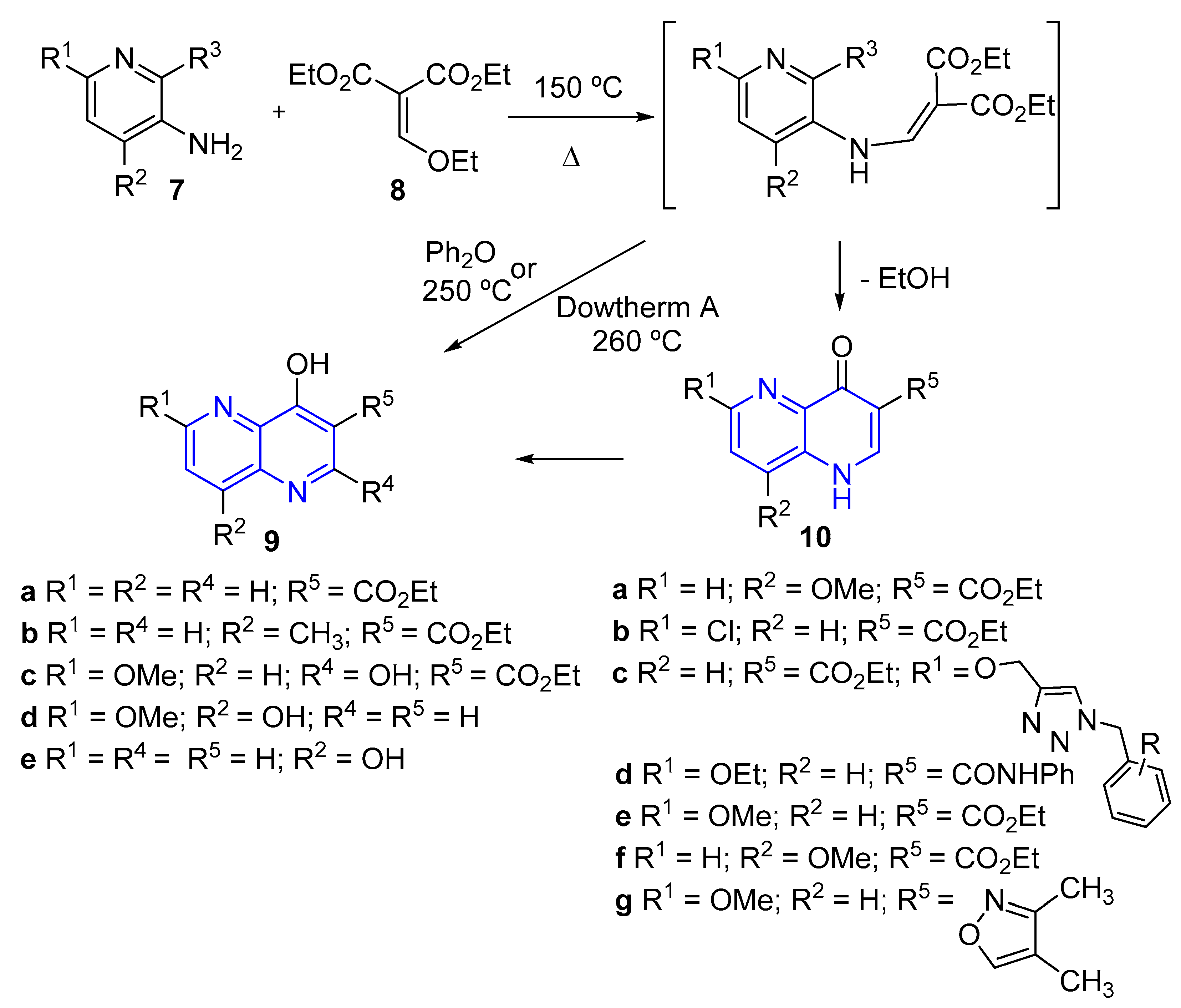
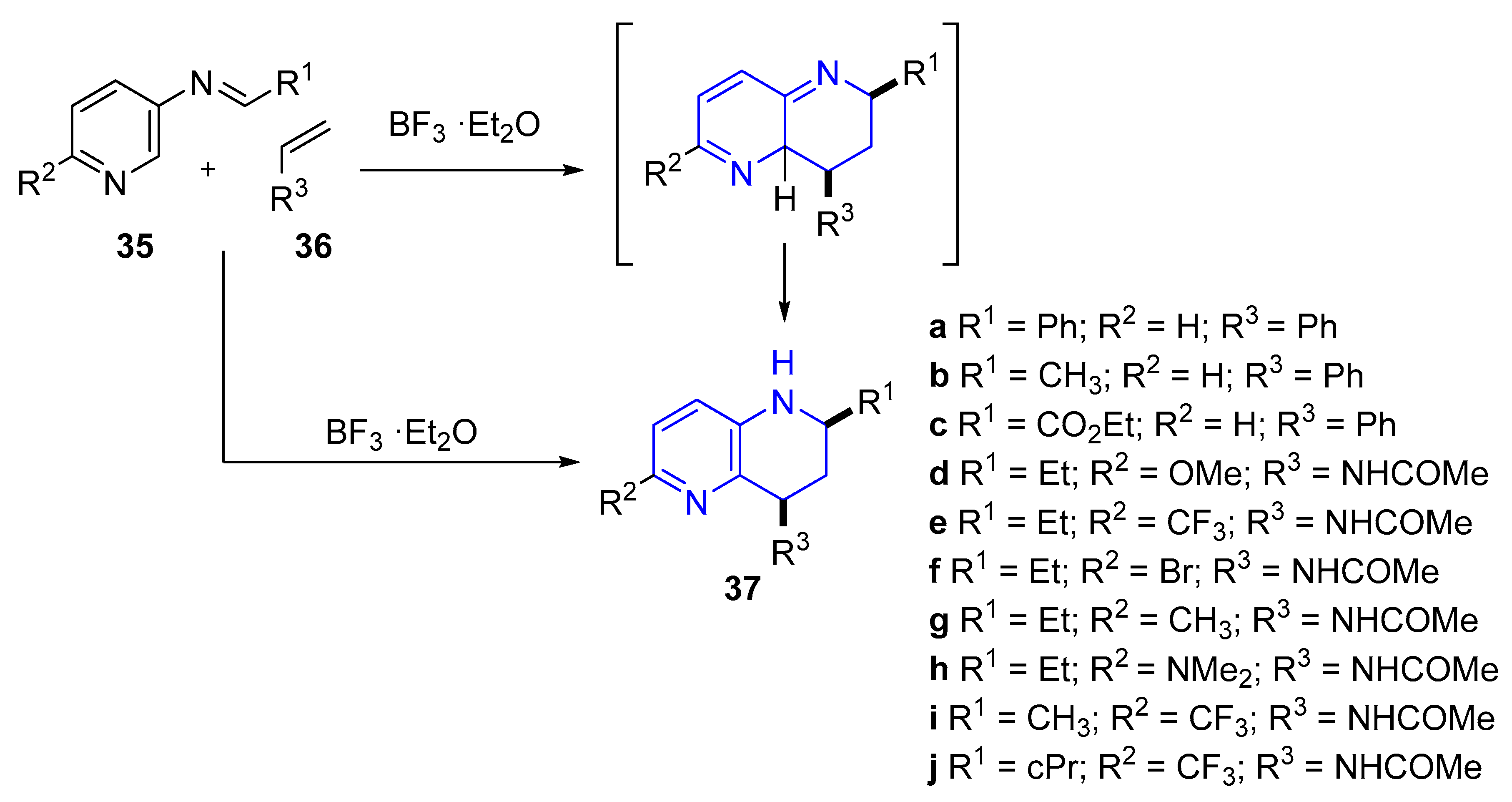
2.2. Synthesis of 1,5-Naphthyridines by Cycloaddition Reactions
3. Reactivity of 1,5-Naphthyridines
3.1. Reactions with Electrophilic Reagents
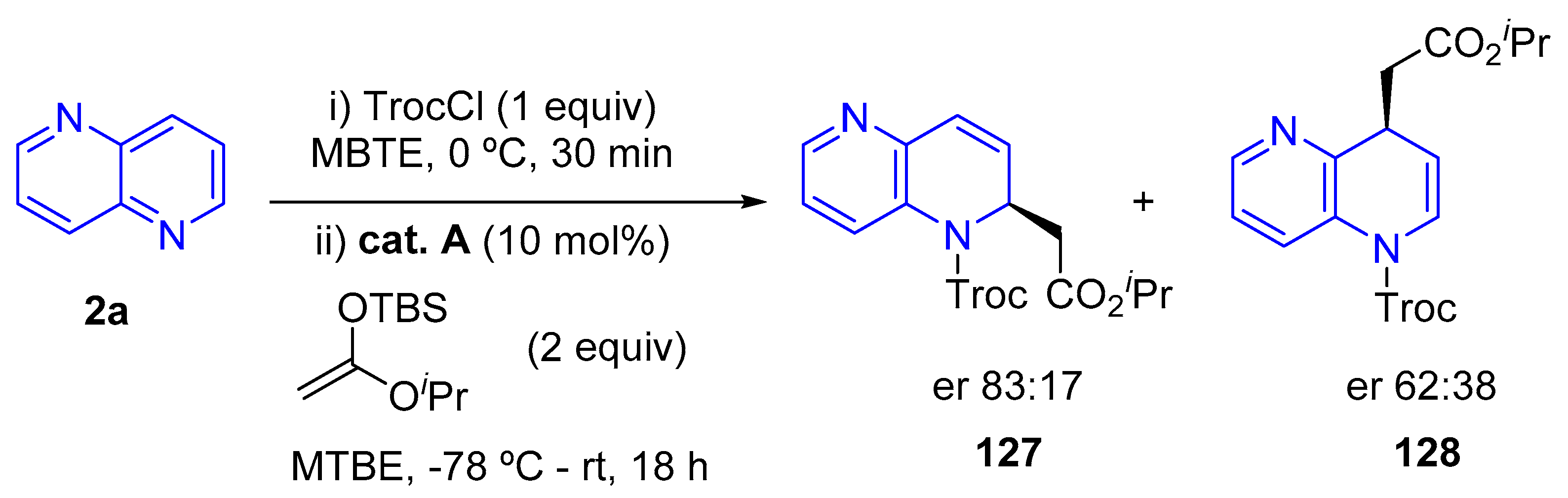
3.1.1. N-alkylation, N-acylation and N-tosylation
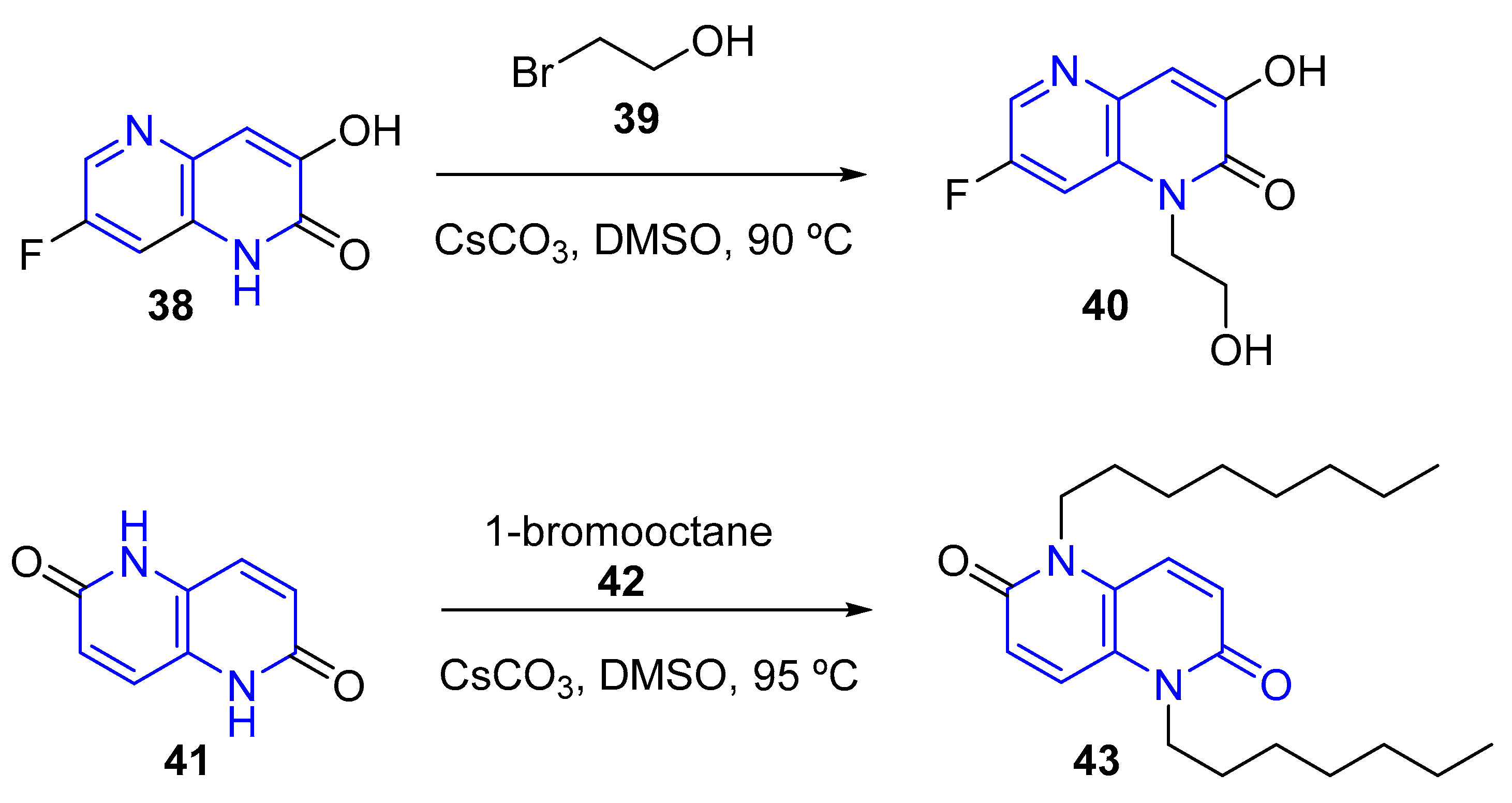
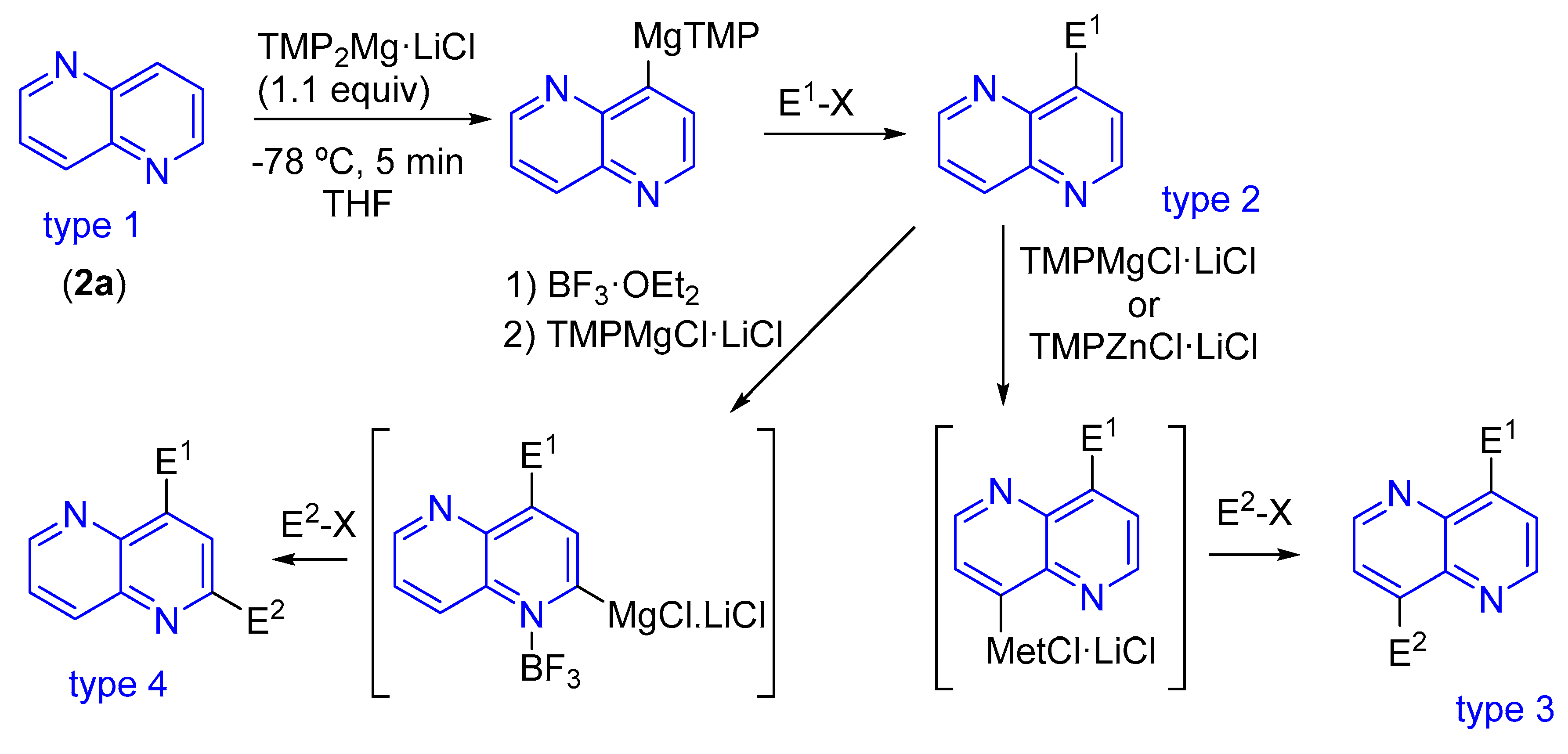
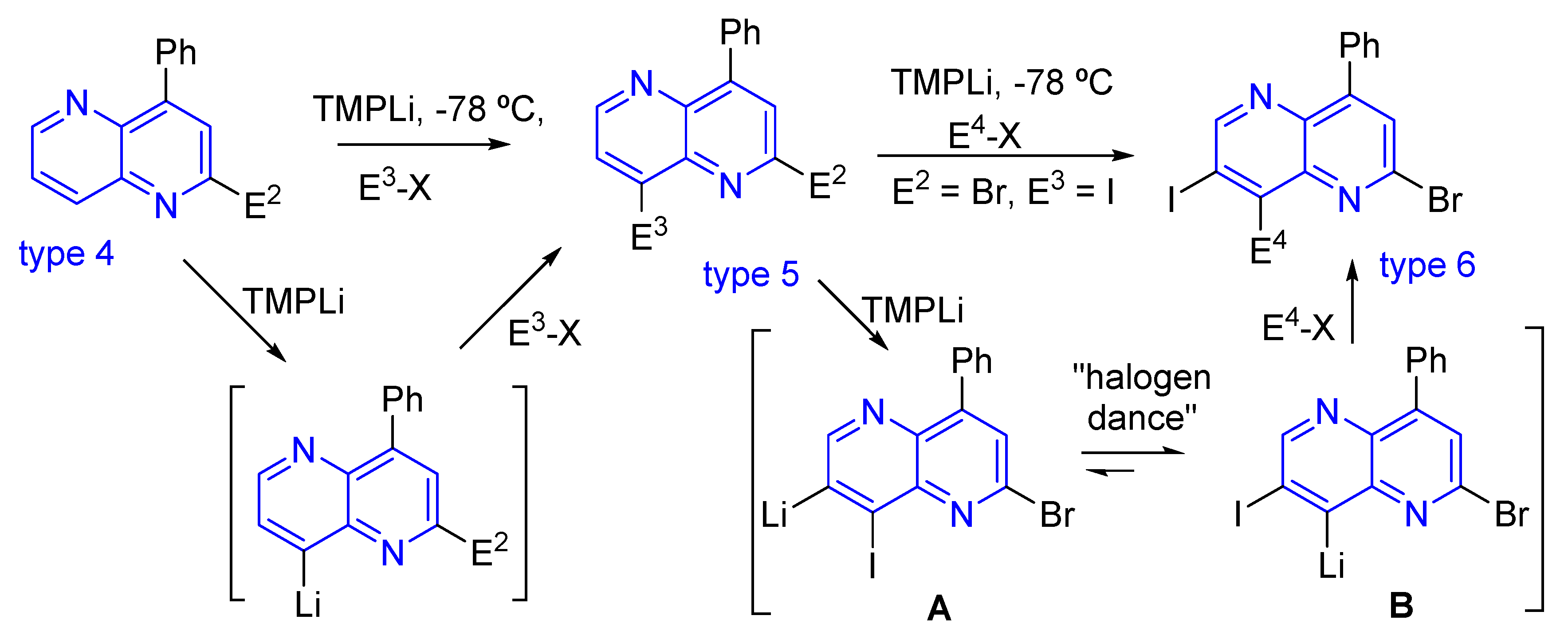
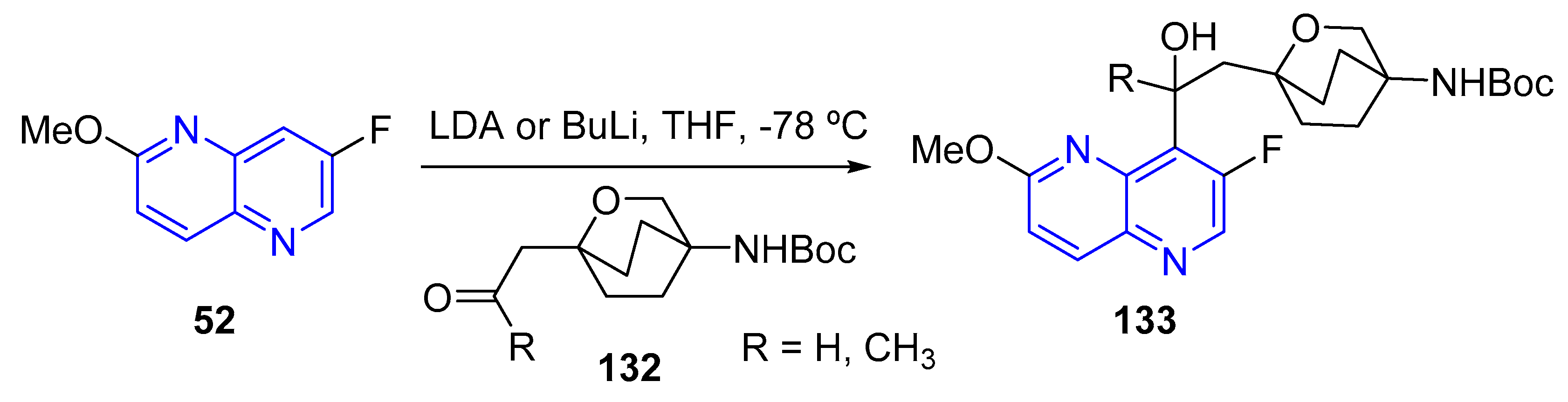
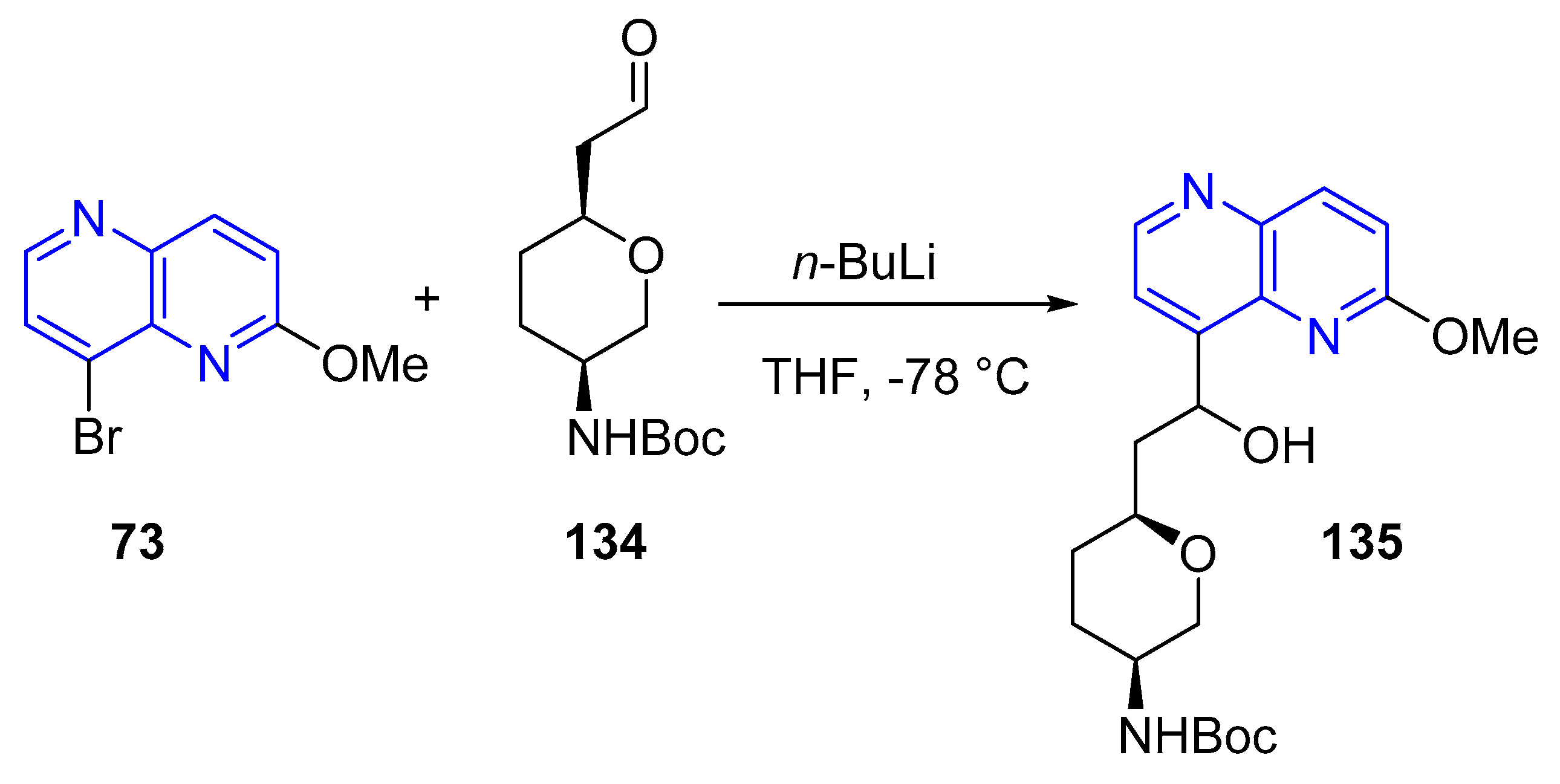
3.1.2. Halogen Substitution at Carbon
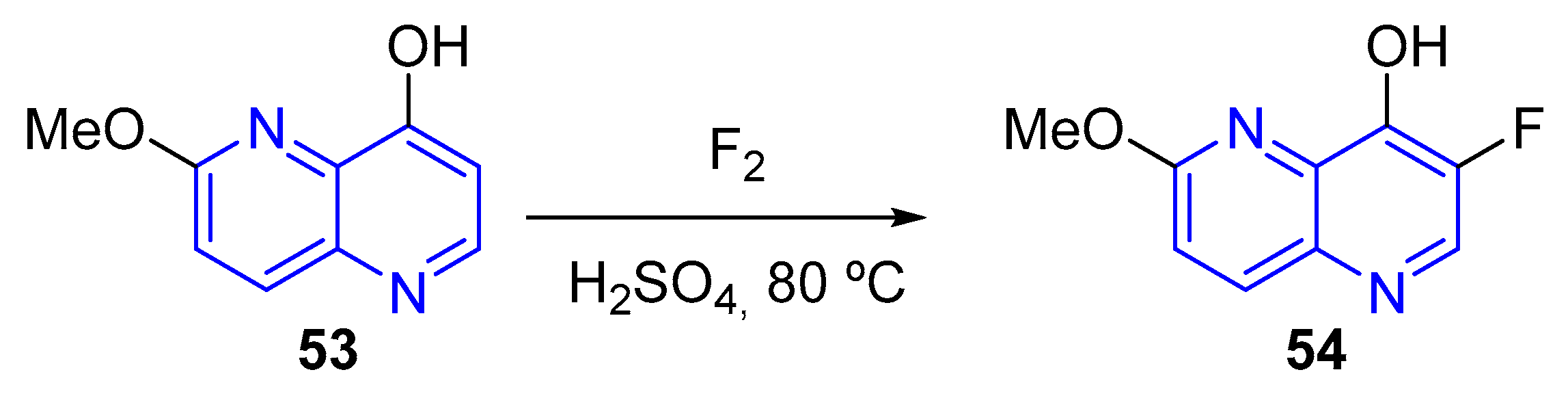
- (a) Fluorination
- (b) Chlorination
- (c) Bromination
3.2. Reactions with Nucleophilic Reagents
3.2.1. Perfluoroalkylation
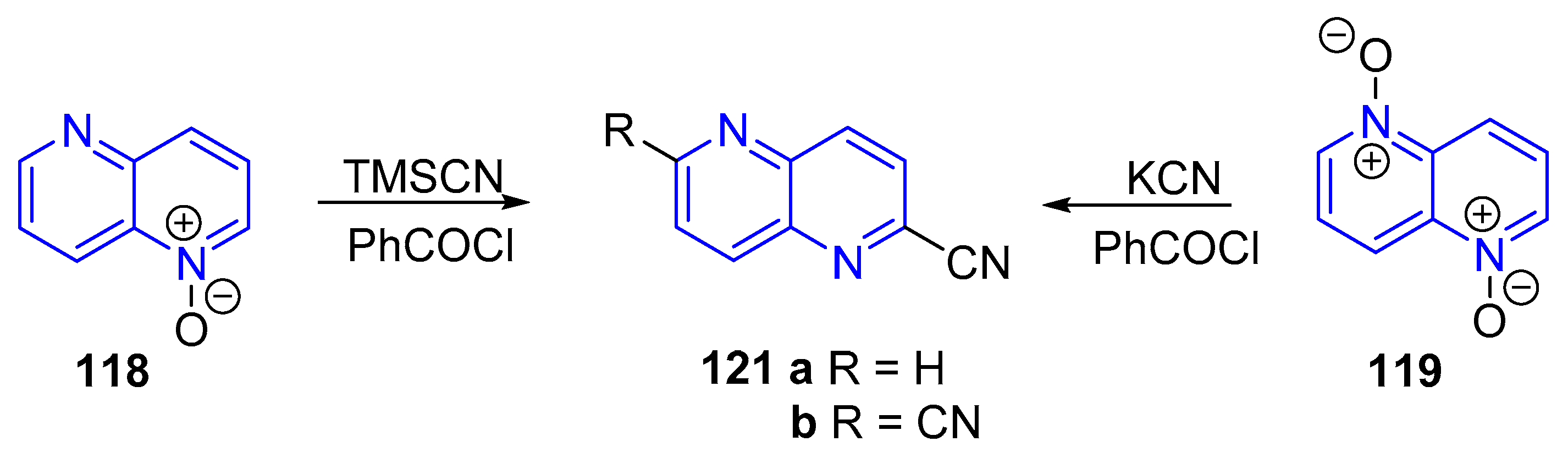
3.2.2. Cyanation
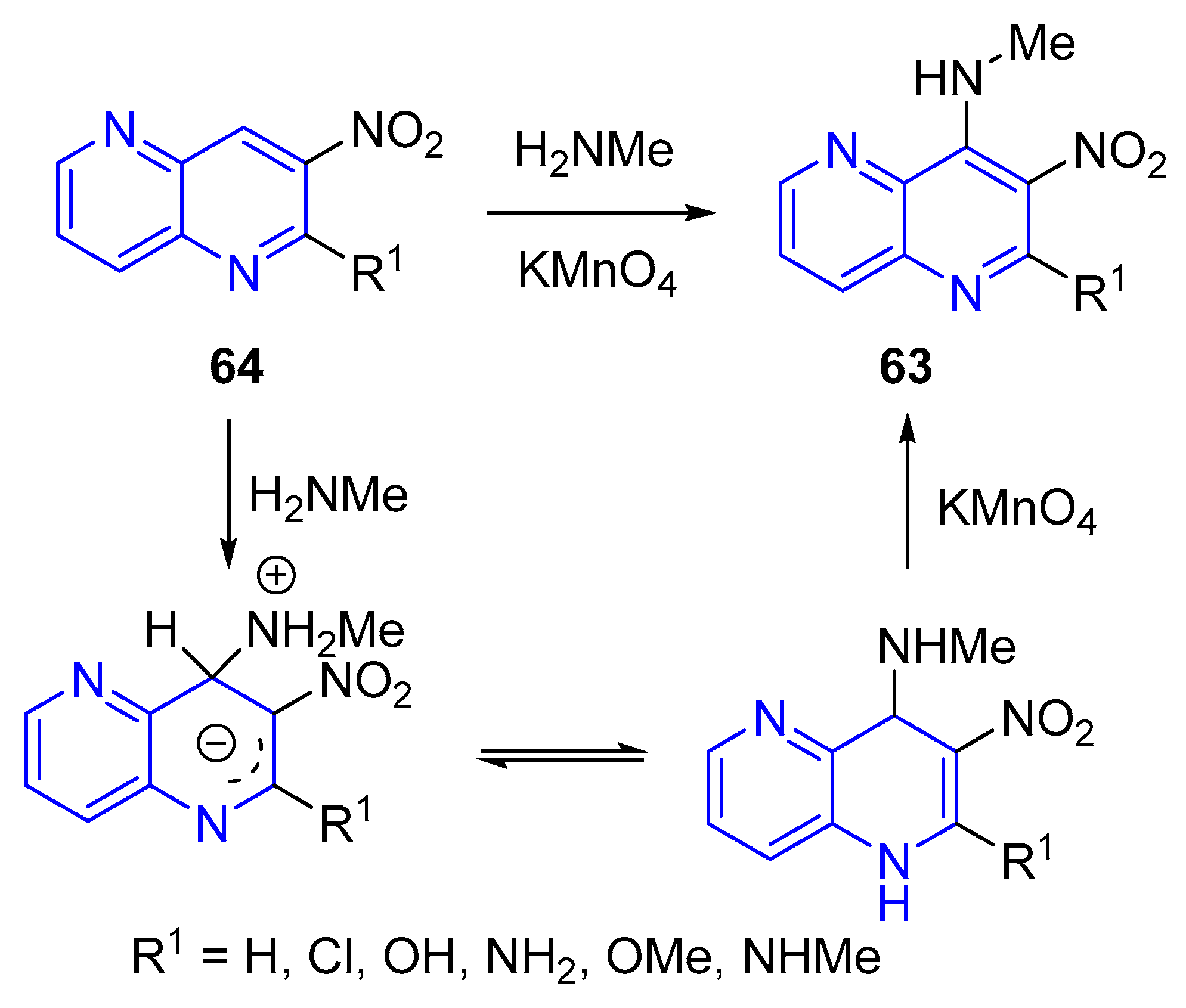
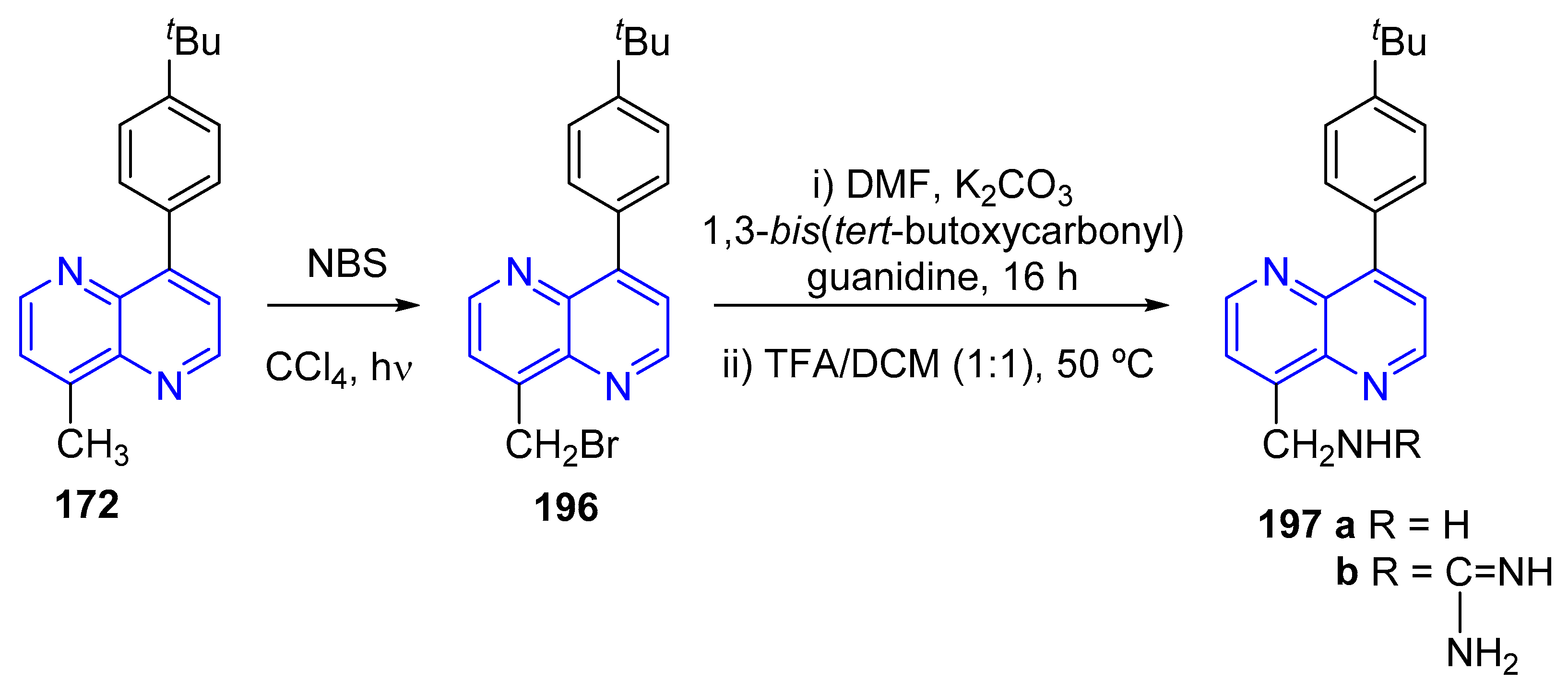
3.2.3. Direct Amination
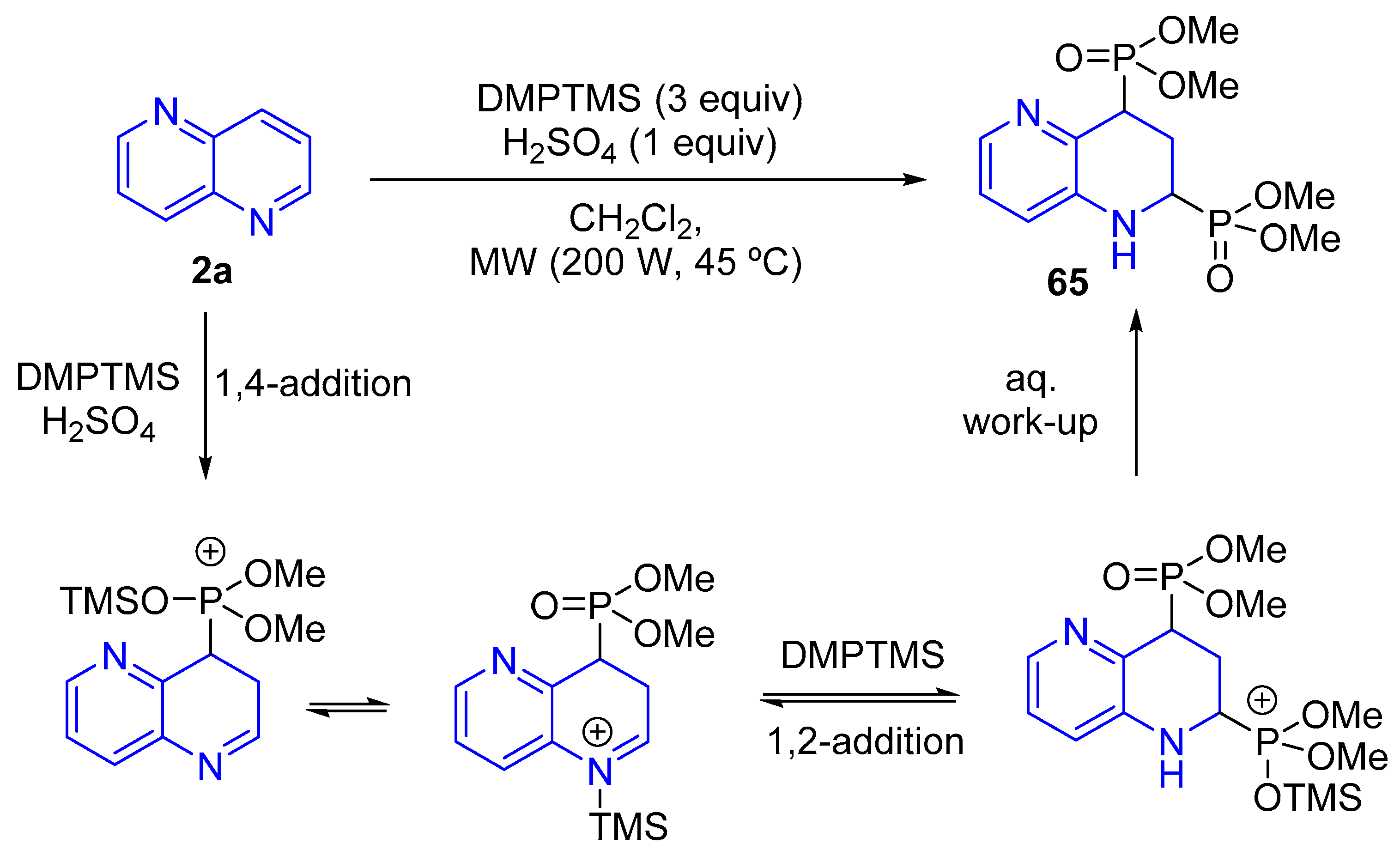
3.2.4. Phosphorylation
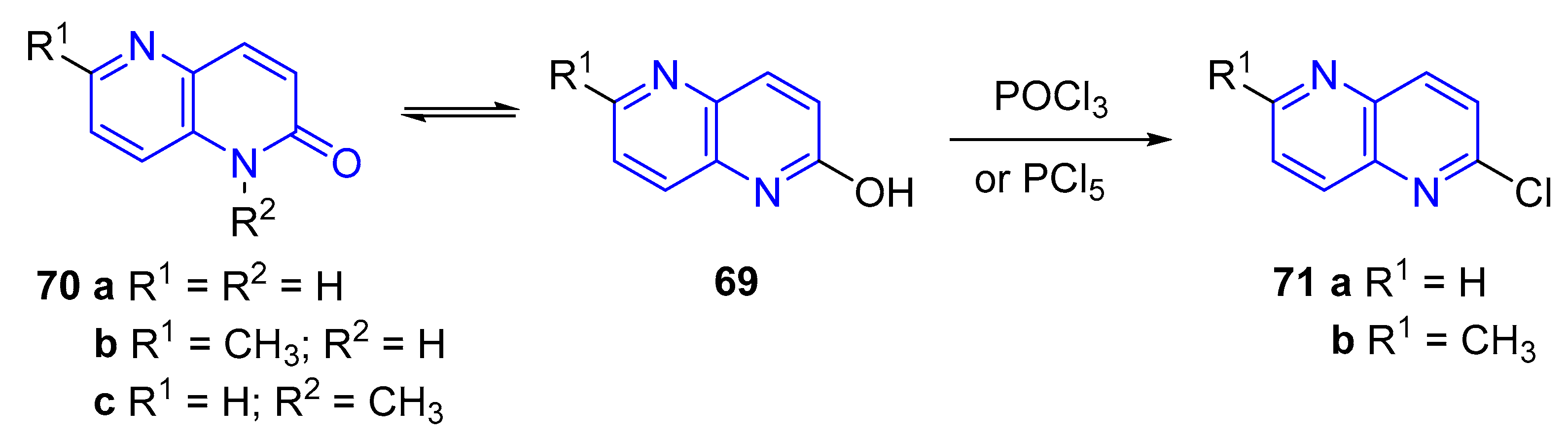
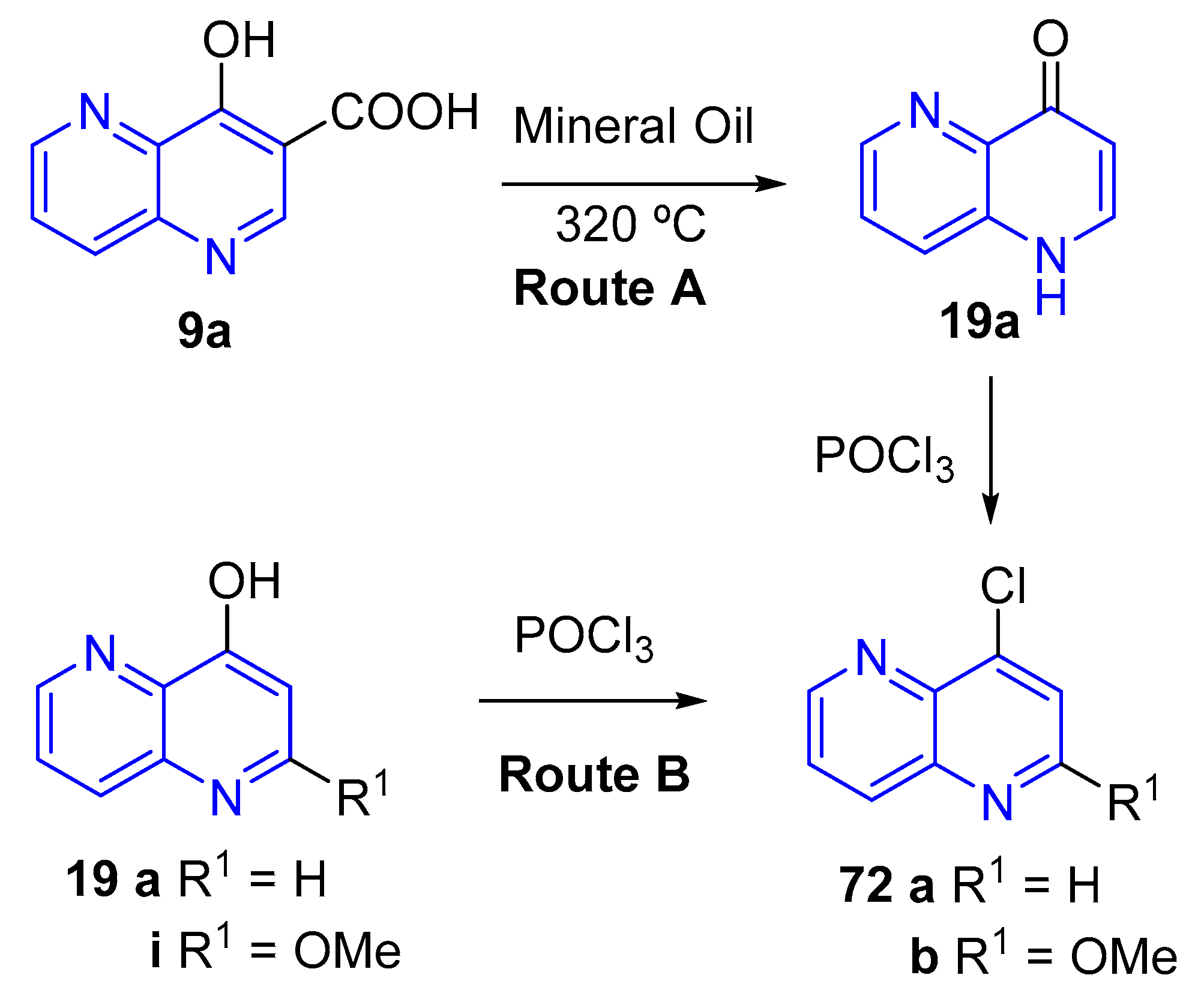
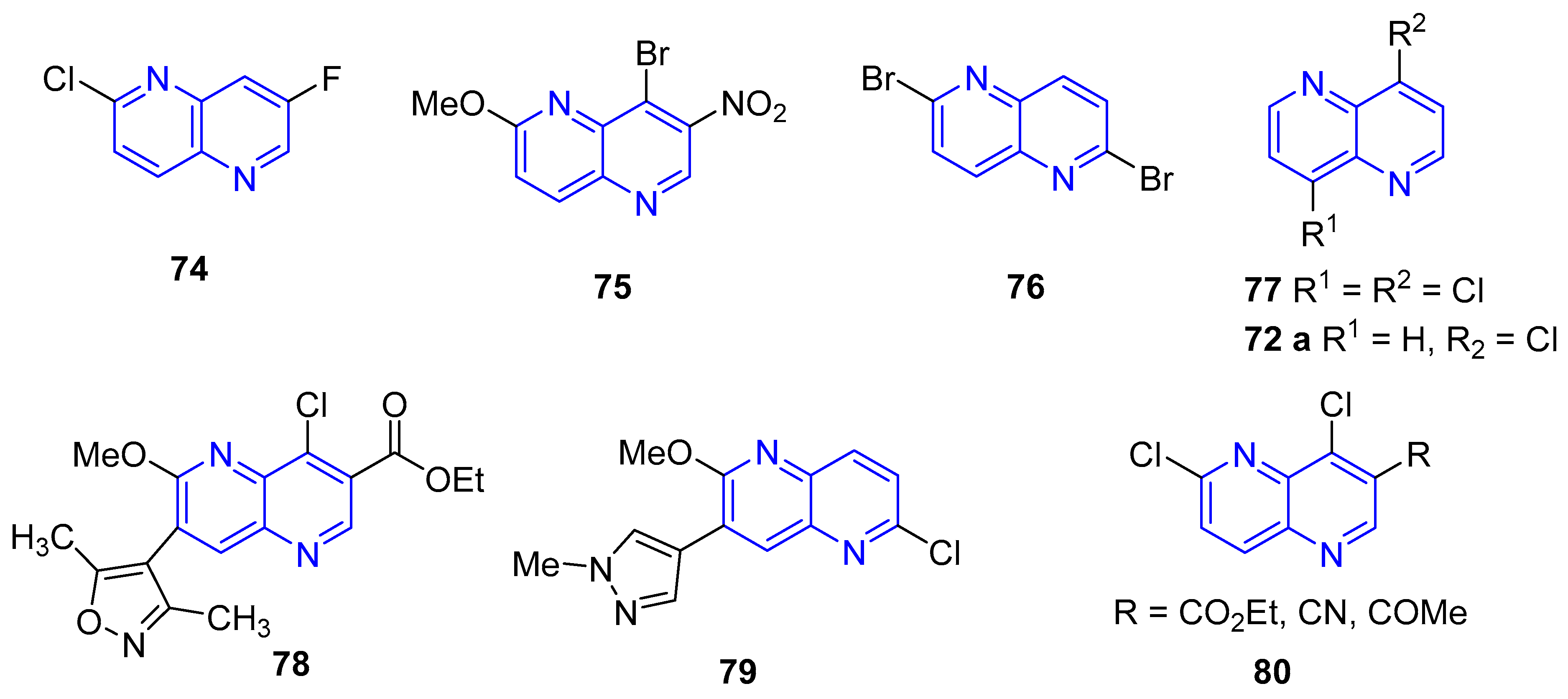
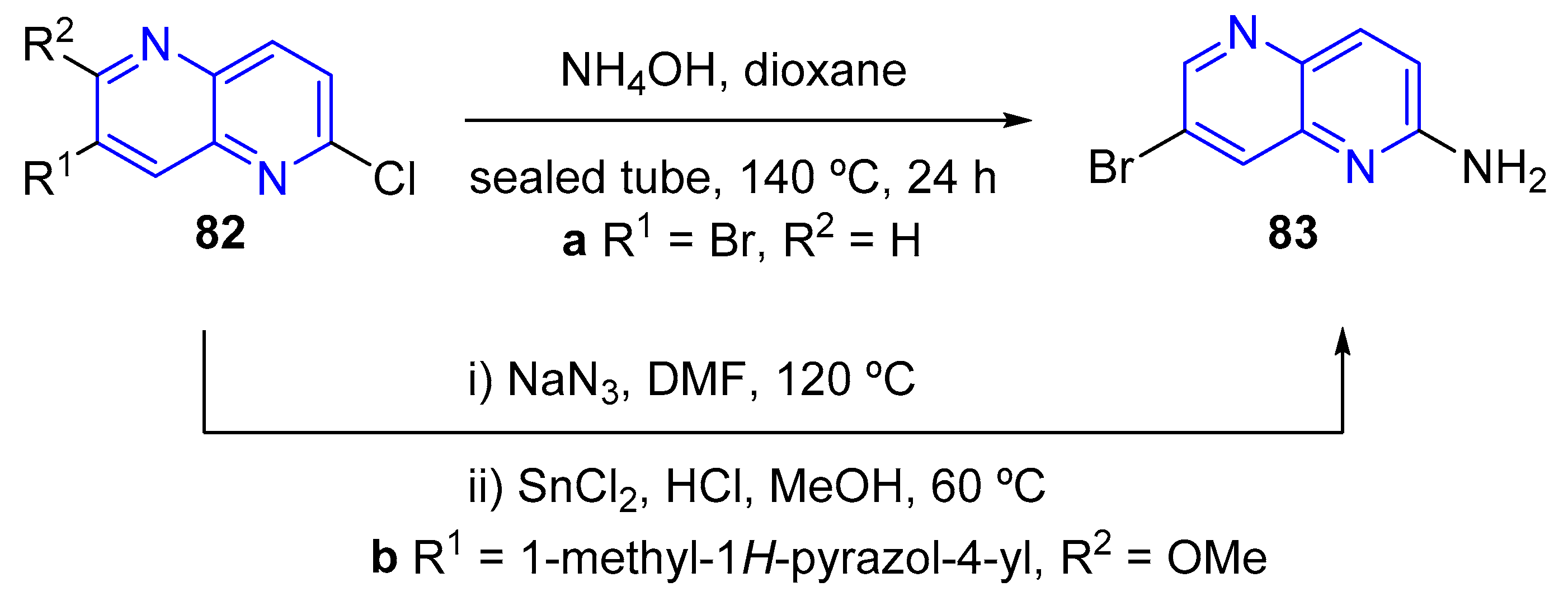
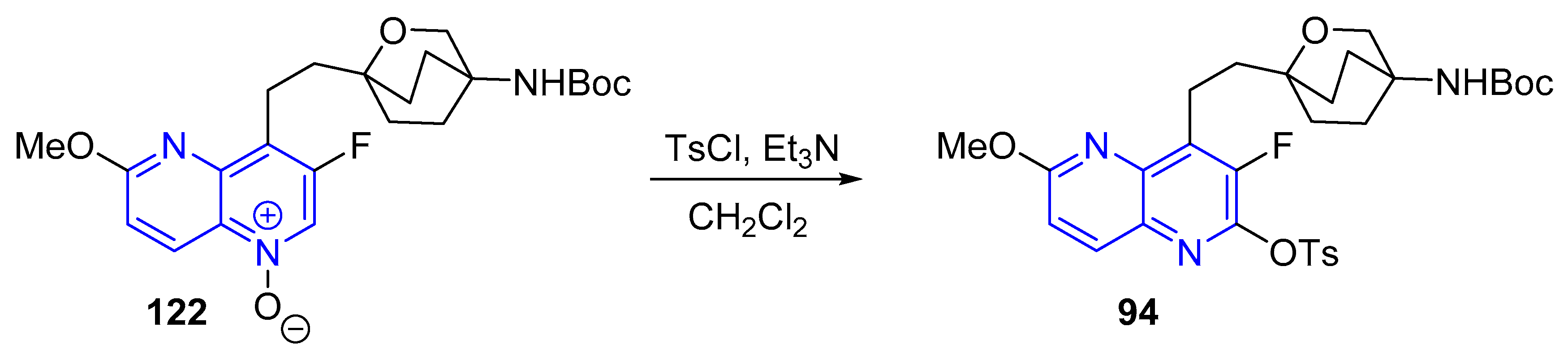
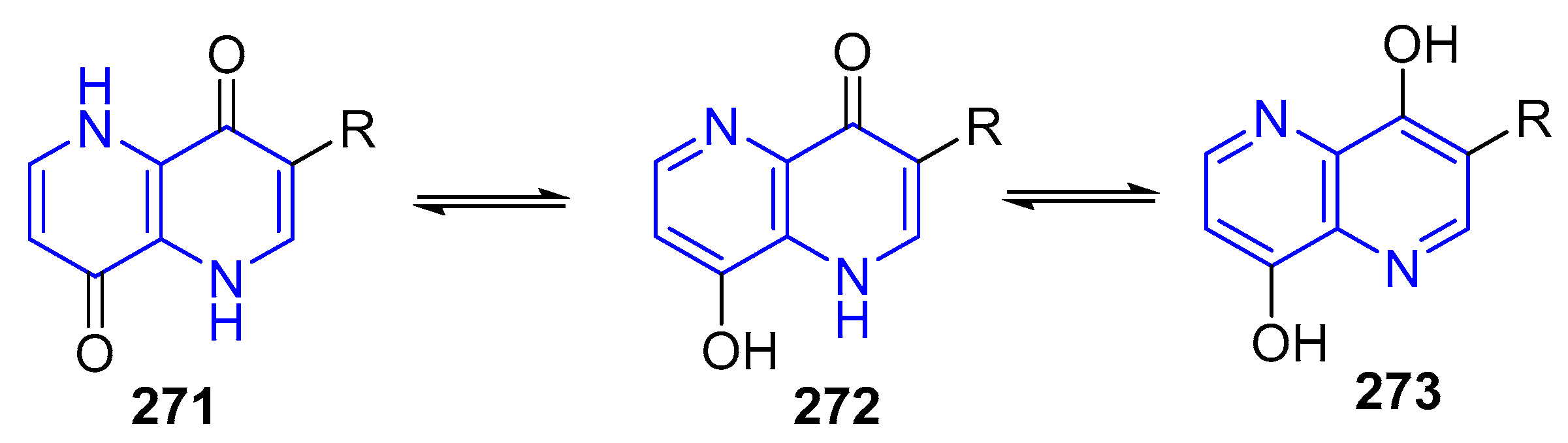
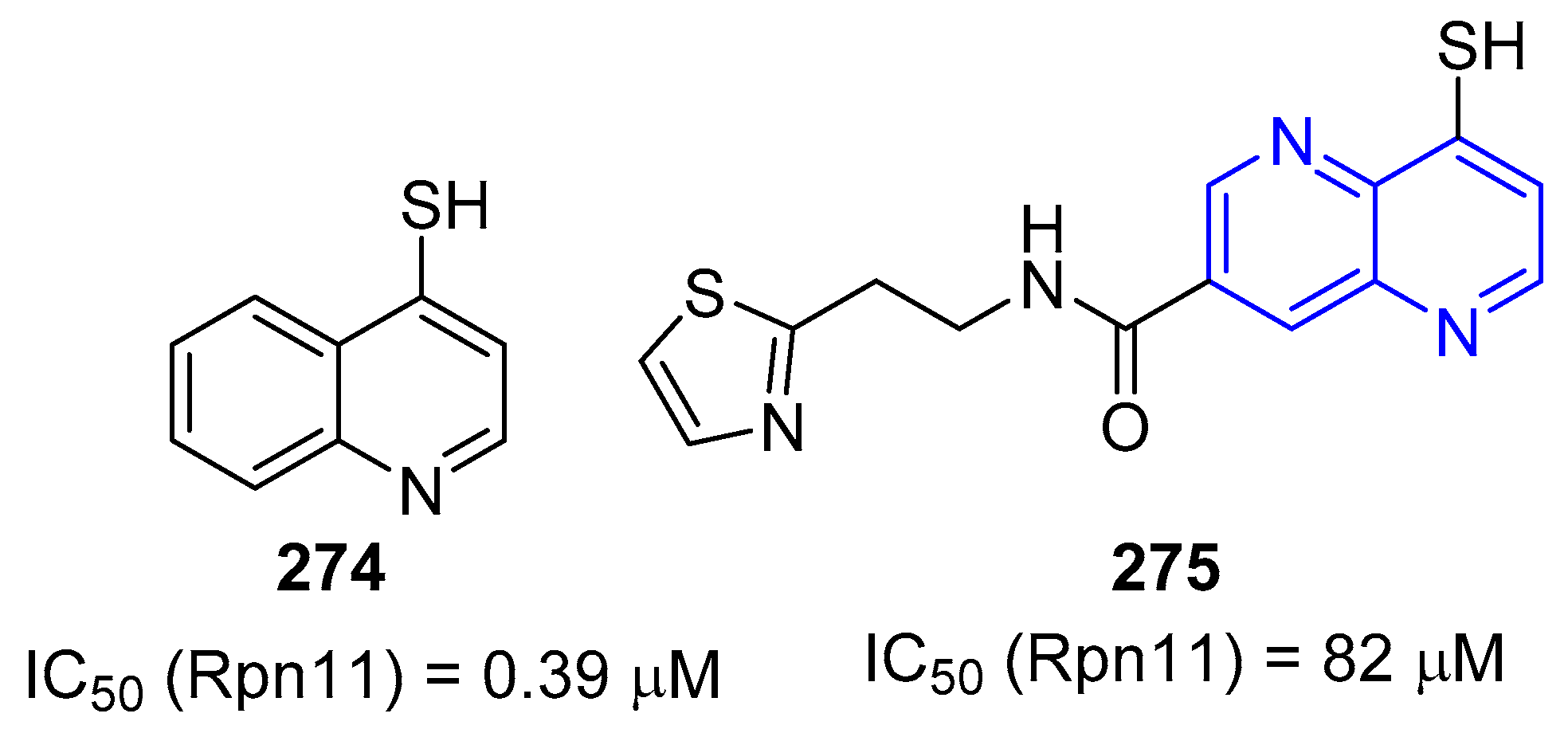
3.2.5. Halogenation of hydroxy-1,5-naphthyridine and/or 1,5-naphthyridinones
3.2.6. Nucleophilic Substitution with Displacement of Good Leaving Groups
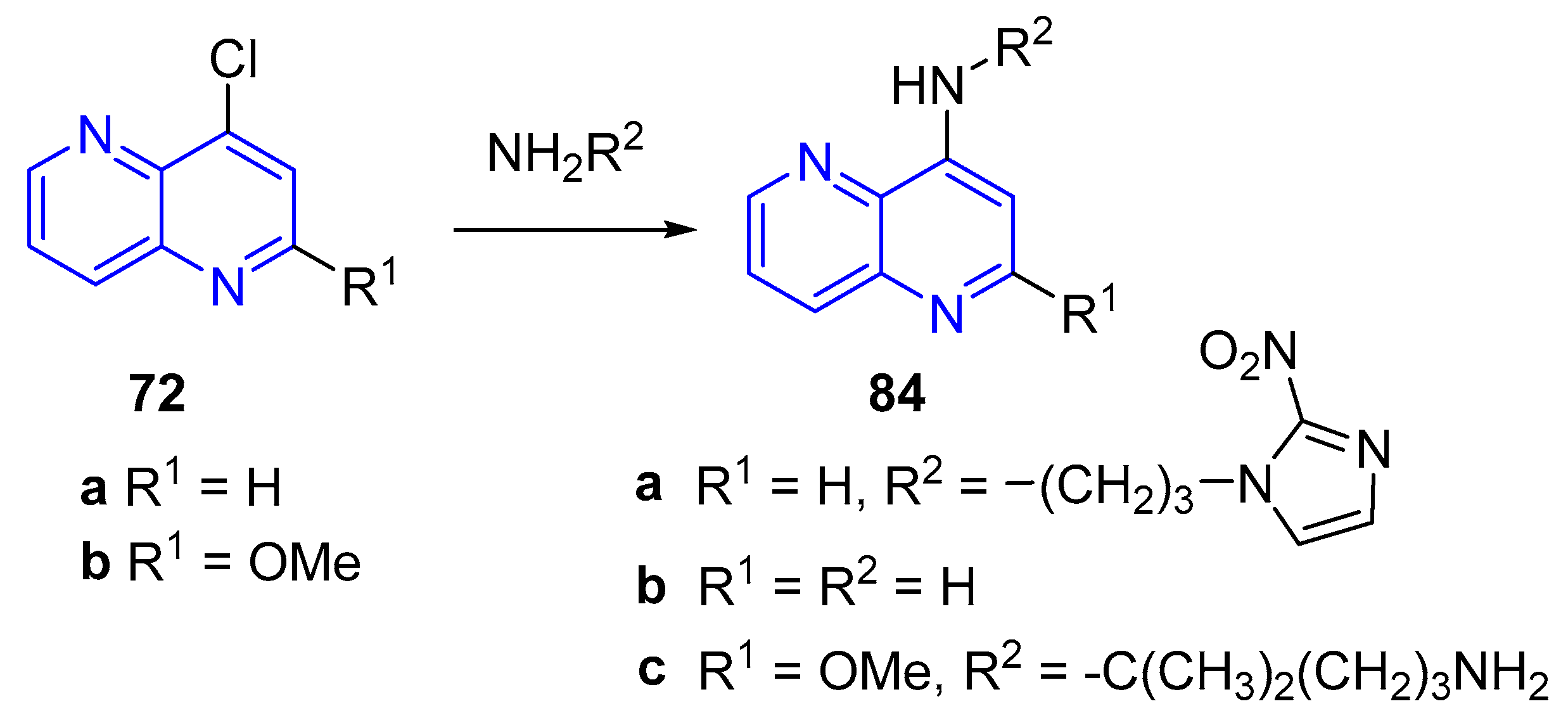
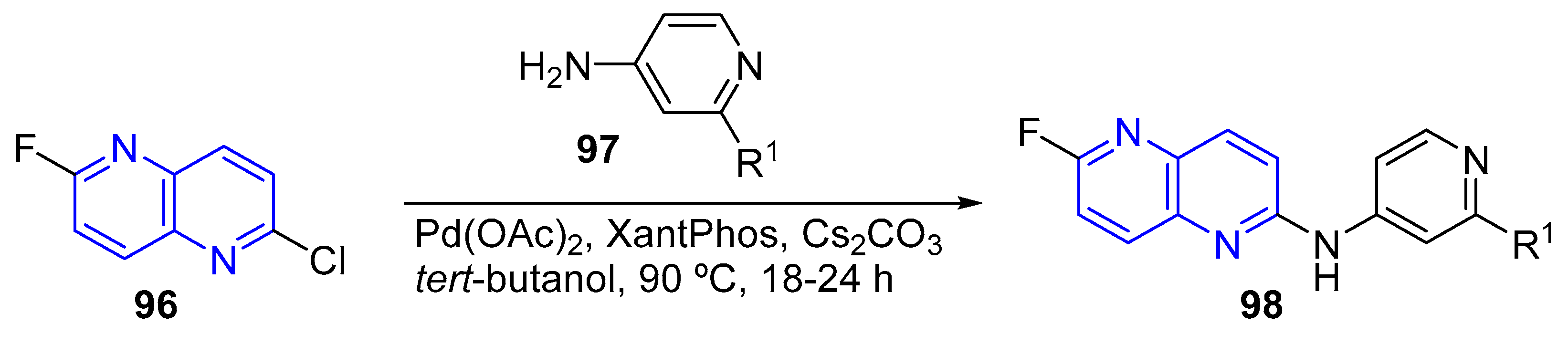
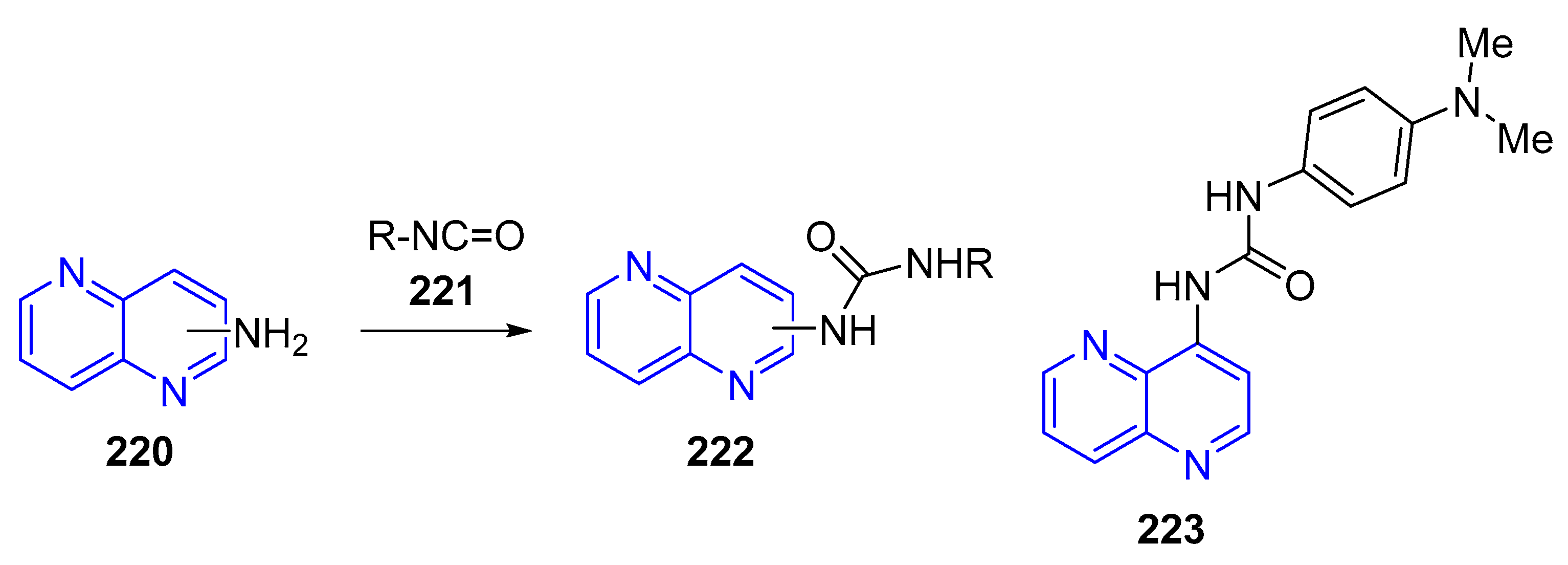
- (a) Amination
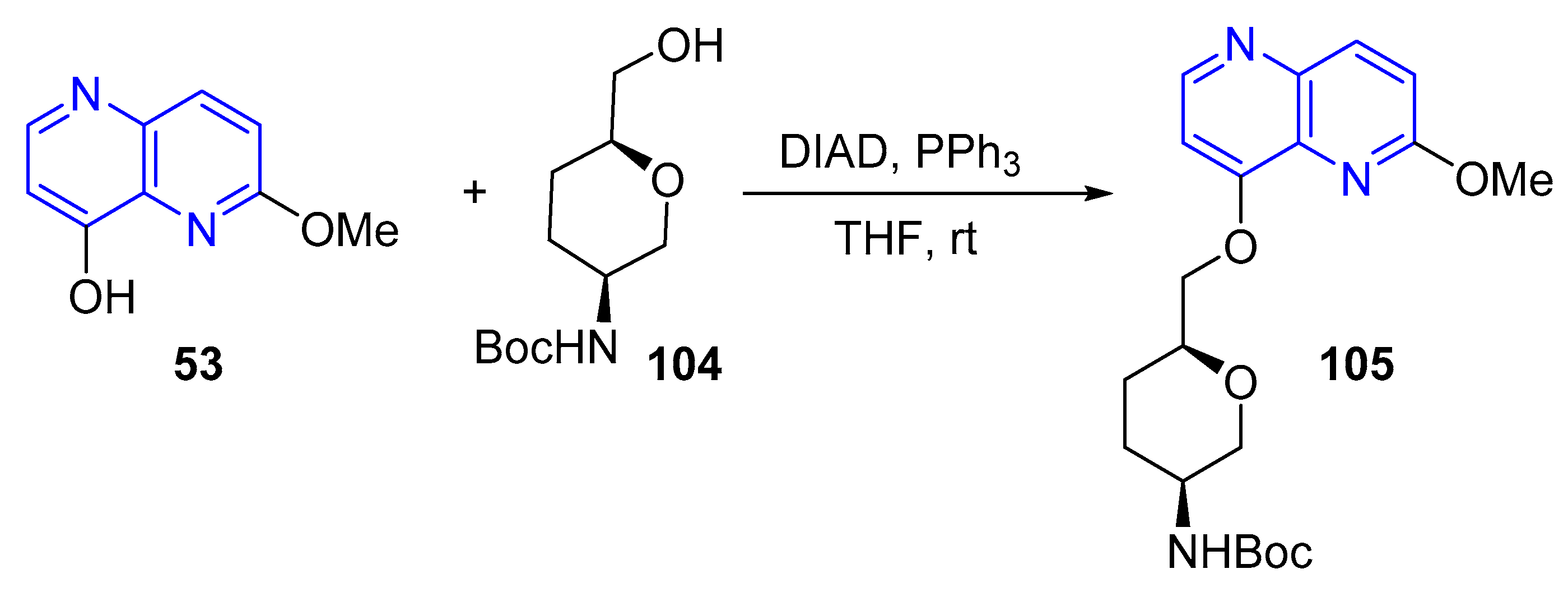
- (b) Alcoxylation, Phenoxylation
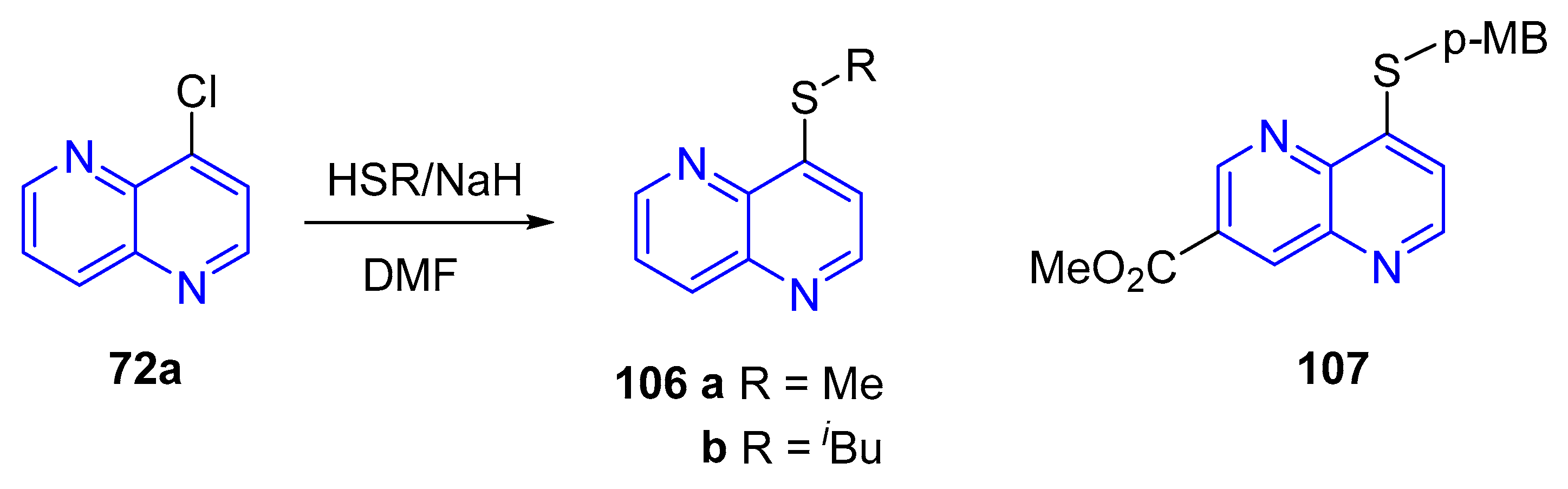
- (c) Sulfurization
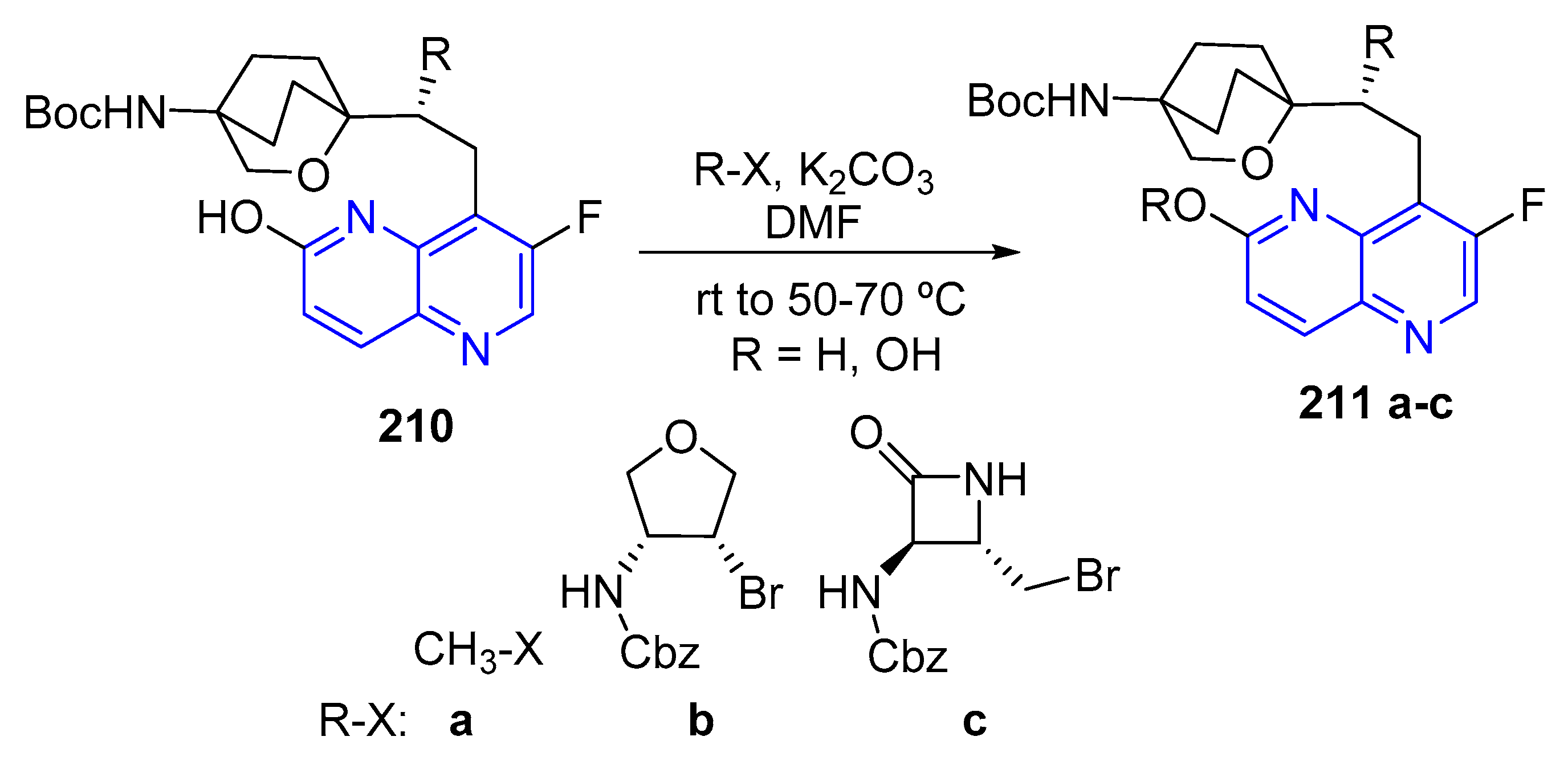
- (d) Alkylation
3.3. Oxidations and Reductions
3.3.1. Oxidation
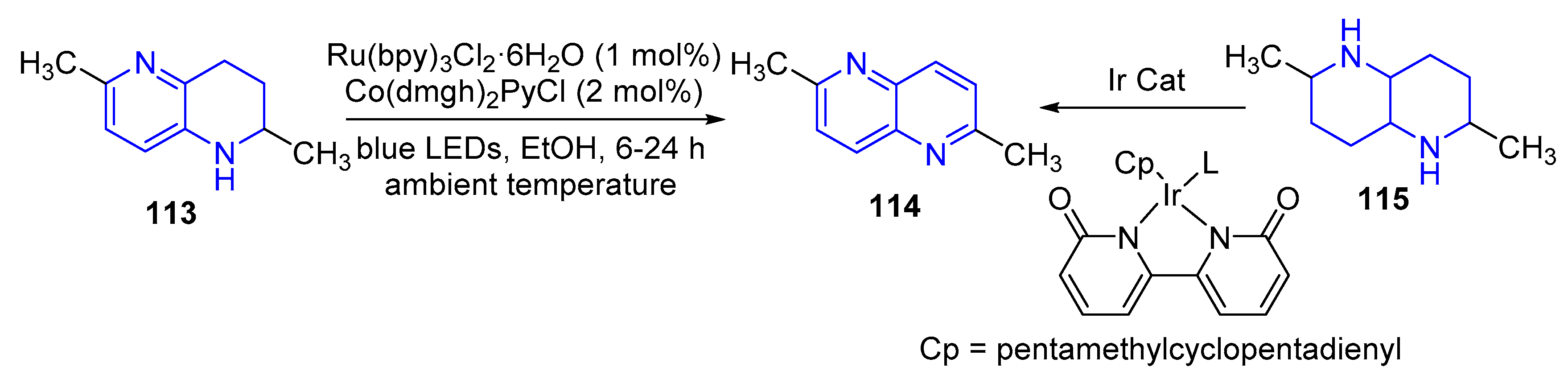
- (a) Aromatization of tetrahydro-1,5-naphthyridines
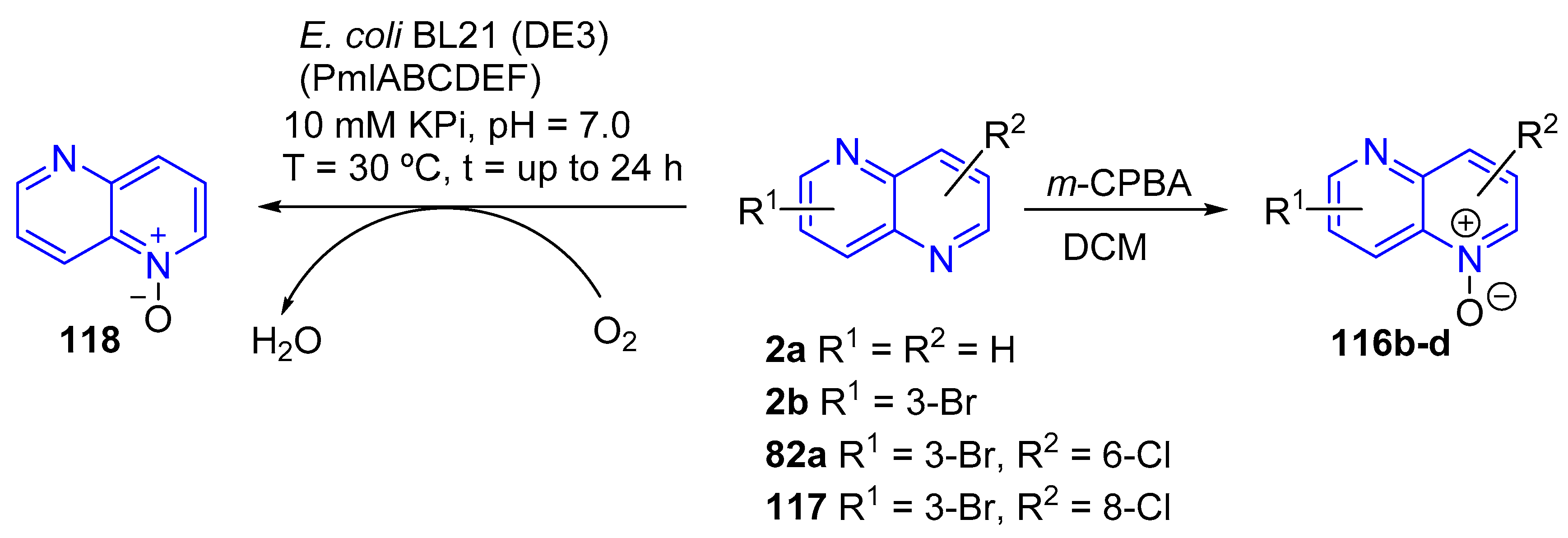
- (b) Preparation of 1,5-Naphthyridine N-oxides and Their Synthetic Application
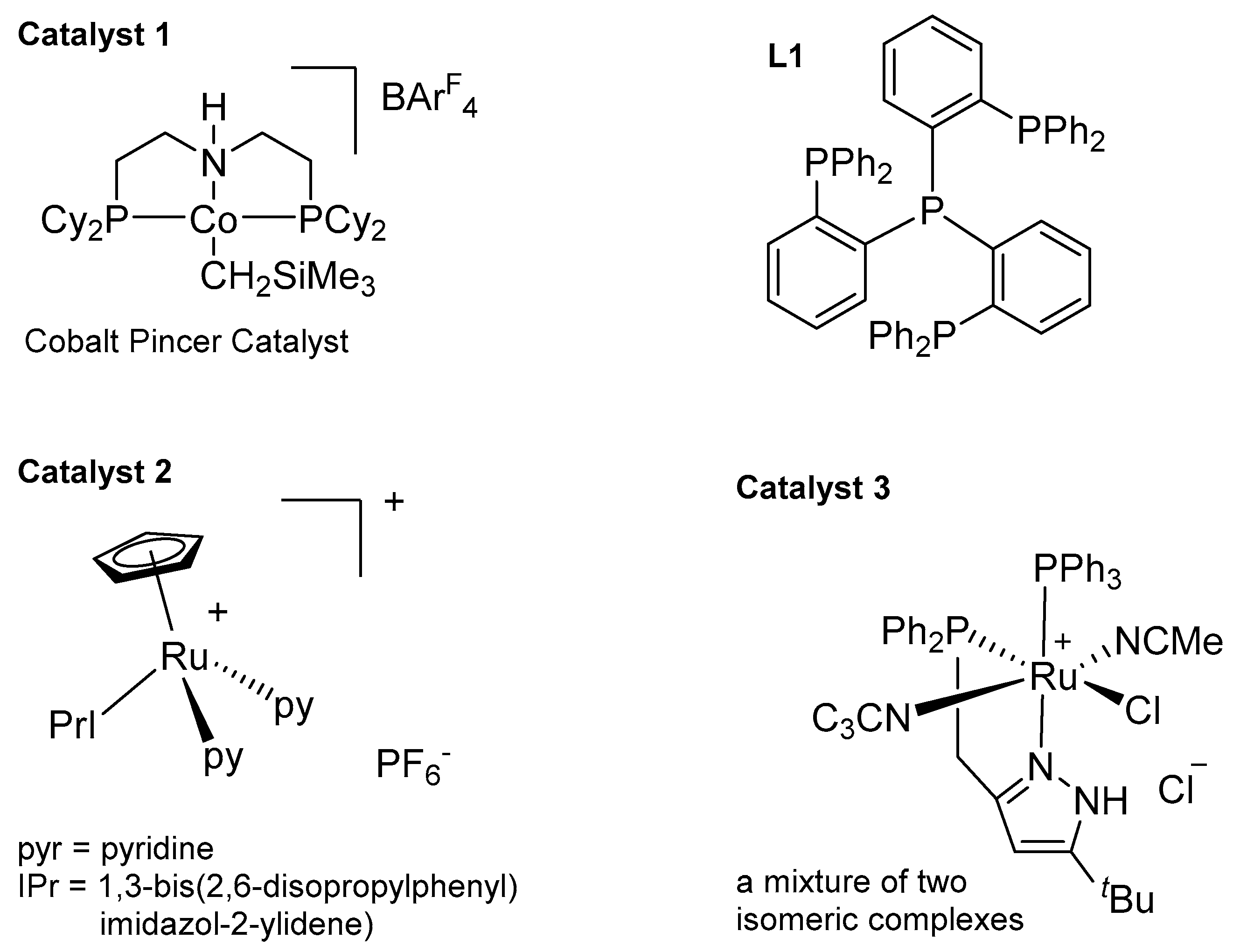
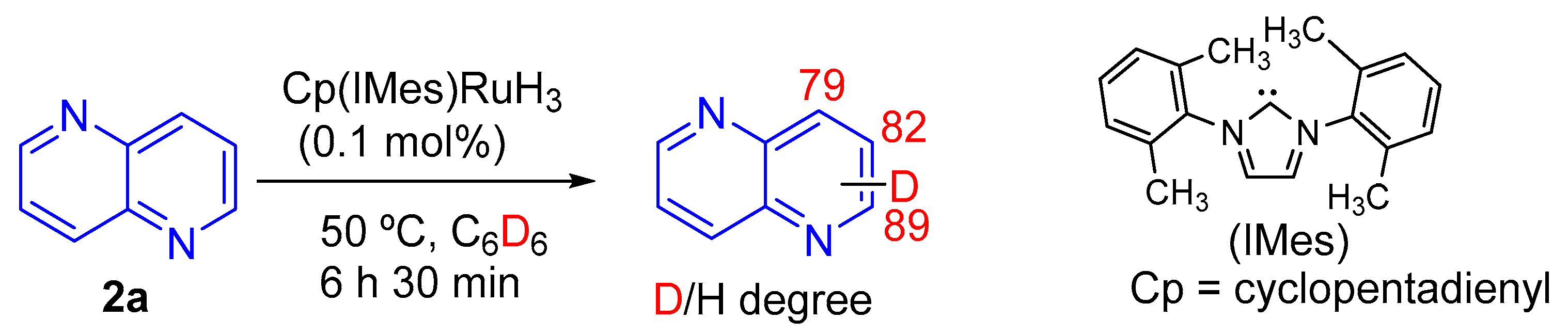
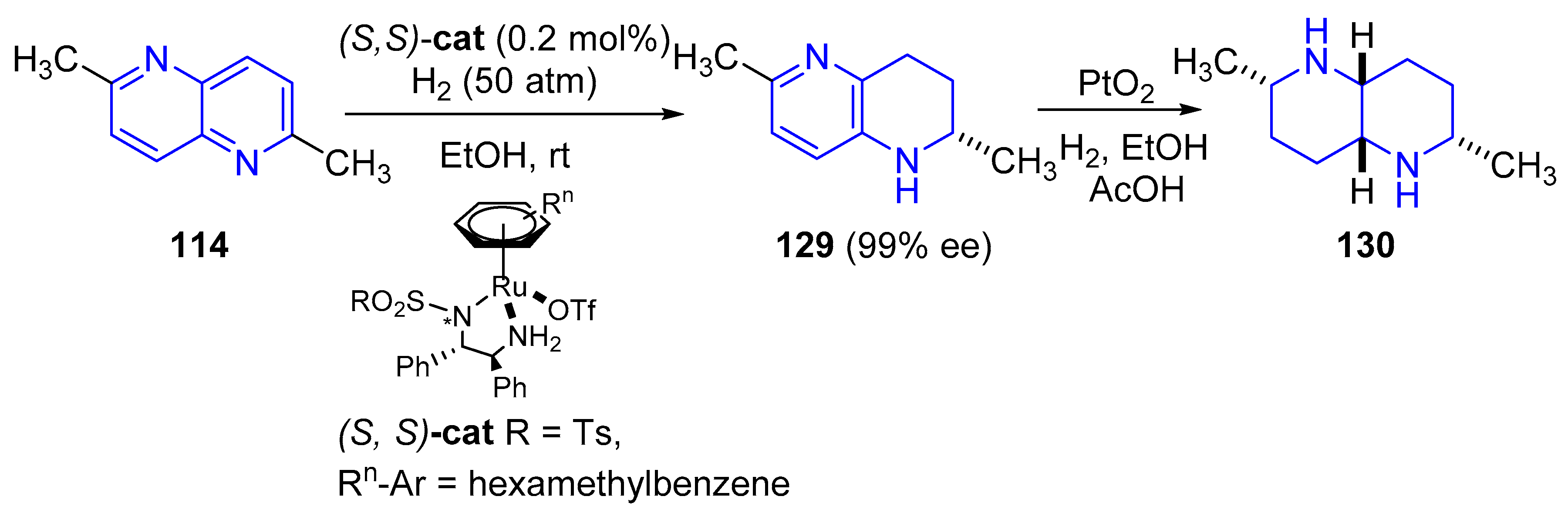
3.3.2. Reduction
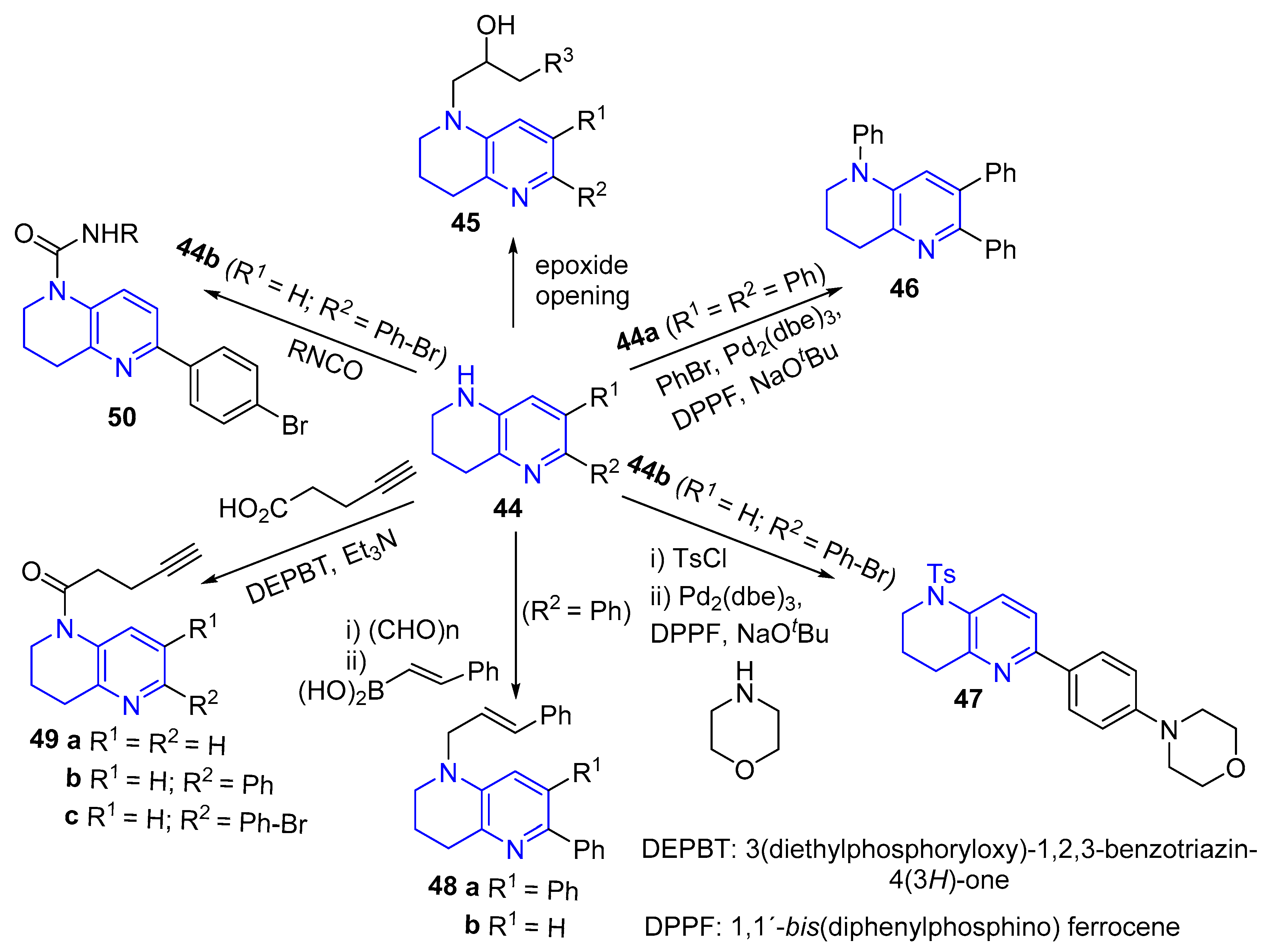
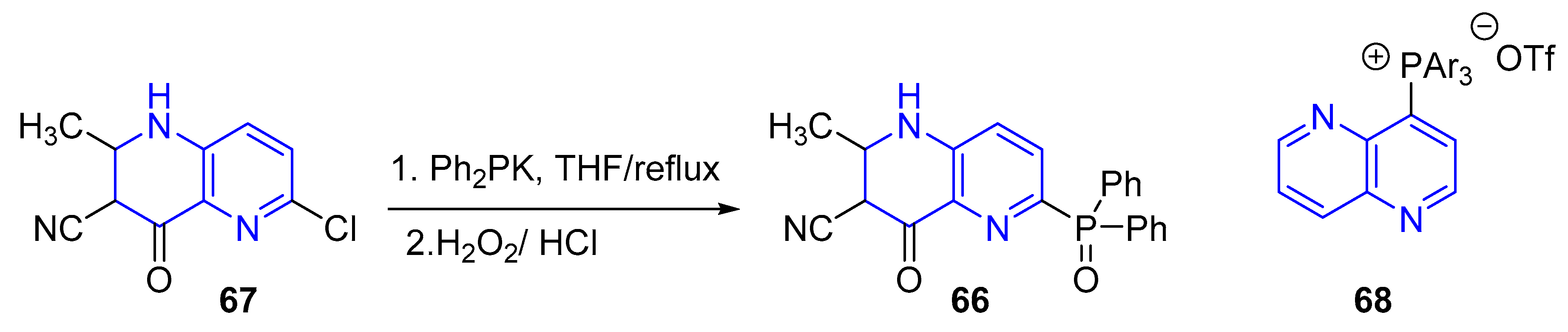
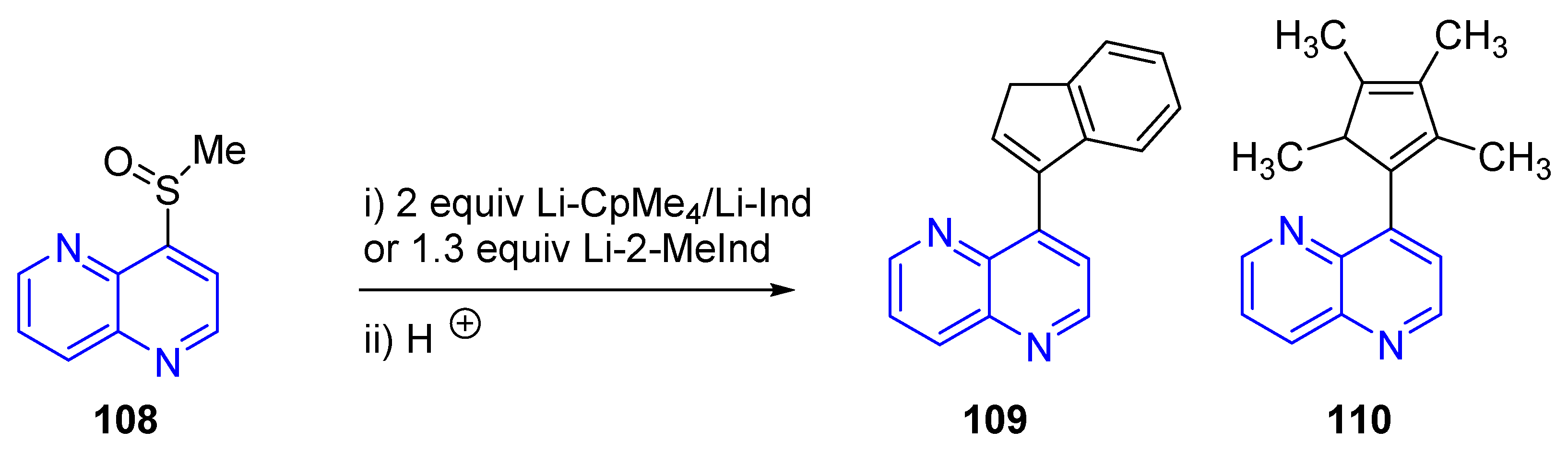
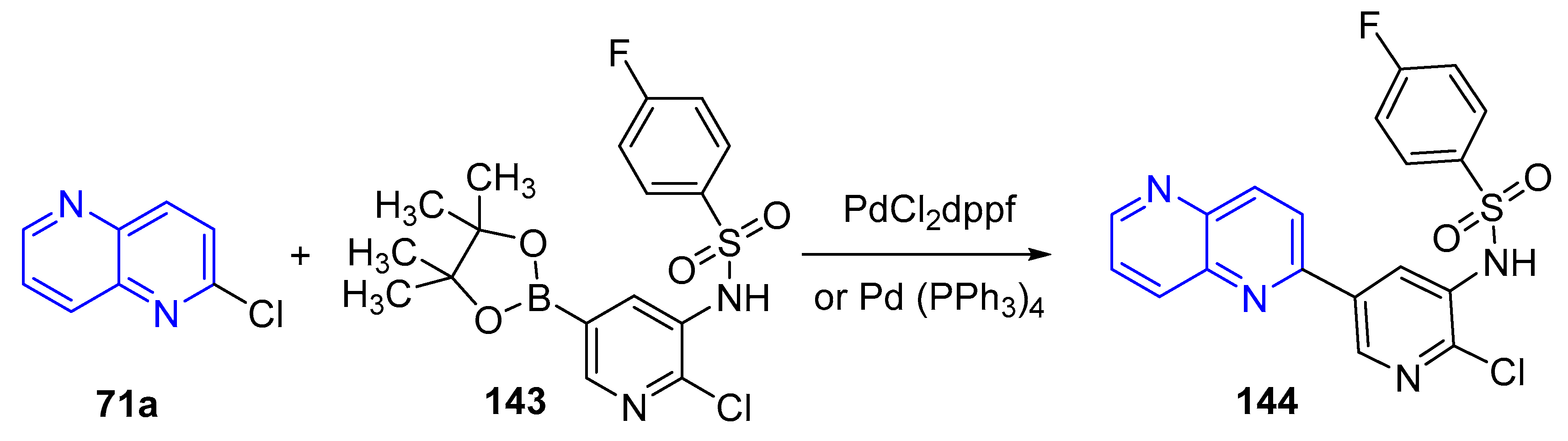
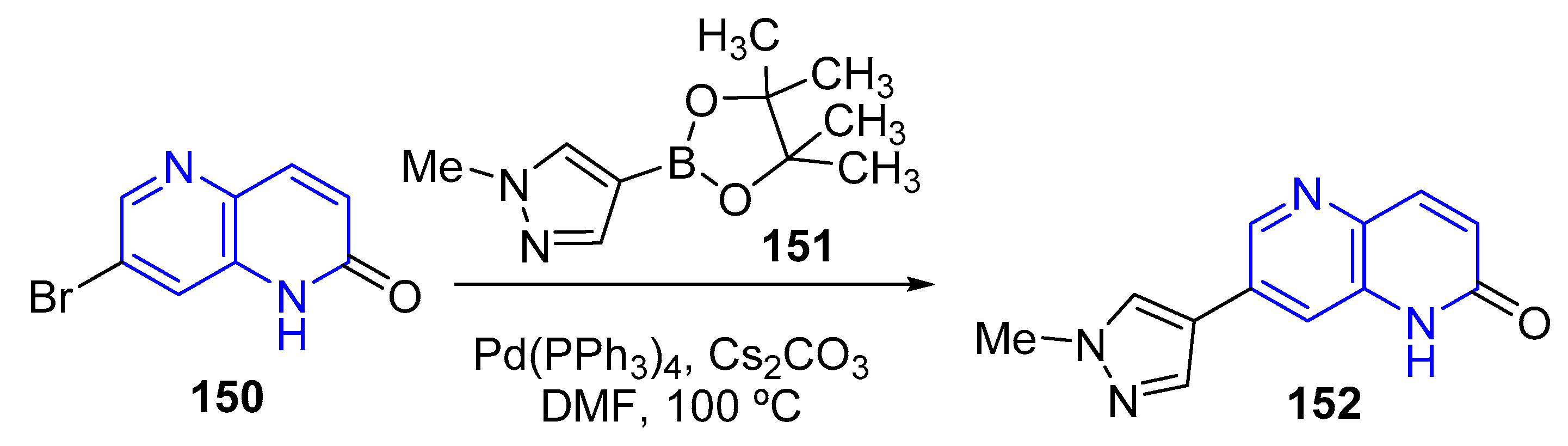
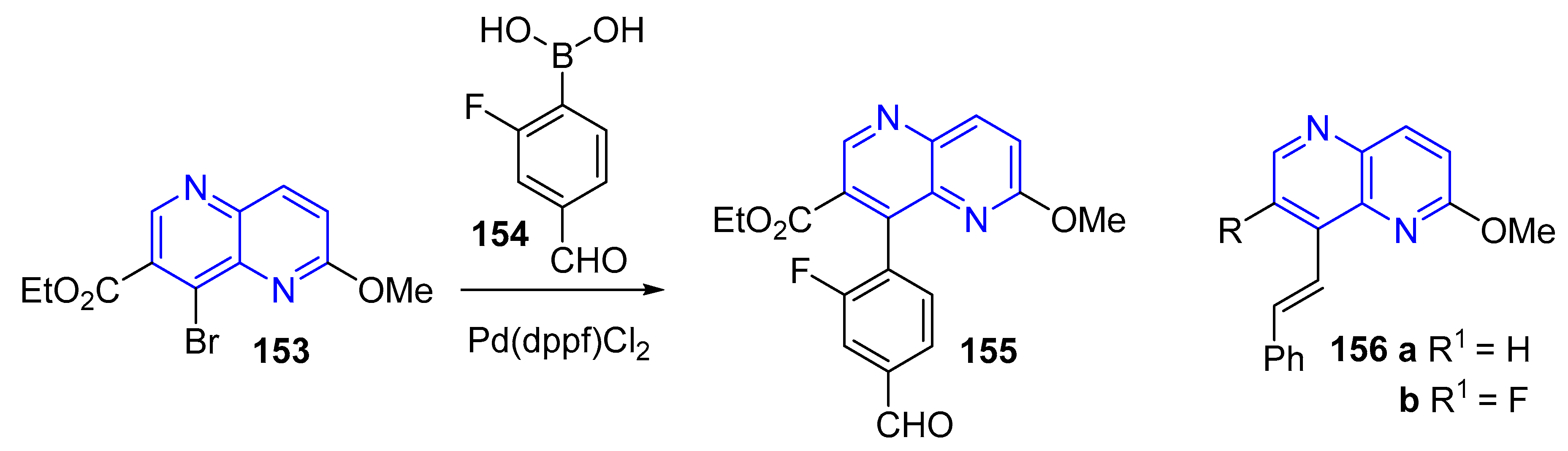
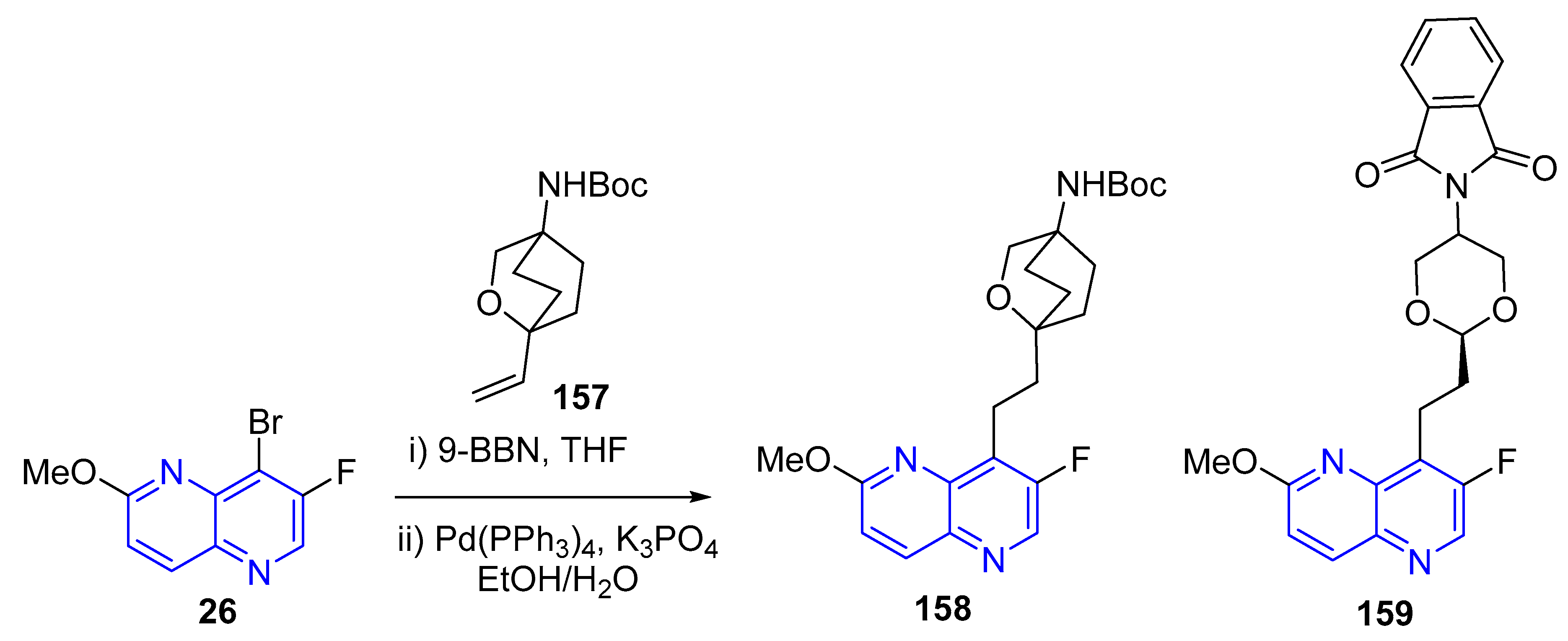
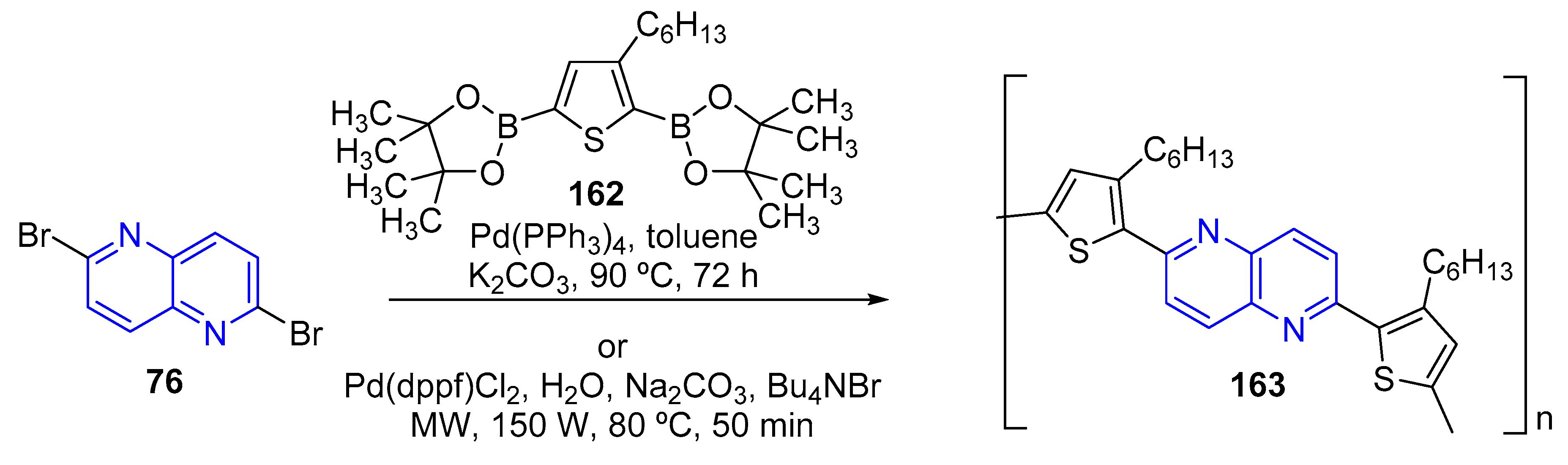
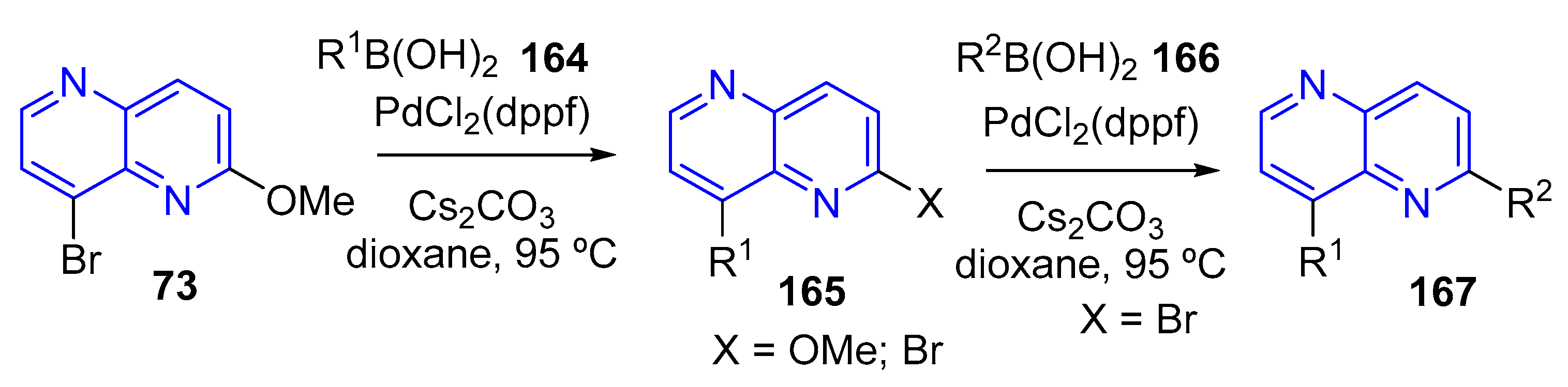
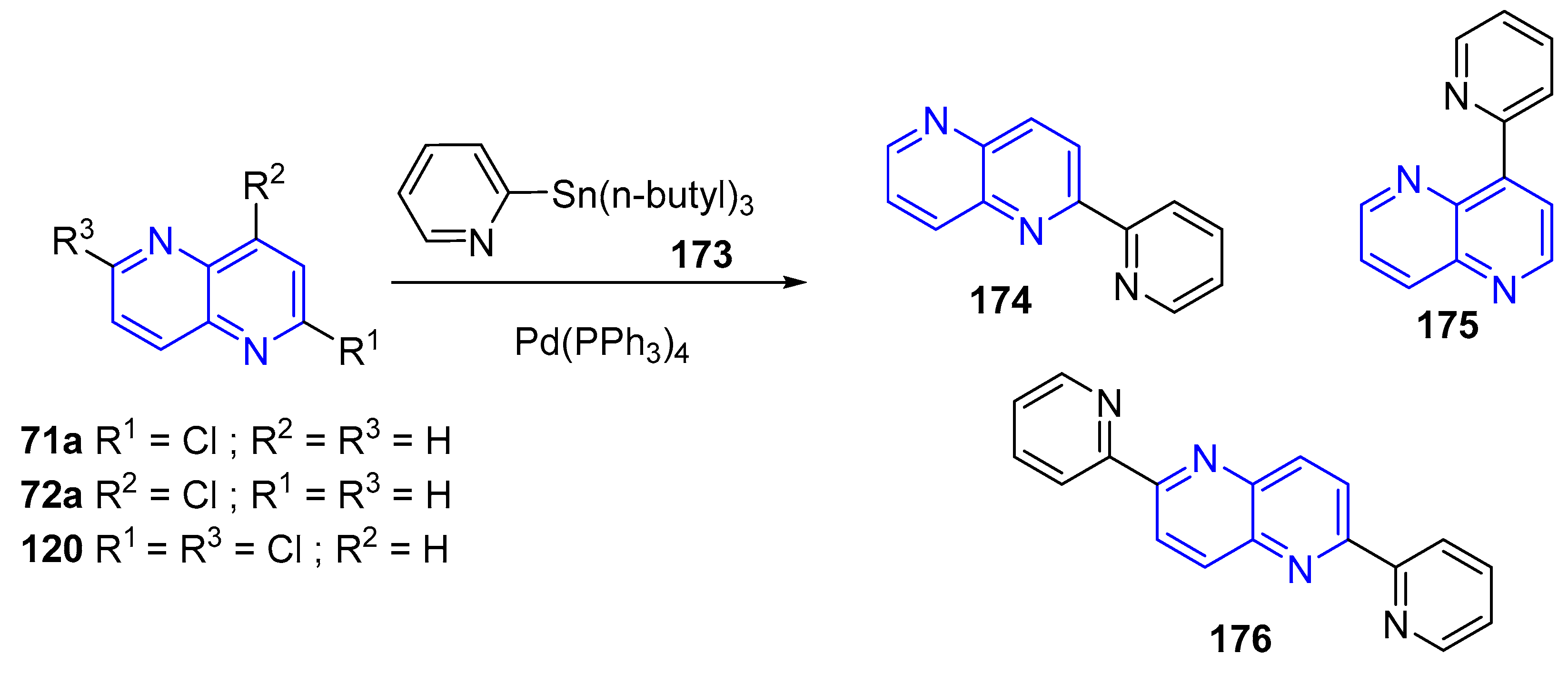
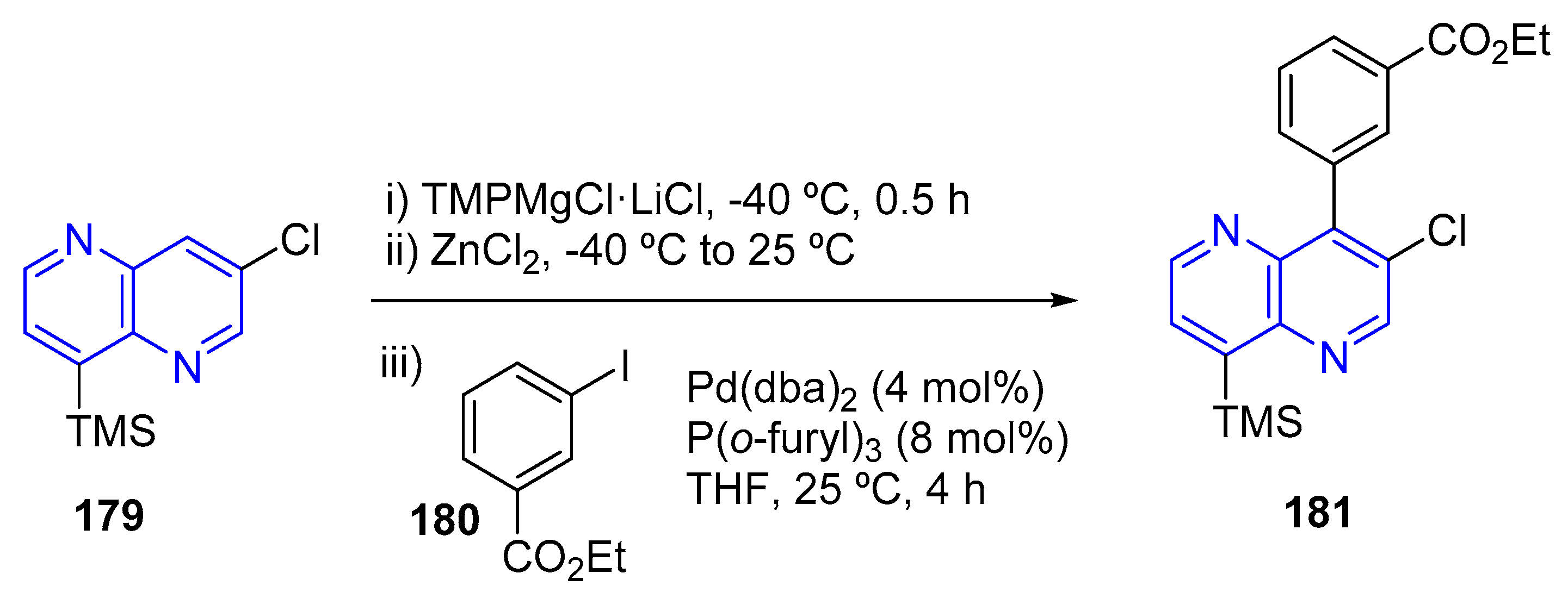
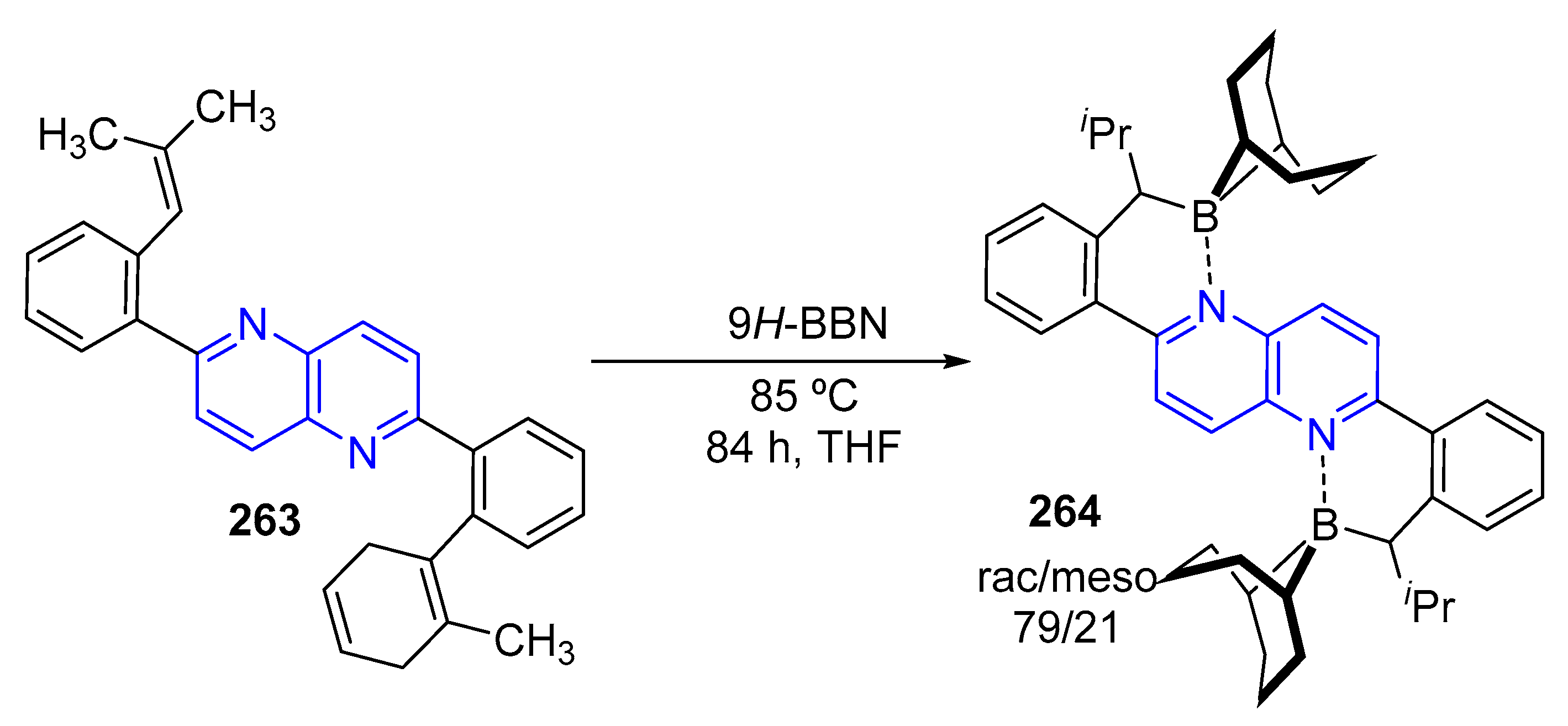
3.4. C-C Bond Constructions, Cross-Couplings
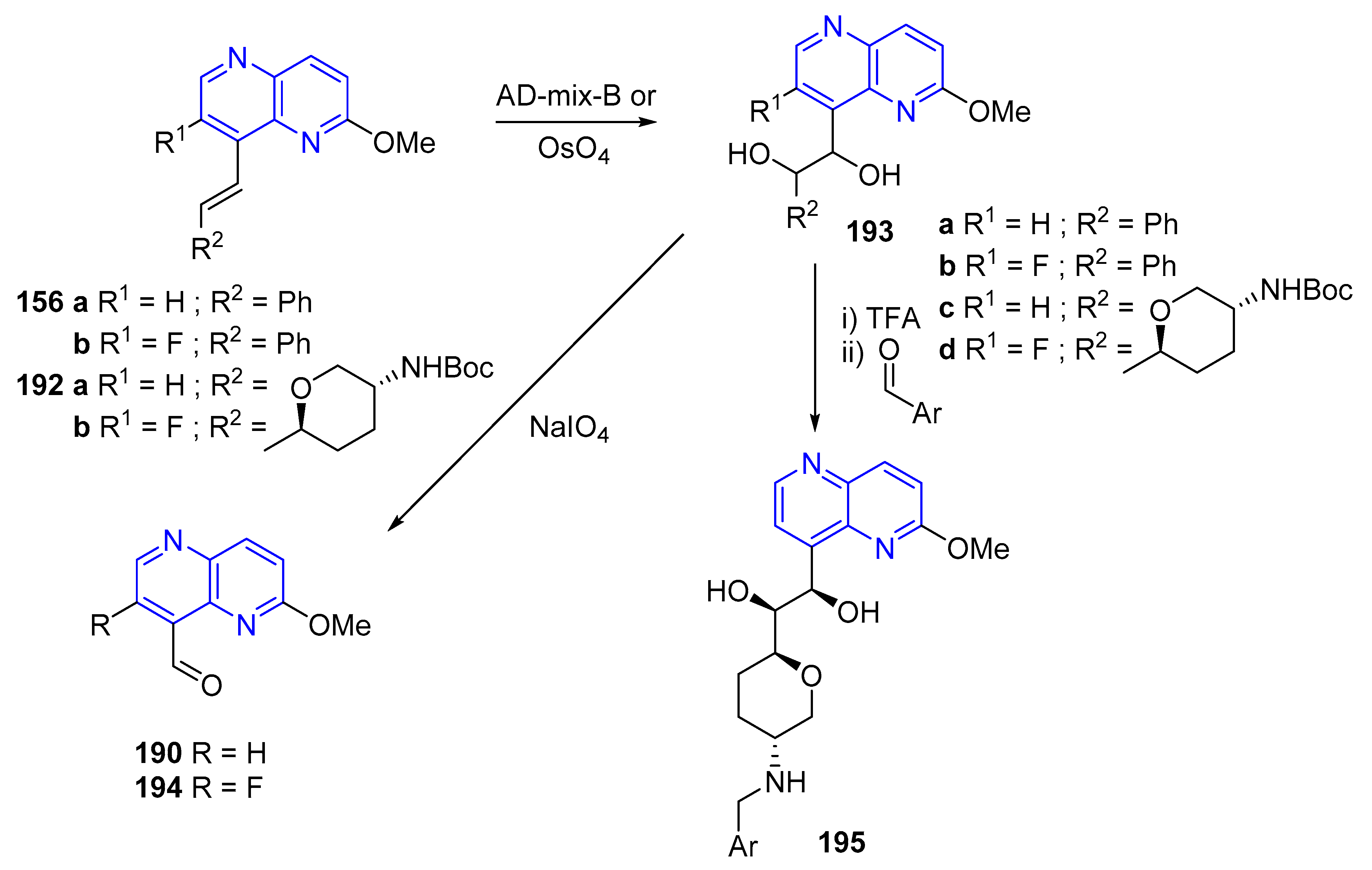
3.5. Modification of the Side Chain
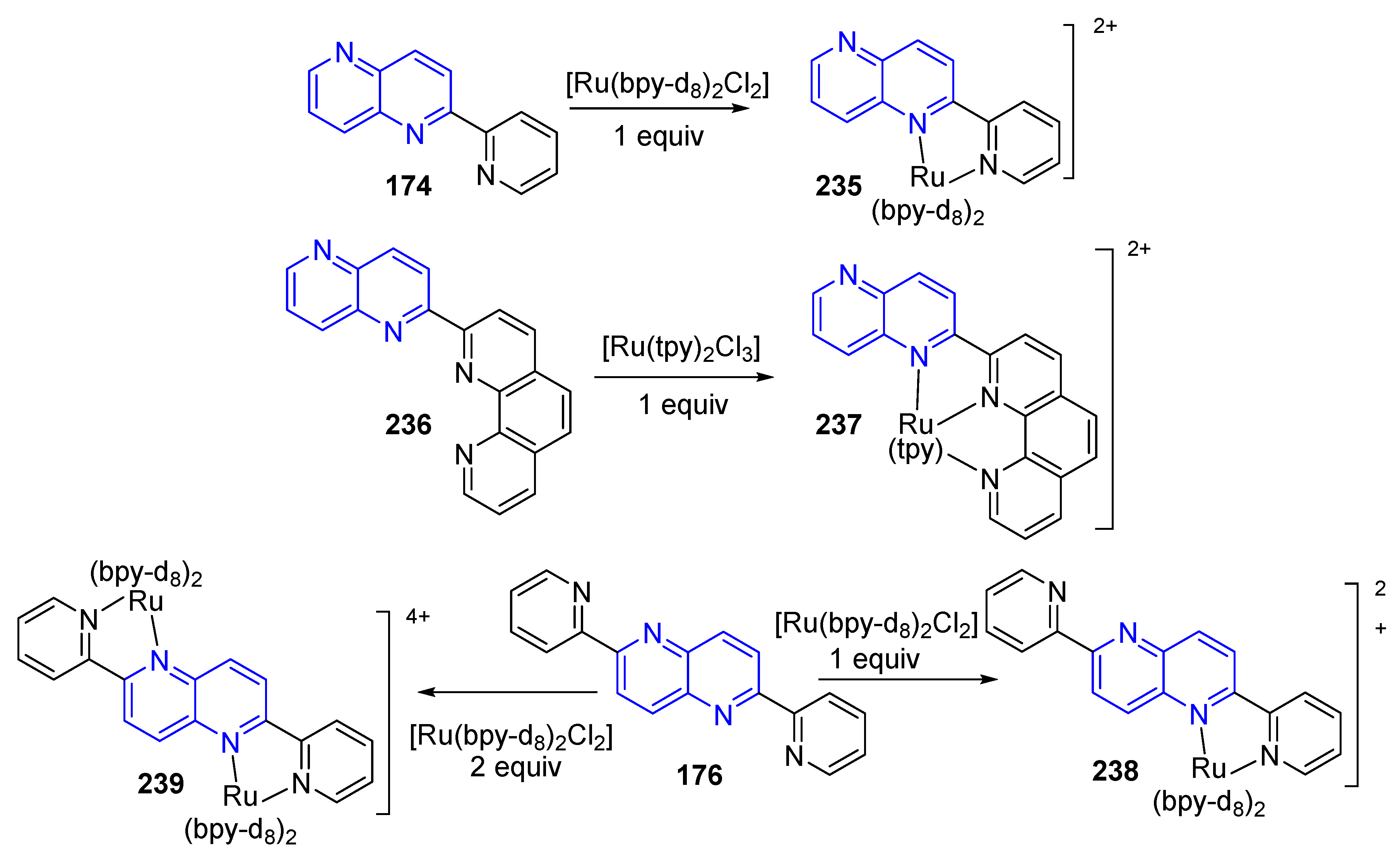
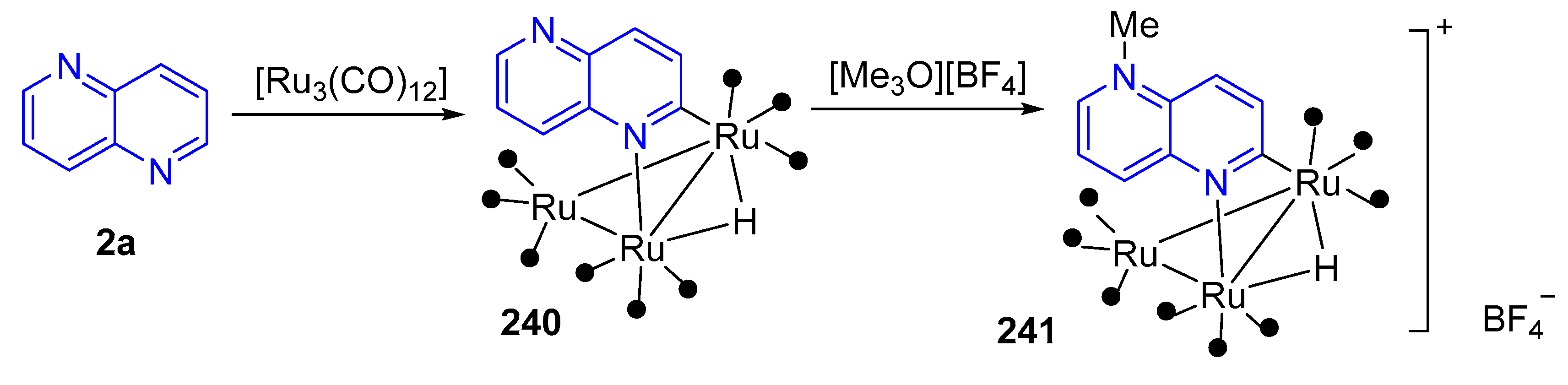
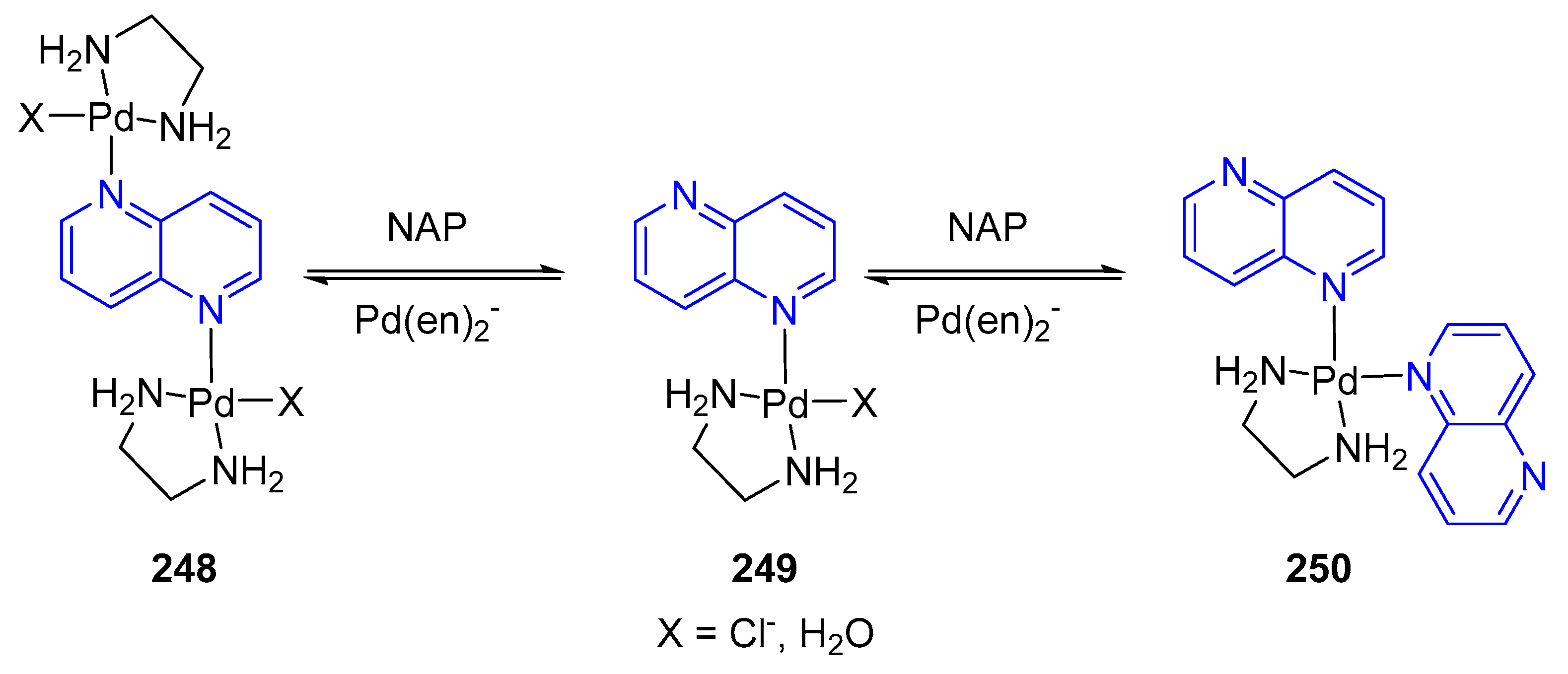
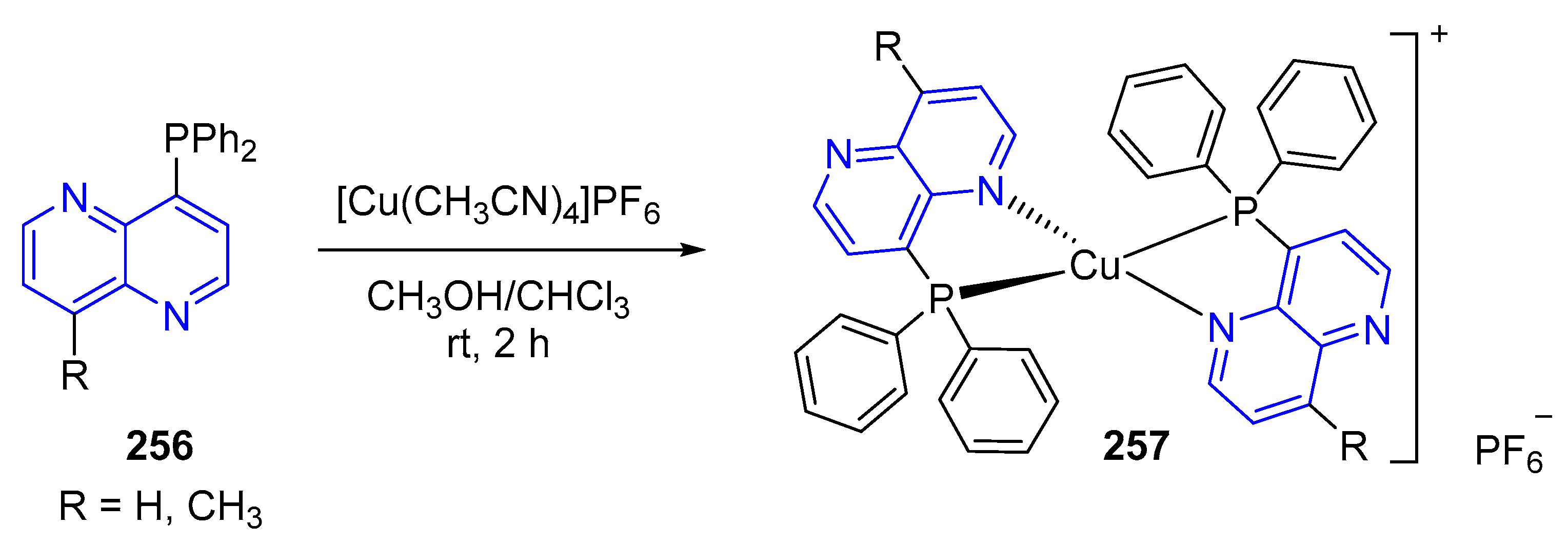
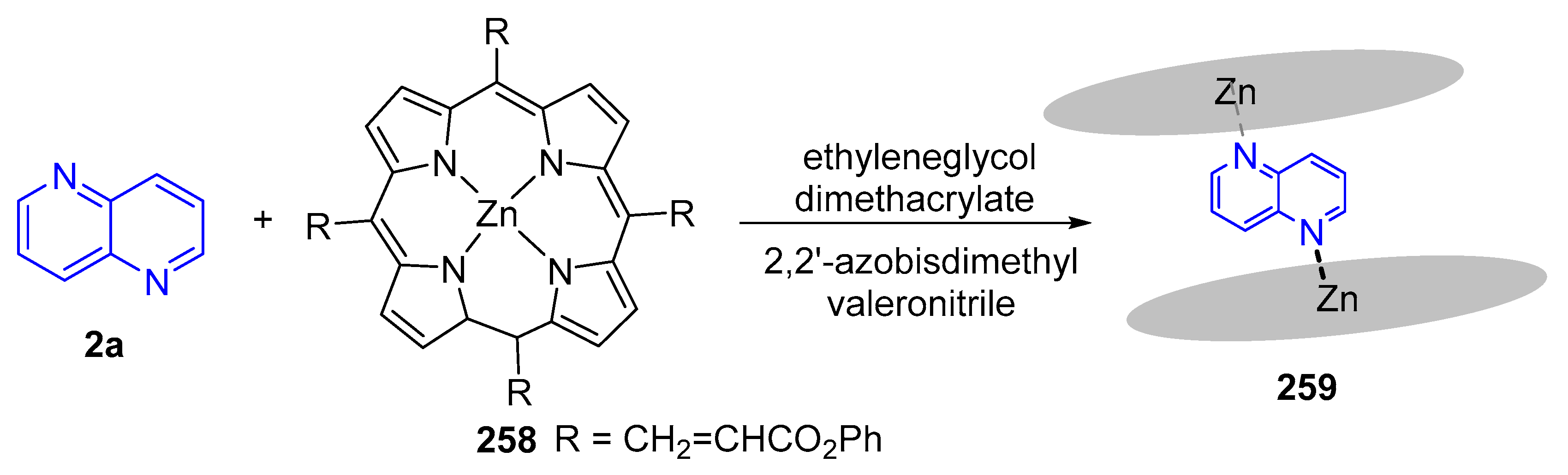
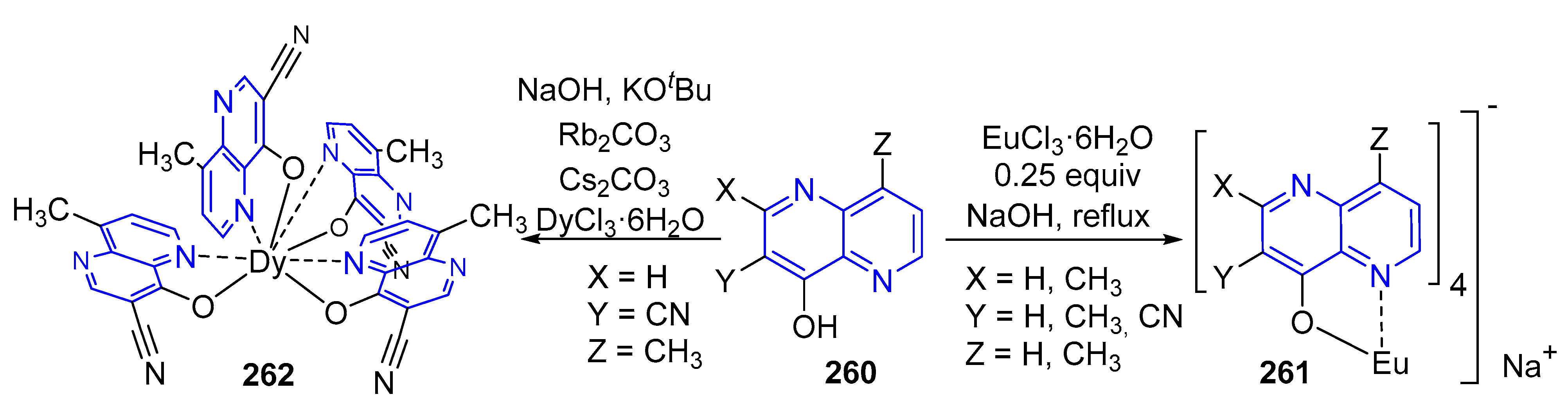
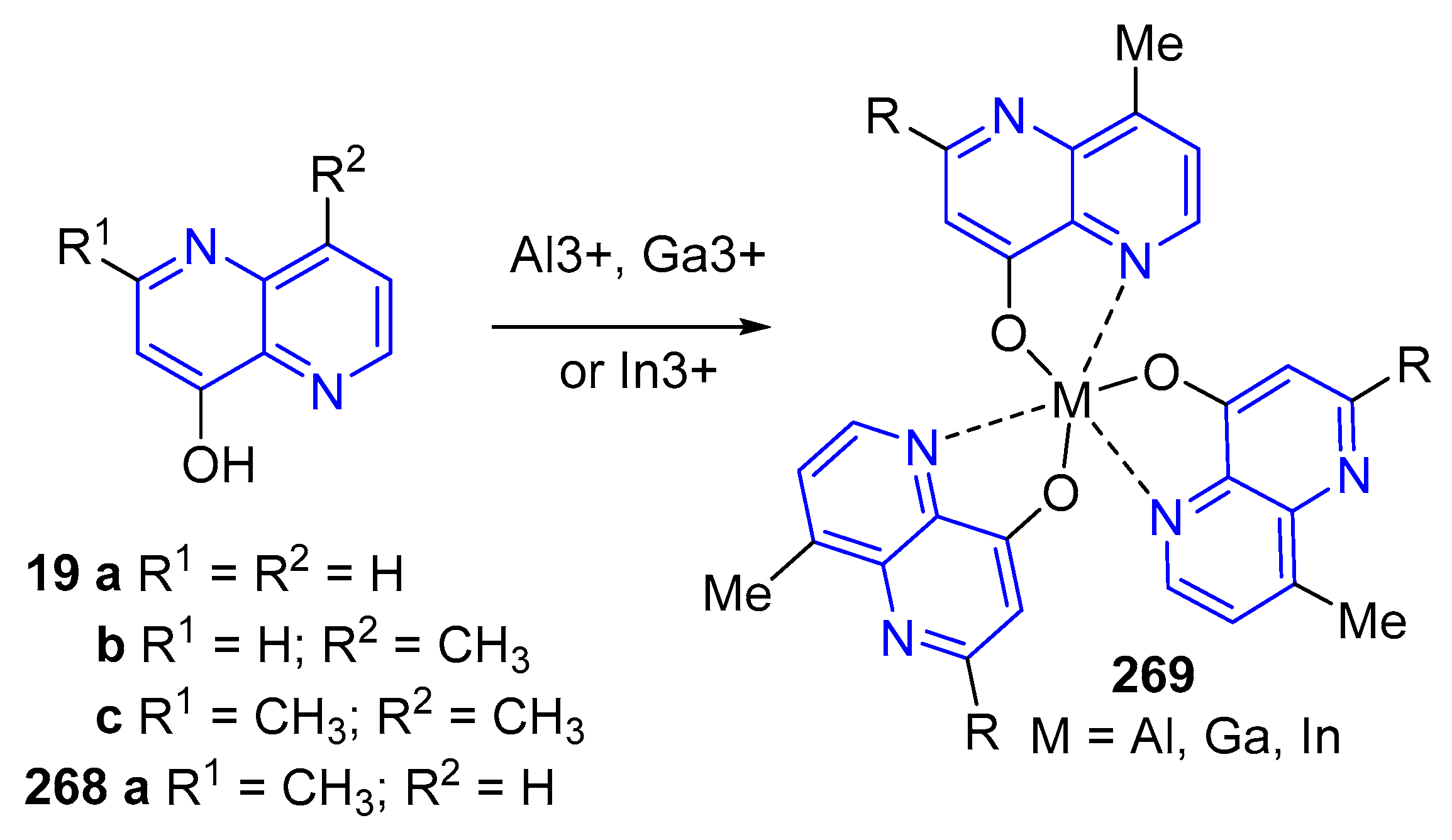
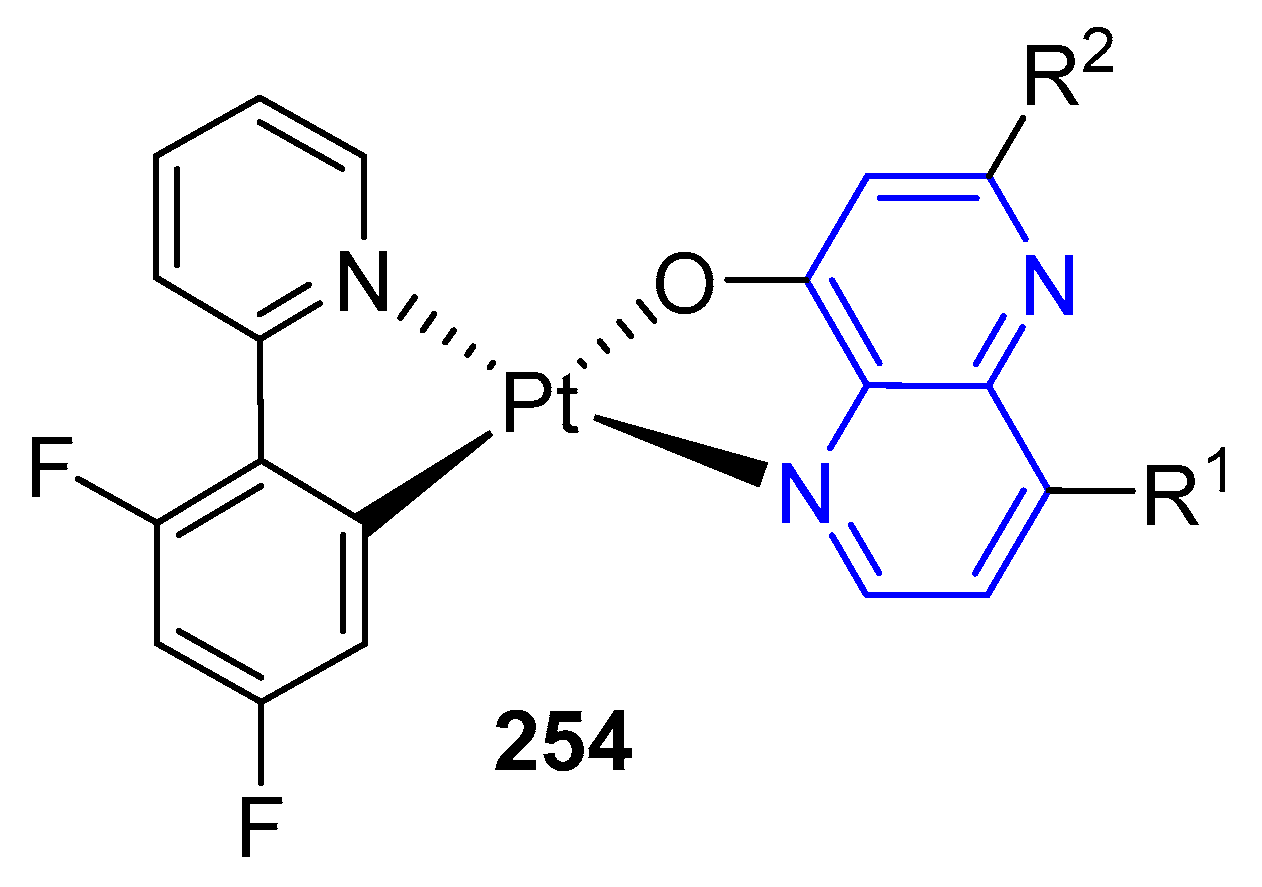
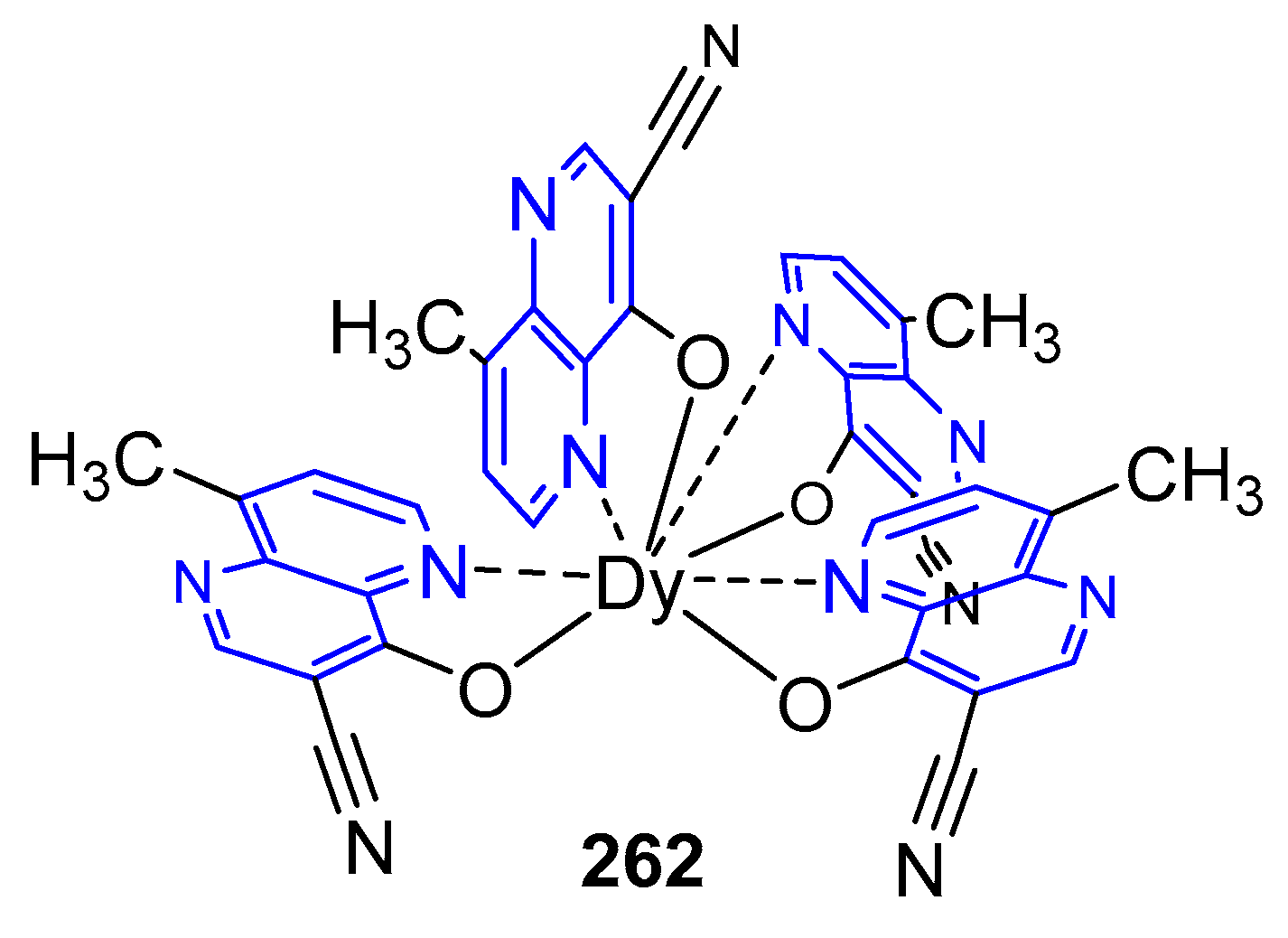
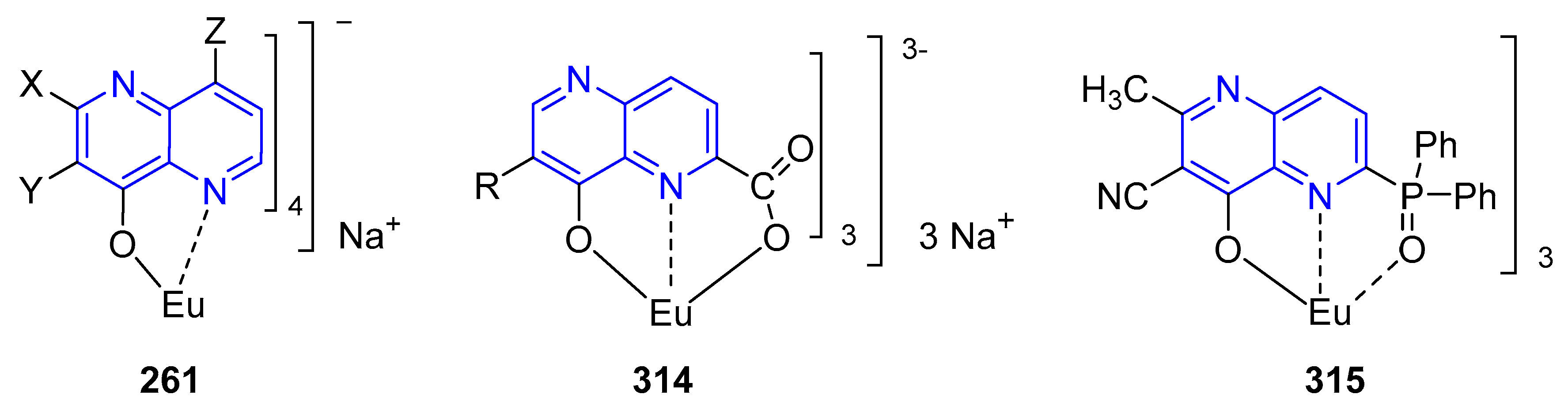
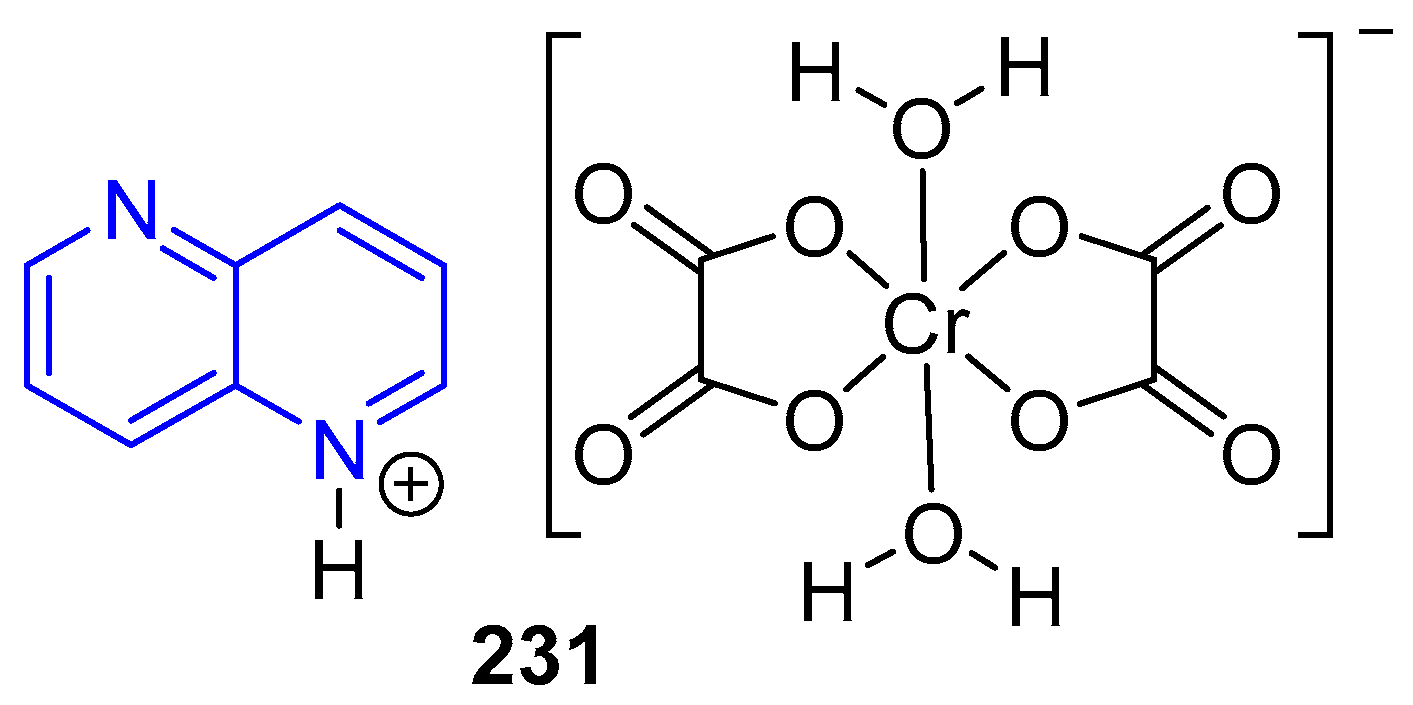
3.6. Formation of Metal Complexes
4. Properties of 1,5-Naphthyridines
5. Applications of 1,5-Naphthyridines
5.1. Biological Activity
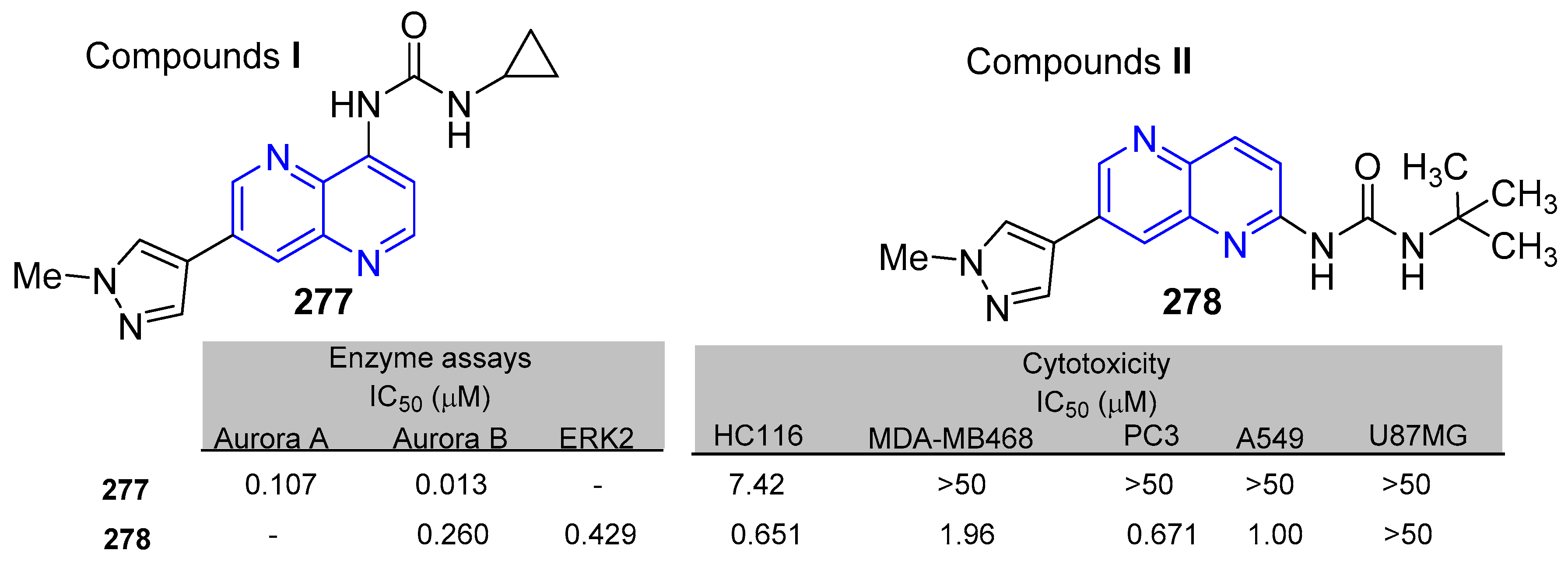
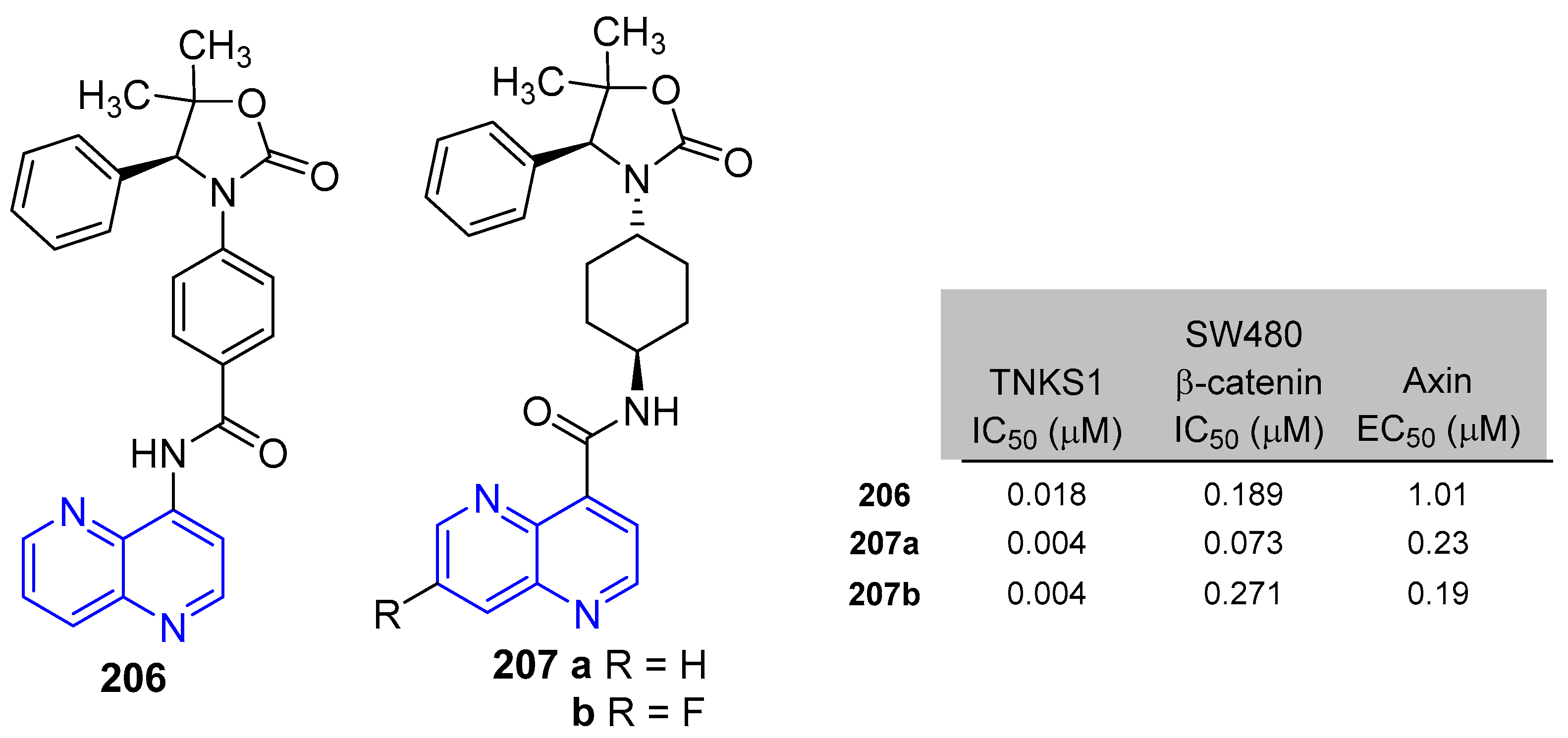
5.1.1. Antiproliferative Activity
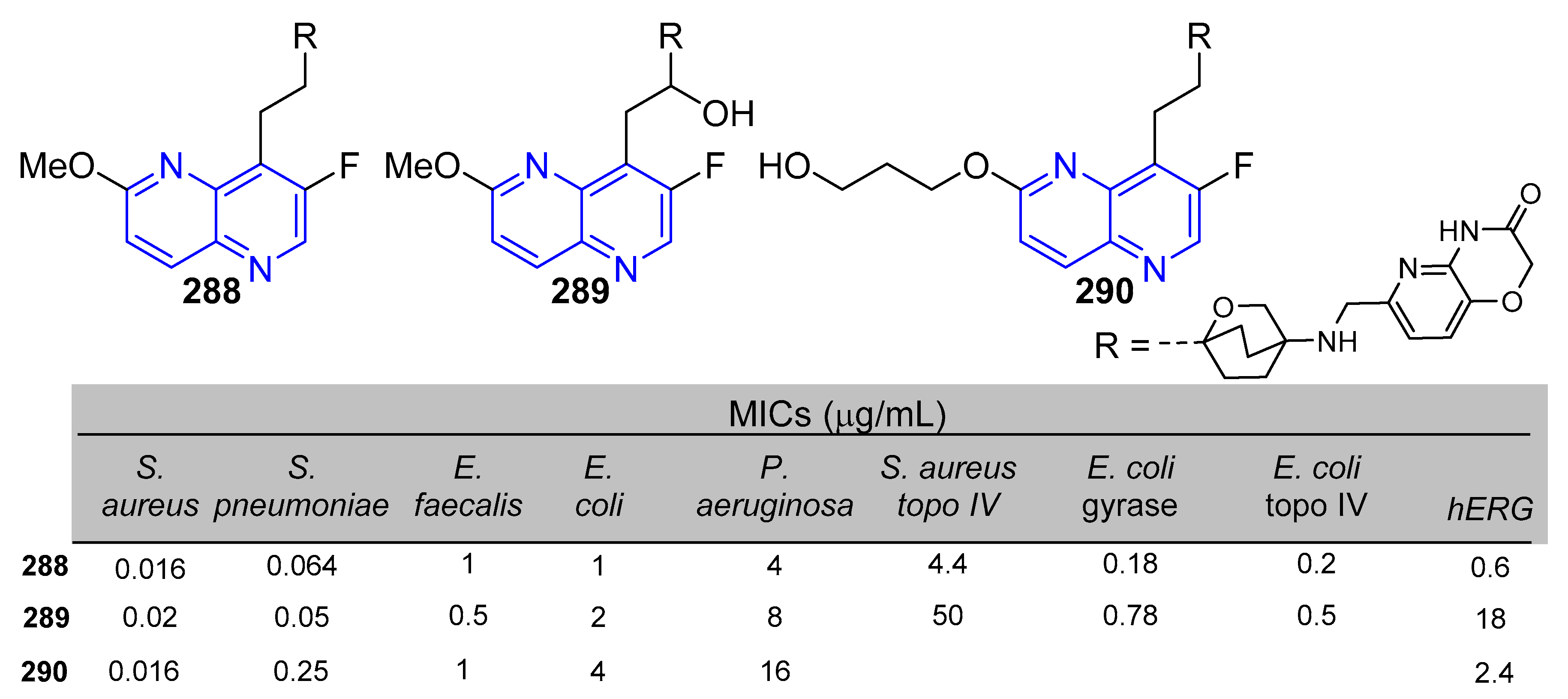
5.1.2. Antibacterial and Antifungal Activity
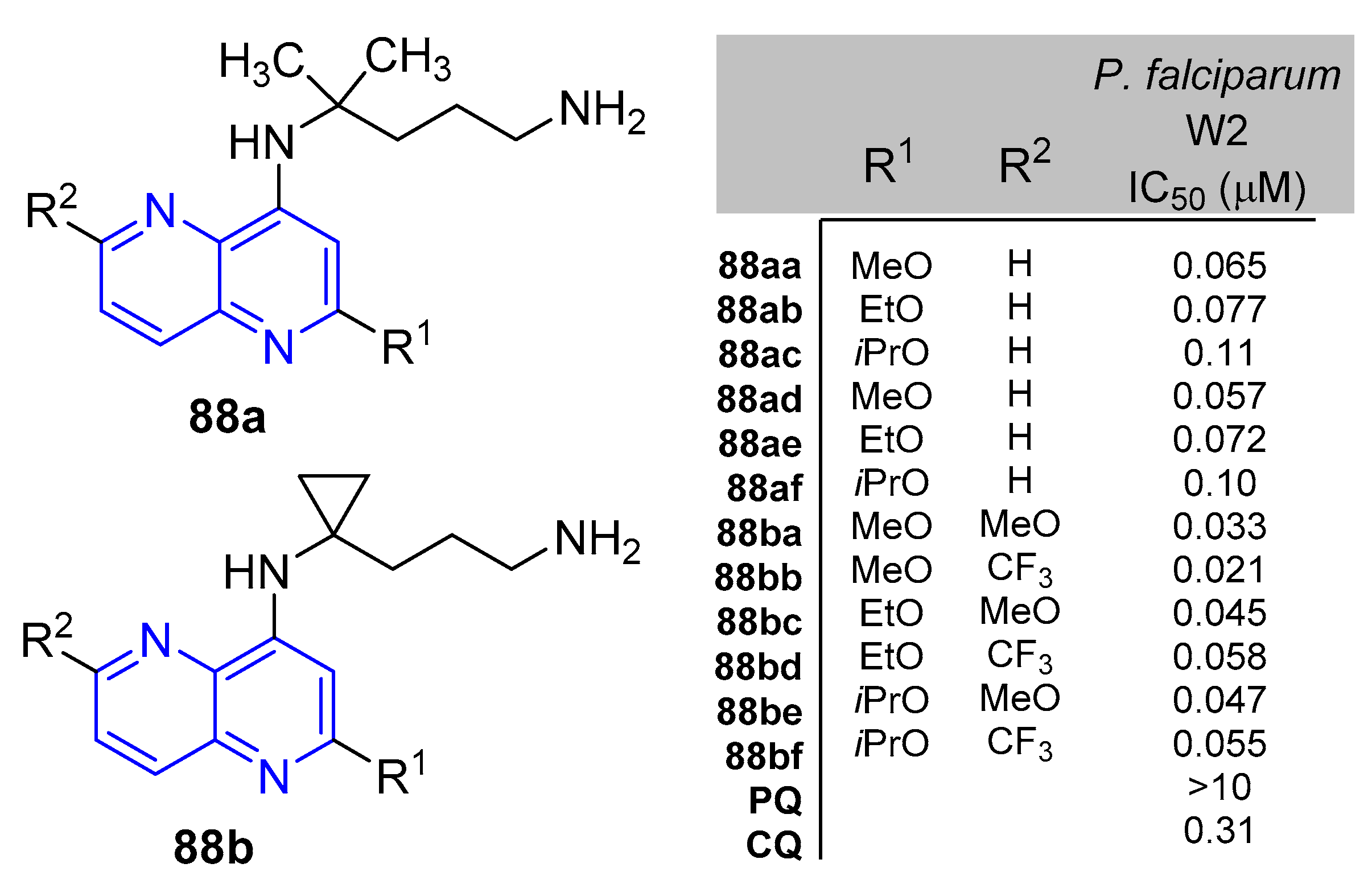
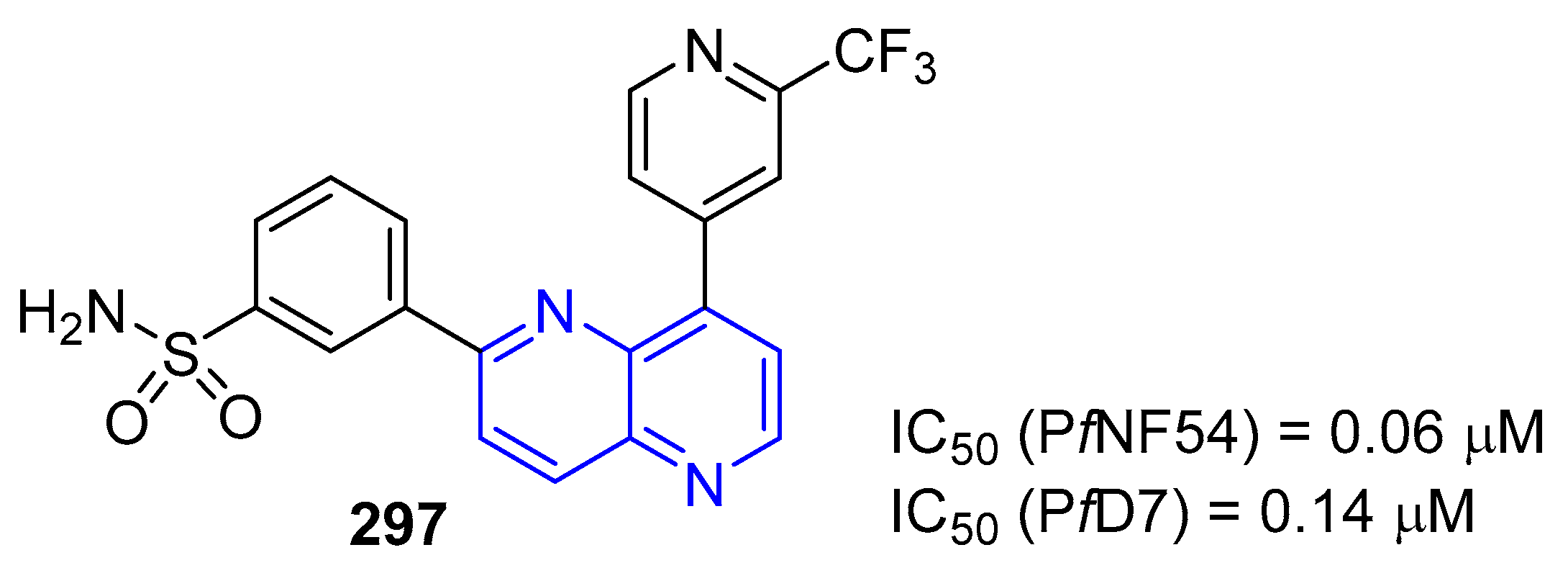
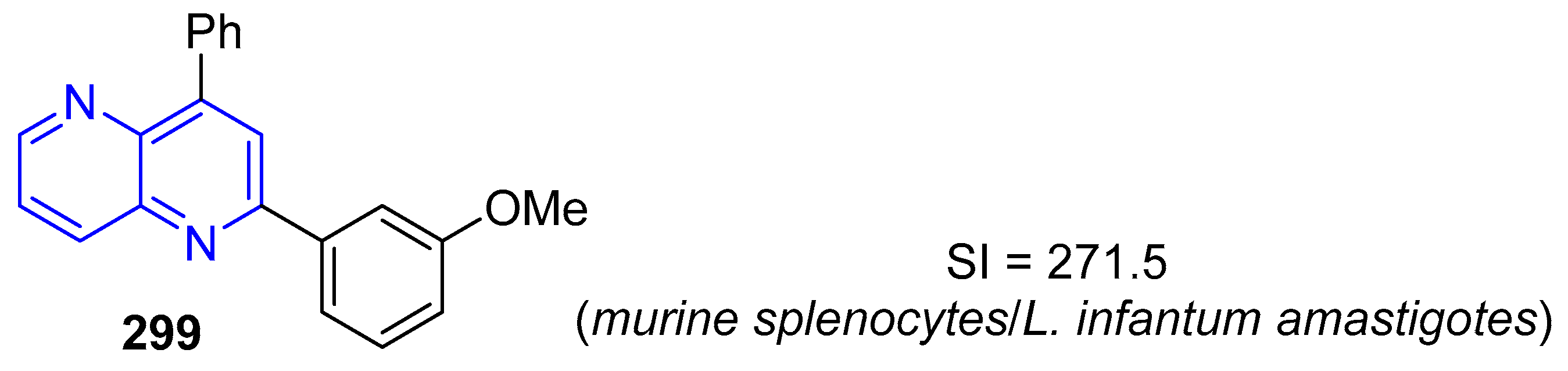
5.1.3. Antiparasitic Activity
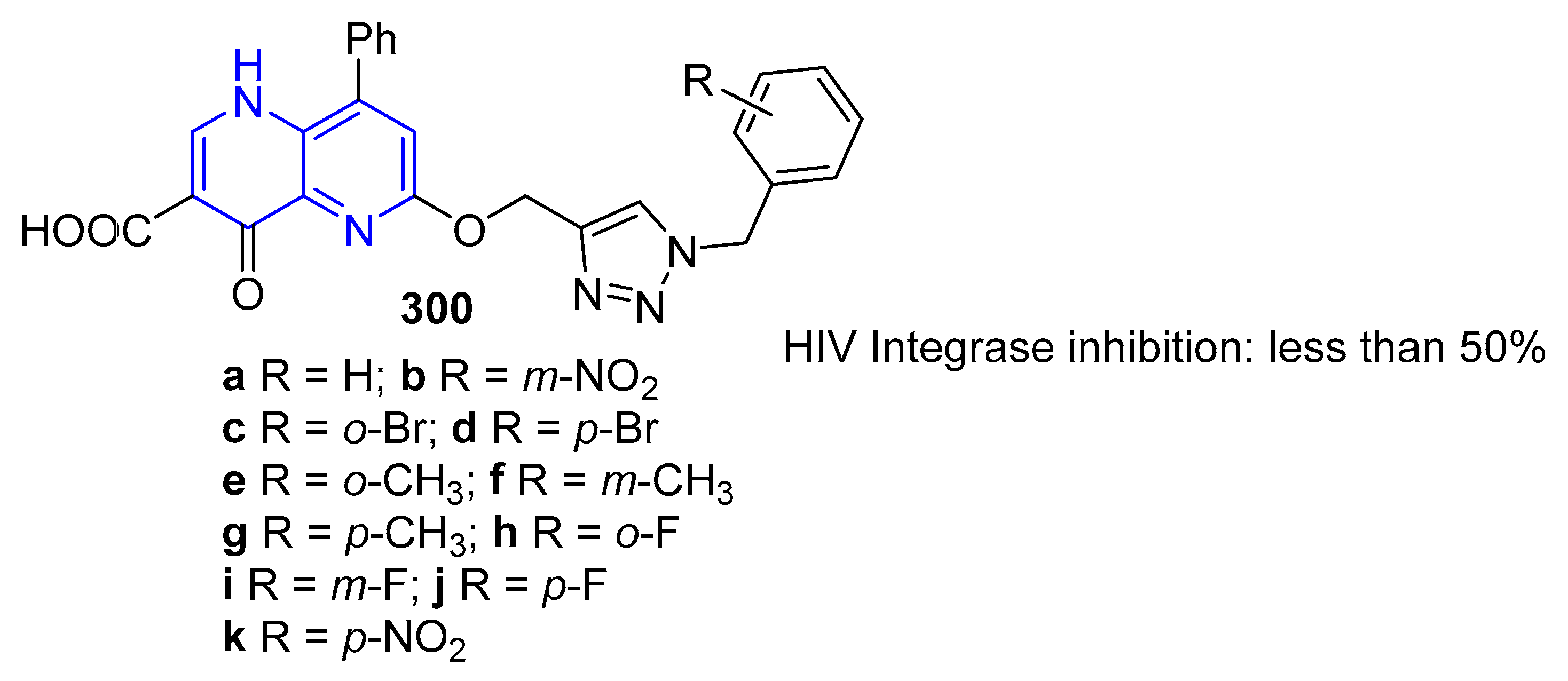
5.1.4. Antiviral Activity
5.1.5. Anti-inflammatory Activity
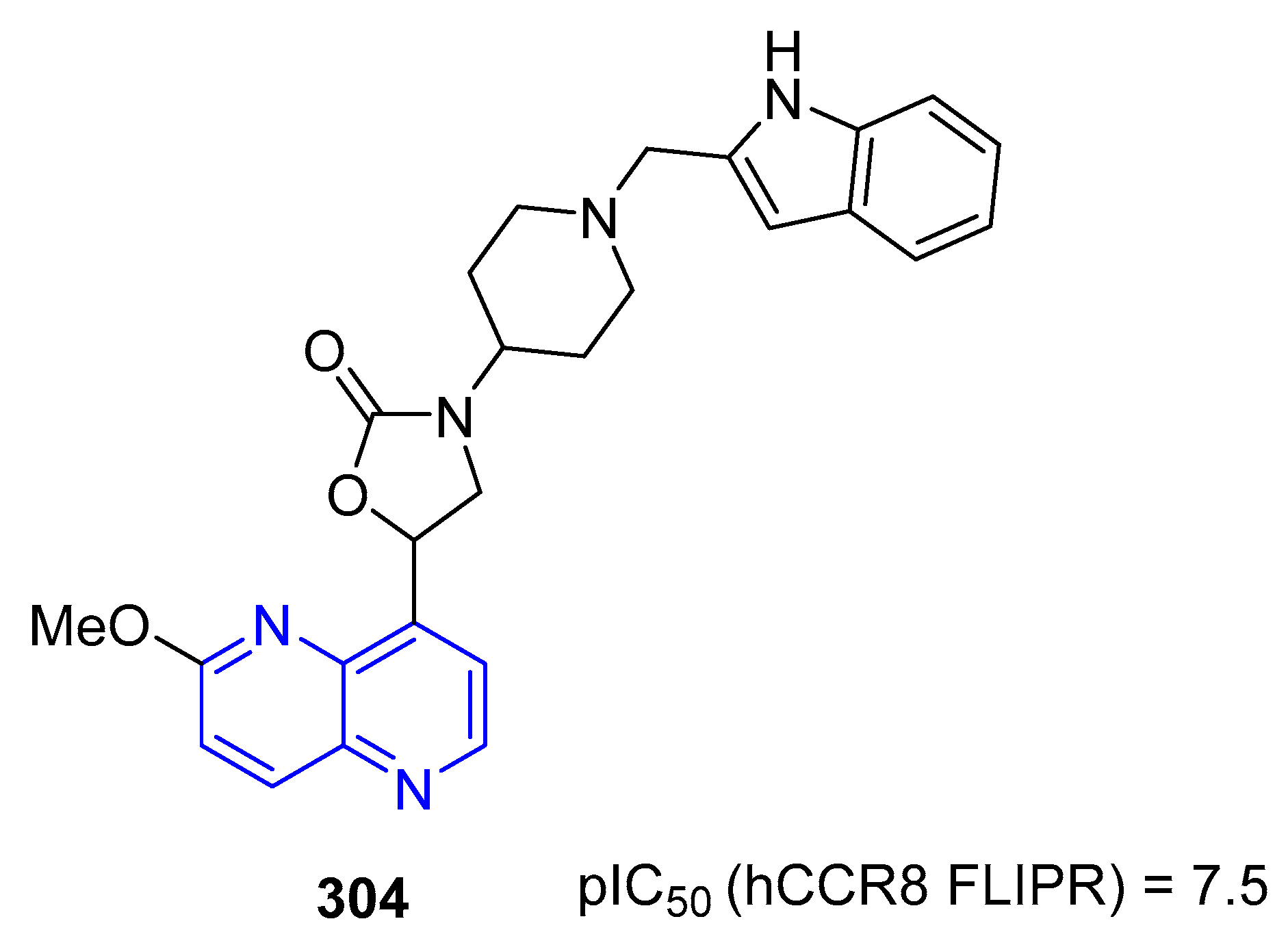
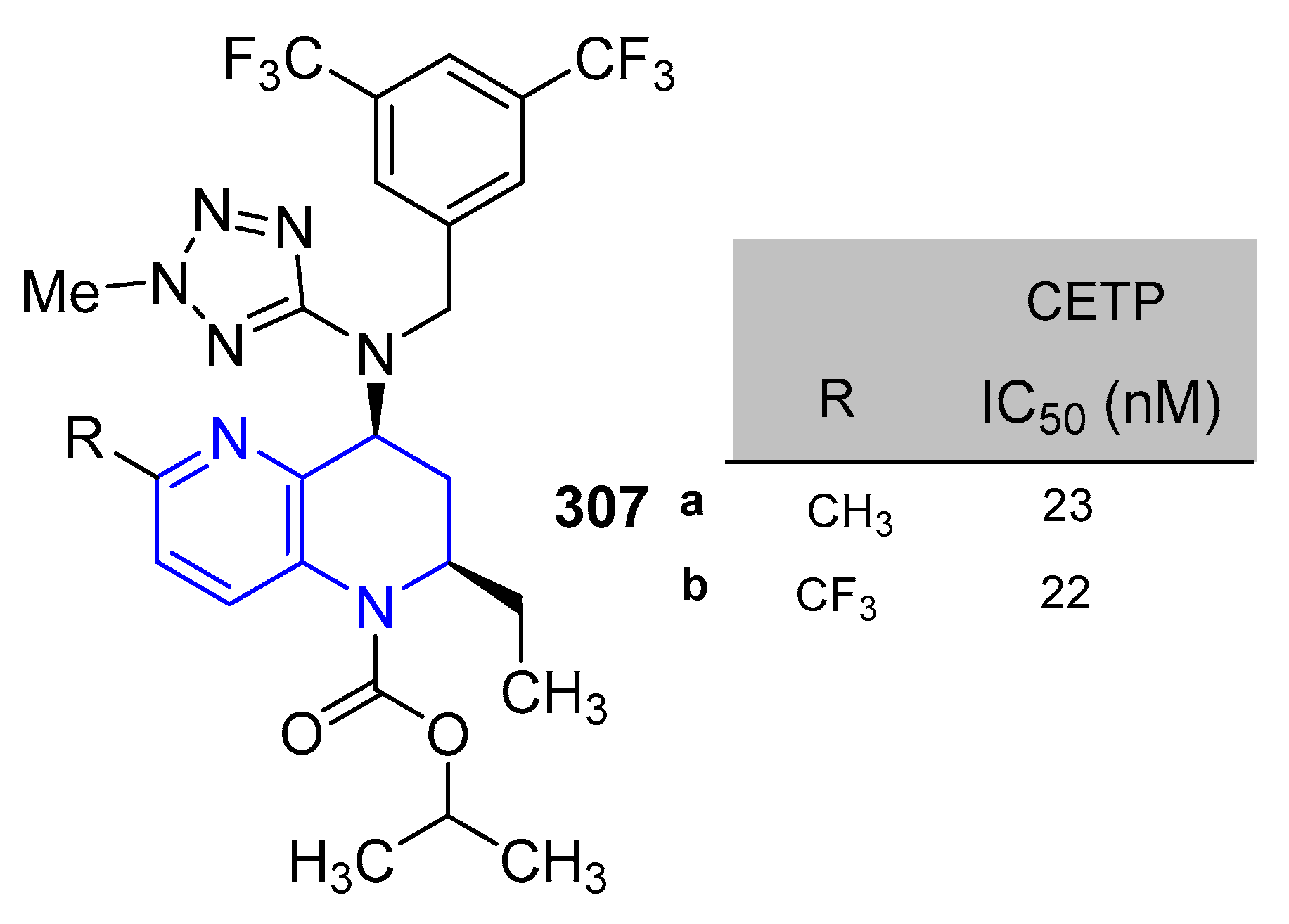
5.1.6. Activity in Cardiovascular Diseases
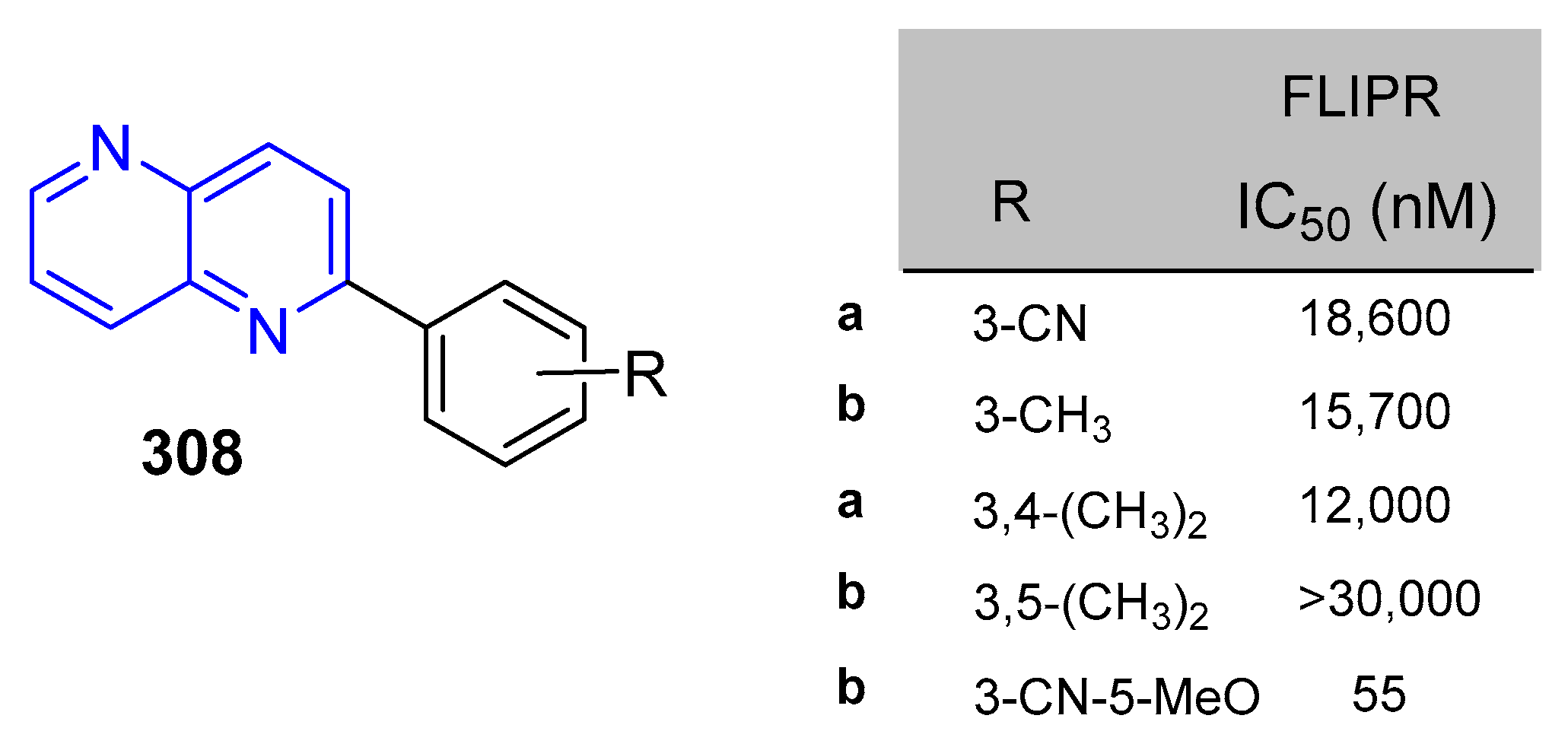
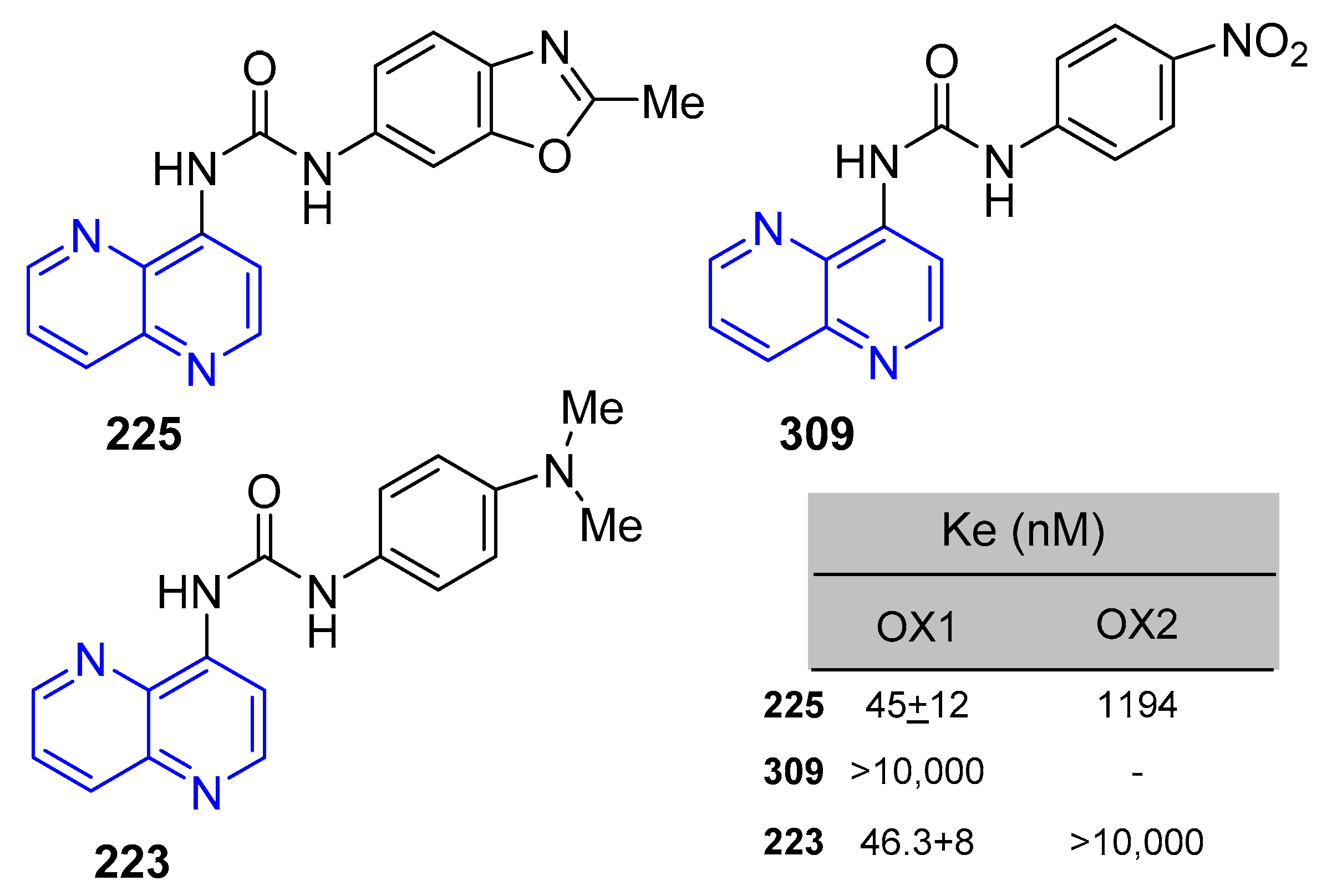
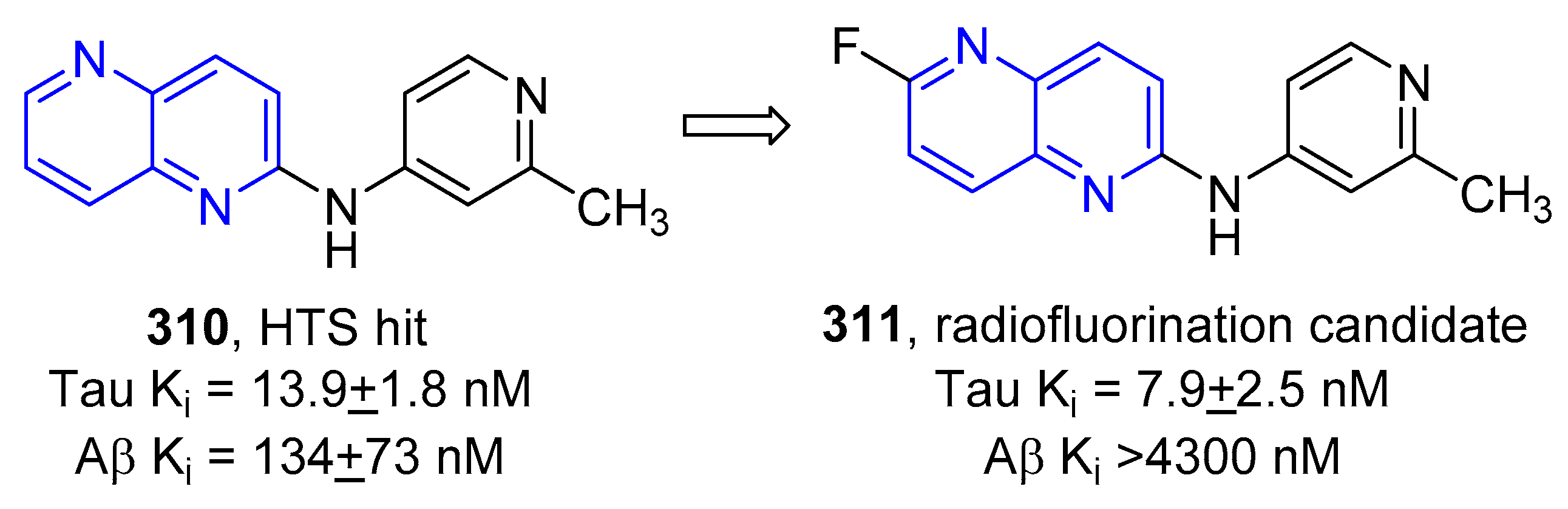
5.1.7. Activity in Central Nervous System (CNS)
5.1.8. Activity in Hormonal Diseases
5.2. Other Applications
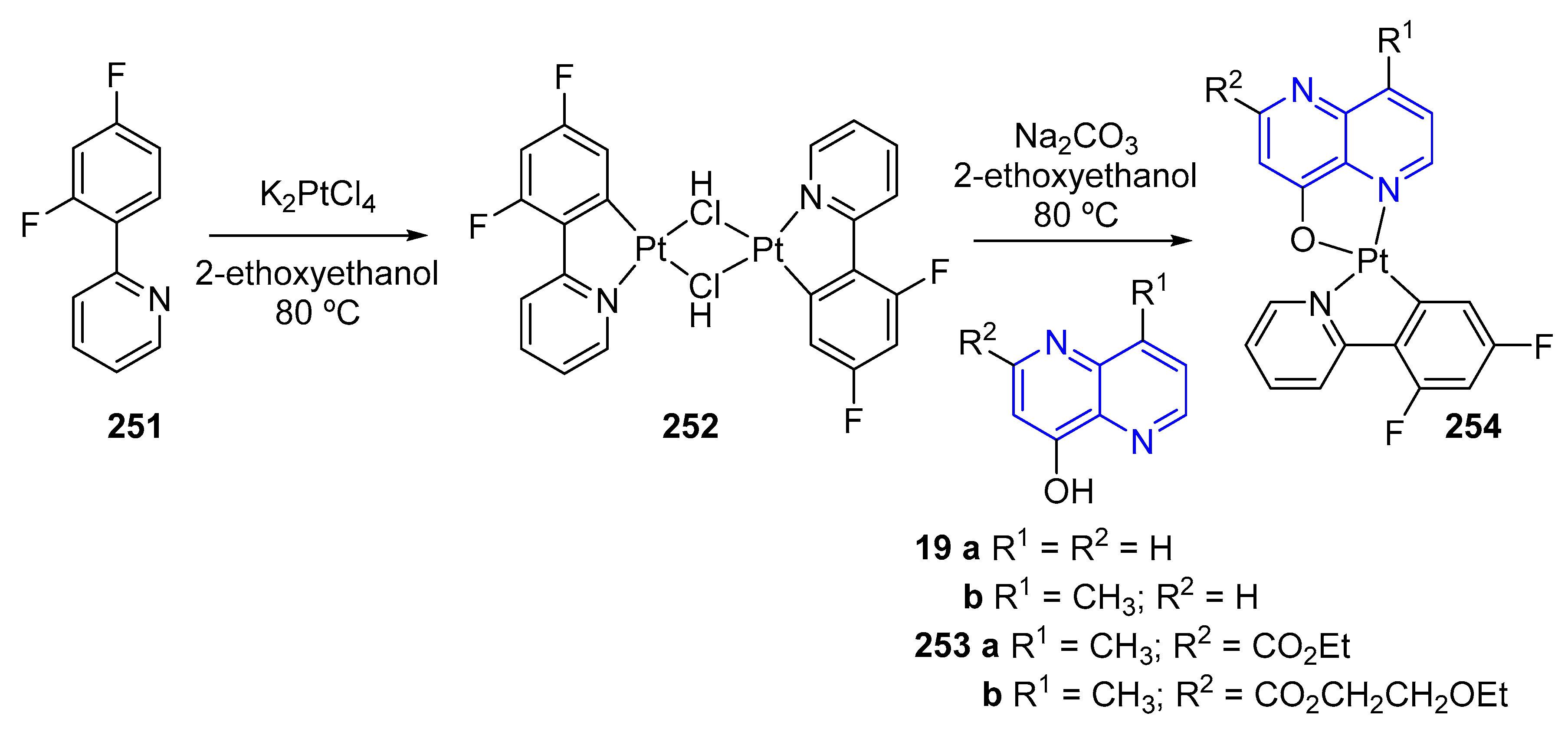
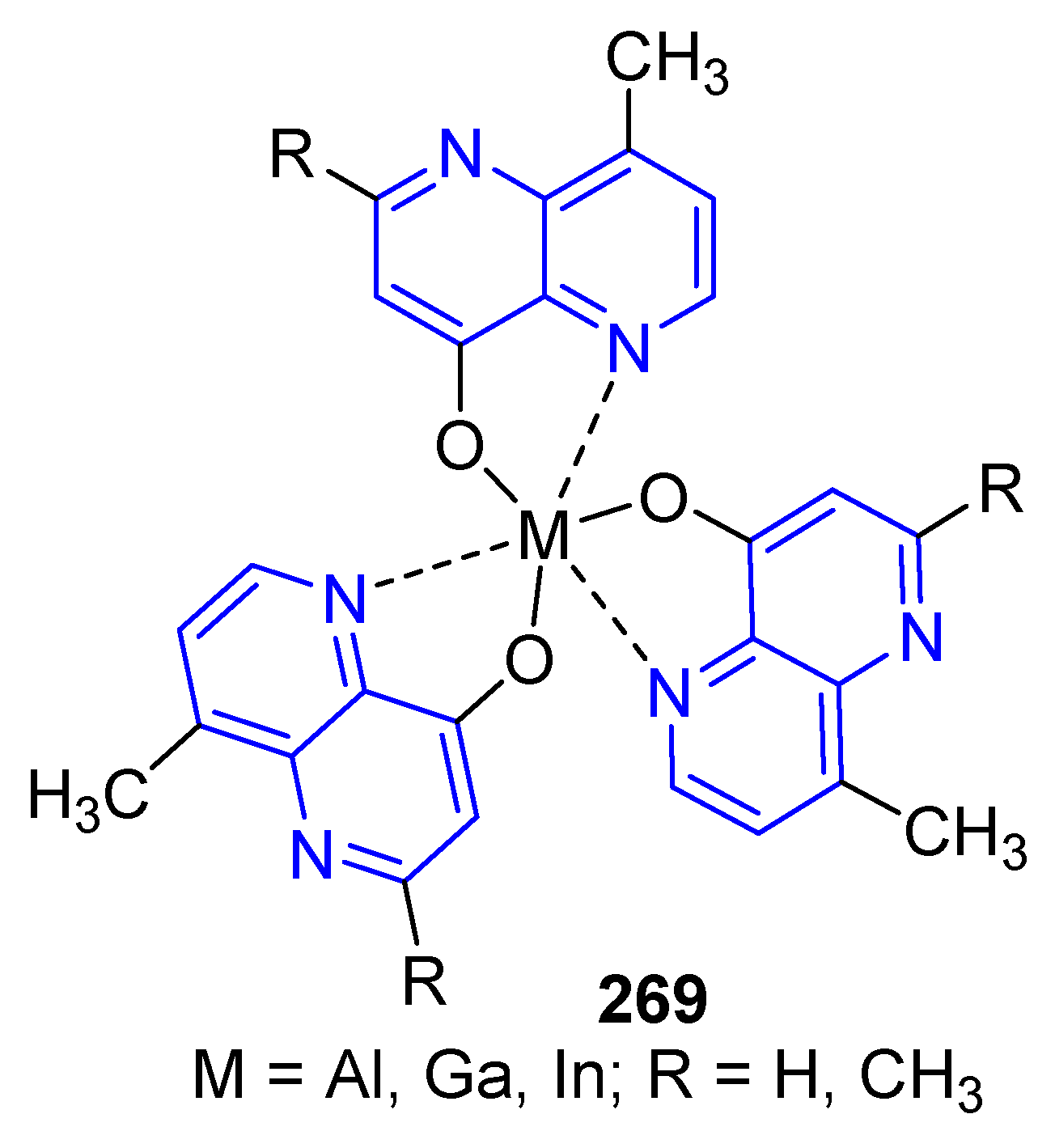
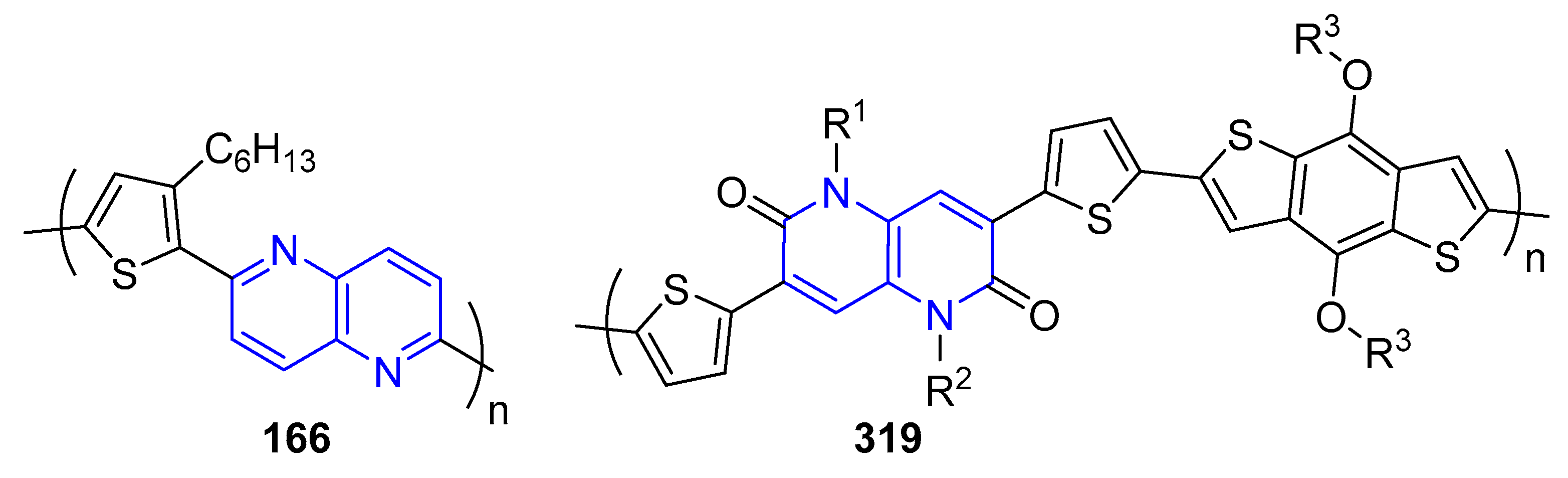
5.2.1. Light-Emitting Compounds
5.2.2. Solar Cells
5.2.3. Energy Storage
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Allen, C.F.H. The naphthyridines. Chem. Rev. 1950, 47, 2, 275–305. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, V.P.; Roman, S.V.; Dyachenko, V.D. Naphthyridines. Structure, physicochemical properties and general methods of synthesis. Russ. Chem. Rev. 2000, 69, 201–220. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Roman, S.V.; Dyachenko, V.D. Pyridopyridines. Russ. Chem. Rev. 2001, 70, 299–320. [Google Scholar] [CrossRef]
- Litvinov, V.P. Advances in the chemistry of naphthyridines. Adv. Heterocycl. Chem. 2006, 91, 189–300. [Google Scholar]
- Reissert, A. Weber di-(y-amidopropyl)esaigeiiure(diamino-1,7-heptanmethylsaure-4) und ihr inneres condeneations product, dee octohydro-1,8-naphthyridine. Berichte 1893, 26, 2137–2144. [Google Scholar]
- Bobranski, B.; Sucharda, E. A synthesis of 1,5-naphthyridine. Berichte 1927, 60, 1081. [Google Scholar]
- Chunavala, K.C.; Adimurthy, S. Iodine- and Indium (III) chloride-catalized facile syntheses of 1,5- and 1,8-naphthyridines. Synth. Commun. 2011, 41, 1843–1851. [Google Scholar] [CrossRef]
- Lee, Y.; Woo, S.-J.; Kim, J.-J.; Hong, J.-I. Linear-shaped thermally activated delayed fluorescence emitter using 1,5-naphthyridine as an electron acceptor for efficient light extraction. Org. Electron. 2020, 78, 105600. [Google Scholar] [CrossRef]
- Wu, J.-F.; Liu, M.-M.; Huang, S.-X.; Wang, Y. Design and synthesis of novel substituted naphthyridines as potential c-Met kinase inhibitors based on MK-2461. Bioorg. Med. Chem. Lett. 2015, 25, 3251–3255. [Google Scholar] [CrossRef]
- Defaux, J.; Maud, A.; Loge, C.; Le Borgne, M.; Schuster, T.; Seipelt, I.; Aicher, B.; Teifel, M.; Gunther, E.; Gerlach, M.; et al. Discovery of (7-aryl-1,5-naphthyridin-2-yl)ureas as dual inhibitors of ERK2 and aurora B kinases with antiproliferative activity against cancer cells. Bioorg. Med. Chem. Lett. 2014, 24, 3748–3752. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Q.; Gudise, C.; Meng, L.; Wei, L.; Smith, E.; Kong, Y. Synthesis and evaluation of naphthyridine compounds as antimalarial agents. Bioorg. Med. Chem. Lett. 2007, 17, 6101–6106. [Google Scholar] [CrossRef]
- Magee, T.V.; Ripp, S.L.; Li, B.; Buzon, R.A.; Chupak, L.; Dougherty, T.J.; Finegan, S.M.; Girard, D.; Hagen, A.E.; Falcone, M.J.; et al. Discovery of azetidinyl ketolides for the treatment of susceptible and multidrug resistant community-acquired respiratory tract infections. J. Med. Chem. 2009, 52, 7446–7457. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Magee, T.V.; Buzon, R.A.; Widlicka, D.W.; Bill, D.R.; Brandt, T.; Cao, X.; Coutant, M.; Dou, H.; Granskog, K.; et al. Process development of a novel azetidinyl ketolide antibiotic. Org. Process Res. Dev. 2012, 16, 788–797. [Google Scholar] [CrossRef]
- Li, B.; Widlicka, D.W.; Buzon, R.A.; Dou, H.; Grasnskog, K.; Flanagan, M.E.; Li, B.; Liu, F.; Liu, W.; Magee, T.V.; et al. A scalable synthesis of 3-hydroxy-1,5-naphthyridine-4-carbaldehyde. Synlett 2010, 2, 250–252. [Google Scholar] [CrossRef]
- Gellibert, F.; Woolven, J.; Fouchet, M.H.; Mathews, N.; Goodland, H.; Lovegrove, V.; Laroze, A.; Nguyen, V.L.; Sautet, S.; Wang, R.; et al. Identification of 1,5-naphthyridine derivatives as a novel serie of potent and selective TGF-β type I receptor inhibitors. J. Med. Chem. 2004, 47, 4494–4506. [Google Scholar] [CrossRef]
- Hirota, J.; Usui, K.; Fuchi, Y.; Sakuma, M.; Matsumoto, S.; Hagihara, R.; Karasawa, S. Fluorescence properties and exciplex formation of emissive naphthyridine derivatives: Application as sensors for amines. Chem. Eur. J. 2019, 25, 14943–14952. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.B.; Dewar, M.J.S. Centrosymmetric 1,5-naphthyridine derivatives: Synthesis, tautomerism, and termal rearrangements. J. Org. Chem. 1978, 43, 1331–1337. [Google Scholar] [CrossRef]
- Papadopoulou, M.V.; Bloomer, W.D. Nitroimidazole-based bioreductive compounds bearing a quinazoline or a napthyridine chromophore. Anti-Cancer Drugs 2009, 20, 493–502. [Google Scholar] [CrossRef]
- Chien, C.T.; Shiu, J.R.; Chang, C.P.; Hon, Y.S.; Huang, D.F.; Chou, P.T.; Liu, C.Y.; Chow, T.J. Platinium complexes of 4-hydroxy-1,5-naphthyridines as emitting dyes. J. Chin. Chem. Soc. 2012, 59, 357–364. [Google Scholar] [CrossRef]
- Wang, K.Y.; Chen, C.; Liu, J.F.; Wang, Q.; Chang, J.; Zhu, H.J.; Li, C. Novel multifunctional organic semiconductor materials base don 4,8-substituted 1,5-naphthyridine: Synthesis, single cristal structures, opto-electrical properties and quantum chemistry calculation. Org. Biomol. Chem. 2012, 10, 6693–6704. [Google Scholar] [CrossRef]
- Allegretti, P.A.; Horton, T.M.; Abdolazimi, Y.; Moeller, H.P.; Yeh, B.; Caffet, M.; Guillermina, M.; Smith, M.; Annes, J.P. Generation of highly potent DYRK1A-dependent inducers of human β-cell replication via multi-dimensional compound optimization. Biorg. Med. Chem. 2020, 28, 115193. [Google Scholar] [CrossRef]
- Zeng, J.; Lü, X.; Zeng, C.; Hu, L.; Zhong, R. Design, synthesis and anti-hiv integrase evaluation of 1,2,3-triazol-4-yl-substituted 1,4-dihydro-4-oxo-1,5-naphthyridine-3-carboxilic acids. Chin. J. Chem. 2009, 27, 953–962. [Google Scholar] [CrossRef]
- Beaudin, J.; Bourassa, D.E.; Bowles, P.; Castaldi, M.J.; Clay, R.; Couturier, M.A.; Karrick, G.; Makowski, T.W.; McDermott, R.E.; Meltz, C.N.; et al. Synthesis and purification of 3-ethoxy-4-oxo-1,4-dihydro-1,5-naphthyridine-3-carboxylic acid benzylamid. Org. Process. Res. Develop. 2003, 7, 873–878. [Google Scholar] [CrossRef]
- Mirguet, O.; Lamotte, Y.; Chung, C.-W.; Bamborough, P.; Delannée, D.; Bouillot, A.; Gellibert, F.; Krysa, G.; Lewis, A.; Witherington, J.; et al. Naphthyridines as novel BET family bromodomain inhibitors. Chem. Med. Chem. 2014, 9, 580–589. [Google Scholar] [CrossRef]
- Singh, S.B.; Kaelin, D.E.; Wu, J.; Miesel, L.; Tan, C.M.; Meinke, P.T.; Olsen, D.; Lagrutta, A.; Bradley, P.; Lu, J.S.; et al. Oxabicyclooctane-linked novel bacterial topoisomerase inhibitors as broad spectrum antibacterial agents. ACS Med. Chem. Lett. 2014, 5, 609–614. [Google Scholar] [CrossRef]
- Abele, S.; Schmidt, G.; Fleming, M.J.; Steiner, H. A one-pot diazotation fluorodediazoniation reaction and fluorine gas for the production of fluoronaphthyridines. Org. Process Res. Dev. 2014, 18, 993–1001. [Google Scholar] [CrossRef]
- Selakovic, Z.; Tran, J.P.; Kota, K.P.; Lazic, M.; Retterer, C.; Besh, R.; Panchal, R.G.; Soloveva, V.; Vantongreen, A.S.; Welles, B.J.; et al. Second generation of diazachrysenes: Protection of ebola virus infected mice and mechanism of action. Eur. J. Med. Chem. 2019, 162, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wei, H.; Yan, W.; Zhao, Z.; Cai, Z.; Sun, B.; Meng, Z.; Liu, Z.; Bian, Z.; Huang, C. Water-soluble and highly luminescent europium (III) complexes with favorable photostability and sensitive pH response behavior. Inorg. Chem. 2016, 55, 10645–10653. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Sun, B.; Cai, Z.; Zhao, Z.; Tan, Y.; Yan, W.; Wei, H.; Liu, Z.; Bian, Z.; Huang, C. Quantum yields over 80% achieved in luminescent europium complexes by employing diphenylphosphoryl tridentate ligands. Inorg. Chem. 2018, 57, 7512–7515. [Google Scholar] [CrossRef]
- Wang, Z. Comprehensive Organic Name Reactions and Reagents; John Wiley & Sons: Hoboken, NJ, USA, 2009; p. 3824. [Google Scholar]
- Liao, S.H.; Shiu, J.R.; Liu, S.W.; Yeh, S.J.; Chen, Y.H.; Chen, C.T.; Chow, T.J.; Wu, C.I. Hydroxynaphthyridine-derived group III metal chelates: Wide band gap and deeep blue analogues of green Alq3(tris(8-hydroxyquinolate)aluminium) and their versatile applications for organic light-emitting diodes. J. Am. Chem. Soc. 2009, 131, 763–777. [Google Scholar] [CrossRef]
- Defaux, J.; Antoine, M.; Le Borgne, M.; Schuster, T.; Seipelt, I.; Aicher, B.; Teifel, M.; Guenther, E.; Gerlach, M.; Marchand, P. Discovery of 7-aryl-substituted (1,5-naphthyridin-4-yl)ureas as aurora kinase inhibitors. ChemMedChem 2014, 9, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Poloek, A.; Lin, C.W.; Chen, C.T. High color rendering index and color stable hybrid white efficient OLEDs with a double emitting layer structure using a single phosphorescence dopant of heteroleptic platinum complexes. J. Mater. Chem. C 2014, 2, 10343–10356. [Google Scholar] [CrossRef]
- Perez, C.; Li, J.; Parlati, F.; Rouffet, M.; Ma, Y.; Mackinnon, A.L.; Chou, T.-F.; Deshaies, R.J.; Cohen, S.M. Discovery of an inhibitor of the proteasome subunit rpn11. J. Med. Chem. 2017, 60, 1343–1361. [Google Scholar] [CrossRef] [PubMed]
- Kandepedu, N.; González-Cabrera, D.; Eedubilli, S.; Taylor, D.; Brunschwig, C.; Gibhard, L.; Njoroge, M.; Lawrence, N.; Paquet, T.; Eyermann, C.J.; et al. Identification, characterization, and optimization of 2,8-disubstituted-1,5-naphthyridines as novel plasmodium falciparum phosphatidylinositol-4-kinase inhibitors with in vivo efficacy in a humanized mouse model of malaria. J. Med. Chem. 2018, 61, 5692–5703. [Google Scholar] [CrossRef]
- Hameed, S.; Raichurkar, A.; Madhavapeddi, P.; Menasinakai, S.; Sharma, S.; Kaur, P.; Nandishaiah, R.; Panduga, V.; Reddy, J.; Sambandamurthy, V.K.; et al. Benzimidazoles: Novel mycobacterial gyrase inhibitors from scaffold morphing. ACS Med. Chem. Lett. 2014, 5, 820–825. [Google Scholar] [CrossRef]
- Yoon, W.S.; Kim, D.W.; Park, J.-M.; Cho, I.; Kwon, O.K.; Whang, D.R.; Kim, J.H.; Park, J.-H.; Park, S.Y. A novel bis-lactam acceptor with outstanding molar extinction coefficient and structural planarity for donor-acceptor type conjugated polymer. Macromolecules 2016, 49, 8489–8497. [Google Scholar] [CrossRef]
- Rombouts, F.J.R.; Andres, J.-I.; Ariza, M.; Alonso, J.M.; Austin, N.; Bottelbergs, A.; Chen, L.; Chupakhin, V.; Cleiren, E.; Fierens, K.; et al. Discovery of N-(pyridin-4-yl)-1,5-naphthyridin-2-amines as potential tau pathology PET tracers for Alzheimer’s disease. J. Med. Chem. 2017, 60, 1272–1291. [Google Scholar] [CrossRef]
- Shen, Z.-L.; Dhayalan, V.; Benischke, A.D.; Greiner, R.; Karaghiosoff, K.; Mayer, P.; Knochel, P. Polyfunctional lithium, magnesium, and zinc alkenyl reagents as building blocks for the synthesis of complex heterocycles. Angew. Chem. Int. Ed. 2016, 55, 5332–5336. [Google Scholar] [CrossRef]
- Siddiqui, M.; Ullah, N.; Al-Betar, A.; Al-Saadi, A. Synthesis of functionalized polythiophene as a potenial organic semi-conductor. MATEC Web Conf. 2016, 49, 02001/1–02001/7. [Google Scholar] [CrossRef]
- Alonso, C.; González, M.; Palacios, F.; Rubiales, G. Study of the hetero-[4+2]-cycloaddition reaction of aldimines and alkynes. Synthesis of 1,5-naphthyridine and isoindolone derivatives. J. Org. Chem. 2017, 82, 6379–6387. [Google Scholar] [CrossRef]
- Ghashghaei, O.; Masdeu, C.; Alonso, C.; Palacios, F.; Lavilla, R. Recent advances of the Povarov reaction in medicinal chemistry. Drug Discov. Today 2018, 29, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Palacios, F.; Alonso, C.; Arrieta, A.; Cossio, F.P.; Ezpeleta, J.M.; Fuertes, M.; Rubiales, G. Lewis acid activated aza-Diels-Alder reaction of n-(3pyridyl)aldimines: An experimental and computational study. Eur. J. Org. Chem. 2010, 2010, 2091–2099. [Google Scholar] [CrossRef]
- Palacios, F.; Alonso, C.; Fuertes, M.; Ezpeleta, J.M.; Rubiales, G. Glyoxalate-derived aldimines in cycloaddition reactions with olefins. Eur. J. Org. Chem. 2011, 23, 4318–4326. [Google Scholar] [CrossRef]
- Alonso, C.; Fuertes, M.; González, M.; Rodríguez-Gascón, A.; Rubiales, G.; Palacios, F. Synthesis and biological evaluation of 1,5-naphthyridines as topoisomerase I inhibitors. A new family of antiproliferative agents. Curr. Top. Med. Chem. 2014, 14, 2722–2728. [Google Scholar] [CrossRef] [PubMed]
- Tejeria, A.; Pérez-Pertejo, Y.; Reguera, R.M.; Balana-Fouce, R.; Alonso, C.; González, M.; Rubiales, G.; Palacios, F. Substituted 1,5-naphthyridine derivatives as novel antileishmanial agents. Synthesis and biological evaluation. Eur. J. Med. Chem. 2018, 152, 137–147. [Google Scholar] [CrossRef]
- Fernandez, M.C.; Escribano, A.; Mateo, A.I.; Parthasarathy, S.; Martin de la Nava, E.M.; Wang, X.; Cockerham, S.L.; Beyer, T.P.; Schmidt, R.J.; Cao, G.; et al. Desing, synthesis and structure-activity-relationship of 1,5-tetrahydronaphthyridines as CEPT inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 3056–3062. [Google Scholar] [CrossRef]
- Woo, G.H.C.; Beeler, A.B.; Snyder, J.K. 1,2,3,4-Tetrahydro-1,5-naphthyridines and related heterocyclic scaffolds: Exploration of suitable chemistry for library development. Tetrahedron 2007, 63, 5649–5655. [Google Scholar] [CrossRef]
- Yoon, W.S.; Won Kim, D.; Choi, M.-W.; Park, J.-M.; Park, S.Y. Designing 1,5-naphthyridine-2,6-dione-based conjugated polymers for higher crystallinity and enhanced light absorption to achieve 9.63% efficiency polymer solar cells. Adv. Energy Mater. 2018, 8, 1701467. [Google Scholar] [CrossRef]
- Alonso, C.; Martinez de Marigorta, E.; Rubiales, G.; Palacios, F. Trifluoromethylation reactions of hydrocarbon derivatives and heteroarenes. Chem. Rev. 2015, 115, 1847–1935. [Google Scholar] [CrossRef]
- Shirai, T.; Kanai, M.; Kuninobu, Y. 2-Position-selective C-H perfluoroalkylation of quinoline derivatives. Org. Lett. 2018, 20, 1593–1596. [Google Scholar] [CrossRef]
- Nagase, M.; Kuninobu, Y.; Kanai, M. 4-Position-selective C-H perfluoroalkylation and perfluoroarylation of six-membered heteroaromatic compounds. J. Am. Chem. Soc. 2016, 138, 6103–6106. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Kaelin, D.E.; Wu, J.; Miesel, L.; Tan, C.M.; Meinke, P.T.; Olsen, D.B.; Lagrutta, A.; Wei, C.; Peng, X.; et al. Structure activity relationship of substituted 1,5-naphthyridine analogs of oxabicyclooctane-linked novel bacterial topoisomerase inhibitors as broad-spectrum antibacterial agents (Part 4). Bioorg. Med. Chem. Lett. 2015, 25, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Bouarfa, S.; Grassl, S.; Ivanova, M.; Langlais, T.; Bentabed-Ababsa, G.M.; Lassagne, F.; Erb, W.; Roisnel, T.; Dorcet, V.; Knochel, P.; et al. Copper- and cobalt-catalyzed syntheses of thiophene-based tertiary amines. Eur. J. Org. Chem. 2019, 20, 3244–3258. [Google Scholar] [CrossRef]
- Grzegozek, M.; Szpakiewicz, B. Methylamination of some 3-nitro-1,5-naphthyridines with liquid methylamine/potassium permanganate. J. Heterocyclic Chem. 2006, 43, 425–430. [Google Scholar] [CrossRef]
- Palacios, F.; Alonso, C.; De los Santos, J.M. Synthesis of β-aminophosphonates and -phosphinates. Chem. Rev. 2005, 105, 899–931. [Google Scholar] [CrossRef]
- Radai, Z.; Keglevich, G. Synthesis and reactions of α-hydroxyphosphonates. Molecules 2018, 23, 1493. [Google Scholar] [CrossRef]
- Van Waes, F.E.A.; Debrouwer, W.; Heugebaert, T.S.A.; Stevens, C.V. On the discovery and development of tandem 1,4- and 1,2-addition of phosphites to 1-azadienes. Arkivoc 2014, 386–427. [Google Scholar] [CrossRef]
- De Blieck, A.; Catak, S.; Debrouwer, W.; Drabowicz, J.; Hemelsoet, K.; Verstraelen, T.; Waroquier, M.; Van Speybroeck, V.; Stevens, C.V. Diphosphonylation of aromatic diazaheterocycles and theoretical rationalization of product yields. Eur. J. Org. Chem. 2013, 1058–1067. [Google Scholar] [CrossRef]
- Levy, J.N.; Alegre-Requena, J.V.; Liu, R.; Paton, R.S.; McNally, A. Selective halogenation of pyridines using designed phosphine reagents. J. Am. Chem. Soc. 2020, 142, 11295–11305. [Google Scholar] [CrossRef]
- Adams, J.T.; Bradsher, C.K.; Breslow, D.S.; Amore, S.T.; Hauser, C.R. Synthesis of antimalarials. Synthesis of certain 1,5- and 1,8-naphthyridine derivatives. J. Am. Chem. Soc. 1946, 68, 1317–1319. [Google Scholar] [CrossRef]
- Sieb, D.; Schuhen, K.; Morgen, M.; Herrmann, H.; Wadepohl, H.; Lucas, N.T.; Baker, R.W.; Enders, M. Synthesis and complexation behavior of indenyl and cyclopentadienyl ligands functionalized with a naphthyridine unit. Organomettalics 2012, 31, 356–364. [Google Scholar] [CrossRef]
- Fitchett, C.M.; Steel, P.J. Synthesis and X-ray structures of two discrete metal complexes of 2,2′-bi-1,5-naphthyridine, a new ambivergent ligand. Polyhedron 2007, 26, 400–405. [Google Scholar] [CrossRef]
- McElhinny, C.J., Jr.; Lewin, A.H.; Mascarella, S.W.; Runyon, S.; Brieaddy, L.; Carroll, F.I. Hydrolytic instability of the important orexin 1 receptor antagonist SB-334867: Possible confounding effects on in vivo and in vitro studies. Bioorg. Med. Chem. Lett. 2012, 22, 6661–6664. [Google Scholar] [CrossRef] [PubMed]
- Surivet, J.-P.; Zumbrum, C.; Rueedi, G.; Hubschwerlen, C.; Bur, D.; Bruyére, T.; Locher, H.; Ritz, D.; Keck, W.; Seiler, P.; et al. Design, synhesis and characterization of novel tretrahydropyran-based bacterial topoisomerase inhibitors with potent anti-gram-positive activity. J. Med. Chem. 2013, 56, 7396–7415. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, H.; Chen, Z.; Gong, S.; Lu, Z.-H.; Yang, C. Naphthyridine-based emitters simultaneously exhibiting thermally activated delayed fluorescence and aggregation-induced emission for highly efficient non-doped fluorescent OLEDs. J. Mat. Chem. C Mater. Opt. Electron. Devices 2019, 7, 6607–6615. [Google Scholar] [CrossRef]
- Boali, A.A.; Mansha, M.; Waheed, A.; Ullah, N. Synthesis and selective colorimetric detection of iodide ion by novel 1,5-naphthyridine-based conjugated polymers. J. Taiwan Inst. Chem. E. 2018, 91, 420–426. [Google Scholar] [CrossRef]
- Mansha, M.; Younas, M.; Gondal, M.A.; Ullah, N. 1,5-Naphthyridine-based conjugated polymers as co-sensitizers for dye-sensitized solar cells. Sol. Energy 2019, 194, 682–687. [Google Scholar] [CrossRef]
- Lahue, B.R.; Lo, S.-M.; Wan, Z.-K.; Woo, G.H.C.; Snyder, J.K. Intramolecular inverse-electron-demand Diels-Alder reactions of imidazoles with 1,2,4-triazines: A new route to 1,2,3,4-tetrahydro-1,5-naphthyridines and related heterocycles. J. Org. Chem. 2004, 69, 7171–71822. [Google Scholar] [CrossRef]
- He, K.-H.; Tan, F.-F.; Zhou, C.-Z.; Zhou, G.-J.; Yang, X.-L.; Li, Y. Acceptorless dehydrogenation of N-heterocycles by merging visible-light photoredox catalysis and cobalt catalysis. Angew. Chem. Intern. Ed. 2017, 56, 3080–3084. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Tanaka, Y.; Kobayashi, M.; Yamaguchi, R. Homogeneous perdehydrogenation and perhydrogenation of fused bicyclic N-heterocycles catalyzed by Iridium complexes bearing a functional bipyridonate ligand. J. Am. Chem. Soc. 2014, 136, 4829–4832. [Google Scholar] [CrossRef]
- Petkevicius, V.; Vaitekunas, J.; Tauraite, D.; Stankeviciute, J.; Sarlauskas, J.; Cenas, N.; Meskys, R. A biocatalytic synthesis of heteroaromatic N-oxides by whole cells of Escherichia coli expressing the multicomponent, soluble di-iron monooxygenase (SDIMO) PmlABCDEF. Adv. Synth. Catal. 2019, 361, 2456–2465. [Google Scholar] [CrossRef]
- Nishimura, N.; Siegmund, A.; Liu, L.; Yang, K.; Bryan, M.C.; Andrews, K.L.; Bo, Y.; Booker, S.K.; Caenepeel, S.; Freeman, D.; et al. Phospshoinositide 3-kinase (PI3K)/mamalian target of rapamycin (mTOR) dual inhibitors: Discovery and structure-activity relationship of a series of quinoline and quinoxaline derivatives. J. Med. Chem. 2011, 54, 4735–4751. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.N.; Thummel, R. 1,5-Naphthyridine as a new linker for the construction of bidging ligands and their corresponding Ru(II) complexes. Inorg. Chem 2009, 48, 6459–6470. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, B.K.; Konwar, M.; Bhattacharyya, D.; Adhikari, P.; Das, A. Regioselective cyanation of six-membered N-heteroaromatic compounds under metal-, activator-, base- and solvent-free conditions. Adv. Synth. Catal. 2019, 361, 5616–5625. [Google Scholar] [CrossRef]
- Liu, C.; Rong, Z.; Sun, Z.; Wang, Y.; Du, W.; Wang, Y.; Lu, L. Quenched skeletal Ni as the effective catalyst for selective partial hydrogenation of polycyclic aromatic hydrocarbons. RSC Adv. 2013, 23984–23988. [Google Scholar] [CrossRef]
- Chen, F.; Surkus, A.-E.; He, L.; Pohl, M.-M.; Radnik, J.; Topf, C.; Junge, K.; Beller, M. Selective catalytic hydrogenation of heteroarenes with N-graphene-modified cobalt nanoparticles (Co3O4-Co/NGr@a-Al2O3). J. Am. Chem. Soc. 2015, 137, 11718–11724. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Chakraborty, S.; Yuan, H.; Jones, W.D. Acceptorless, reversible dehydrogenation and hydrogenation of N-heterocycles with a cobalt pincer catalyst. ACS Catal. 2015, 5, 6350–6354. [Google Scholar] [CrossRef]
- Cabrero-Antonino, J.R.; Adam, R.; Junge, K.; Jackstell, R.; Beller, M. Cobalt-catalysed transfer hydrogenation of quinolines and related heterocycles using formic acid under mild conditions. Catal. Sci. Technol. 2017, 7, 1981–1985. [Google Scholar] [CrossRef]
- Mai, V.H.; Nikonov, G.I. Transfer hydrogenation of nitriles, olefins, and N-heterocycles catalyzed by an N-heterocyclic carbene-supported half-sandwich complex of ruthenium. Organometallics 2016, 35, 943–949. [Google Scholar] [CrossRef]
- Alshakova, I.D.; Gabidullin, B.; Nikonov, G.I. Ru-catalyzed transfer hydrogenation of nitriles, aromatics, olefins, alkynes and esters. ChemCatChem 2018, 10, 4874–4883. [Google Scholar] [CrossRef]
- Xuan, Q.; Song, Q. Diboron-assisted palladium-catalyzed transfer hydrogenation of N-heteroaromatics with water as hydrogen donor and solvent. Org. Lett. 2016, 18, 4250–4253. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, J. HMPA-catalyzed transfer hydrogenation of 3-carbonyl pyridines and other N-heteroarenes with trichlorosilane. Molecules 2019, 24, 401. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Rahaman, S.M.W.; Fayzullin, R.R.; Khusnutdinova, J.R. Transfer hydrogenation of carbonyl groups, imines and N-heterocycles catalyzed by simple, bipyridine-based MnI complexes. ChemCatChem 2019, 11, 3844–3852. [Google Scholar] [CrossRef]
- Papa, V.; Yixuan, C.; Spannenberg, A.; Junge, K.; Beller, M. Development of a practical non-noble metal catalyst for hydrogenation of N-heteroarenes. Nat. Catal. 2020, 3, 135–142. [Google Scholar] [CrossRef]
- Mai, V.H.; Gadzhiev, O.B.; Ignatov, S.K.; Nikonov, G.I. H/D exchange in N-heterocycles catalysed by an NHC-supported ruthenium complex. Catal. Sci. Technol. 2019, 9, 3398–3407. [Google Scholar] [CrossRef]
- Chen, F.; Sahoo, B.; Kreyenschulte, C.; Lund, H.; Zeng, M.; He, L.; Junge, K.; Beller, M. Selective cobalt nanoparticles for catalytic transfer hydrogenation of N-heteroarenes. Chem. Sci. 2017, 8, 6239–6246. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Bamberger, J.; Mancheno, O. Asymmetric nucleophilic dearomatization of diazarenes by anion-binding catalysis. Org. Biomol. Chem. 2016, 14, 5794–5802. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, F.; He, Y.-M.; Fan, Q.-H. Asymmetric ruthenium-catalyzed hydrogenation of 2,6-disubstituted 1,5-naphthyridines: Access to chiral 1,5-diaza-cis-decalins. Angew. Chem. Int. Ed. 2015, 54, 4622–4625. [Google Scholar] [CrossRef]
- Balkenhohl, M.; Greiner, R.; Makarov, I.S.; Heinz, B.; Karaghiosoff, K.; Zipse, H.; Knochel, P. Zn-, Mg-, and Li-TMP bases for the successive regioselective metalations of the 1,5-naphthyridine scaffold (TMP=2,2,6,6-Tetramethylpiperidyl). Chem.-Eur. J. 2017, 23, 13046–13050. [Google Scholar] [CrossRef]
- Singh, S.B.; Kaelin, D.E.; Wu, J.; Miesel, L.; Tan, C.M.; Meinke, P.T.; Olsen, D.B.; Lagrutta, A.; Wei, C.; Liao, Y.; et al. C1-C2-linker substituted 1,5-naphthyridine analogues of oxabicyclooctane-linked NBTIs as broad-spectrum antibacterial agents (part 7). MedChemComm 2015, 6, 1773–1780. [Google Scholar] [CrossRef]
- Norman, P. Novel 1,5-naphthyridine PI3Kδ inhibitors, an evaluation of WO2011075628. Expert Opin. Ther. Patents 2011, 21, 1805–1810. [Google Scholar] [CrossRef]
- Mohammed, S.; Maher, K.A. Synthesis and spectral characterization of 1,5-naphthyridine derivatives through cross-coupling Suzuki reaction. Indian J. Heterocy. Chem. 2019, 29, 199–203. [Google Scholar]
- Galatsis, P.; Yamagata, K.; Wendt, J.A.; Connolly, C.J.; Mickelson, J.W.; Milbank, J.B.J.; Bove, S.E.; Knauer, C.S.; Brooker, R.M.; Augelli-Szafran, C.E.; et al. Synthesis and SAR comparasion of regioisomeric aryl naphthyridines as potent mGlu5 receptor antagonists. Bioorg. Med. Chem. 2007, 17, 6525–6528. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Long, C.; Forster, R.J.; Keyes, T.E. Near IR emitting BODIPY fluorophores with mega-stokes shifts. Chem. Commun. 2012, 48, 5617–5619. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Okumu, A.A.; Nolan, S.; English, A.; Vibhute, S.; Lu, Y.; Hervert-Thomas, K.; Seffernick, J.T.; Azap, L.; Cole, S.L.; et al. 1,3-Dioxane-linked bacterial topoisomerase inhibitors with enhanced antibacterial activity and reduced hERG inhibition. ACS Infec. Dis. 2019, 5, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.N.; Mansha, M.; Mehmood, U.; Ullah, N.; Al-Betar, A.F.; Al-Saadi, A.A. Synthesis and characterization of functionalized polythiophene for polymer-sensitized solar cell. Dye. Pigment. 2017, 141, 406–412. [Google Scholar] [CrossRef]
- Parhi, A.K.; Zhang, Y.; Saionz, K.W.; Pradhan, P.; Kaul, M.; Trivedi, K.; Pilch, D.S.; LaVoie, E.J. Antibacterial activity of quinoxalines, quinazolines, and 1,5-naphthyridines. Bioorg. Med. Chem. Lett. 2013, 23, 4968–4974. [Google Scholar] [CrossRef]
- Capani, J.S.; Cochran, J.E.; Liang, J. CsF-mediated in situ desilylation of TMS-alkynes for Sonogashira reaction. J. Org. Chem. 2019, 84, 9378–9384. [Google Scholar] [CrossRef]
- Bosset, C.; Beucher, H.; Bretel, G.; Pasquier, E.; Queguiner, L.; Henry, C.; Vos, A.; Edwards, J.P.; Meerpoel, L.; Berthelot, D. Minisci-photoredox-mediated a-heteroarylation of N-protected secondary amines: Remarkable selectivity of azetidines. Org. Lett. 2018, 20, 6003–6006. [Google Scholar] [CrossRef]
- Osman, K.T.; Ye, J.; Shi, Z.; Toker, C.; Lovato, D.; Jumani, R.S.; Zuercher, W.; Huston, C.D.; Edwards, A.M.; Lautens, M.; et al. Discovery and structure activity relationship of the first potent cryptosporidium FIKK kinase inhibitor. Bioorg. Med. Chem. 2017, 25, 1672–1680. [Google Scholar] [CrossRef]
- Bregman, H.; Chakka, N.; Guzman-Perez, A.; Gunaydin, H.; Gu, X.; Huang, X.; Berry, V.; Liu, J.; Teffera, Y.; Huang, L.; et al. Discovery of novel, induced-pocket binding oxazolidinones as potent, selective, and orally bioavailable tankyrase inhibitors. J. Med. Chem. 2013, 56, 4320–4342. [Google Scholar] [CrossRef]
- Golec, B.; Kijak, M.; Vetokhina, V.; Gorski, A.; Thummel, R.P.; Herbich, J.; Waluk, J. Solvent-induced changes in photophysics and photostability of indole-naphthyridines. J. Phys. Chem. B 2015, 119, 7283–7293. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Kaelin, D.E.; Meinke, P.T.; Wu, J.; Miesel, L.; Tan, C.M.; Olsen, D.B.; Lagrutta, A.; Fukuda, H.; Kishii, R.; et al. Structure activity relationship of C-2 ether substituted 1,5-naphthyridine analogs of oxabicyclooctanelinked novel bacterial topoisomerase inhibitors as broad-spectrum antibacterial agents (Part-5). Bioorg. Med. Chem. Lett. 2015, 25, 3630–3635. [Google Scholar] [CrossRef] [PubMed]
- Perrey, D.A.; Gilmour, B.P.; Runyon, S.P.; Thomas, B.F.; Zhang, Y. Diaryl urea anologues of SB-334867 as orexin-1 receptor antagonists. Bioorg. Med. Chem. Lett. 2011, 21, 2980–2985. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Golec, B.; Nawara, K.; Thummel, R.P.; Waluk, J. Photoinduced oxidation of an indole derivative: 2-(1′H-indol-2′-yl)-1,5-naphthyridine. Photoch. Photobio Sci. 2019, 18, 2225–2231. [Google Scholar] [CrossRef] [PubMed]
- Dridi, R.; Dhieb, C.; Cherni, S.N.; Boudjada, N.C.; Zouaoui, N.S.; Faouzi Zid, M. A new supramolecular chromium(III) complex: Synthesis, structural determination, optical study, magnetic and antibacterial activity. J. Mol. Struct. 2018, 1152, 294–302. [Google Scholar] [CrossRef]
- Huang, P.-J.; Natori, Y.; Kitagawa, Y.; Sekine, Y.; Kosaka, W.; Miyasaka, H. One-dimensional chains of paddlewheel-type dichromium(II,II) tetraacetate complexes: Study of electronic structure influenced by s- and p-donation of axial linkers. Inorg. Chem. 2018, 57, 5371–5379. [Google Scholar] [CrossRef]
- Sieb, D.; Baker, R.W.; Wadepohl, H.; Enders, M. Naphthyridine cyclopentadienyl chromium complexes as single-site catalyst for the formation of ultrhigh molecular weight polyethylene. Organometallics 2012, 31, 7368–7374. [Google Scholar] [CrossRef]
- Cabez, J.A.; del Rio, I.; Peréz-Carreño, E.; Pruneda, V. Reductive dimerization of triruthenium clusters containing cationic aromatic N-heterocyclic ligands. Chem. Eur. J. 2010, 16, 5425–5436. [Google Scholar] [CrossRef]
- Hideki, O.; Tsubasa, S.; Kiyoshi, T. A novel photo-driven hydrogenation reaction of an NAD(+)-type complex toward artificial photosynthesis. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Zhu, S.; Moreno, K.X.; Jenkins, R.M.; Walmsley, J.A. Interactions of 1,5-naphthyridine with Pd(en)Cl2 or [PD(en) (H2O)2](NO3)2 in aqueous solution. Dalton Trans. 2008, 6401–6408. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poloek, A.; Chen, C.-T.; Chen, C.-T. New platinum complexes for hybrid white organic light-emiting diodes. Proc. SPIE 2019, 8829, 88291R-1. [Google Scholar]
- Araki, H.; Tsuge, K.; Sasaki, Y.; Ishizaka, S.; Kitamura, N. Luminiscence ranging, from red to blue: Aseries of cooper (I)-halide complexes having rhombic {Cu2(n-X)2} (X=Br and I) units with N-heteroaromatic ligands. Inorg. Chem. 2005, 44, 9667–9675. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, K.; Jiang, P.; Song, G.; Zhu, H. Synthesis, cristal structures and photophysical propierties of novel copper (I) complexes with 4-diphenylphosphino-1,5-naphthyridide ligands. Inorg. Chem. Commun. 2012, 17, 116–119. [Google Scholar] [CrossRef]
- Djuric, S.; Vojnovic, S.; Pavic, A.; Mojicevic, M.; Wadepohl, H.; Savic, N.D.; Popsavin, M.; Nikodinovic-Runic, J.; Djuran, M.I.; Glisic, B.D. New polynuclear 1,5-naphthyridine-silver(I) complexes as potential antimicrobial agents: The key role of the nature of donor coordinated to the metal center. J. Inorg. Biochem. 2020, 203, 110872. [Google Scholar] [CrossRef]
- Pyykkö, P.; Zaleski-Ejgierd, P. From nanostrip to nanorings: The elastic propierties of gold-glued polyauronaphthyridines and polyacenes. Phys. Chem. Chem. Phys. 2008, 10, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Matsui, J.; Sodeyama, T.; Saiki, Y.; Miyazawa, T.; Yamada, T.; Tamaki, K.; Murashima, T. Face to face porphyrin moieties assembled with spacing for pyrazine recognition in molecularly imprinted polymers. Biosens. Bioelectron. 2009, 25, 635–639. [Google Scholar] [CrossRef]
- Mobinikhaledi, A.; Foroughifar, N. Preparation and characterization of CO (II), Ni (II) and Zn (II) complexes containing diazine ligands. Asian J. Chem. 2003, 15, 455–458. [Google Scholar]
- Wei, H.; Zhao, Z.; Wei, C.; Yu, G.; Liu, Z.; Zhang, B.; Bian, J.; Bian, Z.; Huang, C. Antiphotobleaching: A type of structurally rigid chromophore ready for constructing highly luminescent and highly photostable Europium complexes. Adv. Funct. Mater. 2016, 26, 2085–2096. [Google Scholar] [CrossRef]
- Huang, C. Rare Earth Coordination Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2010. [Google Scholar]
- Bi, Y.; Chen, C.; Zhao, Y.-F.; Zhang, Y.-Q.; Jiang, S.-D.; Wang, B.-W.; Han, J.-B.; Sun, J.-L.; Bian, Z.-Q.; Wang, Z.-M.; et al. Thermostability and photoluminescence of Dy(III) single-molecule magnets under a magnetic field. Chem. Sci. 2016, 7, 5020–5031. [Google Scholar] [CrossRef]
- Grandl, M.; Sun, Y.; Pammer, F. Electronic and structural properties of N→B-ladder boranes with high electron affinity. Org. Chem. Front. 2018, 5, 336–352. [Google Scholar] [CrossRef]
- Tai, C.-K.; Chou, Y.-M.; Wang, B.-C. Investigation of photophysical propierties of mer-tris(8-hydroxyquinolinato)aluminium (III) and its derivatives: DFT and TD-DFT calculations. J. Lumin. 2011, 131, 169–176. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, K.; Cho, S.W.; Yi, Y. Theoretical study on the effects of nitrogen and methyl substitution on tris-(8-hydroxyquinoline) aluminium: An efficient exciton blocking layer for organic photovoltaic cells. J. Chem. Phys. 2012, 137, 034704. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H. Planar mono-, di-, aza- and phospha-naphthalene: Structure and aromaticity. J. Quantum Chem. 2007, 107, 1846–1855. [Google Scholar] [CrossRef]
- Bootsma, A.N.; Wheeler, S.E. Tuning Stacking Interactions between Asp-Arg Salt Bridges and Heterocyclic Drug Fragments. J. Chem. Inf. Model. 2019, 59, 149–158. [Google Scholar] [CrossRef]
- Lu, R.-F.; Boëthius, G.; Wen, S.-H.; Su, Y.; Deng, W.-Q. Improved organic hydrogen carriers with superior thermodynamic propierties. Chem. Commun. 2009, 1751–1753. [Google Scholar] [CrossRef] [PubMed]
- Maclagan, R.G.A.R.; Gronert, S.; Meot-Ner, M. Protonated polycyclic aromatic nitrogen heterocyclics: Proton affinities, polarizabilities, and atomic and ring charges of 1-5-ring ions. J. Phys. Chem. A 2015, 119, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Whyte, S.A.; Mosey, N.J. Behavior of two-dimensional hydrogen-bonded networks under shear conditions: A first-principles molecular dynamics study. J. Phys. Chem. C 2015, 119, 350–364. [Google Scholar] [CrossRef]
- Kenny, P.W.; Montanari, C.A.; Prokopczyk, I.M.; Ribeiro, J.F.R.; Sartori, G. Rodrigues hydrogen bond basicity prediction for medicinal chemistry design. J. Med. Chem. 2016, 59, 4278–4288. [Google Scholar] [CrossRef]
- Toulmin, A.; Wood, J.M.; Kenny, P.W. Toward prediction of alkane/wáter partition coefficients. J. Med. Chem. 2008, 51, 3720–3730. [Google Scholar] [CrossRef]
- Heidarnezhad, Z.; Ganiev, I.; Obidov, Z.; Heidarnezhad, F.; Sharifi, M.S. A theoretical study of NBO analysis and solvation effects on tautomerism stability of 4,8-dioxygenated 1,5-napththyridine. Orient. J. Chem. 2012, 28, 1597–1604. [Google Scholar] [CrossRef][Green Version]
- Tsukasaki, M.; Yamada, A.; Yoshimura, K.; Miyazono, A.; Yamamoto, M.; Takama, M.; Miyamoto, Y.; Morimura, N.; Kamijo, R. Nephronectin expression is regulated by SMAD signaling in osteoblast-like MC3T3-E1 cells. Biochem. Biophys. Res. Commun. 2012, 425, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Konovalov, B.; Zivkovic, M.D.; Milovanovic, J.Z.; Djordjevic, D.B.; Arsenijevic, A.N.; Vasic, I.R.; Janjic, G.V.; Franich, A.; Manojlovic, D.; Skrivanj, S.; et al. Synthesis, cytotoxic activity and DNA interaction studies of new dinuclear platinum(II) complexes with an aromatic 1,5-naphthyridine bridging ligand: DNA binding mode of polynuclear platinum(II) complexes in relation to the complex structure. Dalton Trans. 2018, 47, 15091–15102. [Google Scholar] [PubMed]
- Nunes, M.C.; Goldring, J.P.; Doerig, C.; Scherf, A. A novel protein kinase family in Plasmodium falciparum is differentially transcribed and secreted to various cellular compartments of the host cell. Mol. Microbiol. 2007, 63, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wang, Y.; Wang, F.; Kerns, J.K.; Vinader, V.M.; Hancock, A.P.; Lindon, M.J.; Stevenson, G.I.; Morrow, D.M.; Rao, P.; et al. Oxazolidinones as novel human CCR8 antagonists. Bioorg. Med. Chem. Lett. 2007, 17, 1722–1725. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Hu, Q.; Gu, C.; Liu, B.; Jin, F.; Yuan, J.; Feng, J.; Zhang, L.; Lan, J.; Dong, Q.; et al. Discovery of potent and orally bioavailable inhibitors of Human Uric Acid Transporter 1 (hURAT1) and binding mode prediction using homology model. Bioorg. Med. Chem. Lett. 2016, 26, 277–282. [Google Scholar] [CrossRef]
- Tang, C.W.; Vanslyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Lee, C.-C.; Yuan, C.-H.; Liu, S.-W.; Shih, Y.-S. Efficient deep blue organic light-emitting diodes based on wide band gap 4-hydroxy-8-methyl-1,5-naphthyridine aluminium chelate as emitting and electron transporting layer. J. Disp. Technol. 2011, 7, 454–458. [Google Scholar] [CrossRef]
- Venkatanarayanan, A.; Martin, A.; Keyes, T.E.; Forster, R.J. Electrochemiluminiscence properties of a carboxy functionalisez BODIPY. Electrochem. Commun. 2012, 21, 46–49. [Google Scholar] [CrossRef]
- Poloek, A.; Wang, C.; Chang, Y.-T.; Lin, C.-W.; Chen, C.-T.; Chen, C.-T. New platinum complexes exhibiting host dependent photoluminescence as single dopants in double emitting layer, voltage independent hybrid white electroluminescence devices. J. Mater. Chem. C 2015, 3, 11163–11177. [Google Scholar] [CrossRef]
- Liu, S.-W.; Lee, C.-C.; Lin, C.-F.; Huang, J.-C.; Chen, C.-T.; Lee, J.-H. 4-Hydroxy-8-methyl-1,5-naphthyridine aluminium chelate: A morphologically stable and efficient exciton-blocking material for organic photovoltaics with prolonged lifetime. J. Mater. Chem. 2010, 20, 7800–7806. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, M.-W.; Yoon, W.S.; Oh, S.; Hong, S.H.; Park, S.Y. Structural and electronic origin of bis-lactam-based high-performance organic thin-film transistors. ACS Appl. Mater. Inter. 2019, 11, 8301–8309. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.M.; Simone, D.L.; Konezny, S.J.; Shim, A.; Crabtree, R.H.; Soloveichik, G.L.; Batista, V.S. Fuel selection for a regenrative organic fuel cell/flow battery: Thermodynamic considerations. Energy Environ. Sci. 2012, 5, 9534–9542. [Google Scholar] [CrossRef]
- Park, M.S.; Kang, Y.-S.; Im, D.; Doo, S.-G.; Chang, H. Design of novel additives and nonaqueous solvents for lithium-ion batteries through screening of cyclic organic molecules: An ab initio study of redox potentials. Phys. Chem. Chem. Phys. 2014, 16, 22391–22398. [Google Scholar] [CrossRef] [PubMed]








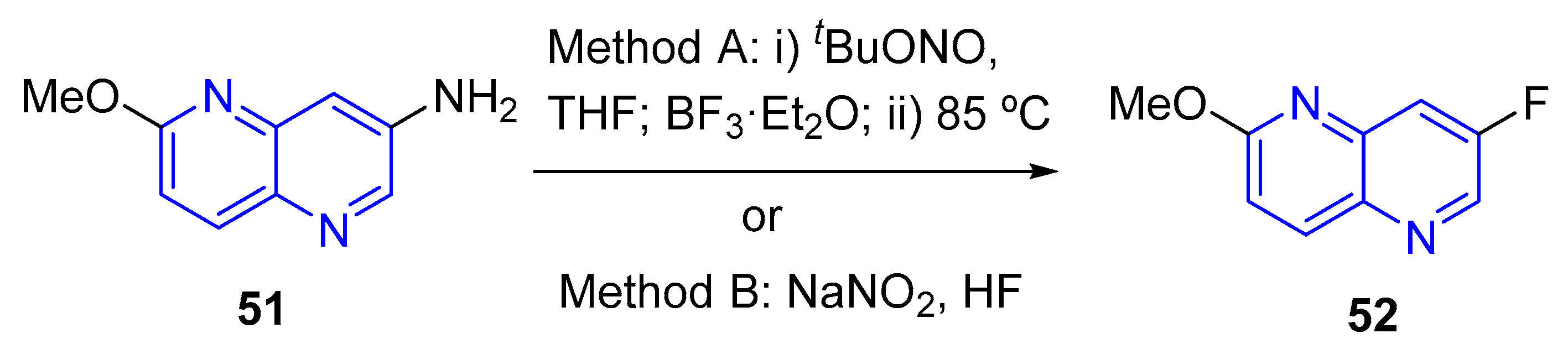
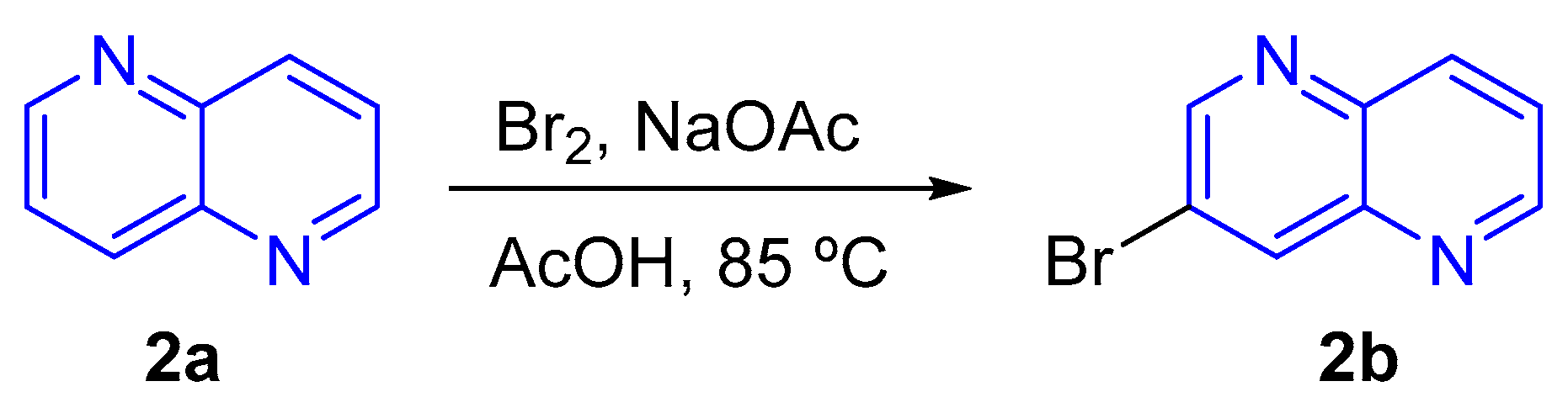
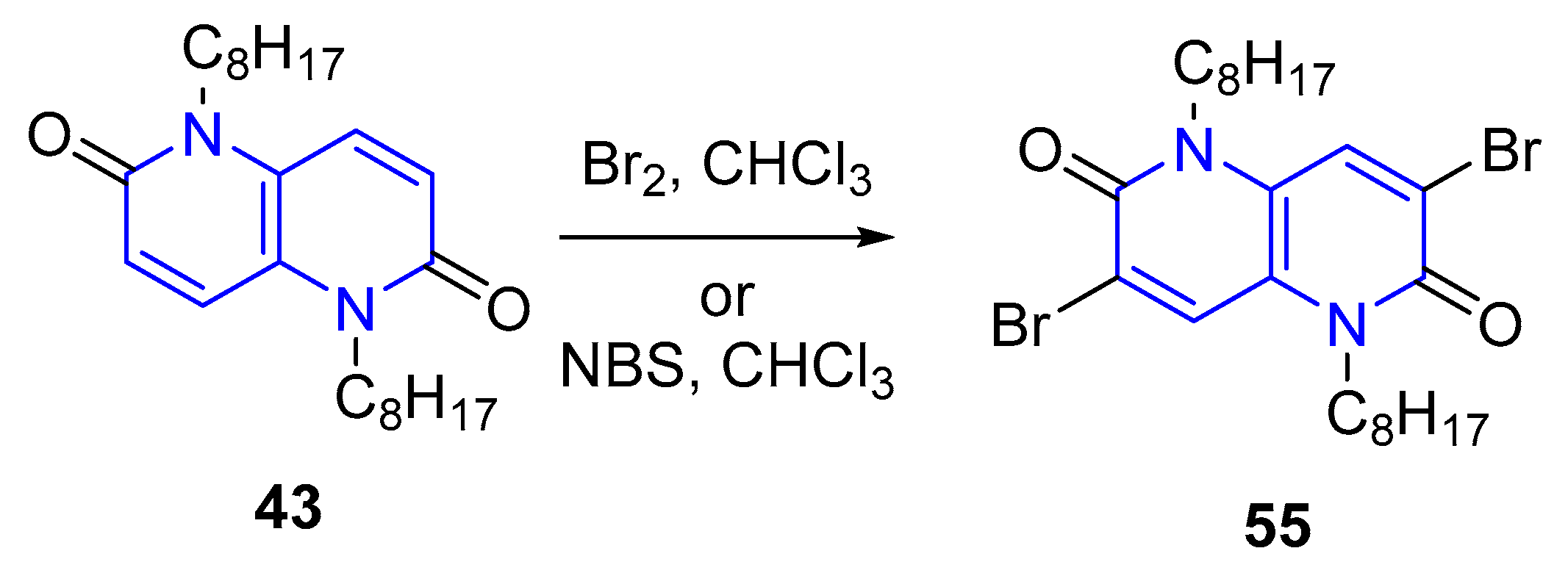
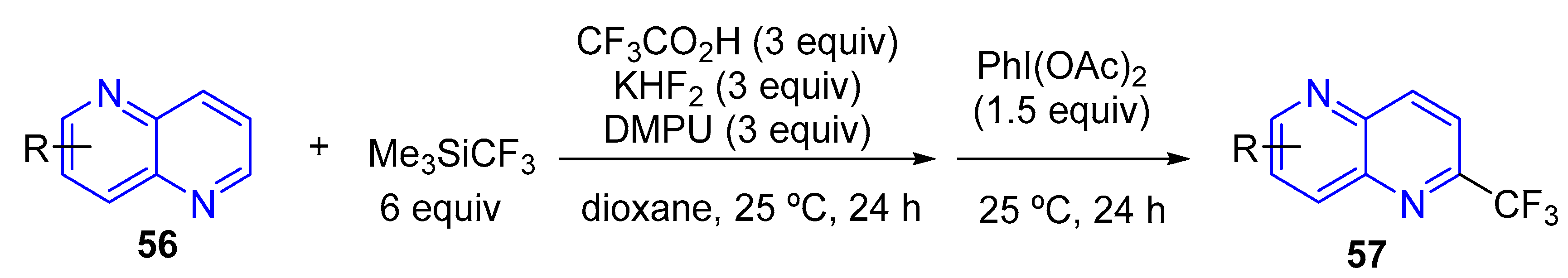

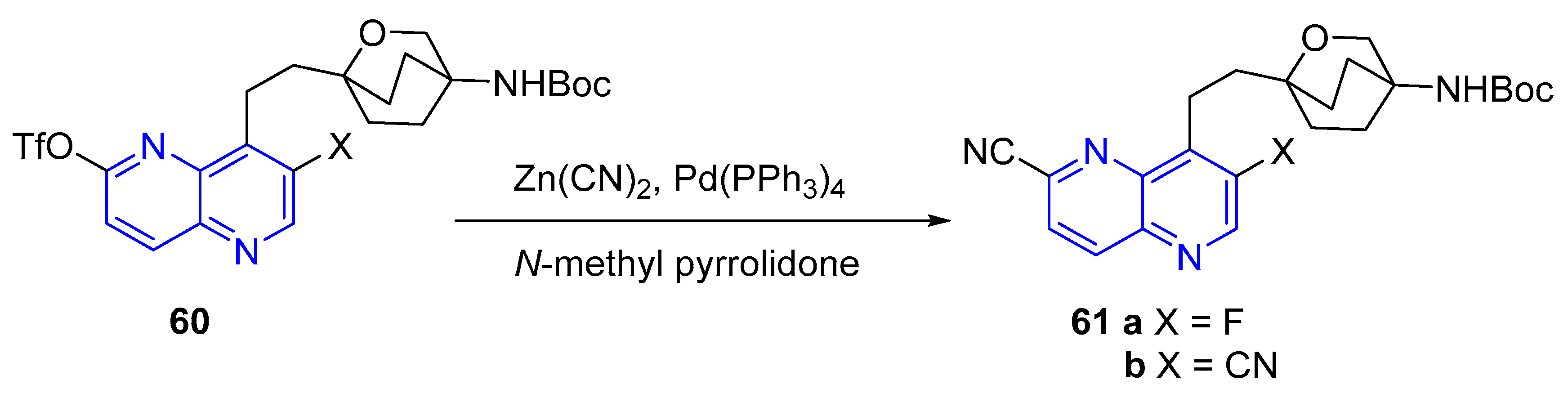













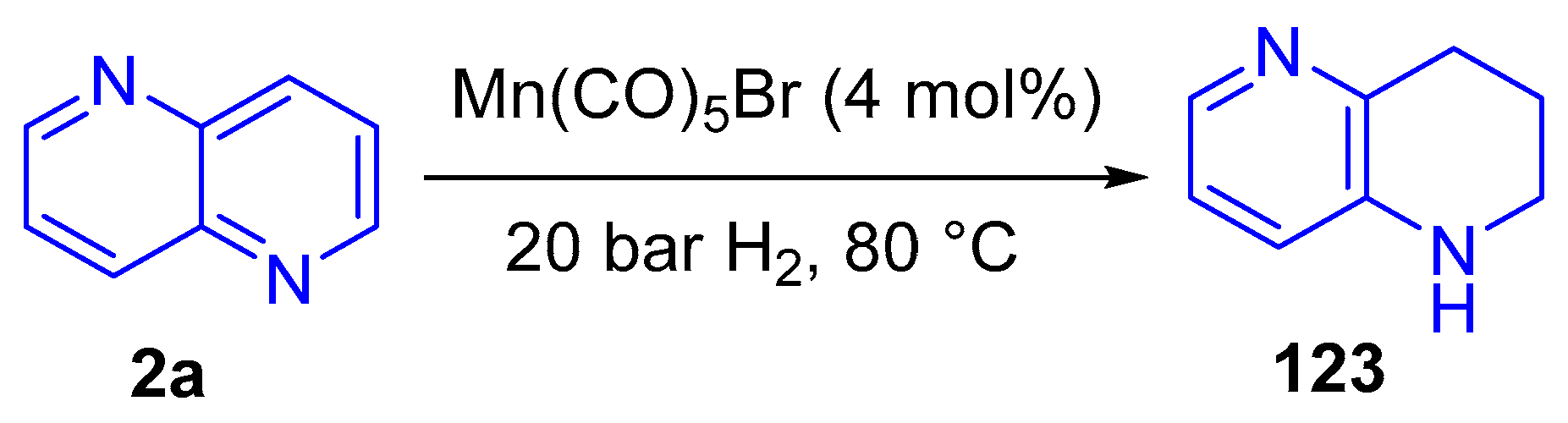
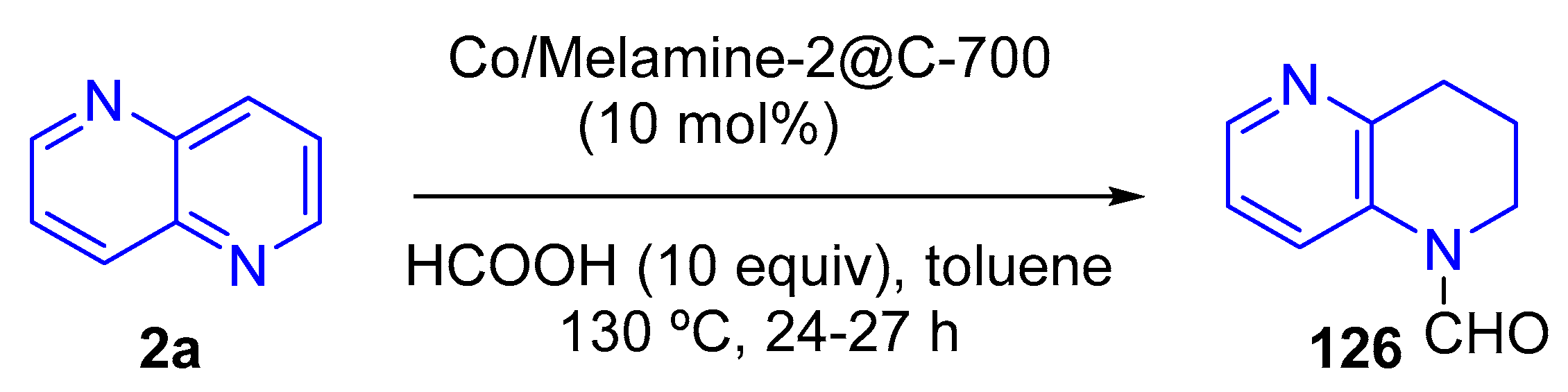

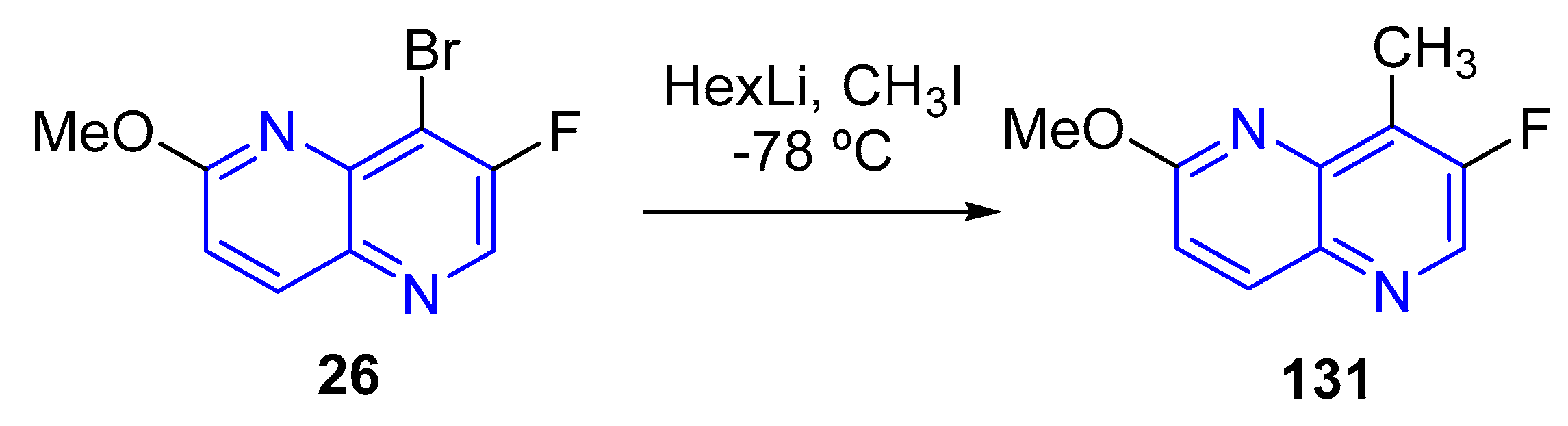
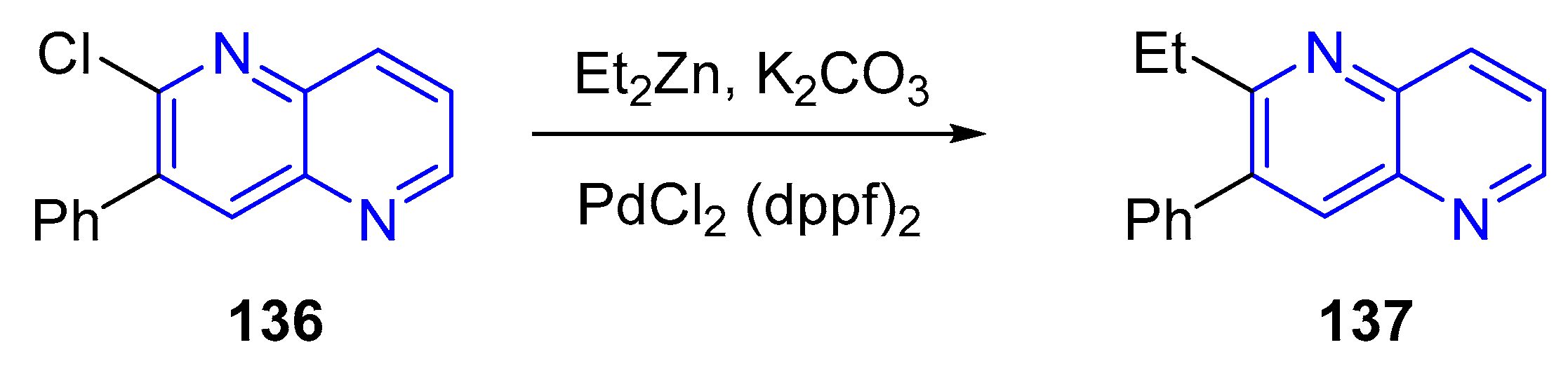



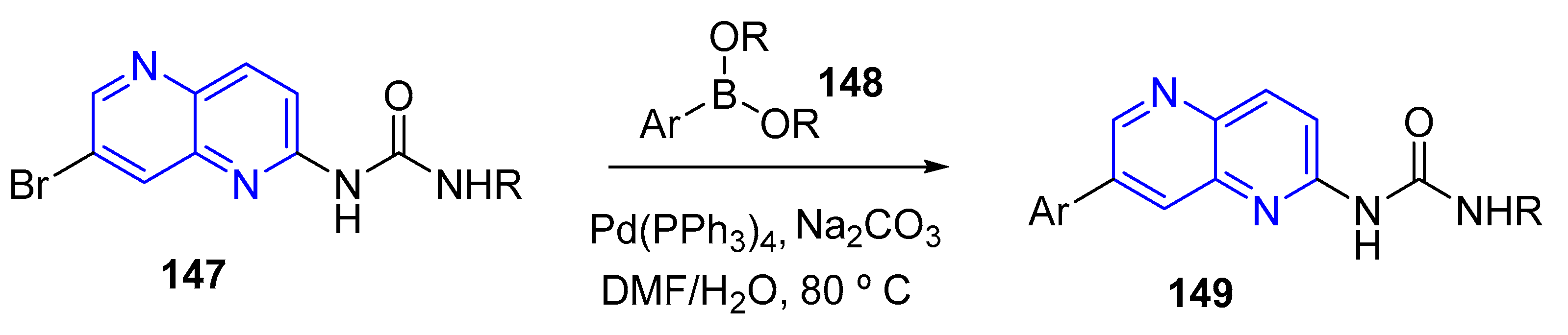
























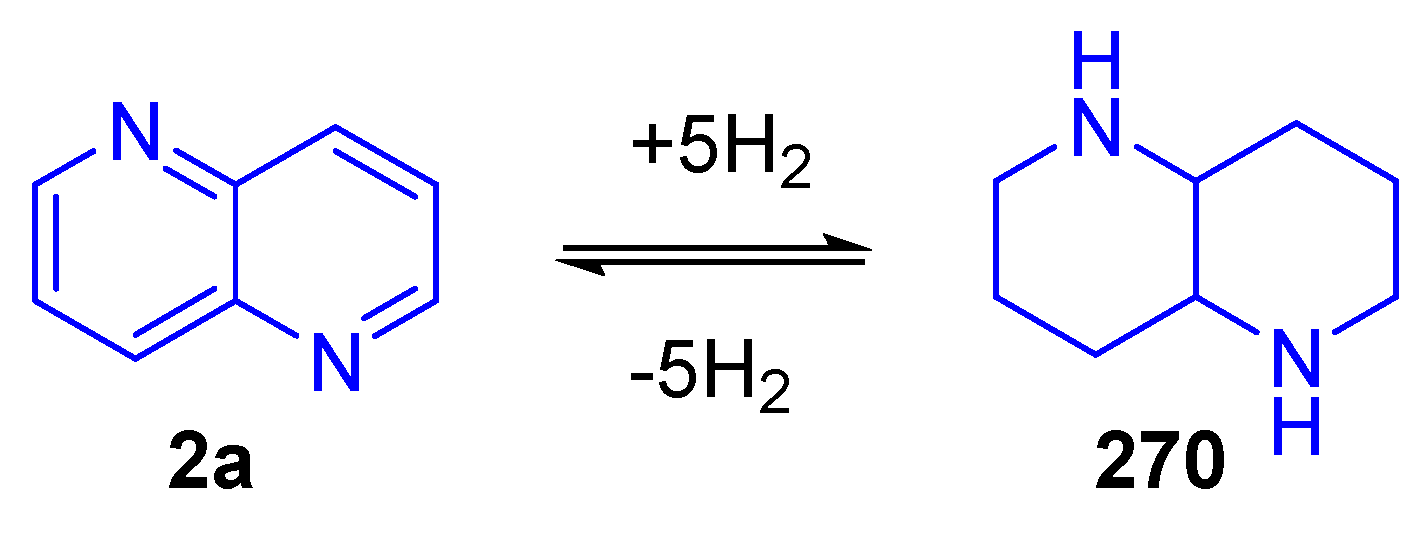




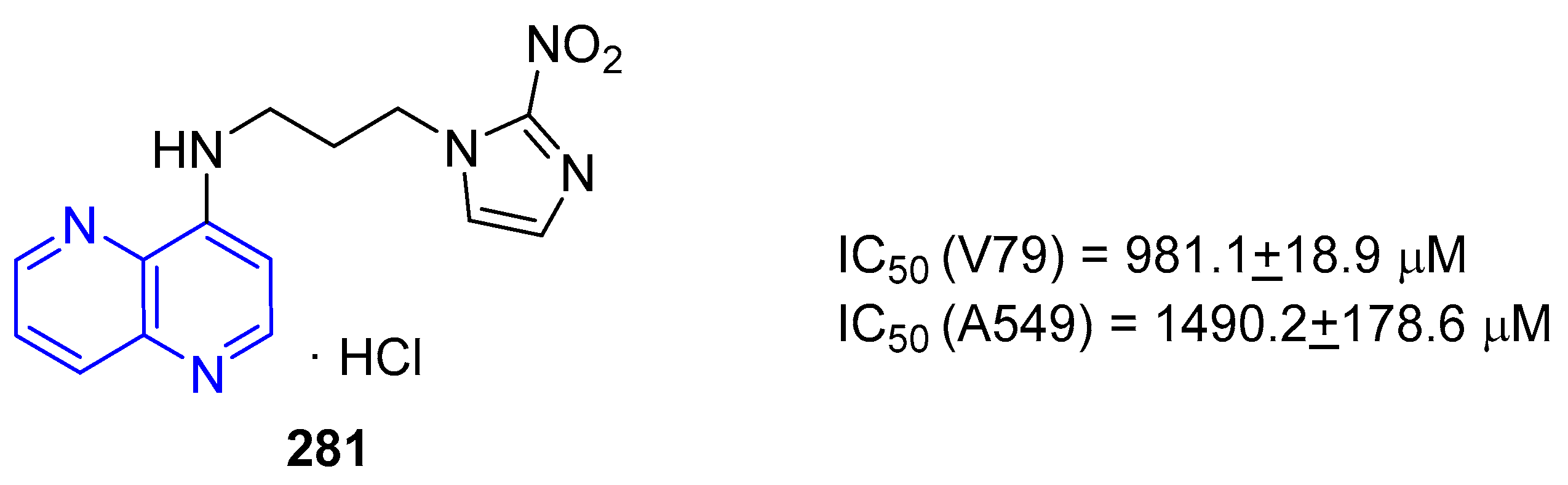
















© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuertes, M.; Masdeu, C.; Martin-Encinas, E.; Selas, A.; Rubiales, G.; Palacios, F.; Alonso, C. Synthetic Strategies, Reactivity and Applications of 1,5-Naphthyridines. Molecules 2020, 25, 3252. https://doi.org/10.3390/molecules25143252
Fuertes M, Masdeu C, Martin-Encinas E, Selas A, Rubiales G, Palacios F, Alonso C. Synthetic Strategies, Reactivity and Applications of 1,5-Naphthyridines. Molecules. 2020; 25(14):3252. https://doi.org/10.3390/molecules25143252
Chicago/Turabian StyleFuertes, Maria, Carme Masdeu, Endika Martin-Encinas, Asier Selas, Gloria Rubiales, Francisco Palacios, and Concepcion Alonso. 2020. "Synthetic Strategies, Reactivity and Applications of 1,5-Naphthyridines" Molecules 25, no. 14: 3252. https://doi.org/10.3390/molecules25143252
APA StyleFuertes, M., Masdeu, C., Martin-Encinas, E., Selas, A., Rubiales, G., Palacios, F., & Alonso, C. (2020). Synthetic Strategies, Reactivity and Applications of 1,5-Naphthyridines. Molecules, 25(14), 3252. https://doi.org/10.3390/molecules25143252






