Biological Activity of Pseudovitamin B12 on Cobalamin-Dependent Methylmalonyl-CoA Mutase and Methionine Synthase in Mammalian Cultured COS-7 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of (Ade)OH-Cba on MS Activity in a Homogenate of Mammalian Cells Grown with or without OH-Cbl
2.2. Effects of OH-Cbl and (Ade)OH-Cba, Added to the Growth Medium, on MS Activity in a Homogenate of MS cDNA-Transfected Mammalian Cells
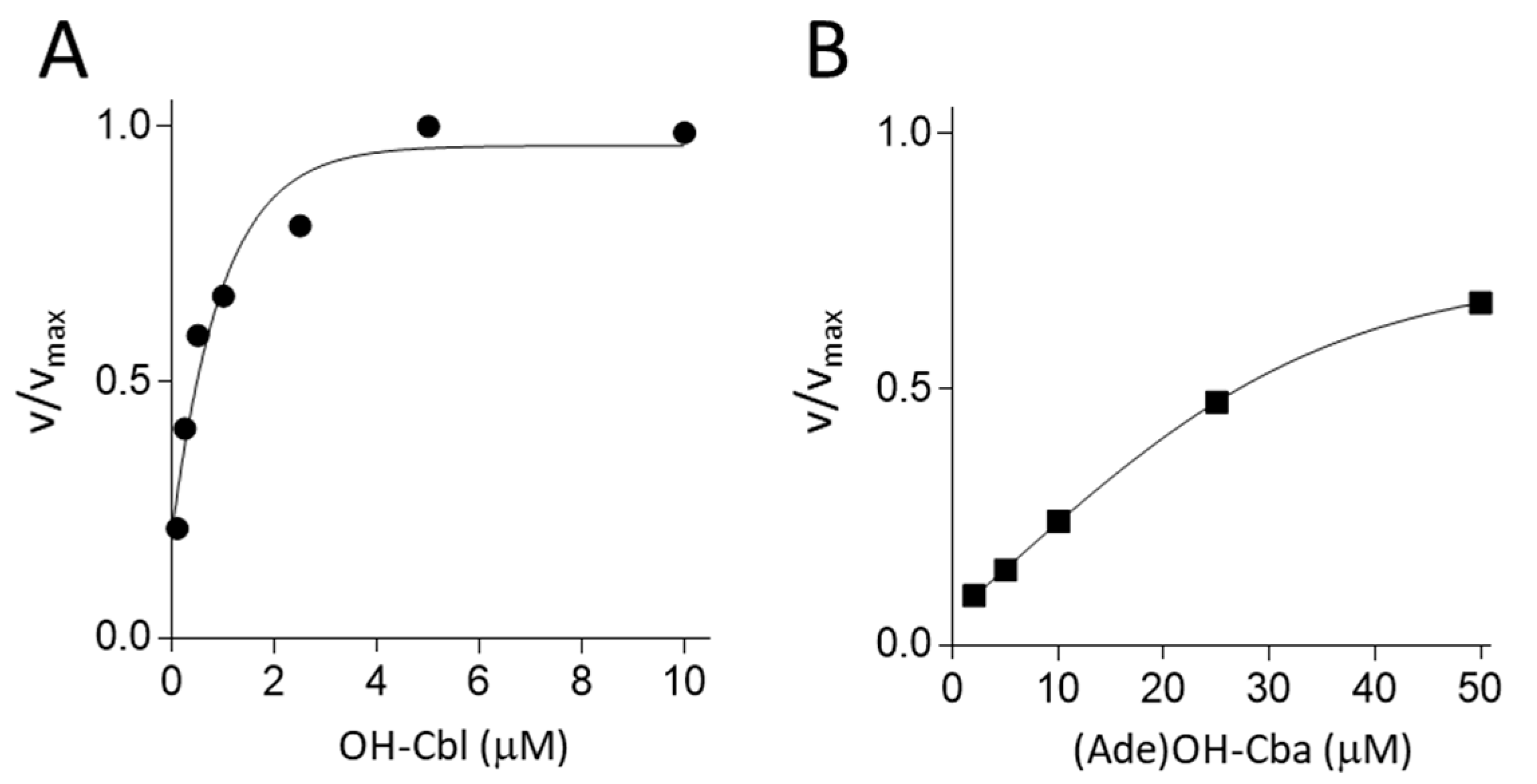
2.3. Effects of Various Concentrations of OH-Cba or (Ade)OH-Cba on the Enzyme Activity of apo-MS
2.4. Effects of (Ade)OH-Cba on MCM Activity in a Homogenate of Mammalian Cells Grown in the Presence or Absence of OH-Cbl
2.5. Effects of (Ade)Ado-Cba on the Activity of apo-MCM in Mammalian Cells
2.6. Effects of TCII on holo-MCM Activity in a Homogenate of Mammalian Cells Grown in the Presence of OH-Cbl
2.7. Biological Properties of (Ade)Cba in Mammalian Cells
3. Materials and Methods
3.1. Materials
3.2. Preparation of (Ade)CN-Cba, (Ade)Ado-Cba, and (Ade)OH-Cba
3.3. Enzyme Assays
3.4. Construction of Vector for Intracellular Expression System of Human MS and Human TCII
3.5. Cell Culture
3.6. Transfection of hMS Plasmid to COS-7 Cells and hTCII Plasmid to HEK293 Cells
3.7. Purification of Recombinant Human TCII from HEK293 Cells
3.8. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blot Analysis
3.9. Protein Quantitation
3.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Watanabe, F.; Bito, T. Determination of cobalamin and related compounds in foods. J. AOAC Int. 2018, 101, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Crippen, K.; Gulati, S.; Banerjee, R. Purification and kinetic mechanism of a mammalian methionine synthase from pig liver. J. Biol. Chem. 1994, 269, 27193–27197. [Google Scholar]
- Fenton, W.A.; Hack, A.M.; Willard, H.F.; Gertler, A.; Rosenberg, L.E. Purification and properties of methylmalonyl coenzyme A mutase from human liver. Arch. Biochem. Biophys. 1982, 214, 815–823. [Google Scholar] [CrossRef]
- Nakao, M.; Hironaka, S.; Harada, N.; Adachi, T.; Bito, T.; Yabuta, Y.; Watanabe, F.; Miura, T.; Yamaji, R.; Inui, H.; et al. Cobalamin deficiency results in an abnormal increase in L-methylmalonyl-co-enzyme-A mutase expression in rat liver and COS-7cells. Br. J. Nutr. 2009, 101, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Oltean, S.; Banerjee, R. Nutritional modulation of gene expression and homocysteine utilization by vitamin B12. J. Biol. Chem. 2003, 278, 20778–20784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozyraki, R.; Cases, O. Vitamin B12 absorption: Mammalian physiology and acquired and inherited disorders. Biochimie 2013, 95, 1002–1007. [Google Scholar] [CrossRef]
- Watanabe, F. Vitamin B12 sources and bioavailability. Exp. Biol. Med. 2007, 232, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Yabuta, Y.; Tanioka, Y.; Bito, T. Biologically active vitamin B12 compounds in foods for preventing deficiency among vegetarians and elderly subjects. J. Agric. Food Chem. 2013, 61, 6769–6775. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Oberhuber, M.; Stupperich, E.; Bothe, H.; Buckel, W.; Konrat, R.; Krӓutler, B. Native corrinoids from Clostridium cochlearium are adeninylcobamides: Spectroscopic analysis and identification of pseudovitamin B12 and factor A. J. Bacteriol. 2000, 182, 4773–4782. [Google Scholar] [CrossRef] [Green Version]
- Santos, F.; Vera, J.L.; Lamosa, P.; de Valdez, G.F.; de Vos, W.M.; Santos, H.; Sesma, F.; Hugenholtz, J. Pseudovitamin B12 is the corrinoid produced by Lactobacillus reuteri CRL 1098 under anaerobic conditions. FEBS Lett. 2007, 581, 4865–4870. [Google Scholar] [CrossRef] [Green Version]
- Stupperich, E.; Eisinger, H.J.; Schurr, S. Corrinoids in anaerobic bacteria. FEMS Microbiol. Rev. 1990, 87, 355–360. [Google Scholar] [CrossRef]
- Allen, R.H.; Stabler, S.P. Identification and quantitation of cobalamin and cobalamin analogues in human feces. Am. J. Clin. Nutr. 2008, 87, 1324–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigger, G.W.; Elliot, J.M.; Rickard, T.R. Estimated fuminal production of pseudovitamin B12, factor A and factor B in sheep. J. Anim. Sci. 1976, 43, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Fedosova, S.N.; Fedosova, N.U.; Kräutler, B.; Nexø, E.; Petersen, T.E. Mechanisms of discrimination between cobalmains and their natural analogues during their binding to the specific B12-transporting proteins. Biochemistry 2007, 46, 6446–6458. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P.; Brass, E.P.; Marcell, P.D.; Allen, R.H. Inhibition of cobalamin-dependent enzymes by cobalamin analogues in rats. J. Clin. Investig. 1991, 87, 1422–1430. [Google Scholar] [CrossRef]
- Sokolovskaya, O.M.; Mok, K.C.; Park, J.D.; Tran, J.L.A.; Quanstrom, K.A.; Taga, M.E. Cofactor selectivity in methylmalonyl coenzyme A mutase, a model cobalamin-dependent enzyme. MBio 2019, 10, e01303-19. [Google Scholar] [CrossRef] [Green Version]
- Sokolovkaya, O.M.; Plessl, T.; Bailey, H.; Mackinnon, S.; Baumgartner, M.R.; Yue, W.W.; Froese, D.S.; Taga, M.E. Naturally occurring cobalamin (B12) analogs can function as cofactors for human methylmalonyl-CoA mutase. bioRxiv 2020. [Google Scholar] [CrossRef]
- Kolhouse, J.F.; Utley, C.; Stabler, S.P.; Allen, R.H. Mechanism of conversion of human apo- to holomethionine synthase by various forms of cobalamin. J. Biol. Chem. 1991, 266, 23010–23015. [Google Scholar]
- Yamada, K.; Kawata, T.; Wada, M.; Isshiki, T.; Onoda, J.; Kawanishi, T.; Kunou, A.; Tadokoro, T.; Tobimatsu, T.; Maekawa, A.; et al. Extremely low activity of methionine synthase in vitamin B12-deficient rats may be related to effects on coenzyme stabilization rather than to changes in coenzyme induction. J. Nutr. 2000, 130, 1894–1900. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Chakraborty, S.; Banerjee, R. Demonstration that mammalian methionine synthases are predominantly cobalamin-loaded. J. Biol. Chem. 1995, 270, 19246–19249. [Google Scholar] [CrossRef] [Green Version]
- Oltean, S.; Banerjee, R. A B12-responsive internal ribosome entry site (IRES) element in human methionine synthase. J. Biol. Chem. 2005, 280, 32662–32668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupperich, E.; Nexø, E. Effect of the cobalt-N coordination on the cobamide recognition by the human vitamin B12 binding proteins intrinsic factor, transcobalamin and haptocorrin. Eur. J. Biochem. 1991, 199, 299–303. [Google Scholar] [CrossRef]
- Balzan, S.; de Almeida Quadros, C.; de Cleva, R.; Zilberstein, B.; Cecconello, I. Bacterial translocation: Overview of mechanisms and clinical impact. J. Gastroenterol. Hepatol. 2007, 22, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Sedman, P.C.; Macfie, J.; Sagar, P.; Mitchell, C.J.; May, J.; Mancey-Jones, B.; Johnstone, D. The prevalence of gut translocation in humans. Gastroenterology 1994, 107, 643–649. [Google Scholar] [CrossRef]
- Engen, P.A.; Green, S.J.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. The gastrointestinal microbiome: Alcohol effects on the composition of intestinal microbiota. Alcohol Res. 2015, 37, 223–236. [Google Scholar]
- de Punder, K.; Pruimboom, L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front. Immunol. 2015, 6, 223. [Google Scholar] [CrossRef] [Green Version]
- Tanioka, Y.; Miyamoto, E.; Yabuta, Y.; Ohnishi, K.; Fujita, T.; Yamaji, R.; Misono, H.; Shigeoka, S.; Nakano, Y.; Inui, H.; et al. Methyladeninylcobamide functions as the cofactor of methionine synthase in a Cyanobacterium, Spirulina platensis NIES-39. FEBS Lett. 2010, 584, 3223–3226. [Google Scholar] [CrossRef] [Green Version]
- Bito, T.; Yabuta, Y.; Ichiyanagi, T.; Kawano, T.; Watanabe, F. A dedecylamine derivative of cyanocobalamin potently inhibits the activities of cobalamin-dependent methylmalonyl-CoA mutase and methionine synthase of Caenorhabditis elegans. FEBS Open Bio 2014, 4, 722–729. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, E.; Tanioka, Y.; Nishizawa-Yokoi, A.; Yabuta, Y.; Ohnishi, K.; Misono, H.; Shigeoka, S.; Nakano, Y.; Watanabe, F. Characterization of methylmalonyl-CoA mutase involved in the propionate photoassimilation of Euglena graculis Z. Arch. Microbiol. 2010, 192, 437–446. [Google Scholar] [CrossRef]
- Tanioka, Y.; Yabuta, Y.; Yamaji, R.; Shigeoka, S.; Nakano, Y.; Watanabe, F.; Inui, H. Occurrence of pseudovitamin B12 and its possible function as the cofactor of cobalamin-dependent methionine synthase in a cyanobacterium Synechocystis sp. PCC6803. J. Nutr. Sci. Vitaminol. 2009, 55, 518–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernάndez, A.; Ruiz, M.T. An EXCEL template for calculation of enzyme kinetic parameters by non-linear regression. Bioinformatics 1998, 14, 227–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 7, 248–254. [Google Scholar] [CrossRef]
Sample Availability: Any samples of the compounds used in this study are not available from the authors. |








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bito, T.; Bito, M.; Hirooka, T.; Okamoto, N.; Harada, N.; Yamaji, R.; Nakano, Y.; Inui, H.; Watanabe, F. Biological Activity of Pseudovitamin B12 on Cobalamin-Dependent Methylmalonyl-CoA Mutase and Methionine Synthase in Mammalian Cultured COS-7 Cells. Molecules 2020, 25, 3268. https://doi.org/10.3390/molecules25143268
Bito T, Bito M, Hirooka T, Okamoto N, Harada N, Yamaji R, Nakano Y, Inui H, Watanabe F. Biological Activity of Pseudovitamin B12 on Cobalamin-Dependent Methylmalonyl-CoA Mutase and Methionine Synthase in Mammalian Cultured COS-7 Cells. Molecules. 2020; 25(14):3268. https://doi.org/10.3390/molecules25143268
Chicago/Turabian StyleBito, Tomohiro, Mariko Bito, Tomomi Hirooka, Naho Okamoto, Naoki Harada, Ryoichi Yamaji, Yoshihisa Nakano, Hiroshi Inui, and Fumio Watanabe. 2020. "Biological Activity of Pseudovitamin B12 on Cobalamin-Dependent Methylmalonyl-CoA Mutase and Methionine Synthase in Mammalian Cultured COS-7 Cells" Molecules 25, no. 14: 3268. https://doi.org/10.3390/molecules25143268





